Abstract
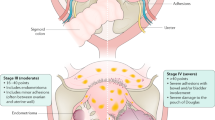
Endometriosis is a common gynecological syndrome associated with pain and infertility and characterized by the growth of hormone-responsive endometrial tissue outside the uterine cavity. Three major subtypes of ectopic endometriotic implants are currently recognized, based on their anatomic location: (1) attached to the peritoneal surface, (2) encapsulated within the ovary, or (3) infiltrating the connective tissues of the rectovaginal septum. However, more widely dispersed lesions have been described in the pleura, the cutaneous skin, and even the lacrimal duct [1]. The high prevalence of endometriosis is broadly recognized, and recent population-based estimates put its overall frequency among reproductive-age women at around 11% [2]. This disease is accompanied by pelvic pain in millions of women worldwide, resulting in work absenteeism, social isolation, and high costs of medical and surgical therapies. In the United States, endometriosis is the third commonest indication for hysterectomy, a procedure that can be particularly devastating in women under age 30, because they are likely to experience residual somatic symptoms and a severe psychological sense of loss.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
Endometriosis is a gynecological syndrome associated with pain and infertility and characterized by the growth of hormoneresponsive endometrial tissue outside the uterine cavity. Recent population-based estimates put its overall frequency among reproductive-age women at around 11%; hence, it afflicts millions of women worldwide, resulting in work absenteeism, social isolation, and high costs of medical and surgical therapies. In this chapter, the authors offer hypotheses as to the role of “neuroangiogenesis” in endometriosis pathogenesis and pathophysiology. Mediators of lesion-associated pain and current and future therapeutic strategies are offered. Evolution of our understanding about the biology of this chronic disease promises to expand treatment choices for women suffering from its complications.
4.2 Clinical Presentation
Endometriosis has been identified in women ranging in age from 12 to 80, with an average of ~28 years old. Exposure to ovarian hormones appears to be essential to stimulating lesion growth. Although its precise mechanisms remain mysterious, the most common symptom in women with endometriosis is progressive, secondary dysmenorrhea. The pain typically begins before menses and continues throughout the duration of menstrual flow. It may be accompanied by dyspareunia, dysuria, dyschezia, or noncyclic pelvic pain [3]. The pain also may be referred to musculoskeletal regions, such as the flank or low back.
Due to the pusillanimous, diffuse nature of common parietal peritoneal endometriosis lesions, physical examination can be unremarkable. However, the astute clinician can sometimes appreciate pain or induration with palpation in the vicinity of the cul-de-sac or rectovaginal septum. Tender nodules along the uterosacral ligaments, especially if the examination is done just before menses, can sometimes be identified. Rarely, impaired renal function and azotemia can occur in women with retroperitoneal ureteric fibrosis that compromises urinary drainage.
Cyclic in situ menstruation at the sites of endometriotic lesions is thought to activate a chronic inflammatory response involving cytokine release and prostaglandin biosynthesis, leading to pain perception. In some cases, direct infiltration of endometriotic cells into afferent nerves has been observed [4]. Furthermore, the enhanced inflammatory milieu may result in sensitization of dorsal root ganglia and central neurons [5]. New hypotheses suggest that coordinated neural and vascular growth, “neuroangiogenesis,” and secondary central neuropathic sequelae contribute to pelvic pain [6, 7].
4.3 Genomics, Genetics, and Epigenetics
Genes strongly influence susceptibility for endometriosis [8] and its heritability is estimated to be as high as 51%. However, the mode of transmission is polygenic and involves multiple gene loci [9]. Early evidence indicated that first-degree female relatives (mothers and sisters) of women with severe endometriosis had a 7% incidence, whereas in primary female relatives of their partners (who typically have similar ethnic and socioeconomic status) <1% had endometriosis [10]. Of interest is the finding that familial cases of endometriosis tend to be more severe and have an earlier onset of symptoms than sporadic cases. Endocrine disruptor compounds in the environment are thought to affect endometriosis, a subject that is presented in Chap. 1 by Palumbo and colleagues.
4.4 Pathogenesis
Descriptions of the symptoms and pathological features of endometriosis date back to Dutch and Belgian publications of the 1600s [11]. The great German pathologists Karl von Rokitansky and Robert Meyer wrote extensively about this disorder in the nineteenth century, and the Canadian physician, Thomas Cullen, is credited for identifying endometriosis as a unique disease in the 1890s. In the 1920s an American gynecologist named John Sampson put forward the hypothesis that pelvic endometriosis lesions arose from endometrial tissue escaping through the fallopian tubes at the time of menstruation. Viable tissue fragments, he postulated, implanted on and invaded peritoneal surfaces, where they regenerated an endometrial epithelial lining [12].
4.4.1 Retrograde Menstruation, Implantation, and Lesion Establishment
Still today, the prevailing hypothesis concerning the histogenesis of endometriosis is Sampson’s implantation theory [12]. This concept is supported by the visual documentation of reflux menstruation [13], intraperitoneal spillage of competent endometrial cells, and the gravity-dependent location of most foci of endometriosis. Furthermore, the incidence is increased in women with Müllerian anomalies and menstrual outflow obstruction [14]. Finally, more than 60% of unilateral ureteral endometriosis lesions occur in the left hemipelvis, which is consistent with the accumulation of refluxed cells in this location, due to the position of the sigmoid colon mesentery [15].
Although some controversy exists as to whether or not lesion implantation requires a breach in the mesothelial surface or if endometriosis cells are capable of invading intact mesothelium, the expression of adhesion molecules on the surface of exfoliated uterine cells is thought to be necessary for nascent lesion attachment and invasion [16]. Cytokines (e.g., interleukin (IL)-lβ, IL-6, and tumor necrosis factor (TNF)-α) and relative steroid hormone sensitivity predispose the adhesion of shed endometrial fragments, facilitating peritoneal invasion.
4.4.2 Angiogenic Factors
Implantation of ectopic endometrium sets into motion a step-wise pathophysiological program involving hormone responsiveness and immune cell activation, as depicted in Fig. 4.1. How these progressive interactions appear to promote the establishment of endometriosis lesions is addressed in this review. Given the universality of retrograde menstruation [13], it is not clear why endometriosis affects only ~11% of women. It is postulated that the intrinsic angiogenic potential of the intraperitoneal environment dictates the likelihood of lesion establishment. At laparoscopy, endometriotic implants are often surrounded by exaggerated vascularity and in the rare occurrence of extrapelvic endometriosis, it is often localized in well-vascularized sites.
Sprouting angiogenesis of new blood vessels from preexisting capillaries is a complex process involving proteolytic degradation of the extracellular matrix, proliferation, and migration of endothelial cells, and ultimately the organization of cell columns into patent capillary tubules. Many angiogenic growth factors and cytokines have been identified in endometriosis and reviewed recently [17]. Vascular endothelial growth factor (VEGF) is primary among those proteins; moreover, it also has neurogenic properties [6]. VEGF is known to be an estrogen-responsive gene, particularly in the uterus [18].
4.4.3 Estrogen Biosynthesis, Receptors, and Action Are Critical for Endometriosis Lesion Growth
One of the defining characteristics of endometriosis is its endocrine responsiveness, particularly with respect to estrogen. Typically, endometriosis pain symptoms begin after the onset of menses and, in the majority of cases, resolve after menopause. The estrogen dependency of this condition led Barbieri to propose the hypothesis that concentrations of estradiol over 50 pg/mL were needed to support the growth of endometriosis lesions [19]. His hypothesis proved to be prescient as more recent and sophisticated pharmacometrics studies of a variety of gonadotropin-releasing hormone analog (GnRHa) drugs have revealed that a threshold of 30–50 pg/mL estradiol is highly correlated with endometriosis symptom recurrence in clinical trials [20]. Promising data are beginning to appear in the literature supporting the use of orally active GnRH antagonists for the treatment of endometriosis-associated pain and improvement in quality of life measures [3]. These therapeutic options will be discussed in more detail elsewhere in this monograph by De Villiers (Chap. 14). Body mass index (BMI) correlates inversely with the risk of endometriosis [21]. Other more sophisticated anthropometric assessment measurements (e.g., skin-fold thickness, arm, waist, chest and hip circumferences) failed to add more predictive power for endometriosis risk than low BMI.
Steroid biosynthesis and metabolism actively occur within these lesions, where an entire range of steroidogenic enzymes are expressed, including steroid acute regulatory (StAR) protein, cholesterol P450 side-chain cleavage, 3β-hydroxysteroid dehydrogenase type 2, 17α-hydroxylase and aromatase. Thus, endometriotic lesions have co-opted the ability to generate their own local estrogenic milieu using cholesterol as a substrate [22].
Like its derivative eutopic endometrium, endometriotic lesions express the gamut of nuclear steroid and isoprenoid receptors, as reviewed elsewhere [22, 23], which impart broad hormone responsivity to the implants. Moreover, the expression of some receptor isoforms differs from those in normal endometrium, providing a growth advantage to the ectopic foci. In particular, estrogen receptor β (ESR2) is highly expressed in endometriosis and concentrations of progesterone receptor B (PGR-B) are downregulated. An apparent epigenetic mechanism in the former case is a result of the hypomethylation of CpG islands in the ESR2 gene promoter [24].
4.4.4 Innervation of Endometriosis Implants
The parietal peritoneum is richly innervated by somatic and visceral afferents arising from branches of the lower intercostal and upper lumbar nerves. Unmyelinated sensory Aδ- and C-nerve endings are exposed to nociceptive biochemical stimuli, which are perceived as sharp pain referred to the periumbilical, suprapubic, or lower abdominal regions. Interestingly, the visceral peritoneum has little innervation, but submesothelial autonomic nerves are present that respond to traction and pressure [25].
Anaf et al. [26] were among the first to postulate that pelvic pain arising from endometriosis might be associated with peritoneal nerve fibers. They observed that subjects with nodular lesions demonstrating perineural invasion had the highest pelvic pain scores. Tulandi et al. [27] used anti-neurofilament antibodies and immunohistochemistry methods to quantify nerve fiber density and neurofilament protein intensity but did not observe more nerves in peritoneal specimens from endometriosis subjects compared to women without endometriosis. Interestingly, in the latter study, they did note more “lymphocytic infiltration” in the histological samples from women with laparoscopically-proven endometriosis than controls. We will discuss this finding in more detail when we address the potential immune modulators of neuroangiogenesis in this setting.
A pioneering study in this field was performed not in women but in rats with surgically induced “endometriosis.” Berkley and colleagues [28] autotransplanted uterine tissue fragments to the bowel mesentery and noted that after ~7.5 weeks, the cystic lesions of ectopic endometrium had acquired a rich plexus of nerves that immunostained positively for neuronal markers indicating the presence of sensory Aδ- and C-fibers along with sympathetic nerves. An additional observation at that time was that neurites growing out of these lesions were accompanied by a dense microvasculature. These findings further support the concept of lesion neuroangiogenesis proposed some years later [6].
By the 2000s, investigators were reporting histological evidence that nociceptive and autonomic afferent nerves were present in endometriotic lesions of women [29]. An important extension of this line of research, first announced by the Australian group [30] and confirmed by Belgian scientists [31], was the discovery that eutopic endometrium from women with endometriosis had a higher density of nerve fibers than the endometrium of unaffected controls. This topic has been argued in the literature, but many studies support neuron density as a distinguishing feature between women with and without endometriosis. As detailed in the introductory part of this chapter, such a finding is consistent with Sampson’s implantation theory that ectopic lesions are derived from shed eutopic endometrium. The idea that eutopic endometrium is the “mother of the implants” in endometriosis is an important contemporary concept in the pathogenesis of this disorder [23].
If ectopic, and even eutopic, endometrial tissues in endometriosis are imbued with a higher density of nociceptive nerves than in unaffected women there must be some trophic factor responsible for this phenomenon. This was a question asked by a number of endometriosis scholars in the early 2000s. Borghese et al. [32] posed this question by assessing a panel of neurotrophin mRNAs in endometriomas and eutopic endometrium using reverse transcription-polymerase chain reaction methods. They reported that transcripts representing nerve growth factor (NGF), brain-derived growth factor (BDNF), neurotrophin-2, neurotrophin-4/5, and the neurotrophin receptor (NTRK2) were all greater in endometrioma samples than in eutopic endometrial tissues from the same subjects. Similar findings reported by Kajitani et al. [33] indicated that NGF mRNA levels were higher in endometrioma and peritoneal lesions than in normal endometrium. In studies from Belgium and France, endometriosis glands at the leading edge of deeply invasive lesions had elevated NGF, N-cadherin, and matrix metalloproteinase 9, and all were accompanied by a high density of nerves [34]. The density of protein gene product (PGP) 9.5-positive nerve fibers also was noted to be associated with deep dyspareunia in cases of endometriosis [35].
Our research team asked a slightly different question and used an agnostic, monoclonal antibody microarray method to compare the expression of neurotrophin proteins in eutopic endometria from women with or without surgically documented endometriosis. Our findings identified many of the same neurogenic factors described above, with BDNF, neurotrophin-3, and neurotrophin-4/5 being the predominant endometrial proteins differentially overexpressed in endometriosis cases [36]. Among these, BDNF concentrations were almost 1000-fold more enriched in endometrial lysates than the other neurotrophins. NGF was present in both sets of tissues but did not differ in concentration between the two patient groups. More recent studies have confirmed that even plasma concentrations of BDNF are increased in women with endometriosis and in some cases, these concentrations correlate with the severity of pelvic pain in affected subjects [37]; however, in most reports, the positive and negative predictive value of discriminating concentrations of BDNF and other biomarkers are not adequate for diagnostic testing or screening purposes [38].
One interesting biochemical aspect of these trophic factors, alluded to above, is that they possess both neurogenic and angiogenic activities. As noted above, the role of angiogenesis in the microenvironment of endometriosis lesions was established in the 1990s [39, 40]. Based on the tightly coupled growth of nerves and capillaries, e.g., during embryonic development and wound healing [41], we proposed that guidance molecules and their receptors common to nociceptive neurons and vascular endothelial cells were expressed in endometriosis tissues, allowing nascent implants to simultaneously recruit nerves and blood vessels through a cooperative process of neuroangiogenesis [6]. Several of these guidance proteins (e.g., BDNF, NGF, VEGF, semaphorin E) and their cognate receptors (e.g., TRK2, VEGFR1, Neuropilin, Plexin-D1, and Robo4 [42]) have been identified in endometriosis [43].
The peritoneal fluid that bathes the superficial lesions also has been identified as a source of neuroangiogenic factors. High levels of NGF were demonstrated in the pelvic fluid of endometriosis cases by Western blotting and its biological activity was confirmed by neurite outgrowth from chicken dorsal root ganglia in vitro. Some studies show correlations among peritoneal fluid cytokine concentrations, nerve fiber density, and pelvic pain [44], but these relationships do not hold true in all studies. In one report, pelvic pain scores were highest in subjects with histologically confirmed perineural invasion in deep endometriosis lesions, and these also demonstrated an increased density of neuroangiogenic activity [45]. A potential mechanism to promote neuroangiogenesis in the vicinity of endometriosis lesions is via exosome secretion. Exosomes derived from endometriosis subjects preferentially induced endothelial tube formation from primary human umbilical vein cells and neurite outgrowth from murine dorsal root ganglia in vitro [46, 47].
4.4.5 Endocrine and Cytokine Regulation of Neuroangiogenic Effects
Estrogenic and inflammatory influences are important modulators of nociception in endometriosis. Some effects are relatively direct; for example, in ovariectomized mice, administration of estradiol increased uterine BDNF and NGF receptor expression and NGF receptor mRNA levels also were upregulated [48]. In an established immunocompetent mouse model of endometriosis, we and our collaborators demonstrated that two selective estrogen receptor modulators (SERMs): oxabicycloheptene sulfonate (an ESR1 ligand) and chlorindazole (an ESR2 selective SERM), arrested cell proliferation, lesion growth, and neuroangiogenesis in surgically induced implants. This murine model corroborated that estrogens and macrophage-derived cytokines interact to promote the growth of peripheral nerves and emphasized that both isoforms of the ESR appear to have important actions on the establishment of endometriosis lesions [22].
Macrophages commonly infiltrate the microenvironment of endometriosis lesions. Strong evidence indicates that these inflammatory phagocytes are recruited to the implants by chemokines such as RANTES (CCL5) or MCP-1 (CCL2). Macrophage-derived cytokines, particularly IL-1β and TNF-α, are enriched in the peritoneal fluid of women with endometriosis [23]. IL-1β was found to directly stimulate BDNF mRNA and protein in endometriosis cell cultures, an effect that was predominantly mediated by c-Jun N-terminal kinase and nuclear factor κB (NF-κB) signaling pathways [49]. More recently, IGF-1 derived from macrophages also was shown to promote neurogenesis within the inflammatory foci of endometriosis implants [50]. Figure 4.2 provides an illustration of invasive endometriosis with classical glandular and stromal elements (panel A). Isolated macrophages, stained with anti-CD68 antibodies (magenta), have infiltrated the lesion (panel B) and BDNF is expressed by glands and stroma (panel C). In panel D, PGP9.5-positive nerve fibers (magenta) are viewed en face in the cut section, innervating the lesion. The regional confluence of endometriotic cells, immune cells, and nerve cells, along with capillaries (not shown in this tableau), supports the hypothesis that chemokines and neuroangiogenic factors produced in situ establish a microenvironment that recruits nociceptive nerves to the growing lesion, which we postulate ultimately effects endometriosis-associated pain.
Endometriosis lesion stained with hematoxylin and eosin (upper left panel), CD68 (anti-macrophage) (upper right panel), BDNF (anti-neurotrophin) (lower left panel), and PGP9.5 (anti-nerve fibers) (lower right panel). Note that nerve fibers in the lower middle panel are mostly viewed en face relative to the orientation of the implant. Magnification × 200. (Reproduced with permission from Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL and Taylor RN. IL-1β stimulates BDNF production in eutopic endometriosis stromal cell cultures: A model for cytokine regulation of neuroangiogenesis. Am J Pathol 188: 2281–2292, 2018. PMCID: 6169127)
4.4.6 Central Sensitization in Endometriosis-Associated Pain
Many of the experiments described in this review were designed to address how peripheral, peritoneal neuroangiogenesis can contribute to nociception of lesions in endometriosis, but another critical component of the processing of pain symptoms, particularly chronic pelvic pain, is mediated via central sensitization. Neuroimaging of the brain in 17 women with surgically confirmed endometriosis and chronic pelvic pain compared with 23 controls without pain revealed decreased grey matter volume in the left thalamus, left cingulate gyrus, right putamen, and right insula of the affected subjects; all four brain regions are known to be involved in pain processing [51]. The stress axis and other psychological factors also can modulate the perception of chronic pain, such that causes as well as consequences of pain and other somatic symptoms are associated with endometriosis [52].
4.5 Medical Therapy for Pain Associated with Endometriosis
Currently approved medications for endometriosis pain, endorsed by the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMEA) include progestins (depot medroxyprogesterone acetate, norethindrone acetate, and dienogest), an androgen (danazol), and GnRHa. In 2018 the FDA also approved the oral GnRH antagonist elagolix for moderate to severe endometriosis pain. In randomized, placebo-controlled trials, medroxyprogesterone acetate, danazol, GnRHa, and the GnRH antagonist were all more effective than placebo. Pain relief of more than 6 months’ duration was noted in 40–70% of women [53]. Although they are not formally FDA endorsed, continuous oral contraceptives also have been found to be efficacious and are widely used [54]. Following surgery for endometriomas, oral contraceptives significantly reduced pain and recurrence when compared to controls. Progestin-containing intrauterine systems have also shown merit to ameliorate post-operative endometriosis-induced dysmenorrhea.
Although highly effective in the management of pain with endometriosis, treatment with GnRHa or the oral antagonist is limited to 6 months by the FDA because of the risk of hypoestrogenic effects induced by ovarian suppression, including loss of bone mineral density. Suppression of the hypothalamic–pituitary axis by these agents can be mitigated with “add-back” of exogenous ovarian steroids. Add-back regimens with norethindrone acetate combined with low-dose estrogen can safely extend pain relief and bone preservation for at least 1 year, and one trial, with a limited number of participants, found no ill effects after 10 years of treatment with add-back therapy [55]. Randomized, clinical trials demonstrated that the 6 months of subcutaneous progestin was as effective as GnRHa in diminishing endometriosis-associated pain, but drop-out rates were significant for both treatment groups. Purported benefits of the progestin are ease of administration, decreased costs, and protection of bone mineral density. The role of the etonogestrel implant in this setting is addressed by Di Carlo in Chap. 7 of this text.
Dopamine receptor-2 agonists, with purported anti-angiogenic properties, have shown some promise in pre-clinical trials. Complementary and alternative medicine (CAM) in the form of medicinal herbs and isoflavones, which are plant-derived nonsteroidal substances, are claimed to have beneficial effects but require more rigorous human studies to further substantiate.
Different classes of immunomodulatory drugs are in stages of development that hold promise as future endometriosis therapeutics. Small molecule anti-rheumatic agents (e.g., hydroxychloroquine), c-Jun N-terminal kinase inhibitors (e.g., bentamapimod), statins (e.g., simvastatin), peroxisome proliferator activated receptor-γ ligands (e.g., pioglitazone) and some biologics (e.g., infliximab, interferon-α) have shown salutary effects in cellular and animal models, although few have been studied in clinical trials [56]. In addition to these, several repurposed medications have been shown in preclinical studies to have activities that are useful for the suppression of inflammation and pain. For example, bortezomib (a proteasome inhibitor that targets multiple myeloma), digitoxin (the cardiac glycoside), and tioconazole (an imidazole antifungal) are all FDA-approved drugs with proven in vitro NF-κB inhibitor activity [57]. This topic is comprehensively reviewed in the accompanying Chap. 5 by Petraglia and colleagues.
4.6 Conclusions
Endometriosis-associated pelvic pain has been recognized for centuries, but its etiology and pathogenesis remain topics of continued debate and investigation. Based on the classical theory first promoted by Sampson, we offer a step-wise hypothesis that explains how interactions of the endocrine and immune systems affect nascent endometriosis lesions (Fig. 4.1). Each of the multiple nodes in this cascade is potential target for new endometriosis treatment strategies. As reviewed in this monograph by Professor Nisolle (Chap. 9), more and more refined surgical methods continue to play a major role in the management of pelvic pain associated with endometriosis. As our knowledge of pain pathophysiology evolves we expect that many, less invasive alternatives can be developed and introduced to clinical care.
References
Laghzaoui O, Laghzaoui M. Nasal endometriosis: apropos of 1 case. J Gynecol Obstet Biol Reprod (Paris). 2001;30:786–8.
Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A, Giudice LC. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96:360–5.
Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Rowan JP, Duan WR, Ng J, Schwefel B, Thomas JW, Jain RI, Chwalisz K. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28–40.
Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–900.
Evans S, Moalem-Taylor G, Tracey DJ. Pain and endometriosis. Pain. 2007;132(Suppl 1):S22–5.
Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–82.
As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, Harris RE. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17:1–13.
Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71:701–10.
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4:9.
Simpson JL, Elias S, Malinak LR, Buttram VC Jr. Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–31.
Knapp VJ. How old is endometriosis? Late 17th- and 18th-century European descriptions of the disease. Fertil Steril. 1999;72:10–4.
Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–69.
Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4.
Stuparich MA, Donnellan NM, Sanfilippo JS. Endometriosis in the adolescent patient. Semin Reprod Med. 2017;35:102–9.
Vercellini P, Pisacreta A, Pesole A, Vicentini S, Stellato G, Crosignani PG. Is ureteral endometriosis an asymmetric disease? BJOG. 2000;107:559–61.
Jiang QY, Wu RJ. Growth mechanisms of endometriotic cells in implanted places: a review. Gynecol Endocrinol. 2012;28:562–7.
Laschke MW, Menger MD. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum Reprod Update. 2018;24(2):207–24.
Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 2000;97:10972–7.
Barbieri RL. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J Reprod Med. 1998;43:287–92.
Riggs MM, Bennetts M, van der Graaf PH, Martin SW. Integrated pharmacometrics and systems pharmacology model-based analyses to guide GnRH receptor modulator development for management of endometriosis. CPT Pharmacometrics Syst Pharmacol. 2012;1:e11. https://doi.org/10.1038/psp.2012.10.:e11.
Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod. 2010;25:1325–34.
Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update. 2019;25:473–85.
Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19:406–18.
Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–7.
Struller F, Weinreich FJ, Horvath P, Kokkalis MK, Beckert S, Konigsrainer A, Reymond MA. Peritoneal innervation: embryology and functional anatomy. Pleura Peritoneum. 2017;2:153–61.
Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15:1744–50.
Tulandi T, Felemban A, Chen MF. Nerve fibers and histopathology of endometriosis-harboring peritoneum. J Am Assoc Gynecol Laparosc. 2001;8:95–8.
Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–8.
Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21:3001–7.
Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21:782–7.
Bokor A, Kyama CM, Vercruysse L, Fassbender A, Gevaert O, Vodolazkaia A, De Moor B, Fulop V, D’Hooghe T. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod. 2009;24:3025–32.
Borghese B, Vaiman D, Mondon F, Mbaye M, Anaf V, Noel JC, de Ziegler D, Chapron C. Neurotrophins and pain in endometriosis. Gynecol Obstet Fertil. 2010;38:442–6.
Kajitani T, Maruyama T, Asada H, Uchida H, Oda H, Uchida S, Miyazaki K, Arase T, Ono M, Yoshimura Y. Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr J. 2013;60:1155–64.
Garcia-Solares J, Dolmans MM, Squifflet JL, Donnez J, Donnez O. Invasion of human deep nodular endometriotic lesions is associated with collective cell migration and nerve development. Fertil Steril. 2018;110:1318–27.
Williams C, Hoang L, Yosef A, Alotaibi F, Allaire C, Brotto L, Fraser IS, Bedaiwy MA, Ng TL, Lee AF, Yong PJ. Nerve bundles and deep dyspareunia in endometriosis. Reprod Sci. 2016;23:892–901.
Browne AS, Yu J, Huang RP, Francisco AM, Sidell N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil Steril. 2012;98:713–9.
Rocha AL, Vieira EL, Ferreira MC, Maia LM, Teixeira AL, Reis FM. Plasma brain-derived neurotrophic factor in women with pelvic pain: a potential biomarker for endometriosis? Biomark Med. 2017;11:313–7.
Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016:CD012179.
Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–8.
Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–90.
Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200.
Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113:607–26.
Asally R, Markham R, Manconi F. The expression and cellular localisation of Neurotrophin and neural guidance molecules in peritoneal ectopic lesions. Mol Neurobiol. 2019;56:4013–22.
McKinnon B, Bersinger NA, Wotzkow C, Mueller MD. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertil Steril. 2012;97:373–80.
Liang Y, Liu D, Yang F, Pan W, Zeng F, Wu J, Xie H, Li J, Yao S. Perineural invasion in endometriotic lesions contributes to endometriosis-associated pain. J Pain Res. 2018;11:1999–2009.
Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor RN, Gold B, Jefferson S, Sidell N, Thompson W. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016;365:187–96.
Sun H, Li D, Yuan M, Li Q, Li N, Wang G. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathwaydagger. Biol Reprod. 2019;100:649–59.
Wessels JM, Leyland NA, Agarwal SK, Foster WG. Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors. Hum Reprod. 2015;30:925–36.
Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL, Taylor RN. IL-1beta stimulates brain-derived neurotrophic factor production in Eutopic endometriosis stromal cell cultures: a model for cytokine regulation of neuroangiogenesis. Am J Pathol. 2018;188:2281–92.
Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW, Saunders PTK, Greaves E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019;33:11210–22.
As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–14.
Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018;51:53–67.
Howard FM. An evidence-based medicine approach to the treatment of endometriosis-associated chronic pelvic pain: placebo-controlled studies. J Am Assoc Gynecol Laparosc. 2000;7:477–88.
Vercellini P, Frontino G, De GO, Pietropaolo G, Pasin R, Crosignani PG. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560–3.
Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol. 2002;99:709–19.
Kotlyar A, Taylor HS, D’Hooghe TM. Use of immunomodulators to treat endometriosis. Best Pract Res Clin Obstet Gynaecol. 2019;60:56–65.
Miller SC, Huang R, Sakamuru S, Shukla SJ, Attene-Ramos MS, Shinn P, Van Leer D, Leister W, Austin CP, Xia M. Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action. Biochem Pharmacol. 2010;79:1272–80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 International Society of Gynecological Endocrinology
About this chapter
Cite this chapter
Taylor, R.N., Yu, J., Francisco, A.M.C., Berga, S.L., Lebovic, D.I. (2021). Neurotrophins and Cytokines in Endometriosis Pain. In: Genazzani, A.R., Nisolle, M., Petraglia, F., Taylor, R.N. (eds) Endometriosis Pathogenesis, Clinical Impact and Management. ISGE Series. Springer, Cham. https://doi.org/10.1007/978-3-030-57866-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-57866-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57865-7
Online ISBN: 978-3-030-57866-4
eBook Packages: MedicineMedicine (R0)