Abstract
Nitric oxide (NO) is an important signaling molecule with broad-spectrum physiological and biochemical properties. It is involved in the induction of various intracellular processes like a defense mechanism of reactive oxygen species (ROS) in challenging environmental conditions. The discovery of NO has led to investigating its synthesis in animals and plants. The primary source for NO synthesis is nitric oxide synthetase (NOS) which regulates nitrite and arginine in plants and arginine to citrulline. Abiotic stress is a major barrier to the defense mechanism and growth of plants. It is defined as the negative impact of some non-living on living organisms in specified environmental conditions. To understand stress tolerance and response, it is important to focus on the cause of stress like herbicides, flooding, heavy metal, high light intensities, salinity, UB radiation, drought, temperature, ozone environmental conditions, and climate change. NO plays an impotent role in managing abiotic stresses that came into the limelight in the last decade. Various studies have been performed to understand the defense mechanism of NO and its biosynthesis in plants, but several queries remain unsolved which can explain the physiological and biochemical mechanism of NO. In this chapter, we compile all the progress of NO researches in abiotic stress tolerance of plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Nitric oxide is an omnipresent molecule and an extremely diffusible gas (Siddiqui et al. 2011). It has drawn much attention in recent years due to its widespread application in processes linked to normal plant physiology. Due to these properties, it has also been termed as the “molecule of the year.” It has been synthesized by plant itself, and it also acts as a gaseous plant growth regulator (Neill et al. 2008). It has been given the name “plant growth regulator” because there are rising evidence reporting its role as mediator of several biological procedures involved in the growth and development of the plant. Seed germination, root organogenesis, photosynthesis, stomatal closure, hypocotyl growth, floral regulation, defense against pathogens, and senescence are the processes included in plant physiology where NO has an extensive role. It has also been reported that nitric oxide is also linked to phytoalexin production and apoptosis (Delledonne et al. 1998). Nitric oxide suppresses growth at higher concentrations, while it provides benefits at lower concentrations. This review puts light on the role of nitric oxide in plants for tolerance of different abiotic stresses (Simontacchi et al. 2013; Wendehenne et al. 2001). Also, the relation between nitric oxide and other phytohormones has been included here. Many recent reports have been found about molecular data, regarding NO-mediated PTMs in animals. This has encouraged botanists to find out the effect of these PTMs in plants when exposed to different stresses. Hence, in the last few years, a great deal of effort were put into the identification of the role of S-nitrosylated, tyrosine nitrate, and S-glutathionylated proteins in plant function modulation (Wendehenne et al. 2001). Also, current knowledge reports about the role of nitric oxide in cell signaling in plants by involvement of oxidative and reductive pathways (Crawford 2006). This review also attempts to impart knowledge on the N-end rule pathway responsible for protein stability, which alters the steadiness of group VII ethylene response factors under hypoxic conditions.
11.2 Antiquity of Nitric Oxide
In 1722, when Joseph Priestly first described nitric oxide, it was known as a highly toxic gas and also a component of industrial wastes and exhaust gas. The model concerning the cytotoxicity of free radical substances was changed with the 1980s’ discovery about the role of nitric oxide signaling in regulating the cardiovascular system by R.F. Furchgott, L.J. Ignarro, and F. Murad (Nobel Prize winners in Physiology and Medicine 1998) (Delledonne et al. 1998). In 1992, nitric oxide was declared as the Molecule of the Year by the journal Science. In that year only, a nitric oxide society was founded, and a scientific journal devoted to nitric oxide was formed. Nitric oxide is a gaseous free radical, and its emission was reported in soybean many years back. Later sunflower and maize were reported to have in vivo and in vitro nitrate reductase (NR)-dependent nitric oxide producing activity (Klepper 1979). Animals possess nitric oxide synthase, and it acts as chief enzyme that catalyzes the in vivo synthesis of nitric oxide. But there is no report of plants having nitric oxide synthase, though it has been proven to be a functional metabolite in plants (Qi and Dong 1999).
11.3 Nitric Oxide Synthesis in Plants
Involvement of NO in various signaling pathways related to plant’s growth and development and biotic and abiotic stress resistance requires quick generation of NO in a site-specific manner. Production of NO in plants has been known to occur through various routes which are broadly classified into enzymatic and non-enzymatic mechanism. Enzymatic route of NO production involves three enzymes, including nitrate reductase, nitrate reductase and animals like nitric oxide synthase (NOS), for the production NO in plants. All routes of NO production in plants are shown in Fig. 11.1.
11.3.1 Nitrate Reductase
Nitrate reductase is a homodimeric protein, containing two subunits of 100 kD. Each subunit constitutes a flavin adenine dinucleotide (FAD), a heme protein containing Fe as a metal cofactor, and a Mo-molybdopterin (Mo-MPT). Nitrate reductase facilitates the first step of nitrogen assimilation in higher plants and is reported to exist dominantly in two forms which are NADH-specific NR forms (EC 1.6.6.1) and NAD(P)H-bispecific forms (EC 1.6.6.2). Reaction catalyzes by nitrate reductase is:
While catalyzing the reduction of nitrate, FAD, Fe, and Mo present in nitrate reductase are cyclically reduced and oxidized. Due to the ability of FAD and Mo in Mo-molybdopterin to attain 3 oxidation states and 2 oxidation states of Fe in heme in collectively results in approximately 12 to 18 oxidized and reduced states of nitrate reductase which are transient in nature and occur in vivo. Under some circumstance, nitrate reductase may also result in the formation of NO (Dean and Harper 1988; Rockel et al. 2002). In Arabidopsis thaliana , nitrate reductase was reported to be present in two isoforms, NIA1 and NIA2. Both isoforms were 83.5% identical to each other in terms of their amino acid constituent, but they had some independent sequence regions including the N-terminal region. Nitrate reductase encoded by NIA1 was demonstrated to control NO emission through stomata guard cells during light to dark transition. In a study on double mutant nia1 and nia2, involvement of nitrate reductase in ABA-induced generation of NO was reported in guard cells (Desikan et al. 2002). Involvement of NO in signaling requires its rapid generation during stimulus, and thus a tight control is required on nitrate reductase to show its activity. In a study on Arabidopsis, modulation of nitrate reductase activity was demonstrated by phosphorylation of a conserved serine residue (Ser 534) present in it (Rockel et al. 2002). Therefore, removal of the phospho-regulation through substitution of the Ser 534 can results in increased NO production by. In another study, the role of 14-3-3 proteins was highlighted in controlling the activity of nitrate reductase (Lillo et al. 2004) probably through its proteolysis (Weiner and Kaiser 1999).
11.3.2 Nitric Oxide Synthase (NOS)
Similar to NOS activity in animals, L-arginine-dependent production of NO has been reported in many plants, which was confirmed by inhibition of NO production with the use of arginine analogues. These inhibitors were also observed to interfere in NO mediated, ABA control over stomata closure. A few examples of the NOS-type activity are shown in Table. 11.1.
Besides the documented NOS-like activity in many plants, no protein homologs to NOS of animals have been yet confirmed in any plant species. In an immunological study, cross-reactivity of anti-mammalian NOS antibodies toward a few plant proteins was demonstrated by Ribeiro et al. (1999). This made researchers to believe that NOS activity in plants is mediated through proteins orthologous to NOS of animals, until a proteomic study was performed in which cross-reacting proteins were confirmed to be heat shock proteins and glycolytic enzymes (Butt et al. 2003). In the same year, a protein (AtNOS1) was identified in Arabidopsis reported to have 16% similarity with a 60 kD protein present in neuron cells of Helix pomatia (snail). This 60 kD protein was demonstrated to have cross-reactivity to anti-human nNOS antibody and was confirmed for its involvement in NO production in snail’s neuron cells. Thus, to find NOS activity of AtNOS1, a mutant Arabidopsis was generated through T-DNA insertion technology, and results of this experiment highlighted the central role of AtNOS1 in NO production in Arabidopsis. Unlike in mammals, NOS activity in plants did not required BH4, FAD, FMN, or heme as cofactors. Rather, amino acid sequence analysis revealed NOS of plants were found similar to proteins with GTP-binding or GTPase activity present in bacteria (Guo et al. 2003).
11.3.3 Nitrite Reductase
In plants, a plasma membrane-bound nitrate-NO oxidoreductase (Ni-NOR) has been reported in roots (Stöhr and Stremlau 2006). This enzyme was reported to utilize cytochrome-c as an electron donor to produce NO under in vitro conditions. Due to lesser studies on Ni-NOR, it was not yet fully identified and characterized and needs to be studied thoroughly for its role in the production of nitric oxide.
11.3.4 Non-enzymatic Production of NO in Plants
Apart from the enzymatic production of NO in plants, it is also produced though non-enzymatic mechanisms . For instance, oxidation of N2O during the nitrification-denitrification cycle results in production of NO as a by-product and is released into the atmosphere (Wojtaszek 2000). It was demonstrated that local acidic condition in the chloroplast and apoplastic spaces where ascorbate is present in abundance promotes chemical reduction of nitrate and produces NO and dehydroascorbic acid (Henry et al. 1997). Another example of reduction of nitrite by ascorbate under acidic pH has been demonstrated in aleurone cells of barley (Beligni et al. 2002). Direct dismutation of nitrate into nitrite or NO under acidic environment is also feasible (StÖhr 2002). In 1994, Cooney et al. reported involvement of carotenoids in the production of NO by reduction of NO2 in a light-dependent manner. In addition to this biotic as well as abiotic stress conditions, interaction between nitrogen species and reactive oxygen also results in the formation of NO.
11.4 Signaling Pathways of Nitric Oxide
Since the orientation of the present chapter is toward the plant kingdom, thus all details further provided in this chapter are related to plants. To act as signaling molecule, it should have the ability of rapid generation during a need to relay the signal quickly to the target protein and shall be rapidly removed when there is no need of the signal. Uncharged free radical entity with small half-life led to identification of NO as a signaling molecule in plants as well as in animals. Also free radical nature of NO favors its existence in three inter-convertible species which, namely, are the radical (NO•), nitrosonium cation (NO+), and the nitroxyl radical (NO¯) (Stamler et al. 1992; Wojtaszek 2000). NO is a gaseous molecule with slight solubility in water as well as lipids. This property of NO allows it to diffuse from one cell to another while travelling through their cell membranes and can also perpetuate signals freely within a cell diffusing through cytoplasm. NO is known to convey the signal through various routes, all of which are displayed diagrammatically in Fig. 11.2.
A previous study on Nicotiana tabacum demonstrated that NO triggers various cellular events by increasing cytoplasmic calcium ion concentration (Besson-bard et al. 2008). Several other studies displayed similar increase in calcium ion concentration, which was mediated due to NO in the hyperosmotic, abscisic acid, and elicitor transduction pathways (Lamotte et al. 2006; Garcia-Mata et al. 2003; Vandelle et al. 2006; Gould et al. 2003). Many other studies have also highlighted the vital role of NO in regulating activities of nearly all cellular channels involved in calcium transportation (Clementi 1998). NO is known to regulate activity of calcium channels either directly by causing post-translational modifications in them, e.g., S-nitrosylation (Stamler et al. 2001; Ahern et al. 2002), or indirectly by activating guanylate cyclase and/or cyclic ADP-ribose (cADPR) resulting in synthesis of cGMP from ADP-ribosyl cyclase and NAD+. cGMP acts as a calcium-mobilizing metabolite and therefore helps in relaying signals (Hanafy et al. 2001). In many studies, presence of plasma membrane and intracellular calcium channel inhibitors resulted in inhibition of NO-mediated accumulation of calcium in the cell cytoplasm. This confirmed the involvement of NO in influx of calcium ions from extracellular and intracellular calcium stores (Lamotte et al. 2006; Gould et al. 2003; Vandelle et al. 2006; Garcia-Mata et al. 2003). Besides, ryanodine receptors are the only calcium channels identified to be involved in NO-mediated mobilization of calcium ion from intracellular calcium reserves (Allen et al. 1995; Fliegert et al. 2007). This increased concentration of calcium ion in the cytoplasm modulates activity of calcium-dependent protein kinases (CDPKs), mitogen-activated protein kinases (MAPKs), and calcium-sensitive channels like Na+ and K+ channels. NO-mediated induction of protein kinases has been demonstrated in many plants including Nicotiana tabacum, Vicia faba, N. plumbaginifolia, etc. (Sokolovski et al. 2005; Lamotte et al. 2006). In a study on Nicotiana tabacum , NO was displayed to induce two protein kinases in it, one of which was identified as NtOSAK (Nicotiana tabacum Osmotic-Stress-Activated protein Kinase) , a 42 kD protein kinase characterized for its activation under abiotic stress conditions such as hyperosmolarity and high salinity (Lamotte et al. 2006). The second protein kinase which was induced by NO was a 48 kD protein and was identified to be to MAPK (Zhang and Liu 2001). NO and its derivatives are also known to perform their biological actions by modulating activities of various target proteins through three different types of post-translational modification, which includes metal nitrosylation, S-nitrosylation, and Tyr-nitration.
11.4.1 Metal Nitrosylation
Plants contain many types of metalloproteins , including hemoglobin (Hb) which has been an important choice of researchers to study its interactions with NO. Most of these studies were focused on the three types of hemoglobin proteins such as leghemoglobin (a symbiotic hemoglobin present in root nodules of leguminous crops), two classes of non-symbiotic hemoglobin (differing in their affinity for oxygen), and truncated hemoglobin. Pioneering studies on leghemoglobin present in root nodules of alfalfa, cowpea, and soybean demonstrated formation of NO nitrosyl-Lb complex (LbFeIINO) and suggested interaction between ferrous-leghemoglobin and NO produced in nodules (Mathieu et al. 1998). In another study, different forms of leghemoglobin, oxy-leghemoglobin and ferryl-leghemoglobin, were demonstrated to scavenge NO and peroxynitrite under in vitro conditions (Herold and Puppo 2005). During the reaction, oxy-hemoglobin gets converted into met-hemoglobin, which is further recycled into oxy-hemoglobin. Similarly, scavenging mechanism was observed to be utilized by class-I hemoglobin in distinct plants, which catalyzed the conversion of NO to nitrate in a NAD(P)-H-dependent manner. In a mutation study on Arabidopsis, it was confirmed that class-1 hemoglobins were also regulated through S-nitrosylation. In other mutagenesis experiments, it was demonstrated that interaction between NO and class-I hemoglobin was not only critical for NO scavenging but also for various physiological conditions like abiotic and biotic stresses (Dordas et al. 2003; Dordas et al. 2004; Perazzolli et al. 2004; Seregélyes et al. 2004).
11.4.2 S-Nitrosylation
S-nitrosylation is a NO-dependent post-translational modification of proteins in which NO is incorporated in the reactive thiol group of cysteine residue to form nitrosothiol. S-nitrosylation is a reversible reaction in which the formation or breakage of the product does not require enzymes, mandatorily. Besides, two enzymes are known to facilitate the forward and backward reactions of S-nitrosylation which are nitrosylase and denitrosylase, respectively. At present thousands of proteins are known in plants which are target for S-nitrosylation, few of these proteins were common in animals which were also characterized for S-nitrosylation. Studies revealed that most of the proteins which were target for S-nitrosylation were involved in major physiological processes including photosynthesis , signaling, genetic information processing, primary and secondary metabolism, cytoskeleton activities, biotic/abiotic stress responses, etc. Few of the proteins which were identified as potential target for S-nitrosylation in previous studies are summarized in Table 11.2.
11.4.3 Tyr-Nitration
Post-translational modification of proteins by covalent addition of a nitro group (-NO2) to tyrosine residue is generally referred to as tyr-nitration. Similar to S-nitrosylation, no enzymes are required for tyr-nitration (Ischiropoulos et al. 1992). Attachment of the nitro group at ortho position of aromatic ring in tyrosine residue is a two-step process. In the first step, the aromatic ring of the tyrosine residue gets oxidized by one electron, which results in the formation of a tyrosine radical. Electrons required for tyrosine oxidation are provided by either hydroxyl (OH•) or carbonate (CO3•¯) radicals, which are themselves derived from peroxynitrite (ONOO¯) (Yeo et al. 2015; Radi 2013) through three different pathways. The second step of tyrosine nitration involves interaction of tyrosine radical with nitrogen dioxide radical, which eventually produces 3-nitrotyrosine (YNO2) (Souza et al. 2000). In this reaction, peroxynitrite is one of the most important molecules required for tyrosine nitration as it produces an oxidant required for the production of tyrosine radical. Peroxynitrite is itself produced through a fast reaction between nitric oxide and super oxide anion radical, and this shows that the peroxynitrite belongs to the reactive nitrogen species (RNS) group which is generated from NO (Patel et al. 1999) and also has its relations with oxidative stress responses. A diagrammatic representation of tyrosine nitrosylation and reactions involved in it is shown in Fig. 11.3.
Few of the proteins which were identified to be the target for tyrosine nitration include methionine synthase (Lozano-Juste et al. 2011), photosystem II protein1 (Galetskiy et al. 2011), abscisic acid receptors (Castillo et al. 2015), monodehydroascorbate reductase (Begara-Morales et al. 2015), isocitrate dehydrogenase (Begara-Morales et al. 2013), mitochondrial Mn superoxide dismutase (Holzmeister et al. 2015), etc.
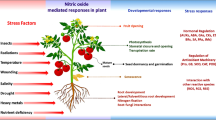
11.5 Abiotic Stress Tolerance and Its Response
Due to immobile nature of plants, they are not capable of changing their place when encountering unfavorable environmental conditions. Unfavorable conditions may be biotic or abiotic. Biotic factors refer to living organisms that are able to threaten plants’ viability via direct physical damage or induce diseases in it, and this category of stress factors include organisms like bacteria, virus, nematodes, fungi, insects, etc. (Atkinson and Urwin 2012). In contrast, abiotic factors are non-living factors like high soil salinity, high/low temperature, high/low light conditions, drought, heavy metals, etc. (Wang et al. 2003; Wani et al. 2016; Nakashima and Yamaguchi-Shinozaki 2006). Among these abiotic factors, high salinity and high temperature are the major role players in decreasing crop yield globally (Grattan et al. 2012; Pereira et al. 2014; Hasegawa et al. 2018; Fischer and Edmeades 2010). Regular irrigation, rainfall, and rock weathering are the main reasons why a normal fertile soil is converted into saline soil (commonly known as user soil). Most of the water leaches down the soil and part of it evaporate either directly or indirectly via transpiration by plants into the atmosphere, leaving salt in the upper layer of the soil (Rengasamy 2006). Thus, over a long period of time, salt keeps on accumulating in the soil and stays unfavorable for plant development. High soil salinity results in several plant changes at physiological, biochemical, and molecular levels. High concentration of salt in the soil lowers its water potential, disabling plants to absorb water from the soil and getting dehydrated Hoffman and Shalhevet (2007). As a consequence of dehydration, growth and metabolism of a plant slows down, eventually leading to its death. Due to salinity, water availability to the plant cell may also become limited even if soil is at saturation level, and the phenomenon is known as “physiological drought.” High salinity results in reduced leaf area, leaf thickening, and stomatal closure, all of which eventually lead to a drastic reduction in its photosynthetic efficiency (Delfine et al. 1998). In order to maintain normal growth and metabolism under highly saline conditions, plants activate cell signaling pathways including those that lead to synthesis of osmotically active metabolites (amino acids, glycine betaine, sugars, sugar alcohols, etc.), plant hormones (ABA), specific proteins (LEA-type proteins, chaperonins), and certain enzymes that control ion and water flux and support scavenging of ROS (Serraj and Sinclair 2002; Kav et al. 2004; Raghavendra et al. 2010). Exposure of plants to high temperature also leads to various morpho-anatomical, physiological, and biochemical changes, affecting plant growth and yield (Long and Ort 2010). The effect of high temperature on plants growth is complex because plant responses are influenced by various factors such as diurnal temperature range and water stress (Paulsen 1994). Heat stress is also known to reduce germination and early growth and lead to accumulation of H2O2 which is able to oxidize various biomolecules in plant cell and leave them inactive (Bhattacharjee 2008). Heat stress is most often associated with drought stress as it impairs physiological processes and plant-water relations of crops (McDonald and Paulsen 1997). Also exposure of high temperature to plants results in a higher production of hydroxyl radical (OḤ) , which is known to initiate peroxidation of thylakoid lipids and thus lead to a decrease in photosynthesis efficiency (Wahid et al. 2007; Mishra et al. 1993).
Most of the pioneering studies were focused on exposure of different plants to a single abiotic stress condition, but their data could not be validated in field conditions, since under field conditions, plants are exposed to different abiotic and biotic stress conditions. This promoted researchers to study plants under combinations of different abiotic stresses, which confirmed that acclimatization done by plants against combinatorial stress conditions was significantly different from that of plants exposed to a single abiotic stress condition (Jiang and Huang 2001; Rizhsky et al. 2004). Similar results of many such studies changed the fashion of experiments, and now most of the abiotic stress studies on plants are done by exposing them to a combination of different abiotic stresses. In a recent study, it was demonstrated that exposure of those seedling to high temperature which were growing on a medium containing high salt concentration resulted in an enhanced thermostability of catalase present in leaves of two varieties of Vigna mungo. In contrast when seedlings of those two varieties were exposed to high temperature ion absence of salt catalase in them was observed to be inactivated (Singh and Mishra 2020).
When plants experience abiotic stress conditions, high-energy electrons which are being transported through electron transport chains of chloroplast and mitochondria spill their energy to nearby oxygen molecules, resulting in an uncontrollable generation of various reactive oxygen species (ROS) in plants (Foyer and Noctor 2003; Asada 2006; Foyer and Noctor 2005; Navrot et al. 2007). ROS are oxidizing in nature with a short life span and are known to exist in various forms including superoxide anion radical (O2¯), hydroxyl radical (OH•), hydrogen peroxide (H2O2), singlet oxygen (1O2), etc. (Wahid et al. 2007). The presence of reactive oxygen species (ROS) generating system in the plants, together with high percentage of polyunsaturated lipids, particularly in the thylakoid membrane, makes them susceptible to oxidative injury. Superoxide anion radical is one of the most commonly generated reactive oxygen species. Although capable of oxidizing various cellular components directly, it exerts most of its damaging effects by initiating the generation of more reactive species including hydroxyl free radicals (Halliwell and Gutteridge 1989). Other ROS are also detrimental to plants as they are highly efficient in generating damages to biomolecules such as proteins, lipid molecules in plasma membrane, and genetic material (DNA) present in a plant cell Mazid et al. (2011). On interaction with ROS, lipid molecules get converted into harmful lipid peroxide and other lipid abducts, and proteins are damaged which results in the reduction of their activity and possibility of DNA molecules getting mutated (Shah et al. 2001).
During the course of evolution, the higher plants have developed an efficient antioxidant defense system, which helps them to cope with environmental stresses. Antioxidant defense system in plants is categorized into two categories. First category of enzymatic defense system is constituted by various enzymes like superoxide dismutase, catalase, peroxidases, glutathione reductase, DHAR, MDHAR, etc. (Asada and Takahashi 1987). These enzymes are involved in detoxification of different ROS, e.g., SOD dismutates superoxide anion radical into H2O2 (Fridovich 1986), which is further detoxified by catalase and various other peroxidases to H2O and O2 (Passardi et al. 2004; Asada 2006). Various enzymes involved in detoxification of different ROS are displayed in Table 11.3. It is generally believed that those plant species or their varieties, which contain antioxidant enzymes possessing high tolerance toward different abiotic stresses, are referred to be resistant. In a recent study on seven varieties of Vigna mungo , it was demonstrated that the extent of thermostability of different SOD isoforms was not similar among varieties and those varieties with thermotolerant SOD isoforms were considered to be more resistant toward heat stress (Singh et al. 2019). Another category of antioxidant defense system constitutes non-enzymatic molecules like ascorbate, reduced glutathione (GSH), α-tocopherol, carotenoids, flavonoids, etc., which contribute to protection of plants by deactivation/quenching of ROS in multiple ways (Asada and Takahashi 1987). In addition to this, proline and glycine betaine are also well-known for their contribution in coping stress conditions such as high temperature and high salinity. Both of these molecules act as an osmolyte, which promotes the entry of water from soil into plant cells. Besides ROS, when plants are exposed to different stress conditions, there occurs a simultaneous increase of nitric oxide and its derivatives in plant cells (Valderrama et al. 2007). Nitric oxide and its derivative are referred to as reactive nitrogen species (RNS) due to the presence of unpaired electron. RNS are known to exist in various inorganic and organic forms which are themselves sub-categorized into radical and non-radical forms (Corpas et al. 2011). Few important examples of RNS are shown in Table 11.3.
Among RNS, peroxynitrite (ONOO¯) has been studied most extensively by researchers due to its involvement in various physiological processes in plants. Peroxynitrite is produced in a very fast reaction between NO and O2•¯, with a rate constant of approximately (1010 M¯1 s¯1) (Estévez and Jordán 2002). Due to the co-existence of NO and O2•¯ in chloroplast, mitochondria, and peroxisomes, these organelles were identified to be the main site of generation in plants (Blokhina and Fagerstedt 2010; Corpas and Barroso 2014). Peroxynitrite , being highly oxidizing in nature, has been demonstrated to cause oxidative damages by interacting with all biomolecules present in the plant cell. Additionally, as described in the previous section of this chapter, RNS species particularly peroxynitrite has been identified to mediate post-translational modifications of various proteins, in turn leading to alterations in the physiological function controlled by them (Szabó et al. 2007; Corpas et al. 2009; Arasimowicz-Jelonek and Floryszak-Wieczorek 2011; Calcerrada et al. 2011; Berton et al. 2012; Szuba et al. 2015). Some researchers proposed that increase in protein nitration could be considered as a biomarker for specific stress conditions in similar to oxidation of proteins, which is utilized as a biomarker for oxidative stress (Corpas et al. 2007; Arasimowicz-Jelonek and Floryszak-Wieczorek 2011).
There are many previous studies which have demonstrated that the effects of ROS and RNS on plants are tightly regulated by each other. It has been shown that RNS are involved in mediating post-translational modifications of various enzymes such as catalase, superoxide dismutase (Mn, Fe, and Cu/Zn isoforms), peroxiredoxin II E and F, DHAR, MDHAR, ascorbate peroxidase, etc., which are known to be involved in ROS detoxifications, and thus RNS regulate their activity during stress conditions (Ortega-Galisteo et al. 2012; Chaki et al. 2015; Holzmeister et al. 2015; Romero-Puertas et al. 2007; Camejo et al. 2015; Clark et al. 2000; Fares et al. 2014; Begara-Morales et al. 2015). On the other hand, generation of one of the most primary RNS “peroxynitrite” is dependent on the presence of a reactive oxygen species “superoxide anion radical.” On observing these regulatory roles of ROS and RNS over each other, Corpas et al. (2013) suggested that using the term nitro-oxidative stress in place of oxidative stress shall be more appropriate.
11.6 Function of Nitric Oxide in Abiotic Stress Tolerance
11.6.1 Salt Stress
Plant suffers osmotic and ionic stress at high salt concentrations. Due to this many chief metabolic processes get affected which in turn limits the growth of plants. Enzyme activities of plants are badly affected under high salinity which leads to alteration in nitrate and sulfate assimilation pathway and a drop in energy. Further, salinity alters the activities of and sulfur & nitrogen demand increases (Vanlerberghe 2013). High salinity leads to oxidative damage due to ROS and causes cellular injury. To avoid such oxidative damage, plants have developed a wide range of defense methods. However, it is a less known fact that plants become tolerant to salinity in the presence of nitric oxide. Arora et al. reported the impacts of exogenous sodium nitroprusside (SNP), a NO donor. It was found that it remarkably reduced the oxidative damage under highly saline conditions. Its effect was observed in rice, cucumber, and lupin seedlings where it enhanced seedling growth and also maize dry weight even under salt stress (Arora et al. 2016). A good stability was observed between carbon and nitrogen metabolism when pretreated with nitric oxide. This effect of nitric oxide was due to increased total soluble protein and enhanced endopeptidase and carboxypeptidase activities in plants under salinity stress (Wojtyla et al. 2016). Salt stress tolerance of roots of red kidney beans was due to the significant role of glucose-6-phosphate dehydrogenase enzyme in NR-dependent nitric oxide production. Prior studies have reported that nitric oxide induces ROS scavenging enzyme activities of CAT, peroxidase (POD), SOD, and ascorbate peroxidase (APX), which in turn reduces membrane permeability, rate of ROS production, and intercellular CO2 concentration under high salinity stress. Nitric oxide also induces the expression of transcripts of genes encoding sucrose-phosphate synthase and Δ′-pyrroline-5-carboxylate synthase, related to stress (Shi and Chan 2014).
11.6.2 Drought Stress
Drought is a key factor responsible for limited crop yield. It has been found that nitric oxide synthesis is dependent on intensity of water deficit, mild or severe. When cucumber roots suffer from mild water deficit (5–10 h), nitric oxide synthesis in root tip cells and the elongation zone surrounding it is slightly increased. But when the duration of this stress increases up to 17 h, it results in rigorous nitric oxide synthesis in the roots of cucumber (Ahmad et al. 2016). Also, pea, wheat, and tobacco have shown enhanced nitric oxide production. Stress due to lack of water induces the production of nitric oxide in mesophyll cells of maize and activity of nitric oxide synthase (NOS) in cytosolic and microsomal fractions of maize leaves. This nitric oxide synthesis was blocked by prior treatment with inhibitors of nitric oxide synthase and nitrate reductase. This suggests that nitric oxide is synthesized from NOS and NR in maize leaves when exposed to water stress. Treatment with nitric oxide synthase and nitrate reductase inhibitors suppressed the activities of chloroplast and antioxidant enzymes present in cytosol, i.e., SOD, and GR. Reduced activities of these enzymes were improved by externally applying NOS and decreasing the buildup of H2O2 due to water stress (Delorge et al. 2014). Due to induction of subcellular antioxidant defense, the possible ability of nitric oxide to scavenge H2O2 is in some part only (Laxa et al. 2019).
11.6.3 Temperature Stress
Crop productivity is equally affected by temperature variations. Plant injuries due to high temperature include damage due to oxidative stress, damage to membrane, lipid peroxidation, degradation of protein, inactivation of enzymes, and disruption of DNA strands. Also cold stress retards biochemical and physiological processes and ROS homoeostasis in plants (Roychoudhury et al. 2013). Nitric oxide has a unique role in the management of heat and cold stress. Lucerne cells have been reported for increased nitric oxide synthesis at high temperatures, while tomato, wheat, and maize developed cold tolerance when NO is applied externally (Parankusam et al. 2017). NO synthesis was augmented by short-time heat stress in alfalfa. It is likely that this result due to antioxidative property of nitric oxide, which raise adverse effects imposed by the intensification of peroxidative metabolism in cold and heat stress. Heat stress stimulated NO synthesis that could play a role in the induction of cell death in Symbiodinium microadriaticum by causing a boost in caspase-like activity (Leterrier et al. 2012).
11.6.4 Ultraviolet Radiation Stress
The injure of the stratosphere ozone layer amplified the radiation of UV-B (250–320 nm) responsible for increase in ion leakage, chlorophyll loss, and reducd efficiency of PS-II photochemistry (Fv/Fm) and the quantum yield PS-II electron transport (ØPS-II), and augmented H2O2 and thylakoid membrane protein oxidation. UV-B radiation leads to a boost in nitric oxide and reactive oxygen species in Arabidopsis (Shi and Chan 2014). Nitric oxide generated from NOS-like activity acts synergistically with ROS to stimulate ethyl synthesis in defense response under UV-B radiation in leaves of maize (Arora et al. 2016). UV-B induced augmentation of NOS activity in maize hypocotyls, indicating that NO may act as a second messenger and perform antioxidant response to UV-B radiation, and SNP-exposed maize plants exhibited increased activity of glucosidase and protein synthesis.
11.6.5 Heavy Metals Stress
As we know heavy metals contamination affects the biosphere in many places worldwide. Several studies have been conducted in order to evaluate the effects and remedy of different heavy metals concentration on plants (Schützendübel and Polle 2002). NO also plays a vital role in the enhancement of antioxidant enzyme activities and alleviating the toxicity of heavy metals. Exogenous application of SNP reduced copper (Cu) toxicity and NH4+ accumulation in rice leaves (Manara 2012a). The protective effect of SNP on the toxicity and NH4+ accumulation can be reversed by c-PTIO, a NO scavenger, suggesting that the protective effect of SNP is attributable to NO released. These results also suggest that reduction of Cu-induced toxicity and NH4+ accumulation by SNP is most likely mediated through its ability to scavenge active oxygen species. SNP pretreatment significantly reduced O2− induced specific fluorescence in Lupinus luteus roots under heavy metal treatment. Results obtained in this study suggest that antioxidant function of NO may be traced by a scavenging of O2−, resulting in a decrease of superoxide anion (Yadav 2010). The detoxification and antioxidative properties of NO are also found in soybean cell cultures under cadmium (Cd) and Cu stress . Moreover, NO decreased the aluminum (Al3+) toxicity in root elongation of Hibiscus moscheutos (Manara 2012b).
11.7 Conclusion
Nitric oxide is a ubiquitous bioactive molecule with diverse roles in a huge spectrum of physiological processes in plants. Even then, there are much more rigorous studies required to understand the complete range of activities being performed by nitric oxide. A huge amount of work is already done about functions of nitric oxide as a signaling molecule interacting with plant hormones, nutrients, or metals. But still much more is to be searched upon the activities of nitric oxide.
References
Abat JK, Deswal R (2009) Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics 9:4368–4380
Abat JK, Mattoo AK, Deswal R (2008) S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata – Ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J 275:2862–2872
Ahern GP, Klyachko VA, Jackson MB (2002) cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25:510–517
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M et al (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813
Allen GJ, Muir SR, Sanders D (1995) Release of Ca2+ from individual plant vacuoles by both InsP 3 and cyclic ADP-ribose. Science 268:735–737
Arasimowicz-Jelonek M, Floryszak-Wieczorek J (2011) Understanding the fate of peroxynitrite in plant cells – from physiology to pathophysiology. Phytochemistry 72:681–688
Arora D, Jain P, Singh N, Kaur H, Bhatla SC (2016) Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic Res 50(3):291–303
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–287
Atkinson N, Urwin P (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp bot 2012 63:3523–3543
Bai X, Yang L, Tian M, Chen J, Shi J, Yang Y et al (2011) Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS One 6:e20714
Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM et al (1999) Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem 274:36729–36733
Begara-Morales JC, Chaki M, Sánchez-Calvo B, Mata-Pérez C, Leterrier M, Palma JM et al (2013) Protein tyrosine nitration in pea roots during development and senescence. J Exp Bot 64:1121–1134
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Mata-Pérez C, Valderrama R, Padilla MN et al (2015) Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J Exp Bot 66:5983–5996
Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL (2002) Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol 129:1642–1650
Berton P, Domínguez-Romero JC, Wuilloud RG, Sánchez-Calvo B, Chaki M, Carreras A et al (2012) Determination of nitrotyrosine in Arabidopsis thaliana cell cultures with a mixed-mode solid-phase extraction cleanup followed by liquid chromatography time-of-flight mass spectrometry. Anal Bioanal Chem 404:1495–1503
Besson-Bard A, Courtois C, Gauthier A, Dahan J, Dobrowolska G, Jeandroz S et al (2008) Nitric oxide in plants: production and cross-talk with Ca2+ signaling. Mol Plant 1:218–228
Bhattacharjee S (2008) Calcium-dependent signaling pathway in the heat-induced oxidative injury in Amaranthus lividus. Biol Plant 52:137–140
Blokhina O, Fagerstedt KV (2010) Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems. Physiol Plant 138:447–462
Butt YKC, Lum JHK, Lo SCL (2003) Proteomic identification of plant proteins probed by mammalian nitric oxide synthase antibodies. Planta 216:762–771
Calcerrada P, Peluffo G, Radi R (2011) Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications. Curr Pharm Des 17:3905–3932
Camejo D, Jiménez A, Palma JM, Sevilla F (2015) Proteomic identification of mitochondrial carbonylated proteins in two maturation stages of pepper fruits. Proteomics 15:2634–2642
Castillo MC, Lozano-Juste J, González-Guzmán M, Rodriguez L, Rodriguez PL, León J (2015) Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci Signal 8:ra89
Chaki M, Álvarez De Morales P, Ruiz C, Begara-Morales JC, Barroso JB, Corpas FJ et al (2015) Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann Bot 116:637–647
Clark D, Durner J, Navarre DA, Klessig DF (2000) Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant-Microbe Interact 13:1380–1384
Clementi E (1998) Role of nitric oxide and its intracellular signalling pathways in the control of Ca2+ homeostasis. Biochem Pharmacol 55:713–718
Cooney RV, Harwood PJ, Custer LJ, Franke AA (1994) Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspect 102:460–462
Corpas FJ, Barroso JB (2014) Peroxynitrite (ONOO-) is endogenously produced in Arabidopsis peroxisomes and is overproduced under cadmium stress. Ann Bot 113:87–96
Corpas FJ, del Río LA, Barroso JB (2007) Need of biomarkers of nitrosative stress in plants. Trends Plant Sci 12:436–438
Corpas FJ, Hayashi M, Mano S, Nishimura M, Barroso JB (2009) Peroxisomes are required for in vivo nitric oxide accumulation in the cytosol following salinity stress of Arabidopsis plants. Plant Physiol 151:2083–2894
Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M, Palma JM et al (2011) Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 181:604–611
Corpas FJ, Palma JM, del Río LA, Barroso JB (2013) Protein tyrosine nitration in higher plants grown under natural and stress conditions. Front Plant Sci 4:29
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
Cueto M, Hernández-Perera O, Martín R, Bentura ML, Rodrigo J, Lamas S et al (1996) Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett 398:159–164
Dean JV, Harper JE (1988) The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H-nitrate reductase enzyme from soybean. Plant Physiol 88:389–395
Delfine S, Alvino A, Zacchini M, Loreto F (1998) Consequences of salt stress on conductance to CO2 diffusion, Rubisco characteristics and anatomy of spinach leaves. Aust J Plant Physiol 25:395–402
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394(6693):585–588
Delorge I, Janiak M, Carpentier S, Van Dijck P (2014) Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front Plant Sci 5:147
Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci U S A 99:16314–16328
Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35:763–770
Dordas C, Hasinoff BB, Rivoal J, Hill RD (2004) Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 219:66–72
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci U S A 95:10328–10333
Estévez AG, Jordán J (2002) Nitric oxide and superoxide, a deadly cocktail. Ann New York Acad Sci 962:207–211
Fares A, Rossignol M, Peltier JB (2011) Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem Biophys Res Commun 416:331–336
Fares A, Nespoulous C, Rossignol M, Peltier JB (2014) Simultaneous identification and quantification of nitrosylation sites by combination of biotin switch and ICAT labeling. Methods Mol Biol 1072:609–620
Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50:85–98
Fliegert R, Gasser A, Guse AH (2007) Regulation of calcium signalling by adenine-based second messengers. Biochem Soc Trans 35:109–114
Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Fridovich I (1986) Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 58:61–97
Galetskiy D, Lohscheider JN, Kononikhin AS, Popov IA, Nikolaev EN, Adamska I (2011) Mass spectrometric characterization of photooxidative protein modifications in Arabidopsis thaliana thylakoid membranes. Rapid Commun Mass Spectrom 25:184–190
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl¯ channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci U S A 100:11116–11121
Gould KS, Lamotte O, Klinguer A, Pugin A, Wendehenne D (2003) Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ 26:1851–1862
Grattan SR, Oster JD, Benes SE, Kaffka SR (2012) Use of saline drainage waters for irrigation. In: Wallender WW, Tanji KK (eds) Agricultural salinity assessment and management, 2nd edn. ASCE, New York, pp 687–719
Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302:100–103
Halliwell B, Gutteridge JMC (1989) Lipid peroxidation: a radical chain reaction. In: Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford, pp 188–276
Hanafy KA, Krumenacker JS, Murad F (2001) NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit 7:801–819
Hasegawa T, Fujimori S, Havlík P, Valin H, Bodirsky BL, Doelman JC et al (2018) Risk of increased food insecurity under stringent global climate change mitigation policy. Nat Clim Chang 8:699–703
Henry YA, Guissani A, Ducastel B, Henry YA, Ducastel B, Guissani A (1997) Basic chemistry of nitric oxide and related nitrogen oxides. In: Henry YA, Guissani A, Ducastel B (eds) Nitric oxide research from chemistry to biology. Landes Co. Biomedical Publishers, Austin, pp 15–46
Herold S, Puppo A (2005) Kinetics and mechanistic studies of the reactions of metleghemoglobin, ferrylleghemoglobin, and nitrosylleghemoglobin with reactive nitrogen species. J Biol Inorg Chem 10:946–957
Hoffman GJ, Shalhevet J (2007) Controlling salinity. In: Hoffman GJ, Evans RG, Jensen ME, Martin DL, Elliot RL (eds) Design and operation of farm irrigation systems, 2nd edn. ASABE, St. Joseph, pp 160–207
Holzmeister C, Gaupels F, Geerlof A, Sarioglu H, Sattler M, Durner J et al (2015) Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J Exp Bot 66:989–999
Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD et al (1992) Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 298:431–437
Jiang Y, Huang B (2001) Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41:436–442
Kav NNV, Srivastava S, Goonewardene L, Blade SF (2004) Proteome-level changes in the roots of Pisum sativum in response to salinity. Ann Appl Biol 145:217–230
Klepper L (1979) Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmos Environ 13(4):537–542
Kuo WN, Ku TW, Jones DL, Jn-Baptiste J (1995) Nitric oxide synthase immunoreactivity in baker’s yeasts, lobster, and wheat germ. Biochem Arch 11:73–78
Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D (2006) Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40:1369–1376
Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ (2019) The role of the plant antioxidant system in drought tolerance. Antioxidants 8:94
Leshem YY, Haramaty E (1996) The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. Foliage. J Plant Physiol 148:258–263
Leterrier M, Valderrama R, Chaki M, Airaki M, Palma JM, Barroso JB et al (2012) Function of nitric oxide under environmental stress conditions. Phytohormones Abiotic Stress Toler Plants:99–113
Lillo C, Meyer C, Lea US, Provan F, Oltedal S (2004) Mechanism and importance of post-translational regulation of nitrate reductase. J Exp Bot 55:1275–1282
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in arabidopsis. Plant Physiol 137:921–930
Lindermayr C, Saalbach G, Bahnweg G, Durner J (2006) Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem 281:4285–4291
Long SP, Ort DR (2010) More than taking heat: crops and global climate change. Curr Opin Plant Biol 13:241–248
Lozano-Juste J, Colom-Moreno R, León J (2011) In vivo protein tyrosine nitration in Arabidopsis thaliana. J Exp Bot 62:3501–3517
Manara A (2012a) Plant responses to heavy metal toxicity. Springer, Dordrecht, pp 27–53
Manara A (2012b) Plants and heavy metals. Signal transduct. [Internet], pp 27–54. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/978-94-007-4441-7
Mathieu C, Moreau S, Frendo P, Puppo A, Davies MJ (1998) Direct detection of radicals in intact soybean nodules: presence of nitric oxide-leghemoglobin complexes. Free Radic Biol Med 24:1242–1249
Mazid M, Khan TA, Mohammad F (2011) Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: a synergistic signaling approach. J Stress Physiol Biochem 7(2):34–74
McDonald GK, Paulsen GM (1997) High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil 196:47–58
Mishra NP, Mishra RK, Singhal GS (1993) Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol 102:903–910
Modolo LV, Cunha FQ, Braga MR, Salgado I (2002) Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol 130:1288–1297
Nakashima K, Yamaguchi-Shinozaki K (2006) Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiol Plant 126:62–71
Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J et al (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59:165
Ninnemann H, Maier J (1996) Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem Photobiol 64:393–398
Ortega-Galisteo AP, Rodríguez-Serrano M, Pazmiño DM, Gupta DK, Sandalio LM, Romero-Puertas MC (2012) S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J Exp Bot 63:2089–2103
Parankusam S, Adimulam SS, Bhatnagar-Mathur P, Sharma KK (2017) Nitric oxide (NO) in plant heat stress tolerance: current knowledge and perspectives. Front Plant Sci 8:1582
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA et al (1999) Biological aspects of reactive nitrogen species. Biochim Biophys Acta Bioenerg 1411:385–400
Paulsen GM (1994) High temperature responses of crop plants. In: Boote KJ, Bennett JM, Sinclair TR, Paulsen GM (eds) Physiology and determination of crop yield. American Society of Agronomy, Madison, pp 365–389
Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M et al (2004) Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 16:2785–2794
Pereira LS, Duarte E, Fragoso R (2014) Water use: recycling and desalination for agriculture. In: van Alfen N (ed) Encyclopedia of agriculture and food systems, vol 5. Elsevier, San Diego, pp 407–424
Qi YC, Dong YS (1999) Nitrous oxide emissions from soil and some influence factors. Dili Xuebao/Acta Geogr Sin 54(6):534–542
Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46:550–559
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Ribeiro EA, Cunha FQ, Tamashiro WMSC, Martins IS (1999) Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett 445:283–S286
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Romero-Puertas MC, Laxa M, Mattè A, Zaninotto F, Finkemeier I, Jones AME et al (2007) S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell 19:4120–4130
Roychoudhury A, Paul S, Basu S (2013) Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep 32:985–1006
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sen S, Cheema IR (1995) Nitric oxide synthase and calmodulin immunoreactivity in plant embryonic tissue. Biochem Arch 11:221–227
Seregélyes C, Igamberdiev AU, Maassen A, Hennig J, Dudits D, Hill RD (2004) NO-degradation by alfalfa class 1 hemoglobin (Mhb1): a possible link to PR-1a gene expression in Mhb1-overproducing tobacco plants. FEBS Lett 571:61–66
Serpa V, Vernal J, Lamattina L, Grotewold E, Cassia R, Terenzi H (2007) Inhibition of AtMYB2 DNA-binding by nitric oxide involves cysteine S-nitrosylation. Biochem Biophys Res Commun 361:1048–1053
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shi H, Chan Z (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 56(2):114–121
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Simontacchi M, García-Mata C, Bartoli CG, Santa-María GE, Lamattina L (2013) Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep 32:853–866
Singh K, Mishra RK (2020) High temperature stress induces conformational change in catalase of Vigna mungo (L.) Hepper which is reversed by simultaneous exposure to salt stress. Plant Physiol Reports. https://doi.org/10.1007/s40502-020-00512-w
Singh K, Yadav SK, Mishra RK (2019) Differential effect of salinity on thermotolerance of SOD isoforms in seven varieties of Vigna mungo (L.) Hepper. Plant Physiol Reports 24:279–288
Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR (2005) Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 43:520–529
Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M et al (2000) Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys 380:360–366
Stamler JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898–1902
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675–683
Stöhr C (2002) Generation and possible roles of NO in plant roots and their apoplastic space. J Exp Bot 53:2293–2303
Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57:463–470
Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680
Szuba A, Kasprowicz-Maluśki A, Wojtaszek P (2015) Nitration of plant apoplastic proteins from stcell suspension cultures. J Proteome 120:158–168
Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C et al (2008) Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321:952–956
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G et al (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60:795–804
Terrile MC, París R, Calderõn-Villalobos LIA, Iglesias MJ, Lamattina L, Estelle M et al (2012) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis transport inhibitor response 1 auxin receptor. Plant J 70:492
Valderrama R, Corpas FJ, Carreras A, Fernández-Ocaña A, Chaki M, Luque F et al (2007) Nitrosative stress in plants. FEBS Lett 581:453–461
Vandelle E, Poinssot B, Wendehenne D, Bentéjac M, Pugin A (2006) Integrated signaling network involving calcium, nitric oxide, and active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses. Mol Plant-Microbe Interact 19:429–440
Vanlerberghe GC (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14:6805–6847
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 28:1–14
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wawer I, Bucholc M, Astier J, Anielska-Mazur A, Dahan J, Kulik A et al (2010) Regulation of Nicotiana tabacum osmotic stress-activated protein kinase and its cellular partner GAPDH by nitric oxide in response to salinity. Biochem J 429:73–83
Weiner H, Kaiser WM (1999) 14-3-3 proteins control proteolysis of nitrate reductase in spinach leaves. FEBS Lett 455:75–78
Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6:177–183
Wojtaszek P (2000) Nitric oxide in plants: to NO or not to NO. Phytochemistry 54:1–4
Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front Plant Sci 7:66
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr J Bot 76:167–179
Yeo WS, Kim YJ, Kabir MH, Kang JW, Kim KP (2015) Mass spectrometric analysis of protein tyrosine nitration in aging and neurodegenerative diseases. Mass Spectrom Rev 34:166–183
Yun BW, Feechan A, Yin M, Saidi NBB, Le Bihan T, Yu M et al (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478:264–268
Zhang S, Liu Y (2001) Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 13:1877–1889
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, K., Shukla, I., Tiwari, A.K., Azmi, L. (2021). Physiological, Biochemical, and Molecular Mechanism of Nitric Oxide-Mediated Abiotic Stress Tolerance. In: Aftab, T., Hakeem, K.R. (eds) Plant Growth Regulators. Springer, Cham. https://doi.org/10.1007/978-3-030-61153-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-61153-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61152-1
Online ISBN: 978-3-030-61153-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)