Abstract
Radiological imaging is essential in complementing clinical data and supporting the urologist in order to establish the proper patient management.
Among the different imaging modalities, Computed tomography (CT) is currently considered the reference standard for the assessment of urologic diseases due to its wide availability, fast scan-times and comprehensive evaluation.
In particular, the term “CT-urography” (CTU) refers to a specific CT protocol aimed at the assessment of the urinary tract that contains an acquisition specifically focused on imaging the collector system (excretory phase).
Nevertheless, CTU protocol is not unique and can be adjusted in terms of scan times, radiations dose and intravenous contrast agent amount according to patient’s characteristics and clinical suspicion.
The other side of this coin is related to the well-known limitations of CT, mainly represented by radiation exposure and nephrotoxicity of contrast medium.
However, technical adjustments and recently developed techniques can be extremely helpful in overcoming such limits.
Therefore, the main purpose of this chapter is to provide the reader the fundamental principles of CTU in evaluating the urinary system, from technical background to future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 History
Radiological techniques have always played a pivotal role in the study of renal diseases.
In the past, intravenous urography (IVU), also known as “excretory urography” and/or “intravenous pyelography”, has been largely performed for assessment of the urinary tract.
This technique includes a first X-ray plain film followed by the intravenous administration of a water-soluble contrast (1.5 ml/kg body weight). Afterwards, a series of images are obtained at specific timepoints: 1–2 min for renal parenchyma visualization, 3 min for the pyelocaliceal system and 10–15 min for ureters and bladder [1]. Moreover, additional images obtained on oblique planes, prone decubitus, delayed or after bladder emptying can be obtained for a more accurate assessment [1]. Principal limitations of this technique include the two-dimensional appraisal and the absent evaluation of the adjacent anatomical structures.
After the introduction of computed tomography, IVU has widely fallen out of favour.
However, only during the 90s, with the introduction of spiral technology, the scanning times were considerably sped up, so that a study of large areas of the body, such as the abdomen, was performable in few seconds. With the advent of multi-detector technology in 2000s, the spatial resolution has been upgraded such that the urothelium of the upper urinary tract and bladder can be appreciated. Moreover, isotropic voxels enabled the creation of equal spatial resolution images in any plane resulting into three-dimensional reconstructions, useful in a more accurate evaluation of anatomic relationships. Consequently, the administration of intravenous contrast medium has been improved as well, leading to the establishment of a specific protocol for urinary tract assessment.
“CT-Urography” (CTU) was established with the aim of a simultaneous morphologic and functional assessment of kidneys, ureters and bladder and therefore consisting in corticomedullary, nephrographic and excretory phases.
Nowadays, CTU is widely performed in the assessment of the kaleidoscopic scenario of urologic diseases, from urolithiasis detection to malignancies staging.
Since the beginnings of CT inception, it was already known that X-ray spectra at different energies were able in differentiating materials with different atomic numbers. Only in 2006, this principle has been successfully applied to the study of human tissues and the first Dual-Energy CT (DECT) system was finally introduced in the daily clinical practice.
DECT has immediately demonstrated its suitableness in evaluation of pathologic conditions of the urinary tract, from material decomposition of urinary stones to iodine uptake of urinary malignancies.
2 Technical Background
The worldwide availability, the anatomic detail resolution and the functional information yielded by the dynamic study led to make CTU the technique of choice in urologic imaging.
Clinical indications for CTU performance include urolithiasis (without contrast medium), trauma, surgical planning or transplantation, vascular damage and iatrogenic complications, characterization of benign lesions from malignancies [2,3,4,5,6].
CTU study must include the whole abdominal cavity preferably using thin slice with a high collimation of the X-ray beam in order to improve spatial resolution, to avoid partial volume artifacts and to obtain multiplanar reconstructions [7].
The 2007 European Society of Urogenital Radiology (ESUR) group meeting underlined the role of CTU in daily clinical practice and led to the creation of an official expert-based guideline for a study protocol standardization [8]. However, this document was not specifically focused on CTU and addressed all indications for abdominopelvic CT.
In October 2018 the French Society of Genitourinary Imaging decided to set up a conference aimed at achieving consensus on patient preparation and CTU imaging protocols for different types of indications [9, 10]. First of all, a strong consensus was reached on patient preparation by recommending only an intravenous injection of 20 mg of furosemide before contrast medium administration. Indeed, the furosemide induces hyper-diuresis within minutes after its injection, which accelerates the opacification of the urinary tract and improves the distension and visualization of the middle and distal ureters. It also induces a dilution of the excreted contrast medium thereby reducing streaking artefacts and improving the visualization of the ureteral wall and the detection of filling defects. The only contraindications to its use are dehydration and acute urinary tract obstruction. Hyper-diuresis can also be obtained by oral or intravenous hydration. A consensus was reached against systematic patient hydration because it seems less effective and reproducible.
Contrast medium (CM) intravenous injection should be tailored according to patient weight and iodine concentration, usually 1.7–2.0 ml/kg of 300 mgI/ml and 1.4–1.6 ml/kg of 370 mgI/ml [8]. Injection rate conventionally employed ranges from 2 to 3.5 ml/s.
According to the clinical suspicion and the information required, CTU can be mainly obtained through two different protocols: conventional multiphasic single-bolus study and biphasic split-bolus technique [8, 11].
The former includes, as a first step, an unenhanced phase meant as a baseline for contrast enhancement assessment, essential in stone detection and hyperdense or hypodense components within masses appraisal. Afterwards, the cortical-medullary phase (obtained at 35–40 s) allows the enhancement of renal cortex and vessels, useful for bleeding detection in trauma cases, hypervascular lesions appraisal, surgical or pre-transplantation assessment. During the nephrographic phase (90–120 s) a homogeneous enhancement of cortical and medullary components of renal parenchyma is obtained, helpful in displaying hypodense nodules. Finally, the excretory phase (7–8 min) permits the opacification of the collecting system, allowing the recognition of filling defects such as stones or urothelial malignancies. Leaks of urinary tract with fluid collections (urinomas) can be detected as well.
For best identification of these findings is mandatory to perform multiplanar reconstruction of excretory phase on coronal view.
As conventional study foresees at least four scans, a considerable radiation dose is delivered to the patient, an important issue especially for young patients. Depending on the clinical scenario, multiple phase imaging may not be required, and some phases may be omitted in order to reduce the amount of radiation delivered to the patient. In this sense, the efforts to further decrease radiation dose led to the development of the so-called “split bolus” technique.
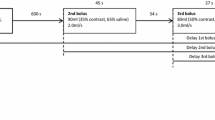
This protocol requires that the CM be administered in two separate injections: after the unenhanced scan, a first CM bolus is followed, after a delay of 8–9 min by a second one.
Currently, a univocal consensus about the amount of contrast agent administered during the first and the second injection has not been reached yet. However, a consensus was reached in favor of injecting two-thirds of the bolus first and using the remaining third for the second injection [10]. A cortico-medullary phase can be performed 30s after the first bolus, whereas a combined nephrographic–excretory phase can be obtained with a delay of 90s after the second contrast bolus [8, 12].
Although different studies have demonstrated high sensitivity and specificity for collecting system and bladder neoplasm detection, this technique seems to be less sensitive for the detection of smaller renal cell carcinomas (RCCs) [12].
Some authors have also proposed a triple-bolus technique [13], with a small bolus of 30 ml was used for opacification of the excretory system followed by 7 min delayed second bolus of 50 ml for parenchyma and veins enhancement and a third 20 s delayed bolus of 65 ml for arterial enhancement.
The choice between different technical approaches should depend on the context. Indeed, if reducing the amount of radiation exposure is crucial for young patients with benign disease, it is less important in patients with severe conditions or malignant tumors.
In conclusion the study by Renard-Penna et al. [10] brings a consensus on CTU technique and strengthen the role of corticomedullary phase. They show that urothelial tumors avidly enhance and in fact may sometimes be better seen with an earlier phase of imaging on a background of normal urothelium and nonopacified urine (Fig. 7.1 and Fig. 7.2). Moreover, small or ‘en plaque’ urothelial lesions may be masked at excretory phase imaging and better detected at corticomedullary or nephrographic phase imaging.
Four-phase CTU after injection of furosemide. (a) Baseline acquisition showing slightly hyperdense material inside the ureter (arrow); (b) Cortico-medullary phase showing pathologic tissue with strong enhancement (arrow) also visible in nephrographic phase (c); (d) Delayed phase show no excretion of urine
2.1 Dual-Energy CT (DECT)
In the last decade the dual energy technology has been extensively employed in urologic imaging [14,15,16,17]. Briefly, DECT technology exploits the exposure of body tissues at two different energy radiation levels, obtained at the time of radiation beam creation and delivery (i.e., “dual sources” or “rapid switching” scanners) or during photon absorption (“double layer” technology) [18]. Hence, DECT is capable to distinguish materials with different atomic number, the so-called “material decomposition”, which results in several advantages.
The first one is achieving image datasets with noise correction and better iodine appraisal, thanks to linear/non-linear blending images and virtual monoenergetic reconstructions. The improved iodine visualization brings the opportunity of reducing injected CM volumes and therefore the risk of renal damage [19].
Moreover, the possibility of virtual non-contrast (VNC) reconstructions, namely the creation of unenhanced scan from the enhanced ones, permits to avoid baseline acquisition with consequent dose reduction [14, 17].
Nevertheless, iodine quantification within lesions can be obtained through quantitative (mg/mL per region of interest, ROI) or qualitative (Colour coded iodine overlay maps) assessment. This is particularly helpful in the evaluation of complex cysts or low attenuating masses and for pseudo-enhancement phenomenon recognition [20] (Fig. 7.3).
Dual Energy application in URO-CT performed with “split bolus” technique. (a) Unenhanced scan reconstruction from enhanced one (VNC) (b) Combined nephrographic-excretory phase showing a mass growth inside the collecting system of the right kidney (c) Color coded iodine overlay map better shows the iodine uptake of the lesion
Finally, material specific analysis of nephrolithiasis, with differentiation of uric acid stones from other types, can provide useful information for the subsequent patient management.
3 Benefits
Although ultrasound is usually performed as the first line imaging modality and its diagnostic potential can be significantly improved through the injection of intravenous contrast medium (CEUS) CT scan is currently considered the gold standard due to the wide availability, the fast scan times, and the comprehensive evaluation. Dedicated diagnostic renal imaging aids in the appropriate treatment planning for renal tumors and may avoid an unnecessary operation.
A conventional CT protocol routinely includes pre- and multiphasic post-contrast images. Volumetric datasets provided by modern CT scanners can be reconstructed in multiple planes and be of variable slices thickness preserving excellent image quality.
CT urography (CTU) also relies on the multiphase principle to focus on an “excretory” phase after the contrast has filtered into the collecting system and bladder, essentially creating an IV urogram with vastly improved tissue contrast.
4 Limitations
Even tough contrast-enhanced computed tomography is the reference standard for primary imaging of urinary tract, intrinsic limitations should be addressed.
Radiation exposure and contrast media nephrotoxicity are considered the main ones.
In a phantom study Vrtiska et al. showed that the range of effective doses for the evaluated CT urography protocols was 20.1–66.3 mSv using 4, 16, and 64 MDCT scanners. The number of phases, anatomic coverage per phase, and scanning parameters all contributed to this variation in dose [21]. The mean effective radiation dose that was reported in vivo in association with four-phase CT scan ranged from 15 to 35 mSv [22, 23].
Radiation dose reduction is extremely important specially in younger patients.
In these cases, first, the use of alternative imaging modalities as ultrasound and MRI must be always kept in consideration. If these techniques cannot provide the requested information than will be necessary to act on CT protocol.
In previous section was already described how radiation dose can be reduced by limiting the number of phases using dual-energy CT or split bolus technique. Radiation dose can be also reduced using low dose unenhanced scan as first step of CTU because the increased image noise is not a problem in stone detection thanks to the marked differences of attenuation between calculi and surrounding soft tissues [22, 24, 25]. Low dose protocol can be also applied on post-contrast phases thanks to machine-related dose reduction algorithms that allow a lower radiation exposure without a significant image quality worsening.
Contrast-enhanced CT studies are contraindicated in patients with an allergy to radiographic contrast media and in patients with impaired renal function.
To minimize contrast-induced nephropathy, contrast material should not be given to patients with Glomerular Filtration Rate (GFR) below 30 ml/min without carefully weighing the risks and benefits and it should be used with caution in patients with GFR ranging from 30 to 60 ml/min [26].
Beyond merely technical limits, other issues can come from image interpretation.
For instance, detection of smaller tumors can especially pose a challenge and differentiation among solid lesions is frequent unclear because of heterogeneous presentation of different histologic subtypes [27].
Distinguish between simple and complex cystic lesions could be also difficult because of low accuracy of CT on thin septa appraisal.
In such cases, different imaging approaches, through US and MRI, can be helpful in obtaining more detailed information about lesion features.
Response assessment following ablative therapies, anti-angiogenic and immunotherapies remains challenging, as reduction in tumor size may not occur. The pattern of enhancement on CT may be a more reliable indicator of treatment success. However, a number of emerging techniques have been investigated.
CT texture analysis using an image processing algorithm to assess heterogeneity in tumor morphology has been proposed as a marker of response. Functional imaging has been investigated, including dynamic contrast-enhanced (DCE) CT, DCE-MRI, DCE-ultrasound and PET. DCE imaging follows the bio-distribution of a contrast agent injected intravenously and then absorbed into the tumor microcirculation, providing information on the tumor microenvironment and vascularity pre- and post-anti-angiogenic therapy [28].
5 Future
In the new era of precision medicine , the ability to extrapolate, from radiological images, quantitative data is the challenge of the present and the next future. This process, known as radiomics, was first invented by Lambin in 2012, it is based on the concept that clinical images contain quantitative features, which may reflect the underlying pathophysiology of a tissue [29, 30].
Machine-Learning (ML) algorithms are emerging tools that may support radiomics. They lead to the selection of appropriate features that can be analysed by dedicated software.
The use of these assays can improve medical decision-making and it finds space especially in oncology, for example, allowing an evaluation of cancer microenvironment and influencing treatment choice. In the last few years, many studies have been carried out on the application of this method even for the evaluation of urothelial cancer, but it is still a prerogative of research.
In a review Zhang et al. summarize their studies and literature about radiomics applied in urothelial cancer to predict pathological grade, clinical stage, lymph node metastasis and treatment response. These studies demonstrate the capability of radiomics to assist more precise characterization and stratification of patients with urothelial cancer.
In particular Zhang et al., Mammen et al., Wang et al. in their papers revealed that texture features extracted from CT or MRI images could reflect the difference between low- and high-grade urothelial carcinoma [31,32,33,34].
Despite the huge potentiality, radiomics still needs improvements such as standardized data collection and evaluation criteria to become applicable in clinical practice.
References
Dyer RB, Chen MY, Zagoria RJ. Intravenous urography: technique and interpretation. Radiographics. 2001;21(4):799–821.
WSES-AAST Expert Panel, Coccolini F, Moore EE, et al. Kidney and uro-trauma: WSES-AAST guidelines. World J Emerg Surg. 2019;14:545.
Ali O, Fishman EK, Sheth S. Correction to: upper urinary tract urothelial carcinoma on multidetector CT: spectrum of disease. Abdom Radiol. 2020;45:889.
Gray Sears CL, Ward JF, Sears ST, et al. Prospective comparison of computerized tomography and excretory urography in the initial evaluation of asymptomatic microhematuria. J Urol. 2002;168:2457–24607.
Albani JM, Ciaschini MW, Streem SB, et al. The role of computerized tomographic urography in the initial evaluation of hematuria. J Urol. 2007;177:644–8.
Ascenti G, Zimbaro G, Mazziotti S, et al. Doppler power with contrast media in the characterization of renal masses. Radiol Med (Torino). 2000;100:168–74.
Fried JG, Morgan MA. Renal imaging: core curriculum 2019. Am J Kidney Dis. 2019;73:552–65.
CT Urography Working Group of the European Society of Urogenital Radiology (ESUR), Van Der Molen AJ, Cowan NC, et al. CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18:4–17.
Hiram Shaish. Making sense of the CT Urogram European. Radiology. 2020;30:1385–6.
Renard Penna R, Rocher L, Roy C, et al. Imaging protocols for CT urography: results of a consensus conference from the French society of genitourinary imaging. Eur Radiol. 2020;30(3):1387–96.
Noroozian M, Cohan RH, Caoili EM, et al. Multislice CT urography: state of the art. Br J Radiol. 2004;77:S74–86.
Cheng K, Cassidy F, Aganovic L, et al. CT urography: how to optimize the technique. Abdom Radiol. 2019;44:3786–99.
Kekelidze M, Dwarkasing RS, Dijkshoorn ML, et al. Kidney and urinary tract imaging: triple-bolus multidetector CT urography as a one-stop shop—protocol design, opacification, and image quality analysis. Radiology. 2010;255:508–16.
Graser A, Johnson TRC, Hecht EM, et al. Dual-energy CT in patients suspected of having renal masses: can virtual nonenhanced images replace true nonenhanced images? Radiology. 2009;252:433–40.
Ascenti G, Mileto A, Gaeta M, et al. Single-phase dual-energy CT urography in the evaluation of haematuria. Clin Radiol. 2013;68:e87–94.
Mileto A, Marin D. Dual-energy computed tomography in genitourinary imaging. Radiol Clin N Am. 2017;55:373–91.
Marino MA, Silipigni S, Barbaro U, et al. Dual energy CT scanning in evaluation of the urinary tract. Curr Radiol Rep. 2017;5:46.
Cicero G, Ascenti G, Albrecht MH, Blandino A, Cavallaro M, D’Angelo T, Carerj ML, Vogl TJ, Mazziotti S. Extra-abdominal dual-energy CT applications: a comprehensive overview. Radiol Med. 2020;125(4):384–97.
Mileto A, Ramirez-Giraldo JC, Marin D, et al. Nonlinear image blending for dual-energy MDCT of the abdomen: can image quality be preserved if the contrast medium dose is reduced? AJR Am J Roentgenol. 2014;203:838–45.
Wang ZJ, Coakley FV, Fu Y, et al. Renal cyst pseudoenhancement at multidetector CT: what are the effects of number of detectors and peak tube voltage? Radiology. 2008;248:910–6.
Vrtiska TJ, Hartman RP, Kofler JM, Bruesewitz MR, King BF, McCollough CH. Spatial resolution and radiation dose of a 64-MDCT scanner compared with published CT urography protocols AJR. Am J Roentgenol. 2009;192(4):941–8.
O’Connor OJ, Maher MM. CT urography. Am J Roentgenol. 2010;195:W320–4.
Caoili EM, Inampudi P, Cohan RH, Ellis JH. Optimization of multi-detector row CT urography: effect of compression, saline administration, and prolongation of acquisition delay. Radiology. 2005;235:116–23.
O’Connor OJ, McSweeney SE, Maher MM. Imaging of hematuria. Radiol Clin N Am. 2008;46:113–32.
Graser A, Johnson TR, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. 2009;19:13–2.
Wymer DC. Imaging. Comp Clin Nephrol. 2010;2010:56–74.
van Oostenbrugge TJ, Fütterer JJ, Mulders PFA. Diagnostic imaging for solid renal tumors: a pictorial review. Kidney Cancer. 2018;2(2):79–93.
Rossi SH, Prezzi D, Kelly-Morland C, Goh V. Imaging for the diagnosis and response assessment of renal tumours. World J Urol. 2018;36(12):1927–42.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–6.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62.
Zhang G, Xu L, et al. Current applications and challenges of radiomics in urothelial cancer. Chin J Acad Radiol. 2020;2:56–62.
Mammen S, Krishna S, Quon M, Shabana WM, Hakim SW, Flood TA, et al. Diagnostic accuracy of qualitative and quantitative computed tomography analysis for diagnosis of pathological grade and stage in upper tract urothelial cell carcinoma. J Comput Assist Tomogr. 2018;42(2):204–10.
Zhang X, Xu X, Tian Q, Li B, Wu Y, Yang Z, et al. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J Magn Reson Imaging. 2017;46(5):1281–8.
Wang H, Hu D, Yao H, Chen M, Li S, Chen H, et al. Radiomics analysis of multiparametric MRI for the preoperative evaluation of pathological grade in bladder cancer tumors. Eur Radiol. 2019.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bottari, A. et al. (2021). CT Scan. In: Huri, E., Veneziano, D. (eds) Anatomy for Urologic Surgeons in the Digital Era. Springer, Cham. https://doi.org/10.1007/978-3-030-59479-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-59479-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59478-7
Online ISBN: 978-3-030-59479-4
eBook Packages: MedicineMedicine (R0)