Abstract
The pancreatic ductal adenocarcinoma (PDAC) microenvironment is a diverse and complex milieu of immune, stromal, and tumor cells and is characterized by a dense stroma, which mediates the interaction between the tumor and the immune system within the tumor microenvironment (TME). The interaction between stromal and tumor cells signals and shapes the immune infiltration of TME. The desmoplastic compartment contains infiltrated immune cells including tumor-associated macrophages (TAMs) and large numbers of fibroblasts/myofibroblasts dominated by pancreatic stellate cells (PSCs) which contribute to fibrosis. The highly fibrotic stroma with its extensive infiltration of immunosuppressive cells forms the major component of the pro-tumorigenic microenvironment (Laklai et al. Nat Med 22:497–505, 2016, Zhu et al. Cancer Res 74:5057–5069, 2014) provides a barrier to the delivery of cytotoxic agents and limits T-cell access to tumor cells (Feig et al. Proc Natl Acad Sci USA 110:20212–20217, 2013, Provenzano et al Cancer Cell 21:418–429, 2012). Activated PSCs reduced infiltration of cytotoxic T cells to the juxtatumoral stroma (immediately adjacent to the tumor epithelial cells) of PDAC (Ene-Obong et al. Gastroenterology 145:1121–1132, 2013). M1 macrophages activate an immune response against tumor, but M2 macrophages are involved in immunosuppression promoting tumor progression (Noy and Pollard Immunity 41:49–61, 2014, Ruffell et al. Trends Immunol 33:119–126, 2012). The desmoplastic stroma is reported to protect tumor cells against chemotherapies, promoting their proliferation and migration. However, experimental depletion of the desmoplastic stroma has led to more aggressive cancers in animal studies (Nielsen et al. World J Gastroenterol 22:2678–2700, 2016). Hence reprogramming rather than simple depletion of the PDAC stroma has the potential for developing new therapeutic strategies for PC treatment. Modulation of PSCs/fibrosis and immune infiltration/inflammation composes the major aspects of TME reprogramming.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumor microenvironment (TME)
- Pancreatic stellate cells (PSCs)
- Extracellular matrix (ECM)
- Gemcitabine
- Pancreatic ductal adenocarcinoma (PDAC)
- Hypoxia
- Hypoxia-inducible factors (HIFs)
- Alpha-smooth muscle actin (α-SMA)
- Collagens
- Tumor immune response
- Tumor infiltrating lymphocytes (TILs)
- Myeloid-derived suppressor cells (MDSCs)
- Tumor-associated macrophages (TAMs)
- Immune checkpoint proteins
- Cytotoxic T cells
15.1 Introduction
Pancreatic cancer (PC) is one of the most lethal malignancies worldwide, with a 5-year survival rate less than 10% [9]. During the last decade, with the progression of pharmaceutics, increasing numbers of novel therapeutic regimens have been used to improve the prognosis of several types of cancers, including melanoma, breast, lung, colorectal, and pancreatic cancers. However, the long-term survival rate in PC has only been marginally improved. More importantly, the death rate of PC continues to increase by 0.3% per annum and is estimated to be the second leading cause of cancer-related death in the United States by 2030 [10].
Currently, gemcitabine-based chemotherapeutic strategy , used as the standard of care for regional and metastatic PC, only delivers modest improvement in patient survival. The more aggressive combination chemotherapy, FOLFIRINOX (folinic acid, fluorouracil, irinotecan, oxaliplatin), has prolonged overall median survival in advanced PC to 11.1 months compared to 6.8 months by gemcitabine single-agent treatment. However, it can only be used in patients with good health condition due to its extremely high toxicity. Moreover, the emerging novel immunotherapies (e.g., anti-PD-1/PD-L1) tested in clinical settings have only shown very limited therapeutic efficacy. Treatment targeting tumor cells (which represent <50% tissue mass in PC) only does not yield a satisfied result. Therefore, a growing research interest is now focused on the tumor microenvironment (TME). Indeed, PC is a stroma-rich cancer, of which over 90% is consisted of cellular (e.g., pancreatic stellate cells, infiltrating immune cells, and endothelial cells) and noncellular (extracellular matrix, cytokines, chemokines, and growth factors) stromal component. Increasing efforts have been made to elucidate the stroma biology to provide more extensive insights into tumor-stroma crosstalk. However, it is still under debate about the stroma in PC being “friend or foe.” Some early studies have revealed that stroma component, served as “partner in crime,” stimulated the initiation and progression of PC [11]. Also, it is believed that therapeutic failures have been attributed to the dense fibrotic stroma and collapsed vasculature resulting from extensive desmoplastic reaction [12, 13]. On the other hand, there are evidences showing that the stroma has protective effects, and the depletion of stroma leads to cancer metastasis [14]. All these findings suggest that TME is not just a static entity, and the role of the stroma is constantly changing in different context and time period [15]. It is postulated that activation and upregulation of the stroma reaction reflect the intention to isolate tumor cells and protect host during the early stage of tumorigenesis; however, with the progression of the tumor, tumor cells could evolve to obtain the ability to induce the stroma cells into criminal accomplice [16].
In this chapter, we will break down the TME of PC into different components and discuss how each component contributes to tumor development as outlined in Fig. 15.1 and some roles of nerves in the TME of PC. We will also discuss existing treatments targeting the stromal components.
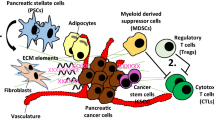
Regulation of pancreatic tumor microenvironment. The pancreatic cancer (PC) stromal sells play important roles in the dynamic changes that stimulate or inhibit the tumor growth and metastasis. The major stromal cells of PC include pancreatic stellate cells (PCSs), immune cells including regulatory T cells (Treg), active T cells, myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAMs) which is further divided into M1 and M2. The active T cells kill tumor cells, and M1 macrophages promote the antitumor immune response, while Th2 Treg, M2, and MDSC inhibit the antitumor immune response and promote tumor development. The extracellular matrix (ECM) functions as an important medium to integrate the interactions between the tumor cells (PC) and their surrounding microenvironment
15.2 Pancreatic Stellate Cells
Pancreatic stellate cells (PSCs) were firstly described as vitamin A-storing cells in the periacinar areas by Watari in 1982 via examination of the pancreas tissue collected from mice with excessive vitamin A administration [17]. Sixteen years later, in 1998, two different research groups published their work about isolation and culture of PSCs from rat and human tissues [18, 19], which was considered as a milestone in pancreatic research as they provided an invaluable method to investigate the biology of PSCs in vitro. In a quiescent state, PSCs comprise approximately 4–7% of the total cells in the pancreas and show a starlike morphology with abundant vitamin A lipid droplets in the cytoplasm surrounding the central nucleus. In an activated state, PSCs exhibit fibroblast-like morphology with the absence of vitamin A lipid droplets, start expressing alpha-smooth muscle actin (α-SMA) and increase secreting extracellular matrix (ECM) proteins including laminins, fibronectins, and collagens (more details in the following ECM section) [20]. As a well-known marker for PSC activation [21], α-SMA was reported to be a negative prognostic factor for patients with resectable pancreatic cancer [22, 23].
During the tumorigenesis of PC, cancer cells activate PSCs through secretion of various cytokines and growth factors such as transforming growth factor-beta1 (TGF-β1), interleukin 6 (IL-6), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), and these cytokines and growth factors also maintain cancer cells in the active state by autocrine mechanisms [24]. When co-cultured with cancer cells directly, PSCs increased cancer cells proliferation via activation the notch signaling pathway [25]. Conditioned medium collected from PSCs stimulated proliferation and invasiveness of cancer cells through upregulation of galectin-3 [26] and PDGF [13]. Some in vivo studies also revealed the critical tumor-stroma interaction in PC progression. Subcutaneous or orthotopic tumor mass isolated from the mice injected with the mixture of cancer cells and PSCs exhibited increased tumor growth, spread, and desmoplasia compared to the mice injected with the cancer cells alone [13, 27, 28]. Xu et al. demonstrated the role of PSCs in facilitating cancer cells metastasis in vivo. Using an orthotopic model, they found that mice injected with the mixture of cancer cells and PSCs showed increased metastasis and that the PSCs migrated along with the cancer cells from the primary site to the distant site [29]. Furthermore, inducing quiescence in PSCs by all-trans retinoic acid (ATRA) attenuated the pro-tumor effect of PSCs on cancer cells and suppressed tumor progression [30, 31]. In this regard, anti-stromal treatment with ATRA has been emerging as an attractive therapeutic approach. A phase 1B clinical trial (NCT03307148) was proposed to repurpose ATRA as an anti-stromal agent to increase the delivery of chemotherapeutic drugs by reprograming the stroma to decrease the desmoplastic reaction. A recent study also showed an important role of activated PSC in pro-angiogenic signaling. Induction of PSC quiescence by ATRA suppressed pro-angiogenesis and thus exerted a significant impact on vascularity in the stromal component [32].
Activated PSCs not only interact with cancer cells to facilitate tumor progression but also modulate the immune response in TME. PSCs inhibit antitumor immunity and facilitate immune evasion by blocking infiltration of T cells, suppressing the activation of effector T cells and stimulating immunosuppressive cells [11, 33]. An early study revealed that PSCs overexpressed Galectin-1 and induced T-cell (CD4+ and CD8+) apoptosis and IL-4 and IL-5 secretion by Th2 T regular cells, which would contribute to the immunosuppressive microenvironment of PC [34]. Moreover, stroma cells with high expression of fibroblast activation protein-alpha (FAP-α) disrupted the antitumor immunity in solid tumors. Depletion of FAP-α+ stroma cells in a subcutaneous model of PC caused tumor regression mediated by an immunogenic environment [35].
15.3 Extracellular Matrix (ECM)
The ECM is a 3D noncellular network of various cross-linked proteins. In normal conditions, quiescent PSCs are responsible for maintaining the balance between synthesis and degradation of ECM proteins. Once PSCs are activated, this physiological balance is impaired, leading to excessive accumulation of ECM proteins [36]. This desmoplastic reaction contributes to the alterations in mechanical and physical features as well as biochemical signaling transduction [1]. PC cells play important roles in mediating ECM production via recruiting and activating PSCs through secretion of sonic hedgehog (SHH) [37]. Additionally, PC cells also secrete various cytokines, e.g., fibroblast growth factor 2 (FGF2), PDGF, and TGFβ1, to stimulate PSC activation and collagen production [27]. Importantly, once activated, PSCs enter a positive feedback loop of maintaining active status by TGFβ1 autocrine, leading to constant desmoplasia [38]. An in vitro study showed that PSCs also maintained their activation through secretion of ECM protein periostin [39].
Except for its key role in tissue integrity, ECM also modulates cell biology by interacting with certain cell surface receptors and is recognized as a hallmark for certain cancer types, including liver, lung, and pancreas [40]. Collagens are the most well-characterized component of ECM in PC. Type I collagen plays an important role in the desmoplastic reaction of PC and is reported to be a potential pro-tumorigenic factor. An early study showed that PC patients with low collagen I deposition had a significantly improved survival compared to those with high collagen I deposition [41]. In contrast, type XV collagen has an antitumor effect. Reduced type XV collagen has been reported to be associated with tumor progression in colon cancer and breast cancer, while increased expression of type XV collagen led to decreased migration of PC cells [40]. By binding to the integrin receptors, type I and V collagens stimulated cell survival, proliferation, and migration and prevented apoptosis in PC cell lines [42, 43]. Knockdown of integrin-β1 reduced tumor growth and metastasis in an orthotopic PC mouse model by blocking the binding of these collagens to PC cells [44]. Focal adhesion kinase (FAK) is an important effector downstream of type I collagen-mediated signaling pathway. Once activated, FAK can regulate epithelial to mesenchymal transition (EMT) by decreasing E-cadherin, increasing N-cadherin, and stimulating Wnt signaling via upregulation of β-catenin phosphorylation [45, 46].
The abundant ECM compartment also contributes to mechanical alterations, such as tissue stiffness. In this regard, elevated interstitial fluid pressure is commonly found in desmoplastic stroma, which can prevent drugs from entering tumor mass. The sparse and collapsed vasculature in stroma can further impair the delivery of antitumor drugs, leading to therapeutic resistance. A previous study was reported that depletion of the interstitial hyaluronan significantly reduced the interstitial pressures and relieved the barrier to drug delivery [47]. In addition, the stiff stroma reduced tissue polarity, disrupted adherence junctions, enhanced tumor cell proliferation, and led to poor patient survival [1]. In contrast, another clinical study suggested that patients with dense stroma had significant improved overall survival compared to those with loose and moderate stroma [23].
15.4 Hypoxic Environment
The accumulation of ECM during the desmoplastic reaction distorts the normal pancreatic structure and compresses blood and lymphatic vessels in the stromal component. Pancreatic ductal adenocarcinoma is characterized by hypovascularity and low level of angiogenesis. In relation to tumor angiogenesis, it is noticed recently that type 2 pericytes promoted the formation of new blood vessels and thus may become a potential target to inhibit the tumor angiogenesis [48]. Microvessel density determined by CD31 immunohistochemistry was found significantly reduced in tumor compartment compared to its normal counterparts [15]. These features contribute to decreased perfusion, which in turn leads to impaired delivery of nutrients, drugs, and oxygen into pancreatic tumor tissues. Consequently, a hypoxic condition within the TME is formed. In an early study with small volume cases of PC patients, the oxygen pressure in the tumor tissues was measured in order to investigate the direct oxygenation in PC. This study revealed a remarkable reduction in oxygenation in tumor compartment compared to normal pancreas tissue [49]. However, most of the studies provide indirect evidence to evaluate the hypoxic status, such as necrotic lesions and hypoxia-inducible factor (HIF)-associated proteins [50].
It is widely accepted that cancer cells under hypoxic condition can orchestrate multiple signaling pathways necessary for regulating cell metabolism, survival, and metastasis. Ultimately these cancer cells become more malignant with high resistance to conventional chemo and radiotherapies [15]. HIF proteins are key mediators in cancer cell response to hypoxia. High expression and activity of HIF-1α enhanced gemcitabine resistance in PC cells by inhibition of gemcitabine uptake through downregulation of the transcription and expression of hENT1 and hENT2 [51]. HIF-1α was also involved in the recruitment of macrophages by promoting C-C motif chemokine ligand 2 secretion, and macrophage infiltration could further stimulate PSCs. In this regard, HIF-1α plays an important role, at least partially, in tumor immune response and desmoplastic reaction in TME [52]. Cells change their way for energy production under hypoxic condition. HIF-1α stimulates glucose supply by increasing transcription of GLUT1 and GLUT3 transporters and synthesis of pyruvate and lactate dehydrogenase [53]. HIF-1α also promotes PC cell survival in hypoxia via upregulation of autophagy [54]. Moreover, HIF-1α can facilitate EMT by regulation of NF-κB, snail, and slug, to increase pancreatic cancer cell migration [55, 56]. Li and colleagues found that HIF-1α knockdown in tumor targeted the delivery of CRISPR/Cas9 system and significantly suppressed the tumor growth and metastasis and improved survival in PC animal model. More importantly, this approach had limited impact on HIF-1α expression in normal cells, which significantly reduced toxicity in vivo [57].
15.5 Infiltrating Immune Cells
Chronic inflammation in the pancreas has been reported to drive pancreatic carcinogenesis. Patients with chronic pancreatitis have an increased risk of developing PC in 10–20 years [11]. Damaged acinar cells by various pancreatic injuries could release multiple inflammation-associated cytokines (e.g., interleukin 6 (IL-6), tumor necrosis factor (TNF)) , which promote the subsequent immune response, and stimulate the growth of precancerous cells [58]. Meanwhile, quiescent PSCs were activated in this inflammatory microenvironment, starting to produce massive amount of ECM proteins and orchestrate the network of acinar cells, infiltrating immune cells (IICs) and cancer cells [13]. IICs have both pro-tumor and antitumor effects. The former class of IICs includes regulatory T cells, myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAM), whereas the latter class includes CD8+ T cells, Th1-type CD4+ T cells, and natural killer cells [33, 59]. At early stage, activated immune cells can eliminate genetically altered precancerous cells. However, this anticancer immune response gradually becomes insufficient to suppress all altered cancer cells, with an increasing number of immunosuppressive cells attracted into the stromal compartment. These immunosuppressive cells can ward off the host immune defense and protect tumor cells from being recognized, leading to immune evasion, even in precancerous lesions such as pancreatic intraepithelial neoplasms (PanINs) and intraductal papillary mucinous neoplasms (IPMN) [60], and eventually a highly immunosuppressive microenvironment is formed. As an important component of the stromal microenvironment, IICs have been characterized as valuable markers in predicting prognosis.
A great attention has been paid to targeting the aberrant immune regulation of the tumor microenvironment, with the intention to remove the suppression of antitumor immunity. A good example is the conversion of pancreatic cancer from “a non-immunogenic malignancy” into “an immunogenic malignancy” by treatment with a novel immunomodulatory vaccine, and the immunotherapy using the checkpoint (PD-1/PD-L1, CTLA-4) blockade was more effective in the vaccine-treated patients than in the untreated patients [61].
15.5.1 Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) , including both granulocytic and monocytic subtypes, are a heterogeneous mixture of activated immature myeloid cells. They can promote tumor progression by inducing regulatory T cells and suppressing the activation of antigen-specific T cells [62, 63]. Oncogenic Kras mutation can reprogram the immunosuppressive microenvironment by recruiting circulating myeloid progenitor cells into the stroma and stimulating its differentiation into MDSCs through upregulation of tumor-derived granulocyte macrophage colony-stimulating factor (GM-CSF) [64]. In other types of cancers, MDSCs can also impair antitumor immunity by suppression of T-cell activation, conversion of macrophages to M2 phenotype, and inhibition of NK cells cytotoxicity [65, 66].
A previous study has reported the immunosuppressive role of MDSCs in the process from pancreatic precancerous lesions to malignant carcinoma using a genetically modified mouse model of PC [33]. This study showed the correlation between intra-tumoral MDSCs and the decreased tumor-infiltrating CD8+ cells. Both circulating and intra-tumoral MDSCs, of the granulocytic subset (Lin-HLA-DR-CD33+CD11b+CD15+), but not the monocytic subset (Lin-HLA-DR-CD14+), are significantly increased in PC patients compared to the healthy population [67]. Stromnes and colleagues reported the potential therapeutic benefits of targeting granulocytic MDSCs by showing that depletion of granulocytic MDSCs enhanced the antitumor T-cell immune response [68]. Additionally, MDSCs were also reported as an independent prognostic factor for patients’ survival as one unit increment in MDSC percentage led to a 22% greater risk of mortality [69].
15.5.2 Tumor-Associated Macrophages
In an established tumor microenvironment, PC cells can mediate differentiation of macrophages which reciprocally facilitate the progression of PC. Using a PC xenograft mouse model, Menen and colleagues have found a significantly increased tumor size and metastasis in the tumor-bearing mice implanted with tumor-educated macrophages compared to those with naive macrophage [70]. Similarly, another study also reported that the interaction between PC cells and macrophages played an important role in tumor progression, especially in those patients with hyperglycemia and diabetes mellitus [71].
Tumor-associated macrophages (TAMs) are divided into two groups: M1 (pro-inflammatory macrophages) and M2 (anti-inflammatory macrophages) . M1 TAMs can suppress tumor development by stimulating a T-cell-mediated antitumor response, whereas the interaction of M2 TAMs with tumor and stellate cells can stimulate secretion of various anti-inflammation cytokines and reprogram the tumor microenvironment to facilitate tumor progression [72]. PC cell-conditioned medium could convert naive macrophages to M2 phenotype in vitro. It has been reported that cytokines including IL-4, IL-10, and IL-13 produced by tumor cells or T cells induced M2 polarization of macrophages, which are predominant in the immunosuppressive tumor microenvironment [71]. Liu et al. reported that activation of toll-like receptor 4 on M2 macrophages induced the EMT in PC cells by increasing the secretion of IL-10 [73]. Clinical studies also revealed the important role of M2 macrophages in PC patient’s survival. PC patients with high infiltration of M2 macrophages had poor prognosis, which was because of the increased nodal lymphangiogenesis and lymphatic metastasis induced by M2 macrophage-induced VEGF-C production [74, 75].
HIF-1α enhances the recruitment of TAMs in the tumor microenvironment by stimulation of C-C motif chemokine ligand 2 (CCL2) secretion which in turn binds to chemokine (C-C motif) receptor 2 (CCR2) [52]. Mitchem et al. have shown that TAMs promoted tumor progression by activating the signal transducer and activator of transcription 3 (STAT3). Targeting TAMs by inhibition of the colony-stimulating factor-1 receptor (CSF1R) or CCR2 improved chemotherapeutic sensitivity, inhibited tumor metastasis, and upregulated antitumor T-cell responses [76]. In agreement, Sanford et al. also revealed the key role of CCL2/CCR2 in TAM recruitment. They found that the migration of circulating CCR2+ monocytes toward the tumor was massively blocked by a CCR2 antagonist (PF-04136309), which in turn led to a consequent depletion of TAMs in a PC mouse model. Their clinical data also showed a significantly decreased survival in patients with a higher level of CCL2 expression and infiltration of immunosuppressive CCR2+ TAMs [77]. Additionally, an early study showed that macrophages originated from tumor-bearing animals rather than those originated from non-tumor-bearing animals expressed VEGF receptor 2 (VEGFR2) , which is associated with VEGF-induced macrophage migration, and selective inhibition of VEGFR2 reduced infiltration of macrophages in orthotopic PC animal model [78].
15.5.3 Tumor-Infiltrating Lymphocytes
Tumor-infiltrating lymphocytes (TILs), including CD4+ helper T cells, CD8+ cytotoxic T cells, regulatory T cells, and B cells, are another class of immune cells, which are critical in modulating the tumor microenvironment in PC [8]. Indeed, an increasing number of studies have revealed the predictive value of stromal TILs in patients with resectable pancreatic cancer. Two of the latest studies have demonstrated that patients with the negative stromal TILs had larger tumor with more advanced stage and liver metastasis and showed worse overall survival [79].
CD8+ T cells are referred as cytotoxic T cells, with the capability of recognizing and killing tumor cells. However, the CD8+ cytotoxic T cells in circulation and tumor microenvironment are diminished in PC patients. Multiple studies have reported that the numbers of CD8+ T cells are reduced in PC patients compared with the normal population [80, 81]. The number of tumor-infiltrating CD8+ T cells is significantly lower in patients with PC than in patients with chronic pancreatitis [82]. Importantly, the infiltration of CD8+ cytotoxic T cells in the tumor microenvironment of PC is predominantly observed in low-grade precancerous lesions and much reduced during tumor development [83]. Ene-Obong and colleagues have revealed that CD8+ T cells were attracted by the activated PSC via secretion of CXCL12, leading to decreased numbers of CD8+ T cells in juxtatumoral compartments and reduced antitumor immunity [5]. Furthermore, tumor-infiltrating CD8+ T cells together with CD4+ T cells have been reported to be associated with tumor stage and act as a favorable predictive factor for survival [84]. It has been shown that increased numbers of tumor-infiltrating CD8+ T cells were significantly and independently related to the improved disease-free survival and overall survival [5, 85, 86].
PC cells can suppress the expression of cytolytic proteins (perforin and granzyme) in CD8+ T cells by producing immunosuppressive cytokine TGF-β [87]. Ellermeier et al. have demonstrated a potent antitumor effect of combining TGF-β gene silencing with activation of retinoic acid-inducible gene I in an orthotopic mouse model of pancreatic cancer [88]. Furthermore, PC cells can modify themselves to regulate the interaction with CD8+ T cells. On one hand, the expression of human leukocyte antigen class I was significantly reduced in PC cells, resulting in an escape from immune response by suppressing the infiltration of CD8+ T cells [86]. On the other hand, PD-L1 expressed and produced by PC cells, can bind to PD-1 on the active CD8+ T cells, inducing the cell death of these CD8+ T cells [89]. PD-1 blockade promotes the infiltration and cytotoxicity of the CD8+ T cells by producing tumor-specific interferon-γ and remarkably improves the mice survival when combined with granulocyte macrophage colony-stimulating factor-secreting PC vaccine (GVAX) [90].
CD4+ T cells play an essential role in activating and modulating both innate and adaptive immune responses. A decreased number of CD4+ T cells were observed in the circulation of PC patients compared to the healthy population [80] and also in PC tumor tissue compared to patients with chronic pancreatitis [82]. Importantly, the number of tumor-infiltrating CD4+ T cells was associated with PC patient’s survival [91].
Naive CD4+ helper T cells can be induced and differentiated into two groups: Th1 and Th2-polarized supopulations. The former contributes to cell-mediated immune response by killing intracellular pathogens, and the latter is involved in humoral immune response and fight against extracellular pathogens. In tumor-associated immune response, Th1 cells are generally related to antitumor activity, and Th2 cells have the potential of inducing immune tolerance and thus promote tumor development. In PC cases, the differentiation is predominantly shifted to Th2 cells due to various cytokines produced in its tumor microenvironment, such as IL-10 and TGF-β [92]. Therefore, reversing Th2 to Th1 phenotype could be used as a potential therapy to improve antitumor immune response. Tassi and colleagues have reported that combination treatment of IL-12 and IL-27 could revert Th2 phenotype to Th1 phenotype in carcinoembryonic antigen-specific CD4+ T cells derived from a PC patient [93].
Regulatory T cells (Tregs) are another subpopulation of CD4+ T cells, which are defined as CD4+CD25+Foxp3+ cells. Tregs are responsible for immunosuppressive activity by expressing CTLA-4 and secreting IL-10 and TGF-β. In homeostasis, Tregs prevent autoimmune response, whereas in tumorigenesis, Tregs facilitate tumor progression via downregulation of antitumor immune response by suppressing tumor-specific CD4+ and CD8+ T cells, TAMs, and NK cells in the tumor microenvironment. Tregs are observed in precancerous lesion and gradually increased through the progression to aggressive malignancy. Hiraoka and colleagues reported that the infiltration of Tregs in PC tissue was significantly increased compared to nonmalignant inflammatory lesions, and a low level of Treg was an independent risk factor in predicting patient’s survival [83]. Circulating Treg level was significantly higher in PC patients than that in the healthy population. High circulating Treg level could serve as a negative prognostic marker and was associated with worse survival in resectable PC patients [94, 95]. Moreover, when it combined with another systemic inflammation marker (neutrophil-lymphocyte ratio), the predictive effectiveness was 3.5-fold increased [95]. PC cells produce abundant ligands that bind and stimulate chemokine receptor type 5 (CCR5) expressed on Tregs. Disruption of this interaction suppressed Treg migration into tumor and reduced tumor growth in a murine PC model [96]. In addition, PC cells recruited Tregs from peripheral blood and induced the differentiation through secreting TGF-β, and polarized Tregs modulated an immunosuppressive tumor microenvironment by secreting TGF-β [97]. Therefore, Tregs have been emerging as a potential target for PC treatment. Blockade of the interaction between CTLA-4 on Tregs and its ligands B7–1 and B7–2 on antigen-presenting cells would suppress the inhibitory signal on antitumor immune response. Unfortunately, an early phase II clinical trial on ipilimumab (an anti-CTLA-4 agent) demonstrated no significant effects in advanced PC patients, except for one case with tumor regression [98]. In a murine PC model, targeting Tregs with anti-CD25 agents or in combination with tumor vaccine stimulated a tumor-specific immune response, which led to significantly reduced tumor volume and improved survival compared to untreated control mice [99]. Anti-CD25 agents combined with tumor vaccine has been under investigation in patients with advanced PC.
15.6 Role of Nerves in the TME of PC
One of the pathological hallmarks of PDAC is perineural invasion (PNI), defined as the presence of cancer cells along nerves and/or within the different layers of nervous fibers: epineural, perineural, and endoneural spaces [100, 101]. PNI is highly prevalence in PDAC. Particularly PNI is detected in 75% of early stages of PDAC, suggesting that it could represent an early event in cancer progression [100,101,102]. Once cancer cells invade the nerves, they create the TME where multiple types of cells including PSCs, immune cells, Schwann cells, and cancer-associated fibroblasts (CAFs) interact to facilitate the growth of cancer cells.
PSCs were suggested to promote neurites’ outgrowth and thus PNI, to reinforce cancer cell migration toward nerves and to facilitate ECM degradation [101, 103]. Inflammation changes observed in nerves of the early stages of PDAC or even in PanIN lesions suggest that nerves may modulate the immune system to support cancer progression [104, 105], resulting in hypertrophy and hypersensitivity of pancreatic afferents and sensory fibers [106, 107]. Interestingly, a recent report has shown that sensory nerves infiltrated within the melanoma TME, and genetic ablation or chemical denervation of sensory nerves accelerated melanoma growth in vivo (DOI: 10.111/jcmm.15381)(Prazeres et al., 2020). Schwann cells support neurons’ integrity. Increasing numbers of studies indicate that Schwann cells have an active role in PNI in PC [108]. Schwann cells have a specific affinity toward preneoplastic and neoplastic PC cells [109]. After direct contact with cancer cells, Schwann cells actively promote cancer invasiveness and stimulate tumor metastasis [110]. Despite the contribution of Schwann cells to neural invasion, their functions in invasiveness and in pain development need further investigation. CAFs have a role in promoting neural invasion via secretion of cytokines and other factors. CAFs can stimulate Schwann cells migration and neural plasticity leading to increased neurite outgrowth. Particularly, CAFs in TME secrete leukemia inhibitory factor (LIF) which positively correlates with intra-tumoral neural density. LIF, in combination with CA19.9, could differentiate PC from other benign pancreatic diseases [111, 112].
15.7 Future Directions
Lack of an effective approach for early stage screening and diagnosis is largely responsible for the dismal prognosis in PC. Most of the patients presented in clinic have already developed advanced and metastatic PC. Therefore, it is extremely urgent to explore a specific method with high efficiency to detect the tumor at an early stage and guide clinical management. Liquid biopsy is now emerging as a promising technology for the diagnosis and the prediction of patient’s outcome. The techniques used in the liquid biopsy in PC include circulating tumor cells (CTCs), cell-free nucleic acid (cfNA), and circulating tumor exosomes. Recently, liquid biopsy that detected epidermal growth factor receptor (EGFR) gene mutations in non-small cell lung cancers has become the first blood-based genetic test approved by the FDA [113]. In PC diagnosis, using various isolation and detection techniques, early studies have reported a significantly increased positivity of CTC in PC patients compared to the healthy population. Ting et al. found that CTCs expressed a high level of the stromal-derived extracellular matrix, which was associated with metastasis to distant lesions and might imply the dynamic change in tumor microenvironment [114]. Tumor cells can act in clusters – circulating tumor micro-embolies (CTMs), in the circulation of PC patients, especially those with metastatic disease [115]. Poly-clonality of CTMs contains various components including cancer-associated fibroblasts, immune cells, platelets, and PSCs, which facilitate distant metastasis [116]. These findings provide insight into clonal diversity and evolution in metastatic disease. In patients with undetectable CTCs, cfNA (e.g., cell-free DNA, miRNA, noncoding RNA) could be used as a powerful and valuable tool for PC diagnosis. Cell-free DNAs are released into the circulation during cancer cell apoptosis and necrosis within the tumor microenvironment. Additionally, the circulating tumor DNAs (ctDNAs) which are released by CTCs are optimal markers to represent the full spatiotemporal heterogeneity of tumor genetics, allowing real-time monitoring of cancer [117]. Furthermore, a significantly higher level of exosome was also observed in PC patients compared to the healthy individuals. Exosomes produced by living cells appear earlier in the blood than cfNA released by apoptotic and necrotic cells [118]. Exosomes play an important role in the intercellular interaction by carrying and transporting proteins, lipids, and nucleic acids (miRNAs, mRNAs, and DNAs). In PC patients, exosomes contain proteomic and genomic elements associated with cancer growth, invasion, metastasis, and chemoresistance [119, 120]. Circulating exosomes could contribute to immunosuppressive response and even prime a distant site for cancer metastasis. Specific exosomes mediate metastasis by interacting with stromal cells and ECM at distant site and stimulating a cascade of signaling pathways and inflammation responses [121]. Other body fluids could also be used as a source of valuable biomarkers for the detection of pancreatic cancer. Pancreatic juice, as an attractive source collected by various endoscopic methods, is reported to improve the sensitivity of the liquid biopsy by providing a higher concentration of components directly associated with tumor [122]. Release of pancreatic juice into the duodenum and intestine makes stool another potential candidate for noninvasive biopsy. Additionally, saliva, urine, and pleural effusion could also be used for monitoring PC. Therefore, combination in application of multiple sources of liquid biopsy would increase the positivity and specificity of this technique in the future.
While the stroma is quite distinctive in PC, its role in tumor development is still controversial. A growing body of evidence suggests that the stroma has both pro- and antitumor effects [123]. The central role of PSCs in modulating the desmoplastic stroma in PC has been well established. Activated PSCs are present in precancerous lesions to advanced malignancy. Extensive experimental evidences support the role of PSCs in interacting with cancer cells and promoting tumor growth and metastasis. On the contrary, Ozdemir et al. observed increased EMT of cancer cells, aggravated hypoxic condition, and decreased survival in a mouse model of pancreatic cancer with conditional depletion of α-SMA-positive myofibroblasts [124]. It is presumably attributed to the dynamic and disease-stage-dependent role of PSCs in tumor initiation and progression. In the early stages of tumorigenesis, PSCs produce stromal components intending to restrict tumor cells, while in the later stages, cancer cells grow aggressively, and turn PSCs into cancer-permissive cells, making the “protective” effect diminished. In the future, more emphasis should be placed on the spatiotemporally dynamic influence of PSC on tumor development.
In previous studies, various parameters are connected to patient’s survival separately. The PSC activation level (α-SMA expression), stroma density, and tumor-infiltrating immune cells are among the significant risk factors associated with prognosis. However, very limited evidence has demonstrated the combined effects of these factors on survival prediction. Erkan et al. combined expression of α-SMA with the expression of collagen to calculate a specific index for the evaluation of the stroma activation in each PC tissue, and they concluded that a high activated stroma index was associated with a poorer outcome and was an independent prognostic marker in the multivariate survival analysis [22]. Importantly, increasing research interests have focused on the presence of lymphocytes within tumor tissues and the location of TILs [125]. The presence of CD8+ T cells in intraepithelial rather than stromal regions was associated with significantly improved survival [126]. The application of multiplex immunohistochemistry to identify the localization and qualities of tumor-infiltrating CD8+ T cells and immunosuppressive cells in resected samples revealed different mechanisms involved in immunotherapy resistance [127], suggesting that TILs in different compartments should be examined separately, and their connection to patient survival and therapeutic efficacy are to be investigated further. In the future, multivariate analysis can be used to evaluate all the factors that have statistical significance in the univariate analysis and to generate a mathematical model including different clinical, pathohistological, and molecular parameters, which can predict patient survival more accurately.
References
Laklai H, Miroshnikova YA, Pickup MW et al (2016) Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 22:497–505
Zhu Y, Knolhoff BL, Meyer MA et al (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74:5057–5069
Feig C, Jones JO, Kraman M et al (2013) Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110:20212–20217
Provenzano PP, Cuevas C, Chang AE et al (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429
Ene-Obong A, Clear AJ, Watt J et al (2013) Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 145:1121–1132
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61
Ruffell B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol 33:119–126
Nielsen MF, Mortensen MB, Detlefsen S (2016) Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol 22:2678–2700
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics 2019. CA Cancer J Clin 69:7–34
Rahib L, Smith BD, Aizenberg R et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921
Neesse A, Algul H, Tuveson DA et al (2015) Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64:1476–1484
Neesse A, Michl P, Frese KK et al (2011) Stromal biology and therapy in pancreatic cancer. Gut 60:861–868
Vonlaufen A, Joshi S, Qu C et al (2008) Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68:2085–2093
Gore J, Korc M (2014) Pancreatic cancer stroma: friend or foe? Cancer Cell 25:711–712
Feig C, Gopinathan A, Neesse A et al (2012) The pancreas cancer microenvironment. Clin Cancer Res 18:4266–4276
Kleeff J, Korc M, Apte M et al (2016) Pancreatic cancer. Nat Rev Dis Primers 2:16022
Watari N, Hotta Y, Mabuchi Y (1982) Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin a administration. Okajimas Folia Anat Jpn 58:837–858
Apte MV, Haber PS, Applegate TL et al (1998) Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43:128–133
Bachem MG, Schneider E, Gross H et al (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115:421–432
Erkan M, Adler G, Apte MV et al (2012) StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61:172–178
Han S, Delitto D, Zhang D et al (2015) Primary outgrowth cultures are a reliable source of human pancreatic stellate cells. Lab Investig 95:1331–1340
Erkan M, Michalski CW, Rieder S et al (2008) The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 6:1155–1161
Sinn M, Denkert C, Striefler JK et al (2014) Alpha-smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer 111:1917–1923
Lunardi S, Muschel RJ, Brunner TB (2014) The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett 343:147–155
Fujita H, Ohuchida K, Mizumoto K et al (2009) Tumor-stromal interactions with direct cell contacts enhance proliferation of human pancreatic carcinoma cells. Cancer Sci 100:2309–2317
Jiang HB, Xu M, Wang XP (2008) Pancreatic stellate cells promote proliferation and invasiveness of human pancreatic cancer cells via galectin-3. World J Gastroenterol 14:2023–2028
Bachem MG, Schunemann M, Ramadani M et al (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128:907–921
Hwang RF, Moore T, Arumugam T et al (2008) Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68:918–926
Xu Z, Vonlaufen A, Phillips PA et al (2010) Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol 177:2585–2596
Froeling FE, Feig C, Chelala C et al (2011) Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 141:1486-97–1497 e1–14
Chronopoulos A, Robinson B, Sarper M et al (2016) ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun 7:12630
Di Maggio F, Arumugam P, Delvecchio FR et al (2016) Pancreatic stellate cells regulate blood vessel density in the stroma of pancreatic ductal adenocarcinoma. Pancreatology 16:995–1004
Clark CE, Hingorani SR, Mick R et al (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 67:9518–9527
Tang D, Yuan Z, Xue X et al (2012) High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer 130:2337–2348
Kraman M, Bambrough PJ, Arnold JN et al (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330:827–830
Apte MV, Pirola RC, Wilson JS (2012) Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol 3:344
Bailey JM, Swanson BJ, Hamada T et al (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004
Shek FW, Benyon RC, Walker FM et al (2002) Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160:1787–1798
Erkan M, Kleeff J, Gorbachevski A et al (2007) Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132:1447–1464
Weniger M, Honselmann KC, Liss AS (2018) The extracellular matrix and pancreatic Cancer: a complex relationship. Cancers (Basel) 10
Whatcott CJ, Diep CH, Jiang P et al (2015) Desmoplasia in primary tumors and metastatic lesions of pancreatic Cancer. Clin Cancer Res 21:3561–3568
Armstrong T, Packham G, Murphy LB et al (2004) Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res 10:7427–7437
Berchtold S, Grunwald B, Kruger A et al (2015) Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Lett 356:721–732
Grzesiak JJ, Tran Cao HS, Burton DW et al (2011) Knockdown of the beta(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int J Cancer 129:2905–2915
Shintani Y, Fukumoto Y, Chaika N et al (2008) Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol 180:1277–1289
Koenig A, Mueller C, Hasel C et al (2006) Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res 66:4662–4671
DuFort CC, DelGiorno KE, Carlson MA et al (2016) Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys J 110:2106–2119
Birbrair A, Zhang T, Wang ZM et al (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307:C25–C38
Koong AC, Mehta VK, Le QT et al (2000) Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 48:919–922
Hiraoka N, Ino Y, Sekine S et al (2010) Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer 103:1057–1065
Wang K, Baldwin GS, Nikfarjam M et al (2018) p21-activated kinase signalling in pancreatic cancer: new insights into tumour biology and immune modulation. World J Gastroenterol 24:3709–3723
Li N, Li Y, Li Z et al (2016) Hypoxia inducible factor 1 (HIF-1) recruits macrophage to activate pancreatic stellate cells in pancreatic ductal adenocarcinoma. Int J Mol Sci 17
Gunda V, Kumar S, Dasgupta A et al (1742) Hypoxia-induced Metabolomic alterations in pancreatic Cancer cells. Methods Mol Biol 2018:95–105
Rausch V, Liu L, Apel A et al (2012) Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment. J Pathol 227:325–335
Cheng ZX, Sun B, Wang SJ et al (2011) Nuclear factor-kappaB-dependent epithelial to mesenchymal transition induced by HIF-1alpha activation in pancreatic cancer cells under hypoxic conditions. PLoS One 6:e23752
Hotz B, Arndt M, Dullat S et al (2007) Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res 13:4769–4776
Li M, Xie H, Liu Y et al (2019) Knockdown of hypoxia-inducible factor-1 alpha by tumor targeted delivery of CRISPR/Cas9 system suppressed the metastasis of pancreatic cancer. J Control Release 304:204–215
Baumgart S, Chen NM, Siveke JT et al (2014) Inflammation-induced NFATc1-STAT3 transcription complex promotes pancreatic cancer initiation by KrasG12D. Cancer Discov 4:688–701
Roghanian A, Fraser C, Kleyman M et al (2016) B cells promote pancreatic tumorigenesis. Cancer Discov 6:230–232
Inman KS, Francis AA, Murray NR (2014) Complex role for the immune system in initiation and progression of pancreatic cancer. World J Gastroenterol 20:11160–11181
Lutz ER, Wu AA, Bigelow E et al (2014) Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2:616–631
Zhan HX, Zhou B, Cheng YG et al (2017) Crosstalk between stromal cells and cancer cells in pancreatic cancer: new insights into stromal biology. Cancer Lett 392:83–93
Huang B, Pan PY, Li Q et al (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66:1123–1131
Pylayeva-Gupta Y, Lee KE, Hajdu CH et al (2012) Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836–847
Sinha P, Clements VK, Bunt SK et al (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179:977–983
Liu C, Yu S, Kappes J et al (2007) Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 109:4336–4342
Gabitass RF, Annels NE, Stocken DD et al (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 60:1419–1430
Stromnes IM, Brockenbrough JS, Izeradjene K et al (2014) Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63:1769–1781
Khaled YS, Ammori BJ, Elkord E (2014) Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res 2014:879897
Menen RS, Hassanein MK, Momiyama M et al (2012) Tumor-educated macrophages promote tumor growth and peritoneal metastasis in an orthotopic nude mouse model of human pancreatic cancer. In Vivo 26:565–569
Karnevi E, Andersson R, Rosendahl AH (2014) Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol 92:543–552
Hu H, Jiao F, Han T et al (2015) Functional significance of macrophages in pancreatic cancer biology. Tumour Biol 36:9119–9126
Liu CY, Xu JY, Shi XY et al (2013) M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig 93:844–854
Kurahara H, Shinchi H, Mataki Y et al (2011) Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res 167:e211–e219
Kurahara H, Takao S, Maemura K et al (2013) M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas 42:155–159
Mitchem JB, Brennan DJ, Knolhoff BL et al (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73:1128–1141
Sanford DE, Belt BA, Panni RZ et al (2013) Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19:3404–3415
Dineen SP, Lynn KD, Holloway SE et al (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68:4340–4346
Lianyuan T, Dianrong X, Chunhui Y et al (2018) The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Cancer Biol Ther 19:296–305
Bang S, Kim HS, Choo YS et al (2006) Differences in immune cells engaged in cell-mediated immunity after chemotherapy for far advanced pancreatic cancer. Pancreas 32:29–36
Xu YF, Lu Y, Cheng H et al (2014) Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology 14:295–301
Helm O, Mennrich R, Petrick D et al (2014) Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One 9:e94357
Hiraoka N, Onozato K, Kosuge T et al (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12:5423–5434
Fukunaga A, Miyamoto M, Cho Y et al (2004) CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28:e26–e31
Lohneis P, Sinn M, Bischoff S et al (2017) Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur J Cancer 83:290–301
Ryschich E, Notzel T, Hinz U et al (2005) Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 11:498–504
Thomas DA, Massague J (2005) TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369–380
Ellermeier J, Wei J, Duewell P et al (2013) Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 73:1709–1720
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Soares KC, Rucki AA, Wu AA et al (2015) PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 38:1–11
Ino Y, Yamazaki-Itoh R, Shimada K et al (2013) Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 108:914–923
Bellone G, Turletti A, Artusio E et al (1999) Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 155:537–547
Tassi E, Braga M, Longhi R et al (2009) Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS One 4:e7234
Yamamoto T, Yanagimoto H, Satoi S et al (2012) Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 41:409–415
Cheng H, Luo G, Lu Y et al (2016) The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 16:1080–1084
Tan MC, Goedegebuure PS, Belt BA et al (2009) Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182:1746–1755
Liyanage UK, Goedegebuure PS, Moore TT et al (2006) Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother 29:416–424
Royal RE, Levy C, Turner K et al (2010) Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 33:828–833
Viehl CT, Moore TT, Liyanage UK et al (2006) Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol 13:1252–1258
Liebig C, Ayala G, Wilks JA et al (2009) Perineural invasion in cancer: a review of the literature. Cancer 115:3379–3391
Demir IE, Ceyhan GO, Liebl F et al (2010) Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel) 2:1513–1527
Hirai I, Kimura W, Ozawa K et al (2002) Perineural invasion in pancreatic cancer. Pancreas 24:15–25
Ceyhan GO, Bergmann F, Kadihasanoglu M et al (2009) Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology 136:177–186. e1
Saloman JL, Albers KM, Rhim AD et al (2016) Can stopping nerves, Stop Cancer? Trends Neurosci 39:880–889
Saloman JL, Singhi AD, Hartman DJ et al (2018) Systemic depletion of nerve growth factor inhibits disease progression in a genetically engineered model of pancreatic ductal adenocarcinoma. Pancreas 47:856–863
Schwartz ES, Christianson JA, Chen X et al (2011) Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 140:1283–1291 e1–2
Schwartz ES, La JH, Scheff NN et al (2013) TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci 33:5603–5611
Deborde S, Wong RJ (2017) How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci 74:4405–4420
Demir IE, Boldis A, Pfitzinger PL et al (2014) Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst 106
Deborde S, Omelchenko T, Lyubchik A et al (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126:1538–1554
Bressy C, Lac S, Nigri J et al (2018) LIF drives neural remodeling in pancreatic Cancer and offers a new candidate biomarker. Cancer Res 78:909–921
Nicola NA, Babon JJ (2015) Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 26:533–544
Voelker R (2016) Liquid biopsy receives approval. JAMA 316:260
Ting DT, Wittner BS, Ligorio M et al (2014) Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep 8:1905–1918
Chang MC, Chang YT, Chen JY et al (2016) Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem 62:505–513
Maddipati R, Stanger BZ (2015) Pancreatic Cancer Metastases Harbor evidence of Polyclonality. Cancer Discov 5:1086–1097
Samandari M, Julia MG, Rice A et al (2018) Liquid biopsies for management of pancreatic cancer. Transl Res 201:98–127
Qi ZH, Xu HX, Zhang SR et al (2018) The significance of liquid biopsy in pancreatic Cancer. J Cancer 9:3417–3426
Harada T, Yamamoto H, Kishida S et al (2017) Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci 108:42–52
Mikamori M, Yamada D, Eguchi H et al (2017) MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci Rep 7:42339
Hoshino A, Costa-Silva B, Shen TL et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335
Yamaguchi Y, Watanabe H, Yrdiran S et al (1999) Detection of mutations of p53 tumor suppressor gene in pancreatic juice and its application to diagnosis of patients with pancreatic cancer: comparison with K-ras mutation. Clin Cancer Res 5:1147–1153
Whatcott CJ, Han H, Von Hoff DD (2015) Orchestrating the tumor microenvironment to improve survival for patients with pancreatic Cancer: normalization, not destruction. Cancer J 21:299–306
Ozdemir BC, Pentcheva-Hoang T, Carstens JL et al (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25:719–734
Peske JD, Woods AB, Engelhard VH (2015) Control of CD8 T-cell infiltration into tumors by vasculature and microenvironment. Adv Cancer Res 128:263–307
Sato E, Olson SH, Ahn J et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543
Stromnes IM, Hulbert A, Pierce RH et al (2017) T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol Res 5:978–991
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, K., He, H. (2020). Pancreatic Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironments in Organs. Advances in Experimental Medicine and Biology, vol 1296. Springer, Cham. https://doi.org/10.1007/978-3-030-59038-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-59038-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59037-6
Online ISBN: 978-3-030-59038-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)