Abstract
Cardiac ischemia/reperfusion (I/R) injury is a serious cardiac complication following acute myocardial infarction, which include myocardial infarct size expansion, left ventricular (LV) dysfunction, and fatal cardiac arrhythmias. Sex hormone deprivation due to aging or other pathological conditions is an independent risk factor for cardiovascular disease. Several scientific research studies have been conducted to investigate the role of sex hormones in myocardial injury during cardiac I/R. Testosterone is the primary sex hormone in men; it regulates male sexual characteristics, and controls muscle and bone mass. In addition, testosterone plays an important role in regulating LV function through multiple mechanisms, including those controlling cellular calcium homeostasis, regulating cardiac mitochondrial function and enhancing antioxidants. Findings from studies regarding the roles of testosterone on the heart during cardiac I/R are controversial; some have reported that decreased testosterone level could impair LV function, whilst others reported the benefits of testosterone deprivation during cardiac I/R. In this chapter, we include evidence regarding the effects of testosterone deprivation and exogenous testosterone administration on myocardial injury in terms of myocardial infarct size, LV function, arrhythmias and molecular alterations. Reports from in vitro, ex vivo, in vivo studies, and clinical reports are summarized and discussed. The contents of this chapter will explain the roles of testosterone during cardiac I/R in preventing cardiac complications. Insights from these reports may help to devise strategies to improve treatment in patients with acute myocardial infarction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Testosterone
- Cardiac ischemia/reperfusion injury
- Myocardial infarction
- Left ventricular function
- Mitochondria
Introduction
Acute myocardial infarction (AMI) remains a global leading cause of death [1]. It is caused by narrowing of the coronary arteries. It has been demonstrated that risk factors associated with AMI are atherosclerosis, hypertension, high cholesterol, diabetes, obesity, smoking and hormonal imbalance [2]. Reperfusion therapy is an effective treatment for AMI as it allows the return of blood to the injured myocardium to resupply essential oxygen and nutrients. However, this intervention has been shown to cause potentially serious adverse effects on the heart, including myocardial infarct size expansion, left ventricular (LV) dysfunction and fatal cardiac arrhythmias, collectively known as cardiac ischemia/reperfusion (I/R) injury [3].
It has been demonstrated that sex hormone dysregulation is associated with the severity of coronary artery disease [4]. Testosterone is a male sex steroid hormone which is secreted mainly by the testicles and, to a lesser extent, by the adrenal glands. Testosterone regulates the development of male reproductive organs and promotes secondary sexual characteristics [4]. In addition, it controls hair growth, muscle growth, collagen synthesis, muscle strength and endurance, memory function, mood, red blood cell production and body mass density [5]. Testosterone deprivation, as seen in male aging, has been associated with the pathophysiology of various organ dysfunctions [4]. In the case of the heart, testosterone deprivation increases the risk of cardiovascular disease by increasing total plasma cholesterol levels and reducing plasma high density lipoprotein (HDL) levels [6]. A report from a clinical study demonstrated that ST−Elevation Myocardial Infarction (STEMI) male patients with hypotestosteronemia had a higher incidence of microvascular obstruction as identified by coronary angiography, compared to STEMI patients with normal plasma testosterone [7]. These findings suggest that testosterone may potentially be involved in the pathogenesis of AMI in male subjects.
In order to understand the mechanisms employed by testosterone in the heart, an animal model of testosterone deprivation has been established. In male rodents, a bilateral orchiectomy (ORX) has been shown to eliminate the endogenous production of testosterone [8, 9]; exogenous testosterone being subsequently given to those rodents as therapy [10, 11]. Exogenous testosterone replacement therapy has been widely used to treat patients with hypogonadism and other low−testosterone related diseases [12]. It has been shown that exogenous testosterone offers various benefits for the human body, for instance it augmented positive mood, increased sexual motivation and performance, increased muscle strength and a lean body mass, reduced body fat mass and improved bone mineral density [5]. However, exogenous testosterone has also been shown to cause adverse effects such as acne, mild fluid retention, increased urination symptoms and worsening of sleep apnea [13]. In accordance with these findings, the Endocrine Society do not recommend treatment with exogenous testosterone in men with heart failure (NYHA class III or IV) [14].
In this chapter, we have summarized and discussed the cumulative data from in vitro, ex vivo, and in vivo studies, and from clinical reports regarding the effects of testosterone deprivation and exogenous testosterone administration on the heart during cardiac I/R injury. This information will provide mechanistic insight for future clinical investigations.
The Effects of Testosterone Deprivation on the Heart with Cardiac I/R Injury
Previous reports have demonstrated that cardiac I/R injury led to myocardial infarct size expansion, LV dysfunction and arrhythmia [10, 15,16,17]. Cardiac I/R injury has been induced in both ex vivo and in vivo settings [18]. For induction in the ex vivo setting, the heart was removed and connected to a Langendorff system [18]. The myocardial ischemia was induced by either stopping the perfusate for several minutes to induce global ischemia or ligating the left coronary artery to induce regional ischemia [18]. After successful myocardial ischemia, the perfusate was opened to initiate reperfusion to the injured myocardium [18]. In the in vivo setting, a thoracotomy was performed and the left coronary artery or left anterior descending (LAD) coronary artery was ligated to induce myocardial ischemia and reperfusion was induced by a loosening of the knot [8, 10, 15]. The alterations in the electrocardiogram were used to confirm a successful cardiac I/R in vivo [8, 10, 15].
The bilateral ORX is a surgical procedure effective in causing a decrease in plasma testosterone level in the body prior to the induction of cardiac I/R [10]. Findings regarding the effects of testosterone deprivation on the heart with I/R injury are inconsistent; some studies have reported that testosterone deprivation led to an aggravation of myocardial damage after cardiac I/R [10, 19,20,21,22,23], whereas several other studies reported contrary findings [24,25,26,27,28,29,30,31,32]. A comprehensive summary of these reports is shown in Table 3.1.
With regards to the deteriorating effects caused as a result of testosterone deprivation on the heart with I/R, results from ex vivo and in vivo studies have shown that testosterone deprivation for at least 2 weeks increased myocardial infarct size after cardiac I/R [10, 21,22,23]. Testosterone deprivation for 3 weeks did not alter LV function [19], however, a worsening of LV dysfunction was initially observed after 7 weeks of ORX as indicated by increased LV end−diastolic pressure (LVEDP), reduced LV developed pressure (LVDP) and ± dP/dt in ex vivo studies [20, 21, 23]. In an in vivo study, after 12 weeks of ORX, increased LVEDP and −dP/dt and decreased stroke volume (SV) were observed [10]. Cardiac arrhythmia following cardiac I/R was investigated in two studies. These both found that the arrhythmia score [10], the number of premature ventricular beats (PVB) and ventricular tachycardia (VT) increased after 9 weeks of ORX [23]. The mechanisms underlying the deteriorating effects of testosterone deprivation on cardiac I/R were determined, and the data showed that testosterone−deprived rats had an increase in lactate dehydrogenase (LDH) [21, 23], apoptosis and cardiac mitochondrial dysfunction [10]. In addition, gap junction protein expression was decreased in ORX rats when compared to their sham operation controls [10], leading to a disturbance of electrical activity in the heart. A comprehensive summary of these reports is shown in Table 3.1.
Mitochondria are vital organelles which control energy metabolism, cell death and cell survival in physiological and pathological conditions [33]. In addition, mitochondria are largely responsible for reactive oxygen species (ROS) production, thereby resulting in a predisposition to oxidative stress [34]. An excessive level of oxidative stress disrupts mitochondrial membrane integrity which will lead to cardiac mitochondrial membrane depolarization and opening of the mitochondrial permeability transition pores (mPTP), followed by mitochondrial swelling [33]. Cytochrome c is released from the mitochondrial intermembrane space to form an apoptosome, which ultimately induces apoptosis [33]. Since cardiac mitochondrial dysfunction was found in cases of both testosterone deprivation and cardiac I/R [15, 17, 35], the severity of cardiac mitochondrial dysfunction was potentially increased in testosterone deprived rats with cardiac I/R [35].
There is only one ex vivo study which reported that testosterone deprivation for 4 weeks did not alter LV function and apoptosis in rats [27]. However, myocardial infarct size and arrhythmia status were not determined in that study [27]. The findings from this ex vivo report were not consistent with the in vivo studies, indicating that the duration of testosterone deprivation as well as the study models could influence the effects of testosterone on the heart.
Although testosterone deprivation has been reported to cause adverse effects on the heart with I/R, inconsistent reports exist. Two studies have reported that myocardial infarct size was decreased in testosterone deprived rodents [24, 28]. Myocardial infarct size was decreased 3 weeks after ORX (in a rat model) [28], and 20 weeks after ORX (in a mouse model) [24]. To the contrary, other studies reported that testosterone deprivation increased myocardial infarct size in rats between 2 and12 weeks after ORX [10, 21,22,23]. The discrepancy in myocardial infarct size might be due to differences in the duration of testosterone deprivation and cardiac I/R protocol. There is an inconsistency in the data in cases of short−term testosterone deprivation. Two weeks of testosterone deprivation increased myocardial infarct size in an in vivo setting after 60 min of ischemia and 4 h of reperfusion. On the other hand, 3 weeks of testosterone deprivation decreased myocardial infarct size in an ex vivo setting after 30 min of ischemia and 150 min of reperfusion. Thus, the difference in cardiac I/R setting such as ischemia and reperfusion duration might involve a discrepancy of myocardial infarct size in testosterone deprived rats with cardiac I/R. A comprehensive summary of these reports is shown in Table 3.1.
As regards LV function, in ex vivo studies testosterone deprivation for 7 weeks has been shown to have both deteriorating and beneficial effects [20, 25, 26, 29, 31, 32]. Testosterone deprived rats who were subjected to 20 min of ischemia resulted in a worsening of LV dysfunction [20]. However, testosterone deprived rats subjected to 25 min of ischemia showed an improvement in LV function during cardiac I/R, all rats undergoing similar reperfusion duration (40 min) [26, 29, 31, 32]. These findings suggest that the ischemic duration could be a key factor in the mediation of LV function in testosterone deprived rats with cardiac I/R. It has been proposed that testosterone deprivation attenuated LV dysfunction during cardiac I/R by increasing the level of survival protein kinase, enhancing antioxidants, reducing apoptosis [26, 29, 32] and inflammation [31]. This finding was supported by another in vivo study in which a short duration of ischemia (5 min) and reperfusion (5 min) was shown to reduce LV dysfunction in testosterone deprived rats by promoting cardiac mitochondrial respiration [30]. A comprehensive summary of these reports is shown in Table 3.1.
Metabolic syndrome is associated with low testosterone levels [36]. It has been shown that the percentage of body fat has a negative correlation with circulating testosterone level [36]. In obese−insulin resistant rats, testosterone deprivation did not increase myocardial infarct size, or arrhythmias, and it did not alter intracellular molecular signaling, compared with their sham operated rats [10, 37]. However, a worsening of LV dysfunction was reported in one study in which it was shown that testosterone deprivation increased LVEDP and left ventricular end systolic pressure (LVESP) in obese−insulin resistant rats, when compared with their sham operated controls [10]. These data indicated that, in a rodent model of metabolic syndrome, although testosterone deprivation did not increase myocardial infarct size, it aggravated LV dysfunction. A comprehensive summary of these reports is shown in Table 3.1.
The Effects of Exogenous Testosterone Administration on the Heart with Cardiac I/R
Testosterone acts via two major pathways: the genomic and the non−genomic pathways [5]. In the genomic pathway, after testosterone enters the cytosol it can be converted to dihydrotestosterone (DHT) by an enzyme 5α−reductase [5]. DHT then binds with an androgen receptor (AR), which forms a homodimer and undergoes a conformational change [5]. This AR complex then binds with other proteins to facilitate their nuclear translocation [5]. After the AR complex enters the nucleus, it binds to the androgen response elements (ARE), which are found on several promoter sites on the DNA [5]. ARA 70, a coactivator, is subsequently recruited for the transcription of AR-regulated genes, which can exert their action on target organs via their protein products [5]. In the non-genomic pathway, this process requires neither AR nuclear translocation nor AR-DNA-binding. The activated AR in the cytoplasm interacts with other signaling pathways such as PI3K/Akt, Ras/Raf and Src [5]. These signaling molecules activate the mitogen-activated protein kinase (MAPK)/extracellular signal−regulated kinase (ERK) pathway [5]. The activation of the MAPK/ERK pathway is associated with cell proliferation gene expression [5]. In this chapter, we have summarized the effects of exogenous testosterone administration on the heart during cardiac I/R in animals with normal plasma testosterone levels.
In an in vitro study, the isolated cardiomyocytes were subjected to 30 min of ischemia (by incubating the cells with an ischemic solution) and 30 min of reperfusion (by incubating the cells with normal culture media) [38]. Testosterone was added to the cells during ischemia until the end of reperfusion. Cellular electrophysiological studies were performed, and the results showed that testosterone reduced action potential duration (APD90) and the number of premature ventricular contractions (PVC) [38]. However, the resting membrane potential, the action potential amplitude, and Vmax were not affected by the application of testosterone [38]. These findings suggested that testosterone could reduce arrhythmias at the cellular level. A comprehensive summary of these reports is shown in Table 3.2.
In the case of ex vivo studies, pretreatment with a very low dose of testosterone (0.001 nM) in isolated rat hearts was not shown to confer any level of protection on the heart regarding cardiac I/R injury [39]. Pretreatment with testosterone at a dose of 3 μg/kg improved LV function, but it did not reduce LDH levels after cardiac I/R [40]. In addition, pretreatment with a high dose of testosterone (5 mg/kg) increased myocardial infarct size by decreasing antioxidant levels, but it did not alter the level of apoptosis [41]. These data suggested that, in rats with a normal circulating testosterone level, the effects of exogenous testosterone were dependent on the dosage of exogenous testosterone. A summary of these reports is shown in Table 3.2.
The Effects of Exogenous Testosterone Administration on the Hearts of Testosterone Deprived Rats with Cardiac I/R
In one ex vivo study, testosterone deprived animals were given exogenous testosterone prior to cardiac I/R in a range of dosages and for various durations of treatment. The study reported that pretreatment with a single dose of testosterone injection (intraperitoneal injection; IP) at 10 mg/kg 6 h prior to cardiac I/R did not reduce LV dysfunction in rats and apoptosis was increased [27]. Another ex vivo study showed that pretreatment with a single dose of testosterone injection (intramuscular injection; IM) at 500 mg/kg, 14 days prior to cardiac I/R significantly attenuated LV dysfunction by reducing intracellular calcium levels, possibly through an increase in sarcoplasmic reticulum calcium ATPase levels [20]. This showed that a single high−dose of exogenous testosterone administration could protect the heart against cardiac I/R injury in testosterone deprived rats. A comprehensive summary of these reports is shown in Table 3.3.
As regards chronic treatment, testosterone was subcutaneously implanted in testosterone deprived rats (100 mg, 3 weeks) prior to cardiac I/R [19, 26, 32]. The results showed that, in the setting of ex vivo cardiac I/R (25 min ischemia and 40 min reperfusion), testosterone attenuated apoptosis but not LV dysfunction [26, 32]. Another study showed that, in an ex vivo setting of cardiac I/R (25 min ischemia and 45 min reperfusion), testosterone attenuated LV dysfunction and increased antioxidant enzyme level [19]. These findings suggested that, with similar doses of testosterone, the duration of reperfusion may affect LV function in testosterone deprived rats with cardiac I/R. A comprehensive summary of these reports is shown in Table 3.3.
The method of regional ischemia performed in some ex vivo studies was ligation of the LAD [21, 23]. Exogenous testosterone (2 mg/kg) was given to the testosterone deprived rats daily for 8 weeks prior to cardiac I/R, and the results demonstrated that testosterone could reduce myocardial infarct size and LV dysfunction [23]. In addition, the numbers of PVC and VT were decreased in testosterone deprived rats with cardiac I/R [23]. Another study showed that testosterone could reduce LDH but it did not affect the heat shock proteins HSP70 and HSF1 [21]. HSP70 and HFS1 are stress inducible proteins which are excessively expressed during cardiac I/R [21]. An activation of the HSP70−HSF1 complex could initiate apoptosis and an inflammatory response [21]. However, the beneficial effects of testosterone were not mediated by this protein. A comprehensive summary of these reports is shown in Table 3.3.
In in vivo studies, the effects of acute and chronic exogenous testosterone have been investigated in testosterone deprived rats. The acute administration of exogenous testosterone (0.2 and 2.0 μg/kg, IV), given to the testosterone deprived rats during cardiac ischemia was shown to reduce myocardial infarct size [22]. However, the precise molecular mechanism responsible for the beneficial effects in acute treatment was not determined [22]. In a chronic pretreatment model, exogenous testosterone (346 ng/kg and 2 mg/kg, 4 weeks) effectively reduced myocardial infarct size, LV dysfunction, and arrhythmias in testosterone deprived rats [10, 35, 42]. These studies proposed that testosterone could reduce cardiac inflammation [42], oxidative stress, apoptosis and cardiac mitochondrial dysfunction [10, 35] in testosterone deprived rats. These beneficial effects of exogenous testosterone were also observed in the obese−insulin resistant rats with testosterone deprivation subjected to cardiac I/R through a similar procedure [10]. A summary of these reports is shown in Table 3.3.
One study used a naturally aged rat model with age related low testosterone levels [43]. Exogenous testosterone was given to the rats at 1 mg/kg for 30 days prior to cardiac I/R, and the results showed that this intervention increased myocardial infarct size, when compared with the vehicle control group [43]. However, the degree of LV dysfunction was decreased. The molecular mechanism demonstrated that exogenous testosterone decreased p-Akt/Akt, which is a survival protein, however it did not affect apoptosis and ER stress levels [43] (Table 3.3). These data suggested that exogenous testosterone reduced LV dysfunction through the activation of this survival protein.
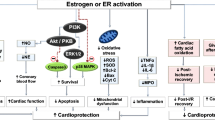
From these reports, it could be concluded that exogenous testosterone application reduced cardiac mitochondrial dysfunction and led to a reduction in cardiac apoptosis, resulting in a reduction in myocardial infarct size and LV dysfunction. In addition, exogenous testosterone reduced matrix metalloproteinase activity, cellular infiltration, and inflammation in testosterone deprived rats. A summary regarding the roles of testosterone on the heart of cardiac I/R is depicted in Fig. 3.1.
The Effects of Testosterone Replacement Therapy (TRT) on Cardiovascular Outcome in Clinical Reports
A previous study has shown that testosterone plays an important role in regulating cardiac damage during MI, specifically that testosterone deprivation led to an increased incidence of microvascular obstruction in STEMI patients [7]. Therefore, TRT is used to prevent cardiac complications in patients with low circulating testosterone levels. There are large cohort studies showing that long−term TRT use (for more than 17 months) could increase cardiovascular event free survival rate, decrease all−cause mortality, and reduce major cardiac adverse events in middle to old aged men with low testosterone levels [44, 45, 47, 48]. However, TRT for 1 year has been shown neither to increase nor decrease MI events in middle to old aged men with low testosterone levels [46]. Although long−term TRT showed benefits as regards the cardiovascular outcomes, two studies have reported adverse effects of TRT in increasing the incidence of obstructive sleep apnea [44] and stroke [48]. In older aged men (the age of all participants was greater than 70 years old), TRT promoted MI risk by increasing noncalcified coronary artery plaque volume, total plaque volume, and coronary artery calcium score [49]. These findings suggested that TRT provided several benefits on cardiovascular outcomes in the middle to old aged male cohort who have low testosterone levels, however, testosterone prescription to the older aged men (subjects over 70 years old) might increase risk for MI. A comprehensive summary of these reports is shown in Table 3.4.
Previous studies also reported the effects of TRT on cardiovascular outcomes in men with low testosterone levels, together with MI or heart failure. In the case of both short-term (1 month) and longer-term (3 months-1 year) treatment, TRT reduced cardiac adverse events by delaying the time to ischemia [50] and time to 1 mm ST segment depression [51], reducing carotid intima−media thickness [50] and QT dispersion [52] in men with low testosterone and heart disease. However, long−term TRT did not affect MI events in aging men (subjects over 70 years old) with MI and low testosterone levels [53]. This information indicated that TRT reduced cardiac adverse events in middle to old aged men who have cardiac complications and low testosterone levels. A summary of these reports is shown in Table 3.4.
Conclusion
Testosterone plays an important role in the regulation of myocardial injury during cardiac I/R, the extent of the impact being dependent on the duration of testosterone deprivation. Exogenous testosterone administration modulates myocardial infarction, LV function, and arrhythmias during cardiac I/R injury, the degree of modulation being dependent on dose and time of administration in both normal and testosterone deprived rats.
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM et al (2018) Heart Disease and Stroke Statistics−2018 Update: A Report From the American Heart Association. Circulation 137:e67–e492
Collins P (2006) Risk factors for cardiovascular disease and hormone therapy in women. Heart 92(Suppl 3):iii24–8
Takawale A, Fan D, Basu R, Shen M et al (2014) Myocardial recovery from ischemia−reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ Heart Fail 7(4):652–662
Ma RC, Tong PC (2010) Testosterone levels and cardiovascular disease. Heart 96(22):1787–1788
Nieschlag E, Nieschlag S (2019) ENDOCRINE HISTORY: The history of discovery, synthesis and development of testosterone for clinical use. Eur J Endocrinol 180(6):R201–R212
Farias JM, Tinetti M, Khoury M, Umpierrez GE (2019) Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab 99(12):4698–4703
Niccoli G, Milardi D, D’Amario D, Fracassi F et al (2015) Hypotestosteronemia is frequent in ST−elevation myocardial infarction patients and is associated with coronary microvascular obstruction. Eur J Prev Cardiol 22(7):855–863
Apaiajai N, Chunchai T, Jaiwongkam T, Kerdphoo S et al (2018) Testosterone Deprivation Aggravates Left−Ventricular Dysfunction in Male Obese Insulin−Resistant Rats via Impairing Cardiac Mitochondrial Function and Dynamics Proteins. Gerontology 64(4):333–343
Pongkan W, Pintana H, Sivasinprasasn S, Jaiwongkam T et al (2016) Testosterone deprivation accelerates cardiac dysfunction in obese male rats. J Endocrinol 229(3):209–220
Pongkan W, Pintana H, Jaiwongkam T, Kredphoo S et al (2016) Vildagliptin reduces cardiac ischemic−reperfusion injury in obese orchiectomized rats. J Endocrinol 231(1):81–95
Arinno A, Apaijai N, Kaewtep P, Pratchayasakul W, et al (2019) Combined low−dose testosterone and vildagliptin confers cardioprotection in castrated obese rats. J Endocrinol (in press)
Osterberg EC, Bernie AM, Ramasamy R (2014) Risks of testosterone replacement therapy in men. Indian J Urol 30(1):2–7
Goodale T, Sadhu A, Petak S, Robbins R (2017) Testosterone and the Heart. Methodist Debakey Cardiovasc J 13(2):68–72
Seftel AD, Kathrins M, Niederberger C (2015) Critical Update of the 2010 Endocrine Society Clinical Practice Guidelines for Male Hypogonadism: A Systematic Analysis. Mayo Clin Proc 90(8):1104–1115
Palee S, McSweeney CM, Maneechote C, Moisescu DM, et al (2019) PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischaemia/reperfusion injury: Benefits beyond lipid−lowering effects. J Cell Mol Med (in press)
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T et al (2019) Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci (Lond) 133(3):497–513
Apaijai N, Chinda K, Palee S, Chattipakorn S et al (2014) Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia−reperfusion injury in obese−insulin resistant rats. PLoS ONE 9(7):e102374
Lindsey ML, Bolli R, Canty JM Jr, Du XJ et al (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314(4):H812–H838
Borst SE, Quindry JC, Yarrow JF, Conover CF et al (2010) Testosterone administration induces protection against global myocardial ischemia. Horm Metab Res 42(2):122–129
Callies F, Stromer H, Schwinger RH, Bolck B et al (2003) Administration of testosterone is associated with a reduced susceptibility to myocardial ischemia. Endocrinology 144(10):4478–4483
Liu J, Tsang S, Wong TM (2006) Testosterone is required for delayed cardioprotection and enhanced heat shock protein 70 expression induced by preconditioning. Endocrinology 147(10):4569–4577
Rubio−Gayosso I, Ramirez−Sanchez I, Ita−Islas I, Ortiz−Vilchis P et al (2013) Testosterone metabolites mediate its effects on myocardial damage induced by ischemia/reperfusion in male Wistar rats. Steroids 78(3):362–369
Tsang S, Wu S, Liu J, Wong TM (2008) Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)−adrenoceptor stimulation. Br J Pharmacol 153(4):693–709
Ghimire A, Bisset ES, Howlett SE (2019) Ischemia and reperfusion injury following cardioplegic arrest is attenuated by age and testosterone deficiency in male but not female mice. Biol Sex Differ 10(1):42
Hadi NR, Yusif FG, Yousif M, Jaen KK (2014) Both castration and goserelin acetate ameliorate myocardial ischemia reperfusion injury and apoptosis in male rats. ISRN Pharmacol 2014:206951
Huang C, Gu H, Zhang W, Herrmann JL et al (2010) Testosterone−down−regulated Akt pathway during cardiac ischemia/reperfusion: a mechanism involving BAD, Bcl−2 and FOXO3a. J Surg Res 164(1):e1−11
Kohno H, Takahashi N, Shinohara T, Ooie T et al (2007) Receptor−mediated suppression of cardiac heat−shock protein 72 expression by testosterone in male rat heart. Endocrinology 148(7):3148–3155
Le TY, Ashton AW, Mardini M, Stanton PG et al (2014) Role of androgens in sex differences in cardiac damage during myocardial infarction. Endocrinology 155(2):568–575
Nam UH, Wang M, Crisostomo PR, Markel TA et al (2007) The effect of chronic exogenous androgen on myocardial function following acute ischemia−reperfusion in hosts with different baseline levels of sex steroids. J Surg Res 142(1):113–118
Pavon N, Martinez−Abundis E, Hernandez L, Gallardo−Perez JC, et al (2012) Sexual hormones: effects on cardiac and mitochondrial activity after ischemia−reperfusion in adult rats. Gender difference. J Steroid Biochem Mol Biol 132(1–2):135–46
Wang M, Tsai BM, Kher A, Baker LB et al (2005) Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia−reperfusion. Am J Physiol Heart Circ Physiol 288(1):H221–H226
Wang M, Wang Y, Abarbanell A, Tan J et al (2009) Both endogenous and exogenous testosterone decrease myocardial STAT3 activation and SOCS3 expression after acute ischemia and reperfusion. Surgery 146(2):138–144
O’Rourke B, Van Eyk JE, Foster DB (2011) Mitochondrial protein phosphorylation as a regulatory modality: implications for mitochondrial dysfunction in heart failure. Congest Heart Fail 17(6):269–282
Bertero E, Maack C (2018) Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res 122(10):1460–1478
Pongkan W, Chattipakorn SC, Chattipakorn N (2015) Chronic testosterone replacement exerts cardioprotection against cardiac ischemia−reperfusion injury by attenuating mitochondrial dysfunction in testosterone−deprived rats. PLoS ONE 10(3):e0122503
Svartberg J (2007) Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res 19(2):124–128
Donner DG, Elliott GE, Beck BR, Bulmer AC et al (2015) Impact of Diet−Induced Obesity and Testosterone Deficiency on the Cardiovascular System: A Novel Rodent Model Representative of Males with Testosterone−Deficient Metabolic Syndrome (TDMetS). PLoS ONE 10(9):e0138019
Alexandre J, Milliez P, Rouet R, Manrique A et al (2015) Aldosterone and testosterone: two steroid hormones structurally related but with opposite electrophysiological properties during myocardial ischemia−reperfusion. Fundam Clin Pharmacol 29(4):341–351
Lauro FV, Francisco DC, Elodia GC, Eduardo PG et al (2014) Activity exerted by a testosterone derivative on myocardial injury using an ischemia/reperfusion model. Biomed Res Int 2014:217865
Kuhar P, Lunder M, Drevensek G (2007) The role of gender and sex hormones in ischemic−reperfusion injury in isolated rat hearts. Eur J Pharmacol 561(1–3):151–159
Seara FAC, Barbosa RAQ, de Oliveira DF, Gran da Silva DLS, et al (2017) Administration of anabolic steroid during adolescence induces long−term cardiac hypertrophy and increases susceptibility to ischemia/reperfusion injury in adult Wistar rats. J Steroid Biochem Mol Biol 171: 34–42
Maldonado O, Ramos A, Guapillo M, Rivera J, et al (2019) Effects of chronic inhibition of Testosterone metabolism on cardiac remodeling after ischemia/reperfusion−induced myocardial damage in gonadectomized rats. Biol Open 8(5)
Seara FAC, Barbosa RAQ, Santos MVN, Domingos AE et al (2019) Paradoxical effect of testosterone supplementation therapy on cardiac ischemia/reperfusion injury in aged rats. J Steroid Biochem Mol Biol 191:105335
Cole AP, Hanske J, Jiang W, Kwon NK et al (2018) Impact of testosterone replacement therapy on thromboembolism, heart disease and obstructive sleep apnoea in men. BJU Int 121(5):811–818
Oni OA, Sharma R, Chen G, Sharma M et al (2017) Normalization of testosterone levels after testosterone replacement therapy is not associated with reduced myocardial infarction in smokers. Mayo Clin Proc Innov Qual Outcomes 1(1):57–66
Li H, Mitchell L, Zhang X, Heiselman D et al (2017) Testosterone Therapy and Risk of Acute Myocardial Infarction in Hypogonadal Men: An Administrative Health Care Claims Study. J Sex Med 14(11):1307–1317
Sharma R, Oni OA, Gupta K, Chen G et al (2015) Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J 36(40):2706–2715
Anderson JL, May HT, Lappé DL, Bair T et al (2016) Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol 117(5):794–799
Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER 3rd et al (2017) Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA 317(7):708–716
Mathur A, Malkin C, Saeed B, Muthusamy R et al (2009) Long−term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol 161(3):443–449
Malkin CJ, Pugh PJ, Morris PD, Kerry KE et al (2004) Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart 90(8):871–876
Malkin CJ, Morris PD, Pugh PJ, English KM et al (2003) Effect of testosterone therapy on QT dispersion in men with heart failure. Am J Cardiol 92(10):1241–1243
Etminan M, Skeldon SC, Goldenberg SL, Carleton B, et al. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy 35(1):72–8
Acknowledgments
This work was supported by grants from Thailand Research Fund (NA: TRG6280005), the Senior Research Scholar grant from the National Research Council of Thailand (SCC), a NSTDA research chair grant from the National Development Agency of Thailand (NC), and the Chiang Mai University Center of Excellence Award (NC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Apaijai, N., Chattipakorn, S.C., Chattipakorn, N. (2020). The Roles of Testosterone in Cardiac Ischemia/Reperfusion Injury. In: Ostadal, B., Dhalla, N.S. (eds) Sex Differences in Heart Disease. Advances in Biochemistry in Health and Disease, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-030-58677-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-58677-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58676-8
Online ISBN: 978-3-030-58677-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)