Abstract
Banco Chinchorro coral reef is an important protected marine area in Mexico, due to its biodiversity and ecosystem services, which has experienced overfishing of Lobatus gigas, Panulirus argus, and several target fish species. We aimed to build a model that was balanced (Ecopath) and spatially-explicit (Ecospace) to assess the application of different management strategies on the ecosystem. The spatial model for Banco Chinchorro reef distinguished the following five subsystems: Cueva de Tiburones, La Caldera, La Baliza, El Colorado, and El Chankay. Outcomes showed that benthic autotrophs comprised of the highest biomass in the whole system, followed by small benthic epifauna and sponges. Concerning ecosystem growth and development, Banco Chinchorro reef achieved a Total System Throughput of 144,980.7 g ww m−2 year−1, an Ascendency of 184,988.0 g ww m−2 year−1*bit, and a Development Capacity equal to 304,287.4 g ww m−2 year−1*bits. The A/C and Ov/C ratios reached 40.69% and 59.31%, respectively. The mean trophic level of the catch was estimated as 3.09, indicating that fishing is concentrated on species located at a high trophic level. Spatial-dynamic simulations showed that exploitation exerted separately, by subsystem, resulted in higher direct and indirect effects than fishing throughout the whole ecosystem. These results suggest that habitat rotation for harvest would not be advised.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Banco Chinchorro coral reef was declared a Biosphere Reserve in 1996 by the Mexican government to protect its biodiversity and ecosystem processes and to manage its natural resources (INE, 2000). This ecological system is located in the northern sector of the Mesoamerican Barrier Reef System and, at 40.7 km long and 18 km wide, is considered to be one of the largest platform coral reefs in the Caribbean Sea (Acosta-González, Rodríguez-Zaragoza, Hernández-Landa, & Arias-González, 2013; Jordán & Martín, 1987). The reef has high biodiversity due to its notable habitat heterogeneity, integrated into surrounding coral reefs are developments of spurs-and-groove habitats, wide stretches of seagrass and algae beds, coral reef patches, and small areas of mangrove (Acosta-González et al., 2013).
However, the reef has historically been exploited by artisanal fishers (>40 years), whose main target species are spiny lobster (Panulirus argus), queen conch snail (Lobatus gigas), and several fish species (Sosa-Cordero, 2003). As a consequence of this intensive period of harvest, L. gigas and P. argus are currently considered to be over-exploited resources (Cala de la Hera, de Jesús-Navarrete, Oliva-Rivera, & Ocaña-Borrego, 2012; de Jesús-Navarrete, Medina-Quej, & Oliva-Rivera, 2003; De Jesús-Navarrete & Valencia-Hernández, 2013; Sosa-Cordero, 2003). In order that these stocks may recover, the Mexican government has established minimum extraction sizes and bans for the fishing cooperatives on the exploitation of L. gigas and P. argus (de Jesús-Navarrete et al., 2003; Rodríguez-Zaragoza et al., 2016). However, poaching activity has caused the situation to reach a critical state, negatively affecting the livelihoods of legal fishers (de Jesús-Navarrete et al., 2003). While the bans have been implemented, the exploitation of reef fish of the Serranidae, Lutjanidae, and Haemulidae families on Banco Chinchorro has increased considerably, impacting ecosystem functioning and properties (resistance) (Rodríguez-Zaragoza et al., 2016). An additional perturbation is the introduction and rapid spread of the alien lionfish Pterois volitans (Ortiz et al., 2015) since its presence may decrease the overall biodiversity of coral reefs and lead to phase-shift transitions from corals to fleshy macroalgae (Albins & Hixon, 2013).
Studies regarding fishing activities have mainly focused on the exploitation of P. argus and L. gigas using classical population analysis (de Jesús-Navarrete et al., 2003; Sosa-Cordero, 2003). Some spatially-explicit predictions and habitat classification models have shown that fish diversity hotspots are highly correlated with reefscapes composed of an aggregation of coral colonies with seagrass beds (Acosta-González et al., 2013). Besides, qualitative and quantitative ecosystem models have been built for analyzing management strategies in Banco Chinchorro from an ecosystem perspective. Rodríguez-Zaragoza et al., (2016) built several stationary trophic models to analyze the multispecies fishery, the structure, trophic functioning, and ecosystem growth and development of five subsystems at Banco Chinchorro reef. Their outcomes showed that, as a consequence of the ecological heterogeneity of this coral reef, a subsystem-level management strategy needs to be designed, particularly because different species or functional groups exhibit a greater sensitivity to human interventions depending on which area they inhabit.
Nowadays, the Ecosystem-Based Fisheries Management (EBFM) is a widely recognized and accepted analytical strategy to assess multispecies fisheries (Pikitch et al., 2004), incorporating the needs of the authorities, fishers, tourism service operators, and others involved, and ensuring the implementation of a holistically sustainable co-management strategy (Ortiz et al., 2013, 2015). Ecosystem mass-balance models may be considered as complementary tools for studies of population dynamics. These models can be constructed using the program EcopathWithEcosim (EwE) (Christensen & Walters, 2004), integrating fishing activities, diet matrices, and network analysis. EwE incorporating the Ecospace routine has frequently been used to build spatially-explicit models based on multi-trophic relationships, assessing the possible effects of applying different management strategies in marine ecosystems (i.e. Walters, Christensen, & Pauly, 1997; Walter, Pauly, Christensen, & Kitchell, 1999; Ortiz and Wolff, 2002; Ortiz, Avendaño, Berrios & Campos 2009; Ortiz, Avendaño, Cantillañez, Berrios & Campos, 2010; Romagnonia, Mackinsonb, Hong & Eikeset 2015; Alexander, Meyjes & Heymans 2016). Nevertheless, few Ecospace models have been built specifically for coral reefs (Gribble, 2005; Okey et al., 2004; Varkey, Ainsworth, & Pitcher, 2012). Recognizing that Ecospace models enable the propagation of higher-order effects as a response to fishing activities to be assessed across spatial scales within marine ecosystems, the main objective of this chapter was to build a mass-balance model using EwE that incorporates the spatial heterogeneity of the coral reef at Banco Chinchorro Biosphere Reserve. To achieve this the five subsystems or habitat types previously described were considered (Rodríguez-Zaragoza et al., 2016), permitting us to assess: (1) biomass distribution and determination of the macroscopic properties of the whole ecological system; (2) spatial changes as responses to the eventual application of different fishing scenarios on commercially interesting species; and (3) the species or functional groups that are most impacted by different spatially-explicit management scenarios.
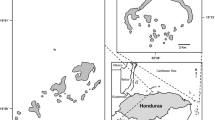
Banco Chinchorro is a coral reef with an ovoid shape (43.2 km long x 18.0 km wide) and platform type, located off the south-west coast of Yucatán Peninsula and separated from the continent by a channel 30.8 km wide and ≈500 m deep (INE, 2000; Vega-Zepeda, Hernández-Arana, & Carricart-Ganivet, 2007) (Fig. 1a). This coral reef has a lagoon with an area >500 km2 and depths varying between 1 and 9 m, surrounded by a semi-continuous barrier reef (~115 km in perimeter), where the seawater is oligotrophic with average surface water temperatures that range between 27 and 29 °C, while salinity varies from 36.6‰ to 36.9‰ (INE, 2000). More details regarding the environmental features of this coral reef are described in Ortiz et al. (2015) and Rodríguez-Zaragoza et al., (2016).
(a) Study area at Banco Chinchorro Biosphere Reserve, Mexico. (b) Trophic model for Banco Chinchorro. Vertical position approximates trophic level. The circle size is proportional to the compartment (species and functional groups) biomass ( g wet weight [ww] m−2). The connections represent the flow of matter among compartments. The number in circle corresponds to the species or functional groups (for details see Table 1). (c) Spatial basemap constructed through Ecospace routine of EwE, showing the five different subsystems, and (d) Spatial fishing effort scenarios simulated by Ecospace. (In the subsystem Cueva Tiburones: fishing on Spiny lobster; in La Caldera: fishing on S. barracuda, E. striatus, M. bonaci, L. analis, BPCF, L. maximus, BCF; in La Baliza: fishing on S. barracuda, E. striatus, M. bonaci, L. griseus, L. analis, BPCF, L. maximus, BCF, Spiny lobster, Queen conch; in El Colorado: fishing on S. barracuda, E. striatus, M. bonaci, PF, L. griseus, L. analis, L. maximus, BCF, Spiny lobster; in El Chankay: fishing on E. striatus, M. bonaci, L. maximus)
2 Modeling Strategy and Assumptions
EcopathWithEcosim (EwE) was initially based on the Polovina (1984) approach, which estimates the biomass and food consumption of several functional groups within an ecosystem. Subsequently, Christensen and Pauly (1992) and Walters et al. (1997) made some extensions to EwE, increasing its capabilities to allow simulations of temporal (i.e. Ecosim) and spatial (i.e. Ecospace) dynamics. EwE permits steady-state ecosystem models to be assessed in terms of matter/energy flow at a particular time, whereas the Ecospace routine provides temporal dynamic simulations of Ecopath, where biomass (B) and consumption (Q) dynamics are evaluated in spatial and temporal dimensions, this means that they vary within the spatial coordinates x, y, and over time (Fig. 1b). For more details of Ecospace theoretical framework see Box 1. Moreover, EwE also includes a network analysis feature called Ascendency (Ulanowicz, 1986, 1997), which allows us to estimate macroscopic properties, such as growth, organization, development, and the “ecosystem health.” In this context, an ecosystem would be considered healthy if it is sustainable because it keeps its organization and processes over time, and is resilient against disturbances (Costanza, Mageau, & Norton, 1998). For more details about Ascendency see chapter “Macroscopic Properties and Keystone Species Complexes in Kelp Forest Ecosystems Along the North-Central Chilean Coast.”
Box 1 Ecospace Theoretical Framework
The Ecospace is a spatially-explicit routine of EcopathWithEcosim program that permits us to define rectangular grids of spatial cells. In this case, the space, time, and state of variables are considered discrete by using the Eulerian approach that considers movement as flow of organisms among fixed cells. The immigration rate by cell is assumed to consist of four emigration flows from the surroundings cells. The emigration flows are represented as instantaneous movement rates (mi) x biomass (Bi) in each cell as follows:
where (x,y) represents cell row and column.
Likewise, fishing mortality (Fi) can be spatially represented by using a gravity function incorporated into Ecospace, by which the proportion of total effort allocated to each cell is considered to be proportional to the sum over groups of biomass multiplied by catchability and market price of the commercial species or functional groups, all is integrated by following algorithm:
where Gkc is weighted attractiveness of cell c to fleet k; Okc = 1 if cell c is open to the fleet and 0 if it is closed to fishing; Ukc = 1 if it was specified that gear k can harvest and 0 otherwise; pki is the relative price assigned for species or functional group i by fleet k fisheries; qki is the catchability of compartment i by fleet k; Bic is the biomass of species or group i in cell c; and Ckc is the relative cost of fishing in cell c by gear k. Finally, the spatial simulation searches for a moving equilibrium for the biomass of each compartment based on the following function:
where Bi(t+∆t) is the biomass of the compartment i moving toward an equilibrium along the time; Bi(t) is the biomass of the compartment i at the initial time of simulation; Bi(e) is the biomass of the compartment i at equilibrium; and Wi(t) is the exponential weight for the compartment i and assumes the following behavior:
where Zi is the total instantaneous mortality rate for the compartment i and Ei is the total instantaneous emigration rate. For more details on Ecospace framework see Walter et al. (1999).
2.1 Data Sources, Model Compartments, and Dynamic Simulations
A global trophic model was constructed of the whole Banco Chinchorro coral reef with compartments representing species and functional groups following the criteria established by Rodríguez-Zaragoza et al. (2016). The functional fish groups were characterized as benthic-pelagic carnivorous fishes, piscivorous fishes, benthic carnivorous fishes, zooplankton feeders, omnivorous fishes, and herbivorous fishes. Other functional groups were the large benthic epifauna, sea urchins, soft corals, small benthic epifauna, zooplankton, stony corals, sponges, benthic autotrophs, symbiotic algae (Symbiodinium spp.), phytoplankton, and detritus. The species were selected for their economic importance: the queen conch snail L. gigas, the spiny lobster P. argus, and the reef fish Mycteroperca bonaci, Sphyraena barracuda, Epinephelus striatus, Lutjanus griseus, Lutjanus analis, and Lachnolaimus maximus. (For more details of the species, functional groups, and sampling procedures in the current study, please see Rodríguez-Zaragoza et al. (2016).
During the balancing process, the model was checked based on the following six guidelines proposed by Heymans et al. (2016): (1) The Ecotrophic Efficiency (EE) of all compartments had to be <1.0 (Ricker, 1968), (2) the Gross Efficiency (GE) of all compartments had to be <0.3 (Christensen & Pauly, 1993). If any inconsistencies were detected, the average biomass was modified within the confidence limits (±1 standard deviation), (3) the Net Efficiency of all compartments had to be >GE, (4) the Respiration/Assimilation (R/AS) had to be <1.0, (5) the Respiration/Biomass (R/B) values for fishes had to be 1–10 year−1 or, for groups with higher turnover, 50–100 year−1, and (6) Production/Respiration (P/R) had to be <1.0 (Table 1).
The Ecospace simulations were performed using EwE software v. 6.4.1. Dispersal rates ranged from 300 km year-1 for fishes to 1.0 km year-1 for species that lived in only one subsystem and for sessile organisms, set based on personal observations made during fieldwork and from the range given by Ortiz et al. (2010) and Varkey et al. (2012). The relative dispersal values in poor habitats (i.e. unsuitable for the taxa) were the highest (factor = 10) for mobile consumers, such as most species and functional groups of fish, medium (factor = 5–8) for spiny lobster, large benthic epifauna, small benthic epifauna, zooplankton, and phytoplankton, and lowest (factor = 2–4) for slow and sessile species or functional groups. Relative vulnerability to predation in poor habitats ranged from 2.0 for top predators (e.g. S. barracuda and M. bonaci) to 100.0 for the species and functional groups of lower trophic levels. Relative feeding rate in poor habitats ranged from 1.0 for top predators, plankton and detritus, to 0.01–0.02 for slow motion and sessile organisms. For all other components an intermediate value (0.5) was used. The spatial distribution of each subsystem in the study area is shown in Fig. 1c. Several fishery scenarios were evaluated over a five-year period, where only the impact on the four most important species was considered in terms of catch and demand (spiny lobster, queen conch, S. barracuda, and L. analis). Spatially-explicit simulations were performed considering exclusive harvest from each subsystem, as well as simultaneous harvests in all subsystems (Fig. 1d). Ecospace simulations were conducted based on three flow controls (i.e. different vulnerabilities, (vij)) that affect the energy transfer rate between two compartments. The following flow controls were used: bottom-up (v = 1.0), mixed (v = 3.0), and top-down (v = 5.0). This approach was used because of the lack of the fishery data’s time-series, making it is impossible to calibrate the EwW model. Market prices and operational costs were not included in the spatial simulations.
3 Macroscopic Network Properties and Dynamic-Spatial Model Responses
The functional groups of benthic autotrophs (BA), small benthic epifauna (SBE), and sponges comprised of the highest biomass of the entire Banco Chinchorro reef (Table 1). The high biomass magnitude for BA has been reported previously for other Mexican coral reefs (Acosta-González et al., 2013; Arias-González, González-Gándara, Cabrera, & Christensen, 2011; Arias-González, Nuñez-Lara, González-Salas, & Galzin, 2004). The size of the autotroph biomass has been conjectured to be a consequence of the lower herbivore pressure exerted by sea urchins, the impact of fishing on large herbivores and the increase in sediments and nutrients from runoff in the seawater (Hughes, 1994; Jackson et al., 2001; Hughes et al., 2003; Fung, Seymour & Johnson, 2011; Arias-González et al., 2017). Similarly, the BA accounted for the highest values of Total System Throughput (TST) and Ascendency (A). However, the fish M. bonaci presented the lowest percentage of Average Mutual Information (AMI), which accounts for the complexity in the entire system (Table 2).
Regarding ecosystem growth and development, the size of the TST for Banco Chinchorro reef was higher than those reported for other coral reef systems, such as those in the Indo-Pacific (Arias-González, Delesalle, Salvat, & Galzin, 1997; Arias-González & Morand, 2006; Liu et al., 2009), Eastern Tropical Pacific (Okey et al., 2004), and the Caribbean Sea (Arias-González et al., 2004; Opitz, 1996; Rodríguez-Zaragoza, 2007). However, our results were similar to those described for the Mahahual and Yuyum reefs located off the Mexican Caribbean coast (facing Banco Chinchorro) (Rodríguez-Zaragoza, 2007) (Table 2). The high biomass of the BA could explain the large size of TST. The Ascendency, Overhead, Development Capacity, A/C, and Ov/C ratios indicated that Banco Chinchorro reef would be a more developed, organized, and healthy ecological system compared to other coastal ecosystems (Baird and Ulanowicz, 1993; Wolff, 1994; Heymans and Baird, 2000; Wolff et al., 2000; Ortiz & Wolff, 2002; Arias-González et al., 2004, 2011; Arias-González & Morand, 2006; Ortiz, 2008; Cáceres et al., 2016; Ortiz et al., 2010, 2015, 2016), but also that this system was less resistant to perturbations. The latter factor could be explained by the lower harvest pressure exerted on this ecosystem.
With regard to the food web structure, Finn’s cycling index (FCI), Finn’s mean path length (FPL), and food web connectance (FWC) were calculated for Banco Chinchorro reef and were higher than those described for some Mexican Caribbean coastal reefs (Rodríguez-Zaragoza, 2007). Nevertheless, the system omnivory index (OI) for the system revealed similar magnitudes compared to models constructed for other coral reefs ecosystems (Arias-González & Morand, 2006), coastal lagoons (Vega-Cendejas & Arreguín-Sánchez, 2001), and benthic communities of temperate systems (Ortiz, 2008; Ortiz et al., 2010; Taylor, Wolff, Mendo, & Yamashiro, 2008). The impact of fishing on the network showed that the mean trophic level of catch in this study was similar to those described for other coral reefs (Arias-González et al., 2004; Liu et al., 2009; Rodríguez-Zaragoza, 2007) and mainly indicated exploitation of organisms from high and intermediate trophic levels. This outcome suggests that the fisheries of Banco Chinchorro reef have not yet generated severe disturbance to the ecosystem, such as would be the case should there be fishing down the food web process, which occurs when there is a considerable reduction in the population size of the large predatory fishes at the top of the food webs, as has been observed in other marine ecosystems (González, Torruco-Gomez, Liceaga-Correa, & Ordaz, 2003; Pauly, Christensen, Dalsgaard, Froese, & Torres, 1998).
The spatially dynamic simulations showed quite similar qualitative and quantitative patterns of direct and indirect effects on the remaining compartments using mixed and top-down flow control mechanisms. Conversely, the magnitude of changes using bottom-up flow control was markedly lower. According to the fishing model scenarios, the subsystems Cueva Tiburones, La Caldera, and El Chankay propagated the highest effects on the other components in the system, thus the harvest trajectory in these areas should be monitored. Likewise, fishing simultaneously in the five subsystems would not spread the greatest impact across the entire ecosystem; therefore, a harvest rotation policy would not be advisable (Fig. 2). It is relevant here to indicate that the validity of these findings is difficult to evaluate because only a few Ecospace models have been constructed for cross-checking between observed and predicted results. Despite this limitation, the dynamic model presented in the current study should be considered as a general (qualitative) strategy for examining the consequences of spatially-explicit fishing pressure, which could be useful for the design of sustainable multispecies fisheries management (Pauly et al., 2002), particularly considering that protected marine areas could restore the populations and ecological networks of adjacent highly exploited systems (Arias-González et al., 2004).
Although we are well aware that the quantitative trophic model constructed and analyzed in this study was a partial representation of the overall trophic makeup and interactions underlying the dynamics within the Banco Chinchorro reef ecosystem, such limitations are common in any type of model and independent of the model´s degree of complexity (Levins, 1966; Ortiz and Levins, 2011, 2017). In the current model, the following constraints were identified: (1) system complexity was reduced concerning the composition of several functional groups, although the most abundant species were considered; (2) regardless of the inherent well-known limitations and shortcomings of the Ecopath and Ecosim theoretical frameworks (Christensen & Walters, 2004), and recognizing that ecological processes occur in changing environments (Levins, 1968), the constructed model and its spatially-explicit simulations represented underlying system processes only when considering their short-term or transient dynamics (Ortiz, 2018; Ortiz et al., 2013, 2015, 2017).
References
Acosta-González, G., Rodríguez-Zaragoza, F. A., Hernández-Landa, R. C., & Arias-González, J. E. (2013). Additive diversity partitioning of fish in a Caribbean coral reef undergoing shift transition. PLoS ONE, 8(6), e65665. https://doi.org/10.1371/journal.pone.0065665.
Albins, M. A., & Hixon, M. A. (2013). Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environmental Biology of Fishes, 96, 1151–1157.
Alexander, K. A., Meyjes, S. & Heymans, J. J. (2016). Spatial ecosystem modelling of marine renewable energy installations: Guaging the utility of Ecospace. Ecological Modelling, 331, 115–128.
Arias-González, J. E., Delesalle, B., Salvat, B., & Galzin, R. (1997). Trophic functioning of the Tiahura sector Moorea Island, French Polynesia. Coral Reefs, 16, 231–246.
Arias-González, J. E., González-Gándara, C., Cabrera, J. L., & Christensen, V. (2011). Predicted impact of the invasive lionfish Pterois volitans on the food web of a Caribbean coral reef. Environmental Research, 111, 917–925.
Arias-González, J. E., & Morand, S. (2006). Trophic functioning with parasites: a new insight for ecosystem analysis. Marine Ecology Progress Series, 320, 43–53.
Arias-González, J. E., Nuñez-Lara, E., González-Salas, C., & Galzin, R. (2004). Trophic models for investigation of fishing effect on coral reef ecosystems. Ecological Modelling, 172, 197–212.
Arias-González, J. E., Fung, T., Seymour, R. M., Garza-Pérez, J. R., Acosta-González, G., Bozec, Y. M. & Johnson C. R. (2017). A coral-algal phase shift in Mesoamerica not driven by changes in herbivorous fish abundance. PLOS ONE, 12, e0174855.
Baird, D. & Ulanowicz, R. (1993). Comparative study on the trophic structure, cycling and ecosystem properties of four tidal estuaries. Marine Ecology Progress Series, 99, 221–237.
Cáceres, I., Ortiz, M., Cupul-Magaña, A. L. & Rodríguez-Zaragoza F. A. (2016). Trophic models and short-term simulations for the coral reefs of Cayos Cochinos and Media Luna (Honduras): a comparative network analysis, ecosystem development, resilience and fishery. Hydrobiologia, 770, 209–224.
Cala de la Hera, Y. R., de Jesús-Navarrete, A., Oliva-Rivera, J. J., & Ocaña-Borrego, F. A. (2012). Auto-ecology of the queen conch (Strombus gigas L. 1758) at Cabo Cruz, Eastern Cuba: Management and sustainable use implications. Proceedings of the Gulf and Caribbean Fisheries Institute, 64, 342–348.
Christensen, V., & Pauly, D. (1992). Ecopath II: A software for balancing steady-state ecosystem models and calculating network characteristics. Ecological Modelling, 61, 169–185.
Christensen, V., & Pauly, D. (1993). Trophic models of aquatic ecosystems. ICLARM Conference Proceedings, 26, 338–352.
Christensen, V., & Walters, C. (2004). Ecopath with Ecosim: Methods, capabilities and limitations. Ecological Modelling, 172, 109–139.
Costanza, R., Mageau, M., & Norton, B. (1998). Patten B predictors of ecosystem health. In D. Rapport, R. Costanza, P. Epstein, C. Gaudet, & R. Levins (Eds.), Ecosystem health (pp. 240–250). Malden: Blackwell Science.
de Jesús-Navarrete, A., Medina-Quej, A., & Oliva-Rivera, J. J. (2003). Changes in the queen conch (Strombus gigas L.) population structure at Banco Chinchorro,Quintana Roo, México, 1990–1997. Bulletin of Marine Science, 73, 219–229.
De Jesús-Navarrete, A., & Valencia-Hernández, A. (2013). Declining densities and reproductive activities of the queen conch Strombus gigas (Mesogastropoda: Strombidae) in Banco Chinchorro, Eastern Caribbean, Mexico. Revista de Biología Tropical, 61, 1671–1679.
Fung, T., Seymour, R. M. & Johnson, C. R. (2011). Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology, 92, 967–982.
González, A., Torruco-Gomez, D., Liceaga-Correa, A., & Ordaz, J. (2003). The shallow and deep bathymetry of the Banco Chinchorro reef in the Mexican Caribbean. Bulletin of Marine Science, 73, 15–22.
Gribble, N. A. (2005). Ecosystem modelling of the great barrier reef: A balanced trophic biomass approach (pp. 2561–2567). Melbourne: Modelling and Simulation Society of Australia and New Zealand.
Heymans, J. & Baird, D. (2000). A carbon flow model and network analysis of the northern Benguela upwelling system, Namibia. Ecological Modelling, 126, 9–32.
Heymans, J. J., Coll, M., Link, J. S., Mackinson, S., Steenbeek, J., Walters, C. & Christensen, V. (2016). Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Ecological Modelling, 331, 173–184.
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science, 265, 1547–1551.
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Gulberg, O., Jackson, J. B. C., Kleypas, J., Lough, J. M., Marshall, P., Myström, N., Palumbi, S. R., Pandolfi, J. M., Rosen, B., & Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science, 301, 929–933.
INE. (2000). Programa de Manejo Reserva Banco Chinchorro. México: Instituto Nacional de Ecología.
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjordal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., Hughes, T. P., Kidwell, S., Lange, C. B., Lenihan, H. S., Pandolfi, J. M., Peterson, C. H., Steneck, R. S., Tegner, M. J., & Warner, R. R. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science, 293, 629–638.
Jordán, E., & Martín, E. (1987). Banco Chinchorro: Morphology and composition of a Caribbean atoll. Atoll Research Bulletin, 310, 1–25.
Levins, R. (1966). The strategy of model building in population biology. Princeton Monographs Series.
Levins, R. (1968). Evolution in changing environments. Princeton, NJ: Princeton University.
Liu, P. J., Kwang-Tsao, S., Rong-Quen, J., Tung-Yung, F., Saou-Lien, W., Jiang-Shiou, H., Jen-Ping, C., Chung-Chi, C., & Hsing-Juh, L. (2009). A trophic model of fringing coral reefs in Nanwan Bay, southern Taiwan suggests overfishing. Marine Environmental Research, 68, 106–117.
Okey, T. A., Banks, S., Born, A. F., Bustamante, R. H., Calvopiña, M., Graham, J. E., Espinoza, E., Fariña, J. M., Garske, L. E., Recke, G. K., Salazar, S., Shepherd, S., Toral-Granda, V., & Wallem, P. (2004). A trophic model of a Galápagos subtidal rocky reef for evaluating fisheries and conservation strategies. Ecological Modelling, 172, 383–401.
Opitz, S. (1996). Trophic interactions in Caribbean coral reefs. Manila, Philippines: ICLARM.
Ortiz, M. (2008). Mass balanced and dynamics simulations of trophic models of kelp ecosystems near the Mejillones Peninsula of northern Chile (SE Pacific): Comparative network structure and assessment of harvest strategies. Ecological Modelling, 216, 31–46.
Ortiz, O. (2018). Robustness of macroscopic-systemic network indices after disturbances on diet-community matrices. Ecological Indicators, 95, 509–517.
Ortiz., M. & Wolff M. (2002). Trophic models of four benthic communities in Tongoy Bay (Chile): comparative analysis and preliminary assessment of management strategies. Journal of Experimental Marine Biology and Ecology, 268, 205–235.
Ortiz M. & Levins, R. (2011). Re-stocking practices and illegal fishing in northern Chile (SE Pacific coast): a study case. Oikos, 120, 1402–1412.
Ortiz, M. & Levins, R. (2017). Self-feedbacks determine the sustainability of human interventions in eco-social complex systems: Impacts on biodiversity and ecosystem health. PLoS ONE; 12, e0176163.
Ortiz, M., Avendaño, M., Berrios, F. & Campos, L. (2009). Spatial and mass balanced trophic models of La Rinconada Marine Reserve (SE Pacific coast), a protected benthic ecosystem: management strategy assessment. Ecological Modelling, 220, 3413–3423.
Ortiz, M., Avendaño, M., Cantillañez, M., Berrios, F., & Campos, L. (2010). Trophic mass balanced models and dynamic simulations of benthic communities from La Rinconada Marine Reserve off northern Chile: Network properties and multispecies harvest scenario assessments. Aquatic Conservation: Marine and Freshwater Ecosystems, 20, 58–73.
Ortiz, M., Campos, L., Berrios, F., Rodríguez-Zaragoza, F. A., Hermosillo, B., & González, J. (2015). Mass balanced trophic models and short-term dynamical simulations for benthic ecological systems of Mejillones and Antofagasta bays (SE Pacific): Comparative network structure and assessment of human impacts. Ecological Modelling, 309–310, 153–152.
Ortiz, M., Levins, R., Campos, L., Berrios, F., Campos, F., Jordán, F., González, J., & Rodríguez-Zaragoza, F. A. (2013). Identifying keystone trophic groups in benthic ecosystems: implications for fisheries Management. Ecological Indicators, 25, 133–140.
Ortiz, M., Berrios F., González, J., Rodríguez-Zaragoza, F. & Gómez I. (2016). Macroscopic network properties and short-term dynamic simulations in coastal ecological systems at Fildes Bay (King George Island, Antarctica). Ecological Complexity, 28, 145–157.
Ortiz, M., Hermosillo-Nuñez, B., Gonzáleza, J., Rodríguez-Zaragoza, F., Gómez, I., Jordán, F. (2017). Quantifying keystone species complexes: Ecosystem-based conservation management in the King George Island (Antarctic Peninsula). Ecological Indicators, 81, 453–460.
Pauly, D., Christensen, V., Dalsgaard, J., Froese, R., & Torres, F. (1998). Fishing down marine food webs. Science, 279, 860–863.
Pauly, D., Christensen, V., Guénette, S., Pitcher, T. J., Sumaila, U. R., Walters, C. J., Watson, R., & Zeller, D. (2002). Towards sustainability in world fisheries. Nature, 418, 689–695.
Pikitch., E. K., Santora, C., Babcock, E. A., Bakun, A., Bonfil, R., Conover, D. O., Dayton, P., Doukakis, P., Fluharty, D., Heneman, B., Houde, E. D., Link, J., Livingston, P. A., Mangel, M., McAllister, M. K., Pope, J. & Sainsbury K. J. (2004). Ecosystem-Based Fishery Management. Science, 305, 346–347.
Polovina, J. J. (1984). Model of a coral reef ecosystem: I the Ecopath model and its application to French Frigate Shoals. Coral Reefs, 3, 1–11.
Ricker, W. E. (1968). Food from the sea. In Committee on Resources and Man (Ed.), Resource and man (pp. 87–108). San Francisco: US National Academy of Sciences/W. H. Freeman.
Rodríguez-Zaragoza, F. A. (2007). Biodiversidad y funcionamiento de los ecosistemas arrecifales costeros del Caribe mexicano. Ph.D. thesis, Centro de Investigación y Estudios Avanzados, Unidad-Mérida, México, p. 344.
Rodríguez-Zaragoza, F. A., Ortiz, M., Berrios, F., Campos, L., de Jesús-Navarrete, A., Castro-Pérez, J., Hernández-Flores, A., García-Rivas, M., Fonseca-Peralta, F. & Gallegos-Aguilar E. (2016). Trophic models and short-term dynamic simulations for benthic-pelagic communities at Banco Chinchorro Biosphere Reserve (Mexican Caribbean): a conservation case. Community Ecology, 17, 48–60.
Romagnonia, G., Mackinsonb, S., Hong, J. & Eikeset A. M. (2015). The Ecospace model applied to the North Sea: Evaluating spatial predictions with fish biomass and fishing effort data. Ecological Modelling, 300, 50–60.
Sosa-Cordero, E. (2003). Trends and dynamics of the spiny lobster, Panulirus argus, resource in Banco Chinchorro, Mexico. Bulletin of Marine Science, 73, 203–217.
Taylor, M. H., Wolff, M., Mendo, J., & Yamashiro, C. (2008). Changes in trophic flow structure of Independence Bay (Peru) over an ENSO cycle. Progress in Oceanography, 79, 336–351.
Ulanowicz, R. (1986). Growth and development: Ecosystems phenomenology. New York: Springer.
Ulanowicz, R. (1997). Ecology, the ascendant perspective. Complexity in ecological systems series. New York: Columbia University Press.
Varkey, D., Ainsworth, C. H., & Pitcher, T. J. (2012). Modelling reef fish population responses to fisheries restrictions in marine protected areas in the coral triangle. Journal of Marine Biology, 2012, 721483. https://doi.org/10.1155/2012/721483.
Vega-Cendejas, M. E., & Arreguín-Sánchez, F. (2001). Energy fluxes in a mangrove ecosystem from a coastal lagoon in Yucatan Peninsula, Mexico. Ecological Modelling, 137, 119–133.
Vega-Zepeda, A., Hernández-Arana, H., & Carricart-Ganivet, J. P. (2007). Spatial and size-frequency distribution of Acropora (Cnidaria: Scleractinia) species in Chinchorro Bank, Mexican Caribbean: implications for management. Coral Reefs, 26, 671–676.
Walter, C. J., Pauly, D., Christensen, V., & Kitchell, J. (1999). Representing density dependent consequences of the life history strategies in aquatic ecosystems: ECOSIM II. Ecosystems, 3, 70–83.
Walters, C. J., Christensen, V., & Pauly, D. (1997). Structuring dynamic models of exploited ecosystems from trophic mass-balance assessments. Reviews in Fish Biology and Fisheries, 7, 139–172.
Wolff, M. (1994). A trophic model for Tongoy Bay – a system exposed to suspended scallop culture (Northern Chile). Journal of Experimental Marine Biology and Ecology, 182, 149–168.
Wolff, M., Koch, V. & Isaac, V. (2000). A trophic flow model of the Caeté mangrove estuary (North Brazil) with considerations for the sustainable use of its resources. Estuarine, Coastal and Shelf Science, 50, 789–803.
Acknowledgements
This study was financed by the World Wildlife Fund (WWF)-México, the Comisión Nacional de Areas Naturales Protegidas (CONANP), and Universidad de Guadalajara. We are grateful to G. Acosta (CICY-Cancún) and R. Hernández (UNAM) for their help in the laboratory work; as well as to M. García, F. Fonseca, G. Muñoz, A. Vega, and J.J. Domínguez at CONANP, A. Hernándezs-Flores (Universidad Martista de Mérida) and the fishing cooperatives of “Langosteros del Caribe” and “Pescadores de Banco Chinchorro” for their help in the fieldwork. We also thank to Pi-Jen Liu (National Chung Hsing University, Taiwan), who help us to estimate the coral and macroalgae biomass. Likewise, thanks to A. de Jesús-Navarrete for the information provided by L. gigas. Finally, we thank F. Berrios and L. Campos for their support in spatial modeling. This work is part of the first author’s postdoctoral research grant at Instituto Antofagasta de Recursos Naturales Renovables, Universidad de Antofagasta, Chile.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rodríguez-Zaragoza, F.A., Ortiz, M. (2021). Macroscopic Network Properties and Spatially-Explicit Dynamic Model of the Banco Chinchorro Biosphere Reserve Coral Reef (Caribbean Sea) for the Assessment of Harvest Scenarios. In: Ortiz, M., Jordán, F. (eds) Marine Coastal Ecosystems Modelling and Conservation. Springer, Cham. https://doi.org/10.1007/978-3-030-58211-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-58211-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58210-4
Online ISBN: 978-3-030-58211-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)