Abstract
Three prevalent neurodegenerative diseases, Parkinson’s, Alzheimer’s, and Huntington’s are in need of symptomatic relief of slowing disease progression or both. This chapter focuses on the potential of cannabinoids to afford neuroprotection, i.e. avoid or retard neuronal death. The neuroprotective potential of cannabinoids is known from the work in animal models and is mediated by the two cannabinoid receptors (CB1/CB2) and eventually, by their heteromers, GPR55, orphan receptors (GPR3/GPR6/GPR12/GPR18), or PPARγ. Now, there is the time to translate the findings into patients. The chapter takes primarily into account advances since 2016 and addresses the issue of proving neuroprotection in humans. One recent discovery is the existence of activated microglia with neuroprotective phenotype; cannabinoids are good candidates to skew phenotype, especially via glial CB2 receptors (CB2R), whose targeting has, a priori, less side effects those targeting the CBs1 receptor (CB1R), which are expressed in both neurons and glia. The fact that a cannabis extract (SativexTM) is approved for human therapy, such that cannabis use will likely be legalized in many countries and different possibilities that cannabinoid pharmacology suggests a successful route of cannabinoids (natural or synthetic) all the way to be approved and used in the treatment of neurodegeneration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurodegenerative diseases
- Dementia
- Drug discovery
- Fatty acid amide hydrolase
- Heteromers
- Therapy
- Microglia
- Nootropics
6.1 Introduction
The history of medicines derived from drugs of abuse is fairly interesting. In the case of natural cannabinoids, i.e. those derived from Cannabis sativa, there has been a huge delay in the approval of “medical cannabis” despite controversy on whether or not Cannabis consumption leads serious dependence. The first cannabinoids approved for therapeutic use were synthetic derivatives of natural compounds: nabilone and dronabinol; they are used for a wide-range of illnesses but mainly to stop nausea and vomiting associated, for instance, with chemotherapy; they are also used to combat anorexia (Fraguas-Sánchez and Torres-Suárez 2018). Following the long-standing and well-known relaxed legislation existing in Holland, Cannabis sativa consumption is now approved in Uruguay, Canada, and several states of EEUU. It is likely that more and more countries will approve ad hoc legislation to allow consumption, not only for recreational purposes but also for medicinal uses. In fact, it is well known that patients of a huge variety of diseases have stuck to the intake of natural cannabinoids: some cases are related to the diseases of CNS, for instance, Parkinson’s disease (PD), some others are related to sclerosis as patients report symptom improvements. Cancer patients even at advanced stages report improvement in cancer-associated pain. Furthermore, medication based on natural cannabinoids has been approved. To our knowledge, there are two cannabinoid-based medicines: Sativex™/nabiximols and Epidiolex™. Epidiolex consists of cannabidiol (CBD), one of the main components of Cannabis sativa, dissolved in sesame oil. Interestingly, Sativex™ is one of the few plant extracts that have been approved as a medicine. It contains several compounds but with enrichment in cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). Sativex™, whose content in CBD and THC is similar, is prescribed for spasticity associated with multiple sclerosis. Rimonabant, a synthetic cannabinoid receptor antagonist was approved for weight loss but was retired after serious adverse events (Carai et al. 2006; Sam et al. 2011). Enhanced cannabinoid action may be afforded by inhibiting the enzyme that degrades endocannabinoids, fatty acid amide hydrolase (FAAH) (Benito et al. 2003; Goncalves et al. 2008; Celorrio et al. 2016) (see below). Unfortunately, a clinical trial using an inhibitor of FAAH led to the death of healthy volunteers (van Esbroeck et al. 2017; Kaur et al. 2018). Despite the issue was independent of enzyme inhibition, this fact has led to some reluctance to develop therapeutic drugs acting on those enzymes. In summary, it is likely that in the future more cannabinoids, natural or synthetic, may be approved for different diseases. Meanwhile, practitioners face the issue of “prescribing” cannabis for patients with neurodegenerative diseases in these countries or in the US States, where consumption is allowed; a review on sources presenting the pros and cons of “medical cannabis” use in patients (Noel 2017) and an account of “appropriate” dosing based on currently approved medicines (MacCallum and Russo 2018) are available. We here report the potential of cannabinoids (natural or synthetic) in neuroprotection related to the three more prevalent neurodegenerative diseases (Alzheimer’s, Parkinson’s, and Huntington’s). Very solid reports have been provided in the last two decades (see (Fernández-Ruiz et al. 2015; Mannucci et al. 2017; Cilia 2018; Fraguas-Sánchez and Torres-Suárez 2018) for review) and, therefore, this chapter focuses on research performed, since 2016.
6.2 Receptors that Respond to Cannabinoids
As of today, two receptors that mediate the physiological effects of cannabinoids are cannabinoid CB1 and CB2 receptors, which belong to the G-protein-coupled receptors (GPCRs) superfamily (https://www.guidetopharmacology.org/). In addition, they interact to each other to form CB1 and CB2 heteromers of proved physiological relevance and with therapeutic potential as the CB1 and CB2 receptors themselves (Callén et al. 2012; Sierra et al. 2015; Navarro et al. 2018, 2018)
The orphan GPCR, GPR55, was at first considered as a third cannabinoid receptor (Ryberg et al. 2007). Although this possibility has not reached consensus and GPR55 may be the receptor of lysophosphatidylinositol, it is known that cannabinoids regulate GPR55 action. In addition, GPR55 may form heteromers with CB1 receptors or with CB2 receptors (Kargl et al. 2012; Balenga et al. 2014; Martínez-Pinilla et al. 2014; García-Gutiérrez et al. 2018). Actually, data indicate that GPR55 may be a target for PD (Celorrio et al. 2017) but, besides complex pharmacology, there are few available tools; therefore, it lacks behind the CB1 and CB2 receptors in the race to find anti-neurodegenerative drugs.
CBD, at high concentrations, activate cannabinoid receptors. Recently, CBD has also been reported as an allosteric modulator of these receptors (Laprairie et al. 2015; Martínez-Pinilla et al. 2017). Interestingly, CBD behaves as an inverse agonist of some orphan GPCRs such as GPR3, GPR6, and GPR12 (Laun and Song 2017; Laun et al. 2019), which share a high degree of homology with the cannabinoid receptors (Morales et al. 2018). GPR18, another orphan of GPCR that may be regulated by cannabinoids, may interact with CB2 (CB2R) but not with the CB1 receptor (CB1R) (Reyes-Resina et al. 2018)
6.3 Addressing Neuroprotection in Humans
Addressing neuroprotection is not easy. Even in the case of laboratory animal models of neurodegenerative diseases, the demonstration that a given drug is neuroprotective poses difficulties. In addition, symptom improvements (in animal models) are quite often considered as neuroprotection and this interpretation is wrong. Yet, preclinical research has led to candidates that seem really neuroprotective, i.e. prevent neuronal death, and cannabinoids are among them.
Demonstrating neuroprotection in humans is a serious concern as there is not any “technique” that can prove it. Food and Drug Administration has no special rules that could serve to address this issue. Furthermore, clinical trials, by concept, and also by the pressure of pharmaceutical companies, are limited in time. Demonstrating neuroprotection requires time and requires safe drugs in chronic administration. In summary, patients are in urgent need of protocols to address neuroprotection. In our understanding, this requires new protocols and the use of drugs that are already considered as safe in chronic usage or of complements that are considered as “generally recognized safe” and are commercially available. This specific issue applied to another promising drug class, antagonists of A2A receptors, has been widely discussed elsewhere (Franco and Navarro 2018). Longitudinal studies are likely required in either i) healthy individuals taking memory enhancers (nootropics) for years and looking for the age of appearance of neurodegenerative signs or ii) patients taking additional medication with a “safe” drug (already approved or provisionally approved on the basis of compassionate drug use) and measuring disease progression using ad hoc scores (Franco et al. 2019). In either case, cannabinoids are candidates that deserve to be tested.
Another advantage of cannabinoids is related to the relatively recent development of PET tracers. Especially, relevant are those that are able to detect CB2R in the brain of living humans (healthy individuals or patients); the very recent papers on tracer development prove the interest of in vivo picturing this receptor (Attili et al. 2019; Kallinen et al. 2019). On the one hand, it is considered that PET for CB2R gives relevant hints for the neuroinflammation extent (see (Kho et al. 2017) for background). On the other hand, it is considered that reducing neuroinflammation in patients reflects less neurodegeneration and hence, reduced the progression of the disease (see (Spinelli et al. 2017) for review). Very importantly, and as pointed out by (Janssen et al. 2018), it would be instrumental to develop a PET tracer for the neuroprotective M2 microglial phenotype. Such a tracer could be a biomarker for neuroinflammation and/or for assessing neuroprotection in humans.
Another issue is related to dosage. Cannabinoids may act in a hormetic-like fashion, i.e. qualitatively different depending on the dose (Calabrese and Baldwin 2002; Calabrese and Rubio-Casillas 2018). Taking a simple example, CBD at high concentrations activates cannabinoid receptors, whereas CBD at lower doses behaves as a negative allosteric modulator (Martínez-Pinilla et al. 2017). See below (section on AD) for another example involving THC (Calabrese and Rubio-Casillas 2018).
6.4 Potential of Cannabinoids in Parkinson’s Disease
Parkinsonian patients are in need of drugs that delay the progression of the disease, i.e. preventing the death of dopaminergic neurons in the substantia nigra. Although there are efficacious interventions to address symptoms, they are not exempt of undesirable effects, mainly dyskinesias, i.e. involuntary movements arising after long periods of chronic pharmacological treatment. There is evidence that cannabinoids may be useful for neuroprotection but also for addressing symptoms and for reducing the chances to suffer from dyskinesias. There are other aspects of the disease, particularly those are known as non-motor symptoms. A recent protocol has been disclosed to address the safety and efficacy of nabilone in a cohort of approximately 38 patients entering into a randomized, placebo-controlled, double-blind clinical study. The primary outcome will be the MDS-UPDRS score and the results are expected by the end of 2019 (Peball et al. 2019).
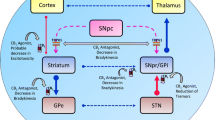
Confirming data in animal and cell models analysis of post-mortem samples and positron emission tomography (PET) in living patients shows that cannabinoid signaling is altered in Parkinson’s disease and that cannabinoid receptors exist in the brain regions susceptible of targeting by therapeutic drugs (Cilia 2018). The expression of CB1R and endocannabinoid synthesizing/degrading enzymes is also altered in the basal ganglia as a consequence of side effects levodopa treatment, more precisely during the active phase of dyskinesia (Rojo-Bustamante et al. 2018). Accordingly, the CB1 and CB2 receptors (individually or forming heteromers with other GPCRs) are potential targets of drugs aimed at affording neuroprotection.
At present, the evidence for efficacy in humans is scarce. The authors for a systematic review on Medical Cannabis and Neurodegenerative and Psychiatric indicated that: “Evaluation of these low-quality trials, as rated on the Cochrane risk of bias tools, was challenged by methodological issues such as inadequate description of allocation concealment, blinding and underpowered sample size. More adequately powered controlled trials that examine the long and short term efficacy, safety and tolerability of cannabis for medical use, and the mechanisms underpinning the therapeutic potential are warranted”. (Lim et al. 2017). In what concerns PD-related pain, prospects are already good; a meta-analysis considering >25 clinical trials (randomized) with idiopathic parkinsonian patients showed that greater pain reductions were achieved with safinamide but followed by cannabinoids and opioids (Qureshi et al. 2018). Therefore, cannabinoids are equipotent as one of the most potent pain relievers, opioids, but with the advantage that cannabinoids have fewer side effects.
Starting with the seminal discoveries of Rafael Mechoulam (Gaoni and Mechoulam 1964; Mechoulam et al. 1970; Mechoulam and Parker 2013) in the cannabinoid field, Israelian laboratories and hospitals have significantly contributed to find evidence for cannabinoid clinical potential. A human-based report by laboratories in this country indicates that cannabinoids are efficacious in “reducing tremor, dyskinesia, rigidity and pain, and improving sleep” (Katz et al. 2017). The authors add that medical cannabis may be useful in dementia “ although clinical data are still inadequate”.
As they are often altered in neurodegenerative models, research on the mitochondrial metabolism and mitochondrial biogenesis is gaining momentum in the field. For instance, THC upregulates proteins involved in biogenesis to MPP+ toxicity in a dopamine transporter-positive cell line (SH-SY5Y) (Zeissler et al. 2016). The mechanisms are dependent on upregulating a PPARγ co-activator 1α, PGC-1α, and a mitochondrial transcription factor, TFAM.
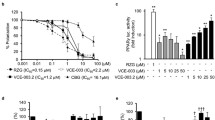
The potential of glia and cannabinoid receptors in glia merits special attention in PD but also in AD (see below). In the rotenone model of PD, a phytocannabinoid, β-caryophyllene reduces, among other, glial activation and this leads to the protection of dopaminergic neurons (Ojha et al. 2016). While these results indicate that glial activation may be detrimental, as it was currently thought, they contrast with those reporting that targeting the CB2R reduces the progression of motor symptoms in the LRRK2-transgenic mice (Palomo-Garo et al. 2016). It is interesting that the authors want to correlate without taking glia into account. Indeed, CB2R expression in CNS neurons (mainly restricted to the globus pallidus and cerebellum) could not explain the results in the LRRK2 mice; therefore, the effects are likely due by CB2R expressed in the activated microglia (see the section on AD, below). In any case, it would be good to know the status of the glia in the LRRK2 mice and the expression of activation markers and of cannabinoid receptors. As of today, there are no hits in Pubmed for “LRRK2-transgenic mice” and “microglia”. However, CB2R in the glia has already been suggested as a pharmacological target against PD-related inflammation (Gómez-Gálvez et al. 2016). The authors were even able to find the upregulation of the receptors in the glial cells of patients using post-mortem samples. It should be noted that VCE-003.2, which is a synthetic quinone derivative of cannabigerol, provided (in a PPARγ receptor-dependent way) benefits against inflammation-driven neuronal deterioration in a PD model in (García et al. 2018).
As also commented below, it is needed to establish not only the target but the pharmacological properties of the “therapeutic” cannabinoid, i.e. whether more efficacious one will be an agonist, an inverse agonist, or an allosteric modulator. In this sense, it is informative the case of the patient displaying mild cognitive impairments and living independent but who became seriously affected when nabilone was administered. To know the reasons of such psychosis, exacerbation would help in designing the most appropriate type of cannabinoid to address PD (Udow et al. 2018).
Familial early-onset PD may be caused by mutations in the (PINK1) gene, which codes for PTEN-induced putative kinase 1. (Madeo et al. 2016) reported in PINK1 knockout mice a CB1 receptor dysfunction that was dependent on dopaminergic transmission. The usefulness of such finding in terms of PD therapy will require further experimental effort.
6.5 Potential of Cannabinoids in Alzheimer’s Disease
Cannabinoids are among the myriad of drugs that transgenic models are efficacious reducing the pathological hallmarks of Alzheimer’s disease (AD) but that, unfortunately, have failed to reach the patient (Franco and Cedazo-Minguez 2014). The handicap is double, i.e. apart from the difficulty in assessing neuroprotection in humans, it turns out that transgenic Alzheimer’s disease models do not display neuronal death. Accordingly, these models serve more for hereditary cases (around 10% of total cases) and less for sporadic non-hereditary cases (around 90% of total cases). Can cannabinoids on delaying disease progression? Part of the answer came from analogies, i.e. if cannabinoids are seemingly neuroprotective in other neurodegenerative diseases they may be also efficacious in Alzheimer’s patients. Recently, the efficacy of some cannabinoids (individually administered or co-administered) has been tested in a so-called phenotypic screening platform that “recapitulate proteotoxicity, loss of trophic support, oxidative stress, energy loss, and inflammation” (Schubert et al. 2019). Compounds were also assayed for “their ability to remove intraneuronal amyloid” (Schubert et al. 2019). The conclusion of synergism upon coadministration is notable (Sativex™/nabiximols is, in fact, a mixture of compounds), but the conclusion that the agonists of the CB1 receptors are affording neuroprotection (Schubert et al. 2019) must be taken with caution as i) previous data do not support this view and ii) phenotypic platforms may not be suitable to measure neuroprotection in this disease. In fact, in one of the newest transgenic models with quicker cognitive impairment onset, the triple 3xTg-AD mice, desensitization of the CB1 receptor may be a “plausible strategy to improve behavior alterations associated with genetic risk factors for developing Alzheimer’s disease” (Llorente-Ovejero et al. 2018). Other recent results on cannabinoid action on animal models of Alzheimer’s disease are provided below.
In what symptoms are concerned, the use of cannabinoids has been suggested to reduce agitation and/or the aggressive behavior found in some patients (Liu et al. 2015). A clinical trial to know the efficacy of a synthetic cannabinoid, nabilone, on agitation in moderate-to-severe Alzheimer’s disease (Ruthirakuhan et al. 2019) has seemingly been completed in March 2019 (https://clinicaltrials.gov/ct2/show/NCT02351882) though no results have been posted. A recent meta-analysis based on double-blind, placebo-controlled trials have retrieved six studies with a total of 251 cases; the conclusion is that the results are inconclusive in what concerns aggression or agitation (Ruthirakuhan et al. 2019).
Cannabidiol, which has recently reported as an allosteric modulator of the CB1 and CB2 receptors (Laprairie et al. 2015; Martínez-Pinilla et al. 2017; Navarro et al. 2018), may activate peroxisome proliferator-activated receptor γ (PPARγ) and via the Wnt/β-catenin pathway, may reduce the oxidative stress and neuroinflammation associated with the disease. The authors propose cannabidiol as a drug to combat Alzheimer’s disease (Vallée et al. 2017). The modulation of genes in the mesenchymal stem cells suggests that cannabidiol leads to an expression pattern that could be more beneficial with any efficacious anti-Alzheimer’s disease therapy (Libro et al. 2016).
Rats with intracerebroventricularly administered okadaic acid appear as a model of Alzheimer’s disease as they present, in some brain regions, pathological hallmarks (phosphorylated tau and ß-amyloid) and display cognition deficits. Consistent with the potentially relevant role of activated microglia in what concerns neuroprotection, a selective CB2 receptor agonist was effective in reducing cognitive impairment, neurodegeneration and neuroinflammation (Çakır et al. 2019). The potential of the receptor as a target for neuroprotection is reinforced by the detection of memory impairment and of Tau pathology in the CB2 receptor knockout mice. Animals presented Tau hyperphosphorylation, on a decrease of AMPK activity and mitochondrial dysfunction (Wang et al. 2018).
Classical activation of microglia has been considered as detrimental but this view has changed. In fact, two different phenotypes arise from the activation of resting M0 microglia such as M1 of proinflammatory and M2 of neuroprotective (see (Franco and Fernández-Suárez 2015) for review). A recent discovery has linked the activated microglia to neuroprotection in Alzheimer’s disease. We found that the primary cultures of microglia from a transgenic AD mouse model present the activated phenotype with an important regulatory role of cannabinoids via cannabinoid receptors and receptor heteromers (Navarro et al. 2018). These results in animals that, unlike human patients, do not present any neuronal death leads to the suggestive hypothesis that microglia may be neuroprotective and prevent neuronal death and consequently, the progression of the disease. Results from the effects of ß-amyloid in a novel immortalized microglial cell line (McCarthy et al. 2016) may help in designing drugs leading to microglial M2 phenotype skewing.
In addition, it should be noted that blood flow is important for any neurodegenerative condition. In this sense, both functional impairments that may be regulated by ligands acting at the cannabinoid receptors have been detected in the vessels from a transgenic AD model (Navarro-Dorado et al. 2016).
The negative regulation of ß-amyloid-activated astroglial hemichannels is seen as a neuroprotective mechanism exerted by cannabinoids (Gajardo-Gómez et al. 2017).
Synthetic cannabinoids constituted by indazolylketones are postulated to be potential to combat Alzheimer’s disease as some of the generated compounds are able to target the CB2 receptor to inhibit ß-secretase 1 (the enzyme that participates in the production of the ß-amyloid toxic peptide) and to inhibit butyryl cholinesterase (one of the enzymes that degrade a neurotransmitter reduced in the disease: acetylcholine) (González-Naranjo et al. 2019).
Finally, an interesting hypothesis has been emitted to explain the biphasic effects of THC that is able to alter short-term memory, while it improves neurological function in old animals and in animal models of Alzheimer’s disease in which the compound prevents neurodegenerative processes. This paradox may be reconciled by one of the properties of hormetic mechanisms, namely different effects depending on the dose (Calabrese and Rubio-Casillas 2018). Interestingly, the benefits of THC on cognitive deficits in transgenic Alzheimer’s disease mice models do not happen in healthy aging of wild-type animals (Aso et al. 2016). In addition, it should be noted that the metabolism of an endocannabinoid, 2-arachidonoylglycerol, is altered by different aggregates of ß-amyloid (Pascual et al. 2017), thus suggesting that endocannabinoid metabolism is altered in patients.
6.6 Potential of Cannabinoids in Huntington’s Disease
Huntington’s disease is caused by neuronal death due to an abnormal protein, consisting of huntingtin with long poly-glutamine expansions. Therefore, it is hereditary and the altered gene contains CAG expansions that may be generated during spermatogenesis. Recent results have shown that torsional stress promote the generation of CAG expansions in the gene coding for huntingtin (Simard et al. 2017).
Cannabinoids may be neuroprotective in this disease as it has been demonstrated in the R6/2 rodent model of the disease. Neuroprotection was achieved by a Sativex™-like combination of compounds, although the motor performance was not improved. The neuroprotective effect was deduced, among others, from dystonia improvement and increased metabolic activity measured by PET in the basal ganglia. These data suggest that an appropriate combination of cannabinoids may affect disease progression (Valdeolivas et al. 2017). In another recent report, a different composition of compounds showed symptoms of improvement. In fact, cannabinoids improved dystonia, gait, and fine motor skills in early-onset Huntington’s disease patients. In some individuals, the treatment was associated with less hypersalivation and less apathy and irritability (Saft et al. 2018).
Spinocerebellar ataxias are hereditary neurodegenerative disorders some of which share with Huntington’s the involvement of proteins with poly-glutamine expansions. From word with both models of spinocerebellar ataxia and post-mortem samples from patients, it is found that the CB1 receptors are upregulated in neurons and the CB2 receptors are upregulated in the Purkinje cells and glial cells present in different regions of the cerebellum. It is thought that activating the CB1 and CB2 receptors or inhibiting the enzymes that degrade endocannabinoids could result in neuroprotection (Gómez-Ruiz et al. 2019). However, it was noted in a rodent model, the targetted deletion of an endocannabinoid-producing enzyme, monoacylglycerol lipase, affords protection for huntingtin-induced medium spiny neuronal loss and motor impairment. The authors suggest that a reduction in the availability of the product of the reaction, 2-arachidonoylglycerol, may be beneficial and that the enzyme is a potential therapeutic target for the disease (Ruiz-Calvo et al. 2019). As it stands, it is not yet clear where, when, and how decreased endocannabinoid tone is neuroprotective and where, when, and how activation or blockage of cannabinoid receptors result in neuroprotection.
One possible approach for drug development is considering functional selectivity and receptor functionality (Franco et al. 2018). In practice, medicinal chemists are designing “biased” agonist, i.e. molecules that upon binding to a given receptor lead to differential signaling that may affect the viability of cells expressing mutant huntingtin (Laprairie et al. 2016). In a recent review, the authors state: “Recent studies have found that Gαi/o-biased CB1 agonists activate extracellular signal-regulated kinase (ERK), increase CB1 (receptor) protein levels, and improve viability of cells expressing mutant huntingtin” (Bagher et al. 2018). Tetrahydrocannabinolic acid, another component of Cannabis sativa, affords neuroprotection in two Huntington’s disease cell models, one expressing a mutated form of huntingtin (STHdhQ111/Q111 cells) and another expressing a protein with 94 poly-glutamine repeats (mHtt-q94). The neuroprotective action is mediated by agonism at PPARγ (Nadal et al. 2017). It remains to be elucidated whether the compound may also act as a biased agonist at the cannabinoid receptors. The VCE-003.2 synthetic molecule, which was above described in the section devoted to Parkinson’s disease, also displays potential to combat Huntington’s disease as it afforded progenitor cell survival without losing the capacity to activate PPARγ. Hence, VCE-003.2 improved motor deficits, reduced neuroinflammation, and prevented medium spiny neuronal loss in the rodent models of the disease (Díaz-Alonso et al. 2016).
It is well-known that Huntington’s disease has no canonical drug to be used for delay neuronal death. Sativex™ was used in a double-blind, randomized, placebo-controlled clinical trial with patients. Symptoms were not improved but the positive aspect is that Sativex™ was safe and well-tolerated (López-Sendón Moreno et al. 2016). Apart from longer treatment and higher doses, the main issue in neurodegenerative diseases is the difficulty in addressing the measure of neuroprotection in humans (see above).
In conclusion, the potential of cannabinoids for neuroprotection is evident but the challenge is to demonstrate select/develop the most appropriate cannabinoid receptor ligand and to demonstrate its usefulness in clinical assays. As pointed out by (Maccarrone et al. 2017): “The continued characterization of individual cannabinoids in different diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and epilepsy) remains important”.
Funding
This work was supported by grants from the Spanish Ministry of Innovation and Competitiveness: Ref. No. BFU2016–64405-R and from the Spanish Ministry of Science, Innovation and Universities: Ref. No. 2019_RTI2018–094204-B-I00; both may include EU FEDER funds, and by the U.S. Alzheimer’s Association, grant Ref. No. AARFD-17-503612.
Conflict of Interests
Authors declare no conflict of interests.
Abbreviations
- AD:
-
Alzheimer’s disease
- CB1R:
-
cannabinoid CB1 receptor
- CB2R:
-
cannabinoid CB2 receptor
- CBD:
-
cannabidiol
- CNS:
-
central nervous system
- FAAH:
-
fatty acid amide hydrolase
- GPCR:
-
G-protein-coupled receptor
- GPRn:
-
orphan GPCR number “n”
- MDS-UPDRS:
-
Scale for non-motor symptoms in parkinsonian patients
- PD:
-
Parkinson’s disease
- PET:
-
positron emission tomography
- PPARγ:
-
peroxisome proliferator-activated receptor γ
- THC:
-
Δ9-tetrahydrocannabinol
References
Aso E, Andrés-Benito P, Ferrer I (2016) Delineating the efficacy of a cannabis-based medicine at advanced stages of dementia in a murine model. J Alzheimers Dis 54:903–912. https://doi.org/10.3233/JAD-160533
Attili B, Celen S, Ahamed M, Koole M, Van Den Haute C, Vanduffel W, Bormans G (2019) Preclinical evaluation of [18 F]MA3: a CB2 receptor agonist radiotracer for PET. Br J Pharmacol 176:1481–1491. https://doi.org/10.1111/bph.14564
Bagher AM, Laprairie RB, Kelly MEM, Denovan-Wright EM (2018) Methods to quantify cell signaling and GPCR receptor ligand bias: characterization of drugs that target the Endocannabinoid receptors in Huntington’s disease. In: Methods in molecular biology. Humana Press, Clifton, N.J, pp 549–571. https://doi.org/10.1007/978-1-4939-7825-0_25
Balenga NA, Martínez-Pinilla E, Kargl J, Schröder R, Peinhaupt M, Platzer W, Bálint Z, Zamarbide M, Dopeso-Reyes IG, Ricobaraza A et al (2014) Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br J Pharmacol 171:1–64. https://doi.org/10.1111/bph.12850
Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci 23:11136–11141
Çakır M, Tekin S, Doğanyiğit Z, Erden Y, Soytürk M, Çiğremiş Y, Sandal S (2019) Cannabinoid type 2 receptor agonist JWH-133, attenuates Okadaic acid induced spatial memory impairment and neurodegeneration in rats. Life Sci 217:25–33. https://doi.org/10.1016/j.lfs.2018.11.058
Calabrese EJ, Baldwin LA (2002) Defining hormesis. Hum Exp Toxicol 21:91–97. https://doi.org/10.1191/0960327102ht217oa
Calabrese EJ, Rubio-Casillas A (2018) Biphasic effects of THC in memory and cognition. Eur J Clin Investig 48:e12920. https://doi.org/10.1111/eci.12920
Callén L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortés A, Mallol J, Casadó V, Lanciego JL, Franco R, Lluis C et al (2012) Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem 287:20851–20865. https://doi.org/10.1074/jbc.M111.335273
Carai MAM, Colombo G, Maccioni P, Gessa GL (2006) Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Rev 12:91–99. https://doi.org/10.1111/j.1527-3458.2006.00091.x
Celorrio M, Fernández-Suárez D, Rojo-Bustamante E, Echeverry-Alzate V, Ramírez MJ, Hillard CJ, López-Moreno JA, Maldonado R, Oyarzábal J, Franco R et al (2016) Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav Immun 57:94–105. https://doi.org/10.1016/j.bbi.2016.06.010
Celorrio M, Rojo-Bustamante E, Fernández-Suárez D, Sáez E, Estella-Hermoso de Mendoza A, Müller CE, Ramírez MJ, Oyarzábal J, Franco R, Aymerich MS (2017) GPR55: a therapeutic target for Parkinson’s disease? Neuropharmacology 125:319–332. https://doi.org/10.1016/j.neuropharm.2017.08.017
Cilia R (2018) Molecular imaging of the cannabinoid system in idiopathic Parkinson’s disease. Int Rev Neurobiol 141:305–345. https://doi.org/10.1016/bs.irn.2018.08.004
Díaz-Alonso J, Paraíso-Luna J, Navarrete C, del Río C, Cantarero I, Palomares B, Aguareles J, Fernández-Ruiz J, Bellido ML, Pollastro F et al (2016) VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci Rep 6:29789. https://doi.org/10.1038/srep29789
Fernández-Ruiz J, Romero J, Ramos JA (2015) Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. Handb Exp Pharmacol 231:233–259. https://doi.org/10.1007/978-3-319-20825-1_8
Fraguas-Sánchez AI, Torres-Suárez AI (2018) Medical use of cannabinoids. Drugs 78:1665–1703. https://doi.org/10.1007/s40265-018-0996-1
Franco R, Aguinaga D, Jiménez J, Lillo J, Martínez-Pinilla E, Navarro G (2018) Biased receptor functionality versus biased agonism in G-protein-coupled receptors. Biomol Concepts 9:143–154. https://doi.org/10.1515/bmc-2018-0013
Franco R, Cedazo-Minguez A (2014) Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Front Pharmacol 5:146. https://doi.org/10.3389/fphar.2014.00146
Franco R, Fernández-Suárez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86. https://doi.org/10.1016/j.pneurobio.2015.05.003
Franco R, Martínez-Pinilla E, Navarro G (2019) Why have transgenic rodent models failed to successfully mimic Alzheimer’s disease. How can we develop effective drugs without them? Expert Opin Drug Discovery 14:327–330. https://doi.org/10.1080/17460441.2019.1581169
Franco R, Navarro G (2018) Adenosine A2A receptor antagonists in neurodegenerative diseases: huge potential and huge challenges. Front Psych 9:1–5. https://doi.org/10.3389/fpsyt.2018.00068
Gajardo-Gómez R, Labra VC, Maturana CJ, Shoji KF, Santibañez CA, Sáez JC, Giaume C, Orellana JA (2017) Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: a neuroprotective mechanism. Glia 65:122–137. https://doi.org/10.1002/glia.23080
Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647. https://doi.org/10.1021/ja01062a046
García C, Gómez-Cañas M, Burgaz S, Palomares B, Gómez-Gálvez Y, Palomo-Garo C, Campo S, Ferrer-Hernández J, Pavicic C, Navarrete C et al (2018) Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: possible involvement of different binding sites at the PPARγ receptor. J Neuroinflammation 15:19. https://doi.org/10.1186/s12974-018-1060-5
García-Gutiérrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, Giner S, Manzanares J (2018) Alterations in gene and Protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics 15:796–806. https://doi.org/10.1007/s13311-018-0610-y
Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C (2016) Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry 64:200–208. https://doi.org/10.1016/j.pnpbp.2015.03.017
Gómez-Ruiz M, Rodríguez-Cueto C, Luna-Piñel E, Hernández-Gálvez M, Fernández-Ruiz J (2019) Endocannabinoid system in Spinocerebellar ataxia Type-3 and other autosomal-dominant cerebellar ataxias: potential role in pathogenesis and expected relevance as Neuroprotective targets. Front Mol Neurosci 12:94. https://doi.org/10.3389/fnmol.2019.00094
Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, Zentar MP, Pollard S, Yáñez-Muñoz RJ, Williams G et al (2008) A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci 38:526–536. https://doi.org/10.1016/j.mcn.2008.05.001
González-Naranjo P, Pérez-Macias N, Pérez C, Roca C, Vaca G, Girón R, Sánchez-Robles E, Martín-Fontelles MI, de Ceballos ML, Martin-Requero A et al (2019) Indazolylketones as new multitarget cannabinoid drugs. Eur J Med Chem 166:90–107. https://doi.org/10.1016/j.ejmech.2019.01.030
Janssen B, Vugts D, Windhorst A, Mach R (2018) PET imaging of microglial activation—beyond targeting TSPO. Molecules 23:607. https://doi.org/10.3390/molecules23030607
Kallinen A, Boyd R, Lane S, Bhalla R, Mardon K, Stimson DHR, Werry EL, Fulton R, Connor M, Kassiou M (2019) Synthesis and in vitro evaluation of fluorine-18 benzimidazole sulfones as CB2 PET-radioligands. Org Biomol Chem 17:5086–5098. https://doi.org/10.1039/c9ob00656g
Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M (2012) The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J Biol Chem 287:44234–44248. https://doi.org/10.1074/jbc.M112.364109
Katz I, Katz D, Shoenfeld Y, Porat-Katz BS (2017) Clinical evidence for utilizing cannabinoids in the elderly. Isr Med Assoc J IMAJ 19:71–75
Kaur RJ, Singh S, Sidhu P, Sharma PK (2018) TGN-1412 and BIA-2474 trials with tragic end: lessons learnt to make clinical trials safer. Rev Recent Clin Trials 13:252–256. https://doi.org/10.2174/1574887113666180521093529
Kho DT, Glass M, Graham ES (2017) Is the cannabinoid CB 2 receptor a major regulator of the Neuroinflammatory Axis of the neurovascular unit in humans? Adv Pharmacol 80:367–396. https://doi.org/10.1016/bs.apha.2017.03.009
Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805. https://doi.org/10.1111/bph.13250
Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM (2016) Biased type 1 cannabinoid receptor signaling influences neuronal viability in a cell culture model of Huntington disease. Mol Pharmacol 89:364–375. https://doi.org/10.1124/mol.115.101980
Laun AS, Shrader SH, Brown KJ, Song Z-H (2019) GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin 40:300–308. https://doi.org/10.1038/s41401-018-0031-9
Laun AS, Song Z-H (2017) GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun 490:17–21. https://doi.org/10.1016/j.bbrc.2017.05.165
Libro R, Diomede F, Scionti D, Piattelli A, Grassi G, Pollastro F, Bramanti P, Mazzon E, Trubiani O (2016) Cannabidiol modulates the expression of Alzheimer’s disease-related genes in Mesenchymal stem cells. Int J Mol Sci 18:26. https://doi.org/10.3390/ijms18010026
Lim K, See YM, Lee J (2017) A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci 15:301–312. https://doi.org/10.9758/cpn.2017.15.4.301
Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N (2015) Cannabinoids for the treatment of agitation and aggression in Alzheimer’s disease. CNS Drugs 29:615–623. https://doi.org/10.1007/s40263-015-0270-y
Llorente-Ovejero A, Manuel I, Lombardero L, Giralt MT, Ledent C, Giménez-Llort L, Rodríguez-Puertas R (2018) Endocannabinoid and muscarinic signaling crosstalk in the 3xTg-AD mouse model of Alzheimer’s disease. J Alzheimers Dis 64:117–136. https://doi.org/10.3233/JAD-180137
López-Sendón Moreno JL, García Caldentey J, Trigo Cubillo P, Ruiz Romero C, García Ribas G, Alonso Arias MAA, García de Yébenes MJ, Tolón RM, Galve-Roperh I, Sagredo O et al (2016) A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J Neurol 263:1390–1400. https://doi.org/10.1007/s00415-016-8145-9
MacCallum CA, Russo EB (2018) Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 49:12–19. https://doi.org/10.1016/j.ejim.2018.01.004
Maccarrone M, Maldonado R, Casas M, Henze T, Centonze D (2017) Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev Clin Pharmacol 10:443–455. https://doi.org/10.1080/17512433.2017.1292849
Madeo G, Schirinzi T, Maltese M, Martella G, Rapino C, Fezza F, Mastrangelo N, Bonsi P, Maccarrone M, Pisani A (2016) Dopamine-dependent CB 1 receptor dysfunction at corticostriatal synapses in homozygous PINK1 knockout mice. Neuropharmacology 101:460–470. https://doi.org/10.1016/j.neuropharm.2015.10.021
Mannucci C, Navarra M, Calapai F, Spagnolo EV, Busardò FP, Cas RD, Ippolito FM, Calapai G (2017) Neurological aspects of medical use of Cannabidiol. CNS Neurol Disord Drug Targets 16:541–553. https://doi.org/10.2174/1871527316666170413114210
Martínez-Pinilla E, Reyes-Resina I, Oñatibia-Astibia A, Zamarbide M, Ricobaraza A, Navarro G, Moreno E, Dopeso-Reyes IGG, Sierra S, Rico AJJ et al (2014) CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp Neurol 261:44–52. https://doi.org/10.1016/j.expneurol.2014.06.017
Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, Oyarzabal J, Canela EI, Lanciego JL, Nadal X et al (2017) Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2receptors. Front Pharmacol 8:744. https://doi.org/10.3389/fphar.2017.00744
McCarthy RC, Lu D-Y, Alkhateeb A, Gardeck AM, Lee C-H, Wessling-Resnick M (2016) Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-beta. J Neuroinflammation 13:21. https://doi.org/10.1186/s12974-016-0484-z
Mechoulam R, Parker LA (2013) The Endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. https://doi.org/10.1146/annurev-psych-113011-143739
Mechoulam R, Shani A, Edery H, Grunfeld Y (1970) Chemical basis of hashish activity. Science (New York, NY) 169:611–612. https://doi.org/10.1126/science.169.3945.611
Morales P, Isawi I, Reggio PH (2018) Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab Rev 50:74–93. https://doi.org/10.1080/03602532.2018.1428616
Nadal X, del Río C, Casano S, Palomares B, Ferreiro-Vera C, Navarrete C, Sánchez-Carnerero C, Cantarero I, Bellido ML, Meyer S et al (2017) Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br J Pharmacol 174:4263–4276. https://doi.org/10.1111/bph.14019
Navarro G, Borroto-Escuela D, Angelats E, Etayo I, Reyes-Resina I, Pulido-Salgado M, Rodríguez-Pérez A, Canela E, Saura J, Lanciego JL et al (2018) Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain Behav Immun 67:139–151. https://doi.org/10.1016/j.bbi.2017.08.015
Navarro G, Reyes-Resina I, Rivas-Santisteban R, Sánchez de Medina V, Morales P, Casano S, Ferreiro-Vera C, Lillo A, Aguinaga D, Jagerovic N et al (2018) Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol 157:148–158. https://doi.org/10.1016/j.bcp.2018.08.046
Navarro G, Varani K, Reyes-Resina I, Sánchez de Medina V, Rivas-Santisteban R, Sánchez-Carnerero Callado C, Vincenzi F, Casano S, Ferreiro-Vera C, Canela EI et al (2018) Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 Heteroreceptor complexes. Front Pharmacol 9:632. https://doi.org/10.3389/fphar.2018.00632
Navarro-Dorado J, Villalba N, Prieto D, Brera B, Martín-Moreno AM, Tejerina T, de Ceballos ML (2016) Vascular dysfunction in a transgenic model of Alzheimer’s disease: effects of CB1R and CB2R cannabinoid agonists. Front Neurosci 10:422. https://doi.org/10.3389/fnins.2016.00422
Noel C (2017) Evidence for the use of “medical marijuana” in psychiatric and neurologic disorders. Ment Health Clin 7:29–38. https://doi.org/10.9740/mhc.2017.01.029
Ojha S, Javed H, Azimullah S, Haque ME (2016) β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem 418:59–70. https://doi.org/10.1007/s11010-016-2733-y
Palomo-Garo C, Gómez-Gálvez Y, García C, Fernández-Ruiz J (2016) Targeting the cannabinoid CB 2 receptor to attenuate the progression of motor deficits in LRRK2-transgenic mice. Pharmacol Res 110:181–192. https://doi.org/10.1016/j.phrs.2016.04.004
Pascual AC, Gaveglio VL, Giusto NM, Pasquaré SJ (2017) 2-Arachidonoylglycerol metabolism is differently modulated by oligomeric and fibrillar conformations of amyloid beta in synaptic terminals. Neuroscience 362:168–180. https://doi.org/10.1016/j.neuroscience.2017.08.042
Peball M, Werkmann M, Ellmerer P, Stolz R, Valent D, Knaus H-G, Ulmer H, Djamshidian A, Poewe W, Seppi K (2019) Nabilone for non-motor symptoms of Parkinson’s disease: a randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (the NMS-nab study). J Neural Transm 126:1061–1072. https://doi.org/10.1007/s00702-019-02021-z
Qureshi AR, Rana AQ, Malik SH, Rizvi SFH, Akhter S, Vannabouathong C, Sarfraz Z, Rana R (2018) Comprehensive examination of therapies for pain in Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology 51:190–206. https://doi.org/10.1159/000492221
Reyes-Resina I, Navarro G, Aguinaga D, Canela EI, Schoeder CT, Załuski M, Kieć-Kononowicz K, Saura CA, Müller CE, Franco R (2018) Molecular and functional interaction between GPR18 and cannabinoid CB2G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem Pharmacol 157:169–179. https://doi.org/10.1016/j.bcp.2018.06.001
Rojo-Bustamante E, Abellanas MA, Clavero P, Thiolat ML, Qin L, Luquin MR, Bezard E, Aymerich MS (2018) The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol Dis 118:64–75. https://doi.org/10.1016/j.nbd.2018.06.019
Ruiz-Calvo A, Bajo-Grañeras R, Maroto IB, Zian D, Grabner GF, García-Taboada E, Resel E, Zechner R, Zimmermann R, Ortega-Gutiérrez S et al (2019) Astroglial monoacylglycerol lipase controls mutant huntingtin-induced damage of striatal neurons. Neuropharmacology 150:134–144. https://doi.org/10.1016/j.neuropharm.2019.03.027
Ruthirakuhan MT, Herrmann N, Gallagher D, Andreazza AC, Kiss A, Verhoeff NPLG, Black SE, Lanctôt KL (2019) Investigating the safety and efficacy of nabilone for the treatment of agitation in patients with moderate-to-severe Alzheimer’s disease: study protocol for a cross-over randomized controlled trial. Contemporary Clin Trial Comm 15:100385. https://doi.org/10.1016/j.conctc.2019.100385
Ruthirakuhan M, Lanctôt KL, Vieira D, Herrmann N (2019) Natural and synthetic cannabinoids for agitation and aggression in Alzheimer’s disease. J Clin Psychiatry 80:18r12617. https://doi.org/10.4088/JCP.18r12617
Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N-O, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152:1092–1101. https://doi.org/10.1038/sj.bjp.0707460
Saft C, von Hein SM, Lücke T, Thiels C, Peball M, Djamshidian A, Heim B, Seppi K (2018) Cannabinoids for treatment of dystonia in Huntington’s disease. J Huntington’s Disease 7:167–173. https://doi.org/10.3233/JHD-170283
Sam AH, Salem V, Ghatei MA (2011) Rimonabant: from RIO to ban. J Obes 2011:432607. https://doi.org/10.1155/2011/432607
Schubert D, Kepchia D, Liang Z, Dargusch R, Goldberg J, Maher P (2019) Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer’s disease. Mol Neurobiol 56(11):7719–7730. https://doi.org/10.1007/s12035-019-1637-8
Sierra S, Luquin N, Rico AJ, Gómez-Bautista V, Roda E, Dopeso-Reyes IG, Vázquez A, Martínez-Pinilla E, Labandeira-García JL, Franco R et al (2015) Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct 220:2721–2738. https://doi.org/10.1007/s00429-014-0823-8
Simard O, Niavarani SR, Gaudreault V, Boissonneault G (2017) Torsional stress promotes trinucleotidic expansion in spermatids. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 800–802:1–7. https://doi.org/10.1016/j.mrfmmm.2017.04.001
Spinelli F, Capparelli E, Abate C, Colabufo NA, Contino M (2017) Perspectives of cannabinoid type 2 receptor (CB2R) ligands in neurodegenerative disorders: structure–affinity relationship (SAfiR) and structure–activity relationship (SAR) studies. J Med Chem 60:9913–9931. https://doi.org/10.1021/acs.jmedchem.7b00155
Udow SJ, Freitas ME, Fox SH, Lang AE (2018) Exacerbation of psychosis triggered by a synthetic cannabinoid in a 70-year-old woman with Parkinson disease. Can Med Assoc J 190:E50–E52. https://doi.org/10.1503/cmaj.170361
Valdeolivas S, Sagredo O, Delgado M, Pozo MA, Fernández-Ruiz J (2017) Effects of a Sativex-like combination of Phytocannabinoids on disease progression in R6/2 mice, an experimental model of Huntington’s disease. Int J Mol Sci 18:684. https://doi.org/10.3390/ijms18040684
Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N (2017) Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin 49:853–866. https://doi.org/10.1093/abbs/gmx073
van Esbroeck ACM, Janssen APA, Cognetta AB, Ogasawara D, Shpak G, van der Kroeg M, Kantae V, Baggelaar MP, de Vrij FMS, Deng H et al (2017) Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356:1084–1087. https://doi.org/10.1126/science.aaf7497
Wang L, Liu B-J, Cao Y, Xu W-Q, Sun D-S, Li M-Z, Shi F-X, Li M, Tian Q, Wang J-Z et al (2018) Deletion of Type-2 cannabinoid receptor induces Alzheimer’s disease-like tau pathology and memory impairment through AMPK/GSK3β pathway. Mol Neurobiol 55:4731–4744. https://doi.org/10.1007/s12035-017-0676-2
Zeissler M-L, Eastwood J, McCorry K, Hanemann OC, Zajicek JP, Carroll CB (2016) Delta-9-tetrahydrocannabinol protects against MPP+ toxicity in SH-SY5Y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget 7:46603–46614. https://doi.org/10.18632/oncotarget.10314
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pérez-Olives, C., Rivas-Santisteban, R., Lillo, J., Navarro, G., Franco, R. (2021). Recent Advances in the Potential of Cannabinoids for Neuroprotection in Alzheimer’s, Parkinson’s, and Huntington’s Diseases. In: Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M. (eds) Cannabinoids and Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology, vol 1264. Springer, Cham. https://doi.org/10.1007/978-3-030-57369-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-57369-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57368-3
Online ISBN: 978-3-030-57369-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)