Abstract
This chapter describes in detail the currently available and evolving endoscopic methods and techniques in the management of leaks and fistulas after laparoscopic sleeve gastrectomy (SG). The incidence of leaks or fistulas after SG is approximately 2 to 5%, and it is the second most common cause of death after bariatric surgery. Surgical re-intervention is associated with increased morbidity, and, therefore non-operative management such as endoscopy is usually preferred. The endoscopic strategies are standardized along two lines of management. The first of these is closure of the leak site, which generally includes the use of a covered self-expanding metallic stent (SEMS) to cover the leak. The second strategy is internal drainage, which aims to guide the drainage of the perigastric collection towards the lumen of the gastrointestinal tract and eventually closure of the fistula tract. Intraluminal pressure in the stomach increases after SG and can lead to a pressure gradient that favors flow through the fistula into the abscess cavity, thus preventing closure. Endoscopic septotomy combined with aggressive sleeve dilatation equalizes cavity pressures and promotes healing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Laparoscopic sleeve gastrectomy (SG) is currently the most popular primary bariatric surgical procedure for morbid obesity [1, 2]. Approximately 10 to 13% of patients undergoing this surgery have complications such as bleeding, stenosis, and leaks [3]. Leaks after SG are typically found at the upper end of the staple line, near the angle of His, where the staple line meets the gastroesophageal junction because of staple line-height mismatch, ischemia, and unfavorable pressure gradients secondary to distal intraluminal narrowing of the sleeve [4, 5]. The incidence of leaks or fistulas after SG is approximately 2 to 5% of the cases, and it is the second most common cause of death after bariatric surgery with an overall mortality rate of 0.4% [6].
The management of post SG leaks is challenging, resource-intensive and invariably requires a multidisciplinary team approach involving surgery, gastrointestinal endoscopy, and interventional radiology. The optimum management of leaks and subsequent intra-abdominal collections following SG is still controversial despite several reported techniques. The American Society for Metabolic and Bariatric Surgery position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and non-operative management was published in 2015 [7]. It states that the initial step in the management of an acute leak is to control the infection secondary to the leak. Thus, surgical washout with drain placement is mandatory in a patient whose condition is unstable, with an acute leak and systemic inflammatory response syndrome or peritonitis, and should not be delayed. In a more stable patient, any collection should be drained whether surgically, radiologically or endoscopically. In addition to adequate drainage, nutritional support and antibiotics are the mainstays of the treatment.
Leaks after SG may be difficult to seal despite adequate drainage because of the higher pressures within the sleeve conduit. Because surgical re-intervention is associated with increased morbidity, non-operative management should be favored whenever possible [7]. Revision surgery before endoscopic management may also delay treatment success [8]. Thus, the role of endoscopy in the management of leaks is usually preferred, and, is being performed more frequently [5].
2 Definitions and Technical Principles of Endoscopic Management
The role of endoscopy in the scenario of leaks is constantly evolving. Endoscopic treatment options of leaks vary widely and currently, there is no consensus on the optimum endoscopic approach to managing SG leaks. In addition, there is an absence of prospective and randomized trials comparing different endoscopic techniques. Primary endoscopic closure is rarely successful or feasible for chronic leak and fistula management. The endoscopic therapeutic strategies have evolved and have increasingly standardized along two lines of management. The first of these is closure of the leak site, which generally includes the use of a covered self-expanding metallic stent (SEMS) to cover the leak [9, 10]. The second strategy is internal drainage, which aims to guide the drainage of the perigastric collection towards the lumen of the gastrointestinal tract and eventually closure of the fistula tract. When using any of the endoscopic methods to treat a post SG leak, it is important to manage any downstream stenosis, twist, or kink within the sleeve that creates an unfavorable pressure gradient to enhance drainage and resolution. Optimizing the pressure gradient allows closure of the cavity by secondary intention, through granulation tissue formation and fibrosis [11].
2.1 Definition of Post SG Leak
The clinical presentation of post SG leak is defined according to the modified UK Surgical Infection Study Group classification [12]. The presence of a leak is confirmed by upper gastrointestinal swallow study or abdominal computed tomography. Leaks are classified as acute (≤1 week), early (1–6 weeks), late (6–12 weeks), and chronic (>12 weeks) according to the Rosenthal classification [1].
2.2 Definition of Post SG Leak Healing
Healing of post SG leak is usually defined as resumption of oral feeding and the absence of (1) percutaneous drainage; (2) leakage of contrast agent seen on upper gastrointestinal swallow study or abdominal computed tomography; (3) intra-abdominal collections; and (4) flow through a previous surgical path (such as gastro-cutaneous fistula).
3 Closure of the Leak Site
The first principle of management of post SG leaks is closure of the leak site, which generally includes the use of a covered SEMS to cover the leak [9, 10] but may also include the use of through-the scope or over-the-scope and clips (OTSC) [13], and endoscopic suturing [14]. Endoscopic treatment of post SG leak with the placement of a covered SEMS or clips should only be done after abdominal collections have been drained either surgically or percutaneously before stent placement. In cases involving inaccessible, especially large, collections, the stenting should be postponed or abandoned.
3.1 Self-Expanding Metal Stents
SEMS have been the most widely studied devices for endoscopic management of SG leaks. SEMS have been used for the palliation of dysphagia in esophageal cancer since the early 1990s [15]. Although primarily used to palliate malignant strictures, other indications for SEMS placement now include strictures from extrinsic compression, malignant perforations and fistulas, and, more recently, benign conditions such as recalcitrant esophageal strictures, perforations, fistulas, post-surgical leaks and bleeding esophageal varices [16].
SEMSs are relatively easy to place and are widely available in most endoscopy units. One benefit to their use, compared to other endoscopic modalities for SG leaks, such as OTSC, suture, and internal drainage, is that SEMS placement does not require endoscopic navigation and identification of the leak or fistula orifice, which can be often difficult to locate. The SEMS coating isolates the leak orifice from gastric contents and allows re-feeding during the healing process (Fig. 1). There is evidence that high intragastric pressure from either mechanical or functional stenosis in the SG may contribute to persistent leak and delayed healing, which can be also be successfully managed by SEMS placement [17, 18]. However, covered SEMS placement for the treatment of post SG leaks should be performed in patients with adequate external drainage of the perigastric collection. It should be mentioned that the use of SEMS for the management of leaks after SG is currently not Food and Drug Administration approved, and is an off-label use of the device.

Reprinted from Vargas EJ, Abu Dayyeh BK. Keep calm under pressure: a paradigm shift in managing postsurgical leaks. Gastrointest Endosc. 2018;87:438–441 [11], with permission from Elsevier
Acute unorganized leak with peritoneal spread treated by covering the leak site with a fully covered self-expanding metallic stent.
3.2 Types of SEMS
Commercially available stents are usually made of a shape-retaining nickel and titanium alloy (nitinol) and covered with polyurethane or silicone. Partially covered SEMSs have a portion of the exposed bare metal at the proximal and distal ends, which allows for ingrowth of surrounding tissue and could increase watertightness. Fully covered SEMSs do not have any exposed bare metal at either end. Partially covered stents have less risk of migration because of hyperplasia and ingrowth of tissue into the uncovered ends [19]. However, tissue ingrowth into the uncovered end also makes their removal more difficult resulting in tissue trauma and limits placement for a longer period. Fully covered stents, on the other hand, are more prone to migration but are easier to remove. New, extra-long, fully covered SEMSs have been developed especially for post SG leaks, such as the MEGA esophageal stent (Taewoong Medical, Gyeonggi-do, South Korea) and the Hanarostent (MITECH, Seoul, South Korea). These SEMS are available in lengths up to 23 cm and 24 cm, respectively, and are associated with less incidence of stent-specific complications such as migration and difficulty with removal [20, 21]. These stents are currently not available in the United States.
3.3 SEMS Insertion Procedure
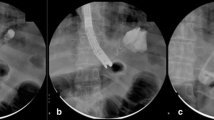
All endoscopic procedures for placement of SEMSs for post SG leaks should be performed under fluoroscopic guidance with patients under general anesthesia. Once the site of the leak is identified, it should be marked with an external radio-opaque marker taped to the patient’s skin (Fig. 2). A stiff guidewire is then passed through the endoscope all the way to the third part of the duodenum. The SEMS is then deployed over the guidewire to cover the leak orifice with the covered part of the SEMS. The longest available stent should be used to provide adequate coverage above and below the leak. In some cases, placement of a second SEMS may be necessary because of liquid reflux from the distal end between the gastric wall and the SEMS, or because of lack of watertightness at the proximal end due to the angle between the proximal end of the stent and the esophagus. If one of the new fully covered, extra-long SEMS, specifically designed for post-SG leaks is used, then it should be placed such that the proximal end is in the mid esophagus and the distal end in the proximal duodenal bulb, just distal to the pylorus. The SEMS are usually left in place from 3 to 4 weeks [19, 20].
Fluoroscopic images of a fully covered SEMS insertion for a post SG leak. A A guidewire has been passed through the endoscope into the duodenum. B External markers are placed on the skin marking the location of the pylorus and the site of the leak. C A fully covered SEMS is passed over the guidewire. D The fully covered SEMS is deployed, coving the leak site
SEMS extraction is usually done by pulling gently but firmly on the proximal end of the stent with a rat − tooth forceps. In case of a partially covered SEMS, argon plasma coagulation may be used to help destroy hyperplasia that develops between the SEMS meshes. This technique is used mainly in patients who had only proximal and mild hyperplasia. For this reason, another extraction technique can be employed, in which a Self-Expanding Plastic Stent (SEPS) is placed into the SEMS in order to induce necrosis of the hyperplastic proliferation. Extraction is then easily performed in a second endoscopic session [19]. In some cases, relapse or persistence of leakage after SEMS extraction justifies another SEMS implantation.
3.4 Outcome of SEMS Placement
The reported overall success rate of SEMS, with percutaneous drainage, in the closure of SG leaks, ranges from 65 to 95% [22, 23]. Table 1 shows the comparison of nine reported series of SEMSs in the management of laparoscopic sleeve gastrectomy leaks. However, these success rates are usually seen after multiple endoscopies (mean 4.7 procedures per patient) until fistula closure is achieved [24]. Permanent closure is usually obtained using only one stent in about 40%, and multiple stents in 20% of patients. In another 20% of patients presenting with a large fistula tract, stenting has to be complemented by another modality, such as insertion of a bio-prosthetic plug into the fistula or use of an OTSC. The success rates of SEMS placement correlates with the duration of treatment with a diminishing chance of fistula closure as the treatment period lengths. Multivariate analysis identified four predictive factors of healing following endoscopic treatment: interval <21 days between fistula diagnosis and first endoscopy, small fistula size (<1 cm), interval between SG and fistula ≤3 days, and, no history of gastric banding [24].
Tolerance to the placement of SEMSs is variable but usually fair. The reported symptoms such as nausea, dysphagia, and retrosternal discomfort are mild and transient, usually resolving within a few days [19]. The adverse events related to SEMS placement for SG leaks include migration, impaction and ulceration, digestive perforation, and incarceration. Stent migration is the most common complication of SEMS placement and is highly dependent on the type of stent used. A large meta-analysis revealed an overall stent migration rate of 16.94% [25]. The migration rate of fully covered SEMS is between 25 and 58% [26, 27]. The SEMSs have been reported to migrate even when they are clipped in position [28, 29]. The migration may require endoscopic stent repositioning, retrieval, or replacement. There have been reports of a number of patients who passed the stent via the rectum without incident [19, 26], but there have also been cases of stents which had to be removed surgically because of migration into the small intestine with subsequent failure to pass the stent through the rectum [26]. When stents migrate, they may become impacted into the wall of the digestive tract, creating a contact ulcer. Gastrointestinal bleeding and intestinal perforations have also been reported, and are due to migration and subsequent impaction of a metallic stent [24].
The other major complication of SEMS placement is incarceration. This is again dependent on the type of stent used and has been reported to occur up 90% of partially covered and about 7% of fully covered SEMS [24]. Removal of an incarcerated partially covered SEMS can even be associated with complications, such as esophageal wall striping and perforation, when not managed properly [30]. Incarcerated SEMS extraction is usually obtained by careful traction, with the help of a SEPS left in place for 1–2 weeks (stent-in-stent technique), or by surgical extraction. The use of a SEPS is an effective technique for the removal of an incarceration SEMS [31]. However, tissue hyperplasia into partially covered SEMS is sometimes responsible for stricture development attributed to a fibrotic healing process after removal in up to 14% of patients and may require endoscopic dilation [31].
3.5 Over-The Scope Clip System
Over-the Scope Clip (OTSC) is a system for endoscopic closure of gastrointestinal leaks and defects after endoscopic or surgical procedures and is a promising option for treatment of leaks and fistula after bariatric surgery. The system is designed to secure larger tissue volume, provide higher stability at the site of leak or perforation, and decrease the strain on the surrounding tissue [32]. It has a very strong grasp to include full wall thickness and can allow closure of defects up to 30 mm [33]. However, simply putting an OTSC at the site of a leak is not usually successful in permanently sealing a leak. Reasons for clip failure include friability of tissue, tissue ischemia, presence of infection, and presence of distal stenosis forming a high-pressure zone at the site of leakage. For this reason, OTSCs are usually placed in combination with a SEMS or just after their removal [34]. A recent systemic review looking at the efficacy and safety of the OTSC system in the management of post SG leaks showed an overall success rate of 86% [32] but success rates are much lower in cases of chronic leaks due to difficulty approximating fibrous tissue [32]. Predictive criteria for fistula closure success using OTSC are as follows: very early fistula (<7 days); fistulas with less fibrosis, leak size 10–30 mm; and leakages after LSG [33].
4 Internal Drainage
Internal drainage is the second principle for management of post SG leaks and aims to guide the drainage of the perigastric collection towards the lumen of the gastrointestinal tract and eventually closure of the fistula tract. This is usually achieved by either endoscopic internal drainage (EID) with biliary double pigtail stents (DPS) [35], or placement of a naso-cystic drain [5] Other, less-often used therapies include endo-luminal vacuum therapy [36].
4.1 Endoscopic Internal Drainage
First described in 2012, EID is a relatively recent strategy in the management of SG leaks [37]. EID is usually performed by the deployment of biliary DPSs across the leak orifice, positioning one end inside the collection and the other end in the lumen of the stomach (Fig. 3a). Alternatively, a naso-cystic tube is placed through the fistula and connected to suction (Fig. 3b). The principle of EID is similar to that of endoscopic cystogastrostomy in pancreatic pseudocyst drainage. The DPS keep the fistula tract between the stomach lumen and the infected para-gastric space open, allowing the para-gastric space to drain and heal by secondary intention progressively reducing it to a “virtual” cavity that is only occupied by pigtail loops.

Reprinted from Vargas EJ, Abu Dayyeh BK. Keep calm under pressure: a paradigm shift in managing postsurgical leaks. Gastrointest Endosc. 2018;87:438–441 [11], with permission from Elsevier
A An organized post SG leak treated by endoscopic internal drainage with two pigtail drains and pneumatic dilation device dilating a twisted and tight distal stomach, facilitating drainage. B An organized post SG leak treated by endoscopic internal drainage with nasocystic drain on low intermittent suction, facilitating drainage.
EID is effective both clinically and from a cost perspective, especially for subacute or chronic leaks with an organized walled-off collection [5, 38,39,40]. Another advantage of EID is that concurrent endoscopic necrosectomy may be performed to remove necrotic infected material from within the cavity and enhance drainage and healing. As experience with EID increases, there is an apparent trend in many centers to move towards early EID, especially in stable patients with a localized perigastric collection and no or minimal signs of sepsis [8, 18]. In some patients, percutaneous drainage may not be possible because of the interposition of spleen or bowel. In these situations, EID offers a viable alternative and may be the only therapy required, precluding the need for external drainage. In those patients who already have external drainage, EID may facilitate its early removal with concomitant capping and slow withdrawal of the percutaneous drain [11]. EID can also be used as a rescue method in cases of failed SEMS-based treatment, with no statistical difference in terms of clinical success between these two groups [41].
4.2 EID Procedure
All endoscopic procedures for EID for post SG leaks should be performed under fluoroscopic guidance with patients under general anesthesia. In the majority of the patients, the fistulous opening is identified in the upper end of the staple line, between the gastric fold, by careful examination. The opening is then cannulated with an ERCP cannula and the leak confirmed by injection of water-soluble contrast into the fistula and extravasation into the para-gastric cavity. A guidewire is passed, through the ERCP cannula, until it looped in the cavity. A double pigtail biliary stent (7–10 Fr, 4–7 cm) is then placed into the cavity, through the fistula, leaving the proximal end of the stent in the stomach or distal esophagus. The process was repeated and a second pigtail stent was placed alongside the first one (Figs. 4 and 5).
Endoscopic images of internal drainage procedure. A A guidewire has been passed into the perigastric collection, through the leak site. B A double pigtail stent deployed in the perigastric collection. The guidewire is reinserted into the perigastric collection. C Two double pigtail stents deployed in the perigastric collection
Fluoroscopic images of endoscopic internal drainage procedure. A Injection of contrast into the perigastric collection, through the leak site. B A guidewire has been passed and looped into the perigastric collection. C Two double pigtail stents deployed in the perigastric collection. D Two pigtail stents deployed along with a naso-jejunal feeding tube
If the fistulous opening is not initially identified during endoscopy then placement of a Savary guidewire or a nasogastric tube may help to open up the gastroesophageal junction and facilitate the identification of the fistula. Sometimes flushing radiographic contrast material through the endoscope in the lower esophagus, under fluoroscopy may show the leak site on fluoroscopy, which can then be identified on endoscopic vision. The leak site may also be identified on endoscopy after methylene blue dye is injected through a percutaneous drain, if available. If the fistulous opening is tight and does not allow passage of the DPS into the cavity, then, biliary dilatation balloon or Soehendra biliary dilation catheter may be used to dilate the track to facilitate the insertion of the stents. Removal of the DPS is performed endoscopically by grasping the proximal end of the stent with a snare and removing it with gentle traction of the endoscope.
4.3 Outcome of EID Procedure
The overall success of EID in healing a post SG leak ranges from 78 to 95% [5, 35, 37, 38, 41, 42], with one small series of nine patients even reporting a 100% success rate [17]. Table 2 shows the comparison of eight reported series of EID in the management of laparoscopic sleeve gastrectomy leaks. This is better with the reported healing rates of 62 to 87% achieved with placement of SEMS with percutaneous drainage [9, 19, 43, 44]. EID has also been shown to be successful in healing post SG leak in patients who have previously failed to respond to covered SEMS placement [35, 37, 41]. Additionally, EID is better tolerated by the patient compared to SEMS, which usually causes symptoms such as pain, nausea, vomiting, and bleeding.
The rate of complications of EID ranges from 4 to 15% [38, 41]. Most of these adverse events are mild and easily tolerated, such as ulceration at the tip of the DPS and bleeding [38]. Migration of the DPS is rare, and if occurs, is usually towards the gastric lumen and spontaneous passage through the rectum. There are reports of distal migration of the DPS. Four of these caused serious complications such as massive upper gastrointestinal bleeding from a pseudoaneurysm of the splenic artery [45] and splenic injury [46,47,48]. The other distal migrations included two patients with the migration of the DPS into the abdominal wall and the other with DPS migrating completely into the perigastric collection. All of these were easily removed endoscopically [41, 49, 50].
The presence of a gastrobronchial fistula is a recognized factor associated with the failure of EID in healing post SG leaks [5]. A statistical analysis evaluating whether other factors such as the type of bariatric surgery, treatment or diagnostic delays, or the use of EID as a primary or secondary treatment demonstrated no significant predictor of success [34, 41, 51]. Success rates were also not influenced by the type of leak according to the Rosenthal classification [1].
A recent study looking at the cost-effectiveness of SEMS placement vs. EID in the endoscopic management of post SG leaks found that EID with DPSs is more effective and reduces the cost by making management easier and shortening hospital stay [40]. The authors recommended that EID should be proposed as standard management for patients with post SG leak.
4.4 Endoscopic Vacuum Therapy
Endoscopic vacuum therapy (EVT), also known as endoscopic negative pressure therapy, involves endoscopic placement of a sponge connected to a nasogastric tube into the defect cavity or gastrointestinal lumen. This promotes healing, which is similar to the mechanisms in which skin wounds are treated with commonly employed wound vacuums [52]. One of the disadvantages of EVT is the need for repeated endoscopic procedures because the sponge needs to be changed every 3 to 5 days.
A recent study, evaluated the use of EVT in patients with early infradiaphragmatic leakage after bariatric surgery, including SG and gastric bypass. In some patients, EVT was performed alone, while others had EVT with a SEMS (stent-over-sponge). In 80% of patients, the leak was connected to abscess cavities. Clinical success, defined as no signs of persistent leakage, was achieved in all patients studied [53]. In another study including patients with acute, early, late, and chronic leaks after SG, the use of EVT was associated with 100% resolution of leaks confirmed by upper GI series, with an average of 10 sponge exchanges over an average of 50 days [36].
In general, EVT is a safe procedure with a low complication rate. The most frequent adverse events are sponge dislocation, minor bleeding after sponge exchange due to ingrowth of granulation tissue into the sponge, and anastomotic strictures. However, major bleeding events have also been reported due to the risk of development of a fistula between the cavity and surrounding major blood vessels and structures due to the ongoing inflammatory process of EVT [54].
5 Septotomy and Pneumatic Balloon Dilatation
Intraluminal pressure in the stomach increases after SG [55] and can lead to a pressure gradient that favors flow through the fistula or leak into the abscess cavity, thus preventing closure. Endoscopic septotomy has been described as a resolution technique that could be useful in the setting of late and chronic leaks. It allows for fluid drainage from the abscess cavity into the stomach by dividing the septum that separates the abscess from the gastric lumen [56], which when combined with aggressive sleeve dilatation, equalizes cavity pressures and promotes secretion flow into the gastrointestinal tract.
Endoscopic septotomy is performed by dividing the septum separating the gastric lumen and the abscess cavity. This is done with a needle knife or a Triangle Tip Knife and electrosurgical energy. The division of the septum is considered complete when the entire abscess cavity communicates with the gastric lumen, thus allowing drainage of secretion into the lumen of the stomach.
When a downstream stenosis is present, the patient may require pneumatic balloon dilation. This can be combined with a septotomy or performed by itself [57]. The dilatation is performed with a large achalasia balloon, usually with a 30 mm diameter balloon, but it may gradually be increased up to 40 mm in case of suboptimal response (Fig. 6).
Fluoroscopic images of balloon dilatation procedure for post SG gastric stenosis. A A guide wire is passed into the duodenum and a balloon is seen going over the guide wire. B The balloon inflation is started by filling it with radiographic contrast. C The balloon is gradually filled with radiologic contrast the stenosis becomes evident in the middle part of the sleeved stomach. D The balloon is gradually filled until the gastric stenosis is dilated
6 Conclusion
As bariatric surgery becomes more prevalent, so will the complications associated with this procedure. Thus, gastroenterologists and endoscopists must become familiar with the types of bariatric surgery, the main complications, and the various endoscopic ways to safely and effectively manage these complications. The optimal approach to managing these patients is through the development of multi-disciplinary teams (MDT) consisting of bariatric surgeons, therapeutic gastroenterologists, interventional radiologist, and intensivists. It is only through following these best practice guidelines that we will be able to provide the best care for these patients.
References
Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M, International Sleeve Gastrectomy Expert Panel, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257(2):231–7.
Trastulli S, Desiderio J, Guarino S, Cirocchi R, Scalercio V, Noya G, Parisi A. Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematic review of randomized trials. Surg Obes Relat Dis. 2013;9(5):816–29.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Eng J Med. 2017;376:254–66.
Lorenzo D, Guilbaud T, Gonzalez JM, Benezech A, Dutour A, Boullu S, Berdah S, Bège T, Barthet M. Endoscopic treatment of fistulas after sleeve gastrectomy: a comparison of internal drainage versus closure. Gastrointest Endosc. 2018;87(2):429–37.
Benedix F, Poranzke O, Adolf D, Wolff S, Lippert H, Arend J, Manger T, Stroh C. Staple line leak after primary sleeve gastrectomy-risk factors and mid-term results: do patients still benefit from the weight loss procedure? Obes Surg. 2017;27:1780–8.
Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11:739–48.
Brethauer SA, Kothari S, Sudan R, Williams B, English WJ, Brengman M, Kurian M, Hutter M, Stegemann L, Kallies K, Nguyen NT, Ponce J, Morton JM. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis. 2014;10(5):952–72.
El Mourad H, Himpens J, Verhofstadt J. Stent treatment for fistula after obesity surgery: results in 47 consecutive patients. Surg Endosc. 2013;27(3):808–16.
Simon F, Siciliano I, Gillet A, Castel B, Coffin B, Msika S. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23:687–92.
Vargas EJ, Abu Dayyeh BK. Keep calm under pressure: a paradigm shift in managing postsurgical leaks. Gastrointest Endosc. 2018;87:438–41.
Surgical Infection Study Group. Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Ann R Coll Surg Engl. 1991;73(6):385–8.
Zacharoulis D, Perivoliotis K, Sioka E, Zachari E, Kapsoritakis A, Manolakis A, Tzovaras G. The use of over-the-scope clip in the treatment of persistent staple line leak after re-sleeve gastrectomy: review of the literature. J Minim Access Surg. 2017;13:228–30.
Schweitzer M, Steele K, Mitchell M, Okolo P. Transoral endoscopic closure of gastric fistula. Surg Obes Relat Dis. 2009;5:283–4.
Lindberg CG, Cwikiel W, Ivancev K, Lundstedt C, Stridbeck H, Tranberg KG. Laser therapy and insertion of Wallstents for palliative treatment of esophageal carcinoma. Acta Radiol. 1991;32:345–8.
Irani S, Kozarek R. Esophageal stents: past, present, and future. Tech Gastrointest Endosc. 2010;12:178–90.
Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25(3):559–63.
Manos T, Nedelcu M, Cotirlet A, Eddbali I, Gagner M, Noel P. How to treat stenosis after sleeve gastrectomy? Surg Obes Relat Dis. 2017;13:150–4.
Eisendrath P, Cremer M, Himpens J, Cadière GB, Le Moine O, Devière J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39(7):625–30.
Galloro G, Magno L, Musella M, Manta R, Zullo A, Forestieri P. A novel dedicated endoscopic stent for staple-line leaks after laparoscopic sleeve gastrectomy: a case series. Surg Obes Relat Dis. 2014;10:607–11.
Bezerra Silva L, Galvão Neto M, Marchesini JC, S N Godoy E, Campos J. Sleeve gastrectomy leak: endoscopic management through a customized long bariatric stent. Gastrointest Endosc. 2017;85:865–6.
Fishman S, Shnell M, Gluck N, Meirsdorf S, Abu-Abeid S, Santo E. Use of sleeve-customized self-expandable metal stents for the treatment of staple-line leakage after laparoscopic sleeve gastrectomy. Gastrointest Endosc. 2015;81:1291–4.
Southwell T, Lim TH, Ogra R. Endoscopic therapy for treatment of staple line leaks post-laparoscopic sleeve gastrectomy (LSG): experience from a large bariatric surgery centre in New Zealand. Obes Surg. 2016;26:1155–62.
Christophorou D, Valats JC, Funakoshi N, Duflos C, Picot MC, Vedrenne B, Prat F, Bulois P, Branche J, Decoster S, Coron E, Charachon A, Pineton De Chambrun G, Nocca D, Bauret P, Blanc P. Endoscopic treatment of fistula after sleeve gastrectomy: results of a multicenter retrospective study. Endoscopy. 2015;47:988–96.
Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287–93.
Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–8.
Leenders BJ, Stronkhorst A, Smulders FJ, Nieuwenhuijzen GA, Gilissen LP. Removable and repositionable covered metal self-expandable stents for leaks after upper gastrointestinal surgery: experiences in a tertiary referral hospital. Surg Endosc. 2013;27:2751–9.
Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech. 2009;19:e1–e4.
Fukumoto R, Orlina J, McGinty J, Teixeira J. Use of polyflex stents in treatment of acute esophageal and gastric leaks after bariatric surgery. Surg Obes Relat Dis. 2007;3:68–71.
Hirdes MM, Vleggaar FP, Van der Linde K, Willems M, Totté ER, Siersema PD. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy. 2011;43:156–9.
Eisendrath P, Jacques D. Major complications of bariatric surgery: endoscopy as first-line treatment. J Nat Rev Gastroenterol Hepatol. 2015;12:701–10.
Shoar S, Poliakin L, Khorgami Z, Rubenstein R, El-Matbouly M, Levin JL, Saber AA. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and Fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27:2410–8.
Mercky P, Gonzalez JM, Aimore Bonin E, Emungania O, Brunet J, Grimaud JC, Barthet M. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27:18–24.
Shehab HM, Hakky SM, Gawdat KA. An endoscopic strategy combining mega stents and over-the-scope clips for the management of post-bariatric surgery leaks and fistulas. Obes Surg. 2016;26(5):941–8.
Gonzalez JM, Lorenzo D, Guilbaud T, Bège T, Barthet M. Internal endoscopic drainage as first line or second line treatment in case of postsleeve gastrectomy fistulas. Endosc Int Open. 2018;6(6):E745–50.
Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12:1278–85.
Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, Bartoli E, Delcenserie R, Yzet T, Regimbeau JM. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22(5):712–20.
Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, Tuszynski T, Pourcher G, Tranchart H, Mariani P, Meduri A, Catheline JM, Dagher I, Fiocca F, Marmuse JP, Meduri B. Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID). Obes Surg 2015;25(7):1293–301.
Donatelli G, Fuks D, Tabchouri N, Pourcher G. Seal or drain? Endoscopic management of leaks following sleeve gastrectomy. Surg Innov. 2018;25(1):5–6.
Cosse C, Rebibo L, Brazier F, Hakim S, Delcenserie R, Regimbeau JM. Cost-effectiveness analysis of stent type in endoscopic treatment of gastric leak after laparoscopic sleeve gastrectomy. Br J Surg. 2018;105(5):570–7.
Bouchard S, Eisendrath P, Toussaint E, Le Moine O, Lemmers A, Arvanitakis M, Devière J. Trans-fistulary endoscopic drainage for post-bariatric abdominal collections communicating with the upper gastrointestinal tract. Endoscopy. 2016;48(9):809–16.
Donatelli G, Ferretti S, Vergeau BM, Dhumane P, Dumont JL, Derhy S, Tuszynski T, Dritsas S, Carloni A, Catheline JM, Pourcher G, Dagher I, Meduri B. Endoscopic internal drainage with enteral nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24(8):1400–7.
Murino A, Arvanitakis M, Le Moine O, Blero D, Devière J, Eisendrath P. Effectiveness of endoscopic management using self-expandable metal stents in a large cohort of patients with post-bariatric leaks. Obes Surg. 2015;25(9):1569–76.
Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73(5):890–9.
Chahine E, D’Alessandro A, Elhajjam M, Moryoussef F, Vitte RL, Carlier R, Alsabah S, Chouillard E. Massive gastrointestinal bleeding due to splenic artery erosion by a pigtail drain in a post sleeve gastrectomy leak: a case report. Obes Surg. 2019;29(5):1653–6.
Marchese M, Romano L, Giuliani A, Cianca G, Di Sibio A, Carlei F, Amicucci G, Schietroma M. A case of intrasplenic displacement of an endoscopic double-pigtail stent as a treatment for laparoscopic sleeve gastrectomy leak. Int J Surg Case Rep. 2018;53:367–9.
Donatelli G, Airinei G, Poupardin E, Tuszynski T, Wind P, Benamouzig R, Meduri B. Double-pigtail stent migration invading the spleen: rare potentially fatal complication of endoscopic internal drainage for sleeve gastrectomy leak. Endoscopy. 2016;48(Suppl 1):E74–5.
Genser L, Pattou F, Caiazzo R. Splenic abscess with portal venous gas caused by intrasplenic migration of an endoscopic double pigtail drain as a treatment of post-sleeve gastrectomy fistula. Surg Obes Relat Dis. 2016;12:e1–3.
Debs T, Petrucciani N, Kassir R, Vanbiervliet G, Ben Amor I, Staccini AM, Sejor E, Gugenheim J. Migration of an endoscopic double pigtail drain into the abdominal wall placed as a treatment of a fistula post revisional bariatric surgery. Obes Surg. 2017;27:1335–7.
AlAtwan AA, AlJewaied A, AlKhadher T, AlHaddad M, Siddique I. A complication of an endoscopic pigtail stent migration into the cavity during deployment as a treatment for gastric leak. Case Rep Surg. 2019;6974527.
Guillaud A, Moszkowicz D, Nedelcu M, Caballero-Caballero A, Rebibo L, Reche F, Abba J, Arvieux C. Gastrobronchial fistula: a serious complication of sleeve gastrectomy. Results of a French multicentric study. Obes Surg. 2015;25(12):2352–9.
de Moura DTH, de Moura BFBH, Manfredi MA, Hathorn KE, Bazarbashi AN, Ribeiro IB, de Moura EGH, Thompson CC. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;16:329–44.
Morell B, Murray F, Vetter D, Bueter M, Gubler C. Endoscopic vacuum therapy (EVT) for early infradiaphragmal leakage after bariatric surgery-outcomes of six consecutive cases in a single institution. Langenbecks Arch Surg. 2019;404:115–21.
Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687–96.
Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, Bernstine H, Singer P, Dickman R, Beglaibter N, Shikora SA, Rosenthal RJ, Rubin M. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg. 2008;18:1083–8.
Guerron AD, Ortega CB, Portenier D. Endoscopic abscess septotomy for management of sleeve gastrectomy leak. Obes Surg. 2017;27:2672–4.
Campos JM, Ferreira FC, Teixeira AF, Lima JS, Moon RC, D’Assunção MA, Neto MG. Septotomy and balloon dilation to treat chronic leak. Obes Surg. 2016;26:1992–3.
Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC, Barthet M. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73(2):238–44.
Alazmi W, Al-Sabah S, Ali DA, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28:3425–8.
Martin Del Campo SE, Mikami DJ, Needleman BJ, Noria SF. Endoscopic stent placement for treatment of sleeve gastrectomy leak: a single institution experience with fully covered stents. Surg Obes Relat Dis. 2018;14:453–61.
Smith ZL, Park KH, Llano EM, Donboli K, Fayad L, Han S, Kang L, Simril RT, Patel R Hollander T, Rogers MC, Elmunzer BJ, Siddiqui UD, Aadam AA, Mullady DK, Lang GD, Das KK, Jamil LH, Lo SK, Gaddam S, Chapman C, Keswani R, Cote G, Kumbhari V, Kushir V. Outcomes of endoscopic treatment of leaks and fistulae after sleeve gastrectomy: results from a large multicenter U.S. cohort. Surg Obes Relat Dis. 2019;15:850–5.
Siddique I, Al-Sabah S, Alazmi W. Endoscopic internal drainage by double pigtail stents in the management of laparoscopic sleeve gastrectomy leaks. Surg Obes Relat Dis. 2020;S1550–7289(20):30169–6. doi: https://doi.org/10.1016/j.soard.2020.03.028.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Siddique, I. (2021). Endoscopic Management of Leak and Abscess Following Laparoscopic Sleeve Gastrectomy. In: Al-Sabah, S., Aminian, A., Angrisani, L., Al Haddad, E., Kow, L. (eds) Laparoscopic Sleeve Gastrectomy. Springer, Cham. https://doi.org/10.1007/978-3-030-57373-7_43
Download citation
DOI: https://doi.org/10.1007/978-3-030-57373-7_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57372-0
Online ISBN: 978-3-030-57373-7
eBook Packages: MedicineMedicine (R0)