Abstract
The recently emerged coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causal agent of COVID-19, is the newest threat to human health. It has already infected more than 54.5 million people worldwide, currently leading to more than 1.3 million deaths. Although it causes a mild flu-like disease in most patients, lethality may increase to more than 20% in elderly subjects, especially in those with comorbidities, like hypertension, diabetes, or lung and cardiac disease, and the mechanisms are still elusive. Common symptoms at the onset of illness are fever, cough, myalgia or fatigue, headache, and diarrhea or constipation. Interestingly, respiratory viruses have also placed themselves as relevant agents for central nervous system (CNS) pathologies. Conversely, SARS-CoV-2 has already been detected in the cerebrospinal fluid. Here, we discuss several clinical features related to CNS infection during COVID-19. Patients may progress from headaches and migraines to encephalitis, stroke, and seizures with leptomeningitis. However, the pathway used by the virus to reach the brain is still unknown. It may infect the olfactory bulb by retrograde neuronal transportation from olfactory epithelium, or it could be transported by the blood. Either way, neurological complications of COVID-19 add greatly to the complex pathophysiology of the disease. Neurological signs and symptoms must alert physicians not only to worst outcomes but also to future possible degenerative diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Viral respiratory diseases are among the most critical problems in public health as every year they are responsible for high rates of mortality [1]. The recently emerged coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the newest threat to human health. SARS-CoV-2 is an enveloped non-segmented positive-sense RNA virus that belongs to the Coronaviridae family [3]. It is closely related to previous coronaviruses of medical relevance, known as SARS-CoV and MERS-CoV. Since December of 2019, the first cases of pneumonia started to be documented in Wuhan, China [1, 2]. It has already infected more than 54.5 million people worldwide, leading to around 1.3 million deaths. The World Health Organization (WHO) officially declared a state of public health emergency of international concern in February 2020 due to the fast spread and lethality of coronavirus disease 2019 (COVID-19) [4, 5].
Although it causes mild flu-like disease in most patients, lethality may increase to 20% in elderly subjects, especially those with comorbidities, like hypertension, diabetes, or lung and cardiac disease [6], and the mechanisms are still elusive [7]. Viral replication in lung tissue leads to direct and indirect pathologies, mainly due to an exacerbated immune response and the cytokine storm produced [5]. Common symptoms at the onset of illness are fever, cough, myalgia or fatigue, headache, and diarrhea or constipation [3, 6]. Severe cases rapidly evolve to pneumonia with “ground-glass opacities” observed by computerized tomography (CT) imaging, evidencing lung infiltration and edema.
Interestingly, respiratory viruses are also capable of causing central nervous system (CNS) pathologies as seen for human respiratory syncytial virus (hRSV) [8] or human metapneumovirus (hMPV) [9]. In fact, several studies have described the association between respiratory viral infections with neurological symptoms as febrile or afebrile seizures, status epilepticus, encephalopathies, and encephalitis [1]. With regard to the recent COVID-19 epidemic, several patients have referred to the loss of the sense of smell and taste during hospitalization. This may be an important feature of COVID-19, but it is still poorly understood.
2 Coronaviruses and the Nervous System
Coronaviruses invade host cells through the interaction of spike proteins (SPs) with membrane receptors, such as angiotensin-converting enzyme 2 (ACE2) [10], dipeptidyl peptidase 4 (DPP4) [11], and, most recently, CD147 [12]. The ACE2 receptor was shown to have an interferon-driven expression that can be used for SARS-CoV-2 to gain access into human cells [13]. A study by Li et al. supported these findings by demonstrating ACE2 expression in many human tissues, including the brain, and this was positively correlated with interferon levels [14]. After attachment, virus particles are internalized and fused with the cell membrane, and the RNA genome is released within the cytoplasm for protein translation and replication. In this context, viral tropism and pathology intimately correlate with the expression of the aforementioned receptors throughout the body [4].
Despite their well-known respiratory effects, coronaviruses are not always confined to the respiratory tract as they may also invade intestine [15], heart tissue [16,17,18], and the CNS [19,20,21]. For example, it is already known that the human coronavirus OC43 (HCoV-OC43) gains access to the CNS through axonal transport and neuron-to-neuron propagation in experimental models [22]. Interestingly, HCoV-43 viral loads in the brain of C57Bl/6 mice reached the same levels when intra-cranioventricular and intranasal delivery were compared. The inoculation of 104 TCID50 led to a time-dependent increase in brain viral load. Moreover, viral N proteins were detected by immunofluorescence, evidencing viral migration through the neurons. Noteworthy, viral proteins were detected as early as 5 days after infection [22].
Interestingly, coronaviruses may reach the CNS through the blood, either by crossing the blood-brain barrier or via the olfactory bulb and retrograde transportation, as previously demonstrated in mice [4, 23, 24]. Additionally, it has been shown that murine hepatitis virus (MHV), another type of coronavirus, may reach the CNS after intranasal delivery. Corroborating this, ablation of the olfactory nerve cells abrogated CNS infection after nasal inoculation with MHV [25]. It is noteworthy that endothelial damage can also facilitate virus access [4].
SPs are the components of SARS-CoV-2 that interact with high affinity to human ACE2 on target cells through its receptor-binding domain (RBD) [4, 26, 27]. SPs consist of an S1 subunit, which is involved in receptor recognition, and an S2 region involved in membrane fusion [26, 27]. This latter subunit must be cleaved to properly interact with ACE2, which can be mediated by transmembrane protease, serine 2 (TMPRRS2) protease, cathepsin B (CTSB), or cathepsin L (CTSL) [28]. The interaction of SARS-CoV-2 with ACE2 receptors in neurons leads to neuronal damage without substantial inflammation [4].
The olfactory epithelium is mainly composed of olfactory sensory neurons (OSNs) responsible for odor detection and transmission to the brain [28]; globose basal cells (GBCs) responsible for neurogenesis, renewing olfactory epithelium and neurons [29, 30]; horizontal basal cells (HBCs), which are quiescent cells and a stem cell reservoir [30]; and sustentacular cells, which act as structural support for OSNs [28]. OSNs in the olfactory bulb form synapses through the cribriform plate [29].
As demonstrated by Brann et al., ACE2 and TMPRSS2 are not expressed in mature OSNs but in sustentacular cells and HBCs instead, which are believed to be the target cells of SARS-CoV-2 infection [28]. Thus, infection of sustentacular cells and HBCs may damage OSNs resulting in anosmia. Another possible pathway for viruses to infect the CNS is through the olfactory bulb [31,32,33]. OSNs connect the nasal cavity to the CNS through the axons, which terminate in the olfactory bulb, transposing the cribriform plate [31]. On the other hand, the olfactory bulb receives dense innervation from higher brain areas to process odor information [33] and is possibly infected by coronaviruses [28]. Therefore, the olfactory deficits may occur due to other mechanisms than olfactory epithelium damage such as higher-order olfactory structures affection, as mentioned by Brann et al. [28]. However, more information is needed to determine this.
There was clear evidence of CNS presence of SARS-CoV in brain tissues of patients with SARS in the early 2000s [19,20,21]. One of these studies found viral RNA of SARS-CoV infection in the brain of eight autopsied patients, and six of these had scattered red degeneration and edema in the cytoplasm of neurons from the hypothalamus and cortex [21].
In addition, Xu et al. reported a case of a 39-year-old doctor that was in contact with SARS-CoV patients and started to experience fever, chills, malaise, dizziness, and myalgia when he was admitted to the hospital [20]. After 35 days of illness onset, he died due to multiple organ failure and brain herniation. The autopsy revealed SARS-CoV RNA in the patient’s brain tissue. While examining autopsied tissue samples from four SARS-CoV patients, Ding and colleagues found evidence of virus infection on the cerebrum and pituitary gland but not in the cerebellum of all four cases [19].
There is additional evidence that human coronaviruses can infect the human brain. For example, Arbour et al. described the presence of coronavirus RNA in autopsied brain samples from patients with multiple sclerosis and other neurological diseases, such as Alzheimer’s, Parkinson’s, schizophrenia, depression, and meningoencephalitis [34].
3 Neurological Manifestations of COVID-19
The manifestations of neurological symptoms in patients with COVID-19 involve the CNS, peripheral nervous system (PNS), and skeletal muscles. Severe patients commonly have neurological symptoms manifested as acute cerebrovascular diseases, consciousness impairment, and muscle injury, leading to a poor prognosis [35]. In a study carried out by Chen and colleagues, 22% of those who died from COVID-19 presented with impaired consciousness, compared with only 1% of the patients that survived [36].
CNS symptoms , such as headache, dizziness, impaired consciousness, ataxia, acute cerebrovascular disease, and epilepsy, were the main form of neurological injury in patients with the COVID-19 virus appearing in 53 out of 218 (24.8%) patients in a Chinese cohort [35]. Interestingly, patients presenting CNS involvement were associated with a more severe course of the disease [35]. On the other hand, PNS involvement occurred in 19 patients (8.9%), and hyposmia and dysgeusia were the most common symptoms, affecting 11 (5.1%) and 12 (5.6%), respectively. No differences in blood parameters were found in patients with or without PNS involvement [35].
There is still no clear evidence of severe neurological COVID-19 infection in children. However, despite the milder course of the disease, it is already known that children are susceptible to the virus with a prevalence of 1.7% in the United States [37]. To the best of our knowledge, Chacón-Aguilar et al. reported the first case of a febrile syndrome associated with neurological symptoms in early childhood [38]. It was a newborn (26 days) with two paroxysmal episodes, a 12 h fever, and nasal discharge and vomiting. On physical examination, the child was alert with mild hypertonia of the limbs and irritability and slightly increased tendon reflexes with normal tone. A nasopharyngeal swab sample tested positive for SARS-CoV-2. There were no changes in the cerebrospinal fluid (CSF). The patient was treated symptomatically and had a good outcome [38]. Considering the current epidemiological situation, fever and convulsive episodes should be suggestive of coronavirus infection and demanding early intervention and extra care of the clinical team.
In 2006, Hwang described a case of complete anosmia 3 weeks after the onset of the first symptoms of SARS-CoV infection [39]. The patient was a 27-year-old woman who presented with fever, cough, headache, myalgia, and diarrhea. Three weeks later, after upper respiratory tract improvement, the patient had complete anosmia for all kinds of odors on both sides of the nasal cavity. Although no abnormal findings that might cause anosmia were found on physical examination or via brain magnetic resonance imaging (MRI), this symptom persisted for the 2 years of follow-up without change [39]. As far as we know, this is the first case report of persisting anosmia after coronavirus infection. Further investigation and patient follow-up studies are necessary given the current reports of anosmia in COVID-19 patients. Moreover, a long period of anosmia may be linked to CNS lesions.
Hyposmia is also gaining the attention of the media and the medical community [40, 41]. A recent study on COVID-19 patients conducted by Leichien and colleagues described olfactory (85.6%) and gustatory (88%) dysfunctions of 417 patients with mild to moderate disease [42]. Among these patients that suffered from olfactory alterations, 12.6% had phantosmia and 32.4% had parosmia, and out of the 76 patients that did not suffer from nasal obstruction or rhinorrhea, 79.7% presented anosmia or hyposmia. This suggests that olfactory neuropathy may play a role in olfactory dysfunction. Smell and/or taste disorders in COVID-19 infections appear to have a variable prevalence between 5 and 48% [43], and the short-term recovery rate from anosmia or hyposmia in 59 recovered patients was 44% [42]. This factor may also be used with a small degree of diagnostic accuracy as sudden loss of smell has shown a specificity of 97% and sensitivity of 65% for COVID-19 infection [44]. Whether or not long-term persistence of anosmia will be observed should be studied further.
Patients with COVID-19 can also present with encephalopathy and other changes in their level of consciousness. Recently, three cases of encephalitis associated with SARS-CoV-2 were described. A study by the Beijing hospital was the first to find the SARS-CoV-2 in a patient’s CSF [45]. In another case, the patient had encephalopathy and was positive for SARS-CoV-2 although no evidence of viral particles were detected in the CSF [46]. Therefore, if actual viral particles are present in CSF, it needs to be evaluated further.
Moriguchi and colleagues reported a case of meningitis/encephalitis in a 24-year-old man with no history of travel [47]. The suspicion of COVID-19 infection was made due to the patient’s poor general condition and altered blood count, as well as a chest CT scan showing small ground-glass opacities on the right superior lobe and both sides of the inferior lobe. The disease was confirmed by means of a polymerase chain reaction (PCR) test for SARS-CoV-2 using a nasopharyngeal swab and CSF. The samples were negative for the swab and positive for the CSF [47]. Neurological findings of coronavirus infections also include cases of acute disseminated encephalomyelitis (ADEM) [48].
Chronic complications have already been described in SARS patients who presented with chronic myalgia and mood and sleep disorders [49]. However, organic neurological damage was not described in these patients. Chronic complications of coronavirus infection in the CNS have already been studied in murine models involving human coronavirus (HCoV-OC43) and mouse hepatitis virus [50, 51].
Thromboembolic and hemorrhagic phenomena can also be secondary to infection by SARS-CoV-2. In a retrospective study of 221 patients in Wuhan, China, Li et al. described 13 patients with acute cerebrovascular disease following COVID-19 infection [52]. The incidence was 5% for acute ischemic stroke, 0.5% for cerebral venous sinus thrombosis, and 0.5% for cerebral hemorrhage. Most of these patients were older (70–91 years old) and therefore had more cardiovascular and cerebrovascular risk factors [52].
Moreover, a new pattern of cerebrovascular condition known as large-vessel stroke appears to affect the young population (33–49 years old). Oxley and colleagues reported five young patients with signs of hemiplegia, dysarthria, and reduced levels of consciousness [53]. Technical imaging examinations of CT and CT angiography scans showed infarction and thrombosis in the right internal carotid artery, left middle cerebral artery, right middle cerebral artery, or right posterior cerebral artery. In addition, at the time of hospital admission of these five patients, the National Institutes of Health Stroke Scale (NIHSS) mean score was 17, consistent with a severe stroke of large vessels [53]. These events are probably related to the prothrombotic effect of the inflammatory response to viral infection [52] and may also justify the use of anticoagulant therapies such as heparin. In fact, this has been an important and critical care observation in COVID-19 patients.
Most recently, Zhao and colleagues described the first association of Guillain-Barré syndrome (GBS) with COVID-19 infection [54]. This was a 61-year-old woman with a complaint of acute weakness in both legs and severe fatigue. Despite the travel history for Wuhan, no respiratory symptoms were reported until 7 days after the onset of GBS symptoms. Oropharyngeal swabs were positive for SARS-CoV-2 by PCR assay [54]. Hence, COVID-19 appears to assume a parainfectious profile, in which GBS and viral infection occur concurrently, instead of the classic postinfectious profile. Curiously, a similar situation has already been described with GBS and Zika virus infection [55]. Cases of Miller Fisher syndrome and polyneuritis cranialis, both rare variants of GBS, have also been reported in patients with COVID-19 [56].
Another possible intriguing neurological association of COVID-19 infection is Takotsubo syndrome , which is characterized by transient left ventricular dysfunction and may be related to dysautonomia of the nervous system [57]. The case involved an 83-year-old woman who presented with typical chest pain and elevation of the ST segment in all precordial leads with deep T-wave inversions on electrocardiogram examination. The highly sensitive cardiac troponin T biomarker was elevated at 1142 ng/L, which is more than 100-fold over normal levels. Imaging tests, such as echocardiography and coronary angiography, were consistent with Takotsubo syndrome, ruling out acute coronary syndrome [58]. During hospitalization, the patient began to experience fever and bilateral opacity on lung radiography. The nasopharyngeal swab was negative for SARS-CoV-2, but the initial positive immunoglobin A and negative immunoglobulin G serology pattern proved acute infection [58]. Considering the association of Takotsubo syndrome with neurological disorders [57, 59], we believe that SARS-CoV-2 infection may induce autonomic dysfunctions. However, more reports are needed to exclude a stress-induced complication.
4 Conclusions
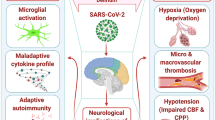
Although COVID-19 is mostly described as a lung disease, causing pneumonia and severe acute respiratory disease, several reports have indicated that patients may also display signs and symptoms related to effects on other organs. Here, we have discussed several CNS-related features, from mild symptoms such as headache, fatigue, and ataxia to more severe conditions as encephalitis, GBS, and stroke (Table 2.1 and Fig. 2.1). These have been observed in several patients, especially at the beginning of the disease. Although the virus has been detected in the CNS of patients, the mechanisms by which it reaches the brain are still unknown. It is possible that it directly infects the CNS through the olfactory epithelium or through the blood, either alone or transported by Trojan horse cells, as T lymphocytes may be infected by the virus with no productive replication (Fig. 2.1).
Scheme of SARS-CoV-2 neurological complications. SARS-CoV-2 may reach the central nervous system either by direct infection and retrograde transportation from olfactory neurons (1) or by a hematogenous route (2). After replication in the lungs, the virus may reach the blood either alone or inside infected cells, such as lymphocytes (3). The virus then target the central nervous system by crossing the blood-brain barrier and reaching the meninges, brain parenchyma, and cerebrospinal fluid (4). The scheme was elaborated by the authors using www.biorender.com
Due to the huge spread of the virus, and even if a small proportion of patients display neurological symptoms, it is important that the medical and research community be aware not only of these acute and critical problems like encephalitis and stroke but also of the possibility of further chronic or degenerative complications. It is important to be prepared to respond to such complications as occurred with the post-epidemic complications of the Zika virus [60,61,62] and von Economo’s famous encephalitis lethargy followed by Parkinsonism symptoms in those who survived the Spanish flu pandemic [63].
The involvement of the CNS was associated with more severe disease when compared to patients with no CNS involvement [35]. This neurotropism characteristic was observed with other human coronaviruses like SARS-CoV [19, 20] and HCoV OC43 [48, 50]. Thus, it is important to prioritize and to individualize treatment protocols based on the severity of the disease and the predominant organ systems involved. We recommend that in the presence of ataxia, loss of consciousness, convulsion, status epilepticus, encephalitis, myelitis, or neuritis [1, 35], differential diagnosis of COVID-19 should be considered, especially during the current pandemic.
The COVID-19 outbreak has spread worldwide, so careful surveillance is essential to monitor and control the disease. This is critical as clinical conditions of the patients can worsen rapidly, leading to respiratory failure. In addition, CNS-related symptoms may indicate a poor prognosis, and it is still not clear whether long-lasting impairments will be observed. Therefore, a fast and accurate diagnosis is necessary to allow the most effective interventions in a precision medicine approach. Such approaches will be more effective when new treatments that prevent or minimize the effects of COVID-19 become available.
Abbreviations
- ACE2:
-
angiotensin-converting enzyme 2
- ADEM:
-
acute disseminated encephalomyelitis
- CD147:
-
CD147-spike protein
- CNS:
-
central nervous system
- COVID-19:
-
coronavirus disease 2019
- CoVs:
-
coronaviruses
- CSF:
-
cerebrospinal fluid
- CTSB:
-
cathepsin B
- CTSL:
-
cathepsin L
- DPP4:
-
dipeptidyl peptidase 4
- ECG:
-
electrocardiogram
- GBC:
-
globose basal cells
- GBS:
-
Guillain-Barré syndrome
- HBC:
-
horizontal basal cells
- HCoV-OC43:
-
human coronavirus OC43
- hMPV:
-
human metapneumovirus
- hRSV:
-
human respiratory syncytial virus
- MERS:
-
Middle East respiratory syndrome
- MHV:
-
murine hepatitis virus
- NIHSS:
-
National Institutes of Health Stroke Scale
- OSN:
-
olfactory sensory neurons
- PNS:
-
peripheral nervous system
- RBD:
-
receptor-binding domain
- SARS:
-
severe acute respiratory syndrome
- SARS-CoV:
-
severe acute respiratory syndrome coronavirus
- SARS-CoV-2:
-
severe acute respiratory syndrome coronavirus 2
- SP or S protein:
-
spike proteins
- STD:
-
smell and/or taste disorders
References
Bohmwald K, Gálvez N, Ríos M, Kalergis A (2018) Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 12:386. https://doi.org/10.3389/fncel.2018.00386
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Baig A, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11(7):995–998
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y et al (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. pii: ciaa248. https://doi.org/10.1093/cid/ciaa248. [Epub ahead of print]
Sun D, Li H, Lu X, Xiao H, Ren J, Zhang F et al (2020) Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. https://doi.org/10.1007/s12519-020-00354-4. [Epub ahead of print]
Pinto B, Oliveira A, Singh Y, Jimenez L, Goncalves A, Ogava R et al (2020) ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. medRxiv. https://doi.org/10.1101/2020.03.21.20040261
Morichi S, Kawashima H, Ioi H, Ushio M, Yamanaka G, Kashiwagi Y et al (2009) Cerebrospinal fluid NOx (nitrite/nitrate) in RSV-infected children with CNS symptoms. J Infect 59(4):299–301
Schildgen O, Glatzel T, Geikowski T, Scheibner B, Simon A, Bindl L et al (2005) Human metapneumovirus RNA in encephalitis patient. Emerg Infect Dis 11(3):467–470
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Raj V, Mou H, Smits S, Dekkers D, Müller M, Dijkman R et al (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495(7440):251–254
Wang K, Chen W, Zhou Y, Lian J, Zhang Z, Du P et al (2020) SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. medRxiv. https://doi.org/10.1101/2020.03.14.988345
Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas C et al (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell (In Press, Journal Pre-proof). https://doi.org/10.1016/j.cell.2020.04.035
Li M, Li L, Zhang Y, Wang X (2020) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9(1):45. https://doi.org/10.1186/s40249-020-00662-x
Leung W, To K, Chan P, Chan H, Wu A, Lee N et al (2003) Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125(4):1011–1017
Dimitrov D (2003) The secret life of ACE2 as a receptor for the SARS virus. Cell 115(6):652–653
Gu J, Korteweg C (2007) Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 170(4):1136–1147
Oudit G, Kassiri Z, Jiang C, Liu P, Poutanen S, Penninger J et al (2009) SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investig 39(7):618–625
Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J et al (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203(2):622–630
Xu J, Zhong S, Liu J, Li L, Li Y, Wu X et al (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41(8):1089–1096
Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y et al (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–424
Dubé M, Le Coupanec A, Wong A, Rini J, Desforges M, Talbot P (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92(17):pii: e00404–pii: e00418. https://doi.org/10.1128/JVI.00404-18
Bleau C, Filliol A, Samson M, Lamontagne L (2015) Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. J Virol 89(19):9896–9908
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–7275
Perlman S, Evans G, Afifi A (1990) Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med 172(4):1127–1132
Li W, Zhang C, Sui J, Kuhn J, Moore M, Luo S et al (2005) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24(8):1634–1643
Hulswit R, de Haan C, Bosch B (2016) Coronavirus spike protein and tropism changes. Adv Virus Res 96:29–57
Brann D, Tsukahara T, Weinreb C, Logan D, Datta S (2020) Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.03.25.009084
Choi R, Goldstein BJ (2018) Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol 3(1):35–42
Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A et al (2017) Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell 20(6):817–830.e8
Durrant DM, Ghosh S, Klein RS (2016) The olfactory bulb: an immunosensory effector organ during neurotropic viral infections. ACS Chem Neurosci 7(4):464–469
Doty RL (2019) Systemic diseases and disorders. Handb Clin Neurol 164:361–387
Wen P, Rao X, Xu L, Zhang Z, Jia F, He X et al (2019) Cortical organization of centrifugal afferents to the olfactory bulb: mono- and trans-synaptic tracing with recombinant neurotropic viral tracers. Neurosci Bull 35(4):709–723
Arbour N, Day R, Newcombe J, Talbot P (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74(19):8913–8921
Mao L, Wang M, Chen S, He Q, Chang J, Hong C et al (2020) Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127. [Epub ahead of print]
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G et al (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ. 2020 Mar 31;368:m1295]. BMJ 368:m1091. https://doi.org/10.1136/bmj.m1091
CDC COVID-19 Response Team (2020) Coronavirus disease 2019 in children – United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 69(14):422–426
Chacón-Aguilar R, Osorio-Cámara J, Sanjurjo-Jimenez I, González-González C, López-Carnero J, Pérez-Moneo-Agapito B (2020) COVID-19: fever syndrome and neurological symptoms in a neonate. An Pediatr (Engl Ed) 27. https://doi.org/10.1016/j.anpede.2020.04.001. [Epub ahead of print]
Hwang C (2006) Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol Taiwanica 15(1):26–28
Stone J (2020) There’s an unexpected loss of smell and taste in coronavirus patients Forbes. https://www.forbes.com/sites/judystone/2020/03/20/theres-an-unexpected-loss-of-smell-and-taste-in-coronavirus-patients/#40567ff45101
Hopkins C, Kumar N (2020) Loss of sense of smell as a marker of COVID-19 infection. London: Ear, nose, and throat surgery professional membership in the United Kingdom (ENT UK). https://www.entuk.org/covid-19
Lechien J, Chiesa-Estomba C, De Siati D, Horoi M, LeBon S, Rodriguez A et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-020-05965-1. [Epub ahead of print]
Beltrán-Corbellini Á, Chico-García J, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J et al Acute-onset smell and taste disorders in the context of Covid-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. https://doi.org/10.1111/ene.14273. [Epub ahead of print]
Haehner A, Draf J, Draeger S, de With K, Hummel T (2020) Predictive value of sudden olfactory loss in the diagnosis of COVID-19. medRxiv. https://doi.org/10.1101/2020.04.27.20081356
Zhou L, Zhang M, Gao J, Wang J (2020) Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis:101642. https://doi.org/10.1016/j.tmaid.2020.101642. [Epub ahead of print]
Filatov A, Sharma P, Hindi F, Espinosa P (2020) Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 12(3):e7352. https://doi.org/10.7759/cureus.7352
Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J et al (2020) A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 94:55–58
Ann Yeh E, Collins A, Cohen M, Duffner P, Faden H (2003) Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 113(1):e73–e76
Moldofsky H, Patcai J (2011) Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 11:37. https://doi.org/10.1186/1471-2377-11-37
Jacomy H, Fragoso G, Almazan G, Mushynski W, Talbot P (2006) Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 349(2):335–346
Hosking M, Lane T (2010) The pathogenesis of murine coronavirus infection of the central nervous system. Crit Rev Immunol 30(2):119–130
Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. https://doi.org/10.2139/ssrn.3550025
Oxley T, Mocco J, Majidi S, Kellner C, Shoirah H, Singh I et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382(20):e60. https://doi.org/10.1056/NEJMc2009787
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19(5):383–384
Siu R, Bukhari W, Todd A, Gunn W, Huang QS, Timmings P (2016) Acute Zika infection with concurrent onset of Guillain-Barre syndrome. Neurology 87:1623–1624
Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R et al (2020) Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. pii: 10.1212/WNL.0000000000009619. https://doi.org/10.1212/WNL.0000000000009619. [Epub ahead of print]
Ramos Ttito A, TipismanaBarbaran M, Najar Trujillo N, Escalaya Advincula A, Icumina Arevalo K, Moscol Ato G et al (2017) Takotsubo cardiomyopathy as a sequel of severe Dysautonomia from Guillain-Barré syndrome. J Neurol Sci 381:919–920
Meyer P, Degrauwe S, Delden C, Ghadri J, Templin C (2020) Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. pii: ehaa306. https://doi.org/10.1093/eurheartj/ehaa306. [Epub ahead of print]
Martins R, Barbarot N, Coquerel N, Baruteau A, Kolev I, Vérin M (2010) Takotsubo cardiomyopathy associated with Guillain–Barré syndrome: a differential diagnosis from dysautonomia not to be missed. J Neurol Sci 291(1–2):100–102
Nunes M, Carlini C, Marinovic D, Neto F, Fiori H, Scotta M et al (2016) Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J Pediatr 92(3):230–240
Cugola F, Fernandes I, Russo F, Freitas B, Dias J, Guimarães K et al (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534(7606):267–271
Figueiredo C, Barros-Aragão F, Neris R, Frost P, Soares C, Souza I et al (2019) Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun 10(1):3890. https://doi.org/10.1038/s41467-019-11866-7
Lutters B, Foley P, Koehler P (2018) The centennial lesson of encephalitis lethargica. Neurology 90(12):563–567
Conflicts of Interest/Competing Interests
The authors report no disclosures.
Availability of Data and Material
We utilized publicly available data.
Funding
JPSP is funded by FAPESP grants #2017/26170–0 and 2017/22504–1 and CNPq (301287/2016–3). No other targeted funding is reported.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bandeira, I.P., Schlindwein, M.A.M., Breis, L.C., Peron, J.P.S., Gonçalves, M.V.M. (2021). Neurological Complications of the COVID-19 Pandemic: What Have We Got So Far?. In: Guest, P.C. (eds) Clinical, Biological and Molecular Aspects of COVID-19. Advances in Experimental Medicine and Biology(), vol 1321. Springer, Cham. https://doi.org/10.1007/978-3-030-59261-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-59261-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59260-8
Online ISBN: 978-3-030-59261-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)