Abstract
Lower extremity arterial disease affects more than 200 × 10e6 people worldwide causing Critical Limb Ischemia (CLI), also referred to as chronic limb-threatening ischemia (CLTI) a life-threatening disease and the major cause of ischemic amputation. For nonrevascularizable patients, the outlook is bleak and novel therapies are needed. This chapter discusses new approaches including gene therapy and stem/progenitor cell (SPC)-based therapies, including autologous bone marrow-derived cells (BM), MB-mononuclear cells (BM-MNC), mesenchymal stem cells (MSC), mobilized bone marrow cell (PB-MNC), allogeneic cells and ex vivo expanded or activated/differentiated cell products. A preliminary first-in-human trial of a novel treatment is presented that combines immune cell therapy and a stepwise activation and differentiation of SPC. Cells from a standard blood draw (with no pretreatment or mobilization) are transformed, within a day, into a therapeutic product (BGC101) composed of endothelial progenitor cells (EPCs), SPCs, dendritic cells (DCs), and T helper cells. BGC101 was found safe and effective in stabilizing and reversing the course of CLI. Controlled studies on a larger population are planned to evaluate this new concept.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Peripheral artery disease
- Critical limb ischemia, chronic limb threatening ischemia, diabetes complications
- Dendritic cells
- Stem/progenitor cells therapy
- Regenerative medicine, personalized cell therapies
- Endothelial progenitor cells, bone marrow cells, mesenchymal stem cells
12.1 Introduction
Peripheral artery disease (PAD) is caused by atherosclerotic occlusion of the arteries of the body. PAD can affect the brain, internal organs, and limbs. Most commonly this vascular disease causes partial or total occlusion of the blood supply to the legs and is sometimes referred to as lower extremity arterial disease (LEAD) . LEAD affects more than 200 million people worldwide with about 12 million people in the USA and 17 million in the EU (https://www.nhlbi.nih.gov/health/educational/pad/materials/pad_extfctsht_general.html, [45, 62]). Clinically, it is characterized by intermittent claudication (IC), pain in the muscles of the lower limb brought on physical activity and rapidly relieved by rest. When PAD worsens, it reaches the stage of Critical Limb Ischemia (CLI) , a life-threatening disease with comorbidities and an extremely low quality of life (QoL). CLI, also referred to as chronic limb-threatening ischemia (CLTI) , is the major cause of ischemic amputation [1, 21, 61]. CLI presents clinically as rest pain, ischemic ulceration, or gangrene of the foot or the leg (Fig. 12.1) and requires immediate treatment [48].
Schematic representation of the response to ischemia in peripheral artery disease [48]
Initially the ischemic limb compensates for the hypoxia by altering the hemodynamics and promoting microvascular adaptations by induction of angiogenesis and/or arteriogenesis. As the severity of the hypoxia increases, the microvascular adaptations are not sufficient. These changes lead to mitochondrial injury, free radical generation and subsequent muscle fiber damage, myofiber degeneration, and fibrosis. Additional decreases in oxygen supply and increased metabolic demands lead to rest pain, chronic non-healing wounds and gangrene, threatening limb function, and viability. In this figure, the blue arrows show the direction of blood flow in the artery, and the white arrows show the increase in severity of disease. Abbreviations: ECs endothelial cells, HIF-1α hypoxia inducible factor-1α, NO Nitric oxide, PAD peripheral artery disease, VEGF vascular endothelial growth factor, WBCs white blood cells
12.1.1 CLI Diagnostics and Current Best Practice
The assessment of the severity of PAD is traditionally based on the Fontaine or Rutherford classifications [31, 37, 72]. Rutherford suggested classification for grading the severity of chronic arterial occlusive disease for the purposes of standardized reporting practices is outlined in Table 12.1, where symptomatic disease is stratified into six categories.
Pressure indexes such as the ankle-brachial index (ABI) may be better for comparing groups of patients, as well as for monitoring a given patient over time after intervention (e.g., after bypass surgery). In addition, to claim cause and effect and attribute the improvement to a treatment, some objective evidence of hemodynamic change needs to be included, and a change in the ABI of more than 0.10 was recommended. This was later adopted by the Trans-Atlantic Inter-Society Consensus (TASC) [25].
Rest pain scores on rating scales ranging from 0 for the best (completely resolved) to 4 points for the worst condition (severe pain unresolved with paracetamol or non-steroidal anti-inflammatory drugs) were suggested by Tateishi-Yuyama [83].
The 6-minute walking test is considered informative and a predictor of further deterioration for CLI patients. Typically, annual decline in 6-minute walk performance (−73.0 ft (~22 meter) is observed in CLI patients with ABI <0.50. Smith et al. defined “mild claudication” as the ability to walk 2 to 3 blocks (900 ft) (~270 meter) before stopping; “moderate claudication ,” 1 or 2 blocks (600 ft) (~180 meter); and “severe claudication,” less than 1 block (300 ft) (~90 meter) [77]. Perera defined a small meaningful change in 6-minute walk as a change of 20 meters and a large meaningful change of 50 meters or more [55, 64].
A more recent classification system (WIfI classification) has been proposed by the Society of Vascular Surgery. This classification evaluates the prognosis of the affected lower limb by considering the following three factors which are graded into four categories (0 = none, 1 = mild, 2 = moderate, 3 = severe): wound (W), ischemia (I), and foot infection (fI) (Table 12.2) [56]. In the last revision of the European Society of Cardiology guidelines, the definition of CLI was replaced by the new concept of CLTI. While the term CLI mainly defined the degree of severe ischemia as the underlying cause of the disease, the CLTI definition also takes into account the degree of infections and wounds, which are perceived as crucial in estimating the prognosis of the lower limb [1, 21]. Unlike Rutherford’s classification, the WIfI does not include pain and walking ability that might also affect CLI prognosis.
CLI is associated with high risks for cardiovascular events, including myocardial infarction, stroke, and death. All current CLI guidelines support the use of statins and medications aimed at improving blood flow (e.g., phosphodiesterase inhibitor cilostazol), reducing blood viscosity (aspirin and anticoagulants), antiplatelet therapy (Plavix), and angiotensin-converting enzyme (ACE) inhibitors to reduce cardiovascular events and mortality [85]. In addition, pain is controlled with several levels of analgesics and narcotic medications. Secondary prevention by lifestyle changes (smoking cessation, healthy diet, weight loss, and regular physical exercise) are also useful [1, 85]. In patients with diabetes, glycemic control is particularly important for improved outcome [1]. While there is no drug specifically approved for the treatment of CLI , promising interventional methods are constantly improving. Veith et al. showed that frequent follow-up of CLI patients and aggressive intervention can dramatically decrease major amputation rates, reporting a decrease from 41% to 5% in primary amputations between the years 1974 and 1989. This approach is now accepted as the standard of care, with further establishment of multidisciplinary wound healing centers in most middle-sized and large hospitals around the world [34, 38, 89]. The therapeutic approach to patients with PAD includes two aspects. The first is to address the risk related to a specific lesion’s symptoms, length, level of occlusion, and localization, while the second is the management of the patients’ increased risk of any cardiovascular (CV) event [1, 21]. A flowchart summarizing the proposed therapeutic strategies is presented in Fig. 12.2. Revascularization should be attempted as much as possible; bypass surgery or angioplasty should be considered depending on the anatomical region and lesion complexity [21]. For some CLI patients, these interventional procedures are not suitable for several of reasons, such as the distribution of the occlusive disease in medium and small vessels, lesions too numerous and too small to revascularize, and comorbidities [48]. Yet, starting from the 1980s, it was shown that with a more aggressive limb salvage approach, less than 6% of CLI patients were not candidates for interventional treatment [88]. According to Aboyans et al. and Conte et al., the majority of CLTI patients are anatomically suitable for revascularization and establishing direct in-line flow to the foot is the primary technical goal [1, 21]. Recently, Abualhin et al. reported that distal anastomosis performed on 73 patients with Rutherford’ categories 5–6 resulted in technical success in 98.6%, with 1-month bypass patency in 87.8% of the patients, bypass assisted patency in 91.9%, and secondary patency in 93.2%. The 1-year results of these parameters were 54.4%, 71.4%, and 75.1%, respectively. Limb salvage and amputation-free survival (AFS) at 1-year were 84.3% and 79.1%, respectively, with most of the failures occurring within the 6-month follow-up (6-month limb salvage 85.8% and AFS 82.1%) [2]. Thus, it seems that despite good technical revascularization, clinical success is considerably less than 100%. Furthermore, due to the ulcers and subsequent gangrene and recurrent infections, these patients are frequently infected and require treatment with antibiotics, often leading to the development of antibiotic resistance. When revascularization attempts fail and for nonrevascularizable patients, new therapies, such as gene or SPC therapy, are required since the current available therapy only includes symptomatic treatments.
Flow diagram for the investigation of patients presenting with suspected chronic limb-threatening ischemia (CLTI) [21]. ABI Ankle-brachial index, PAD peripheral artery disease, TBI toe-brachial index, WIfI Wound, Ischemia, and foot Infection
12.1.2 Demographic Data
From January 2007 to December 2008, the prevalence and incidence of CLI in the USA was 0.23% and 0.20%, respectively. Overall, the success rates of the current therapies and prevention measures are limited, and once PAD progresses to CLI, the risks of limb loss and mortality increase. It is estimated that 220,000–240,000 amputations are carried out in the USA and Europe annually due to failure of revascularization [4]. The risk of amputations because of vascular diseases is dramatically increased in diabetes, which affects more than 230 million people worldwide [10, 94].
It is estimated that CLI will affect more than 3 million patients in the western world with an annual 2% US growth rate [53]. Based on historical data, within 6–12 months after the diagnosis of CLI, approximately 30% of patients will undergo amputation and 20% will die [23, 61, 76]. The 2-year and 5-year mortality rates are approximately 35% and 70%, respectively [66]. Despite advances in medical and interventional therapies, the amputation rate has increased from 19 per 100,000 person/year to 30–50 per 100,000 person/year over the past decades, mainly driven by an increase in the number of diabetics and older patients [3, 62]. Successful rehabilitation is achieved in less than two-thirds and one-half of patients after below-knee and above-knee amputations, respectively. Fewer than 50% of amputees achieve full mobility [4], and in patients who survive the first major amputation, a second amputation is required in 30% of the cases. Amputations cause devastating psychological effects and diminished QoL and also have a negative impact on survival. Even with the current best practice, the 5-year mortality rate for CLI is >60%, exceeding that of prostate cancer (<1%), breast cancer (11%), acute MI (20%), colorectal cancer (36%), and stroke (41%) [9].
12.1.3 Cost of CLI
The estimated total costs of treating CLI in the USA alone are $10 to $20 billion per year [4, 10, 42]. The cost of follow-up, long-term care, and treatment for an amputee who remains at home is $49,000 per year versus only $600 to $800 per year after limb salvage. Amputations are associated with substantial costs (e.g., hospitalization, surgery, fitting and building of prosthesis, rehabilitation process, home health aides, adaptations at the patients’ homes, influence on family and economic productivity, long-term healthcare costs, etc.) [5]. The CLI economic burden is very high. Thus a 25% reduction in amputations could save $2.9 to $3.0 billion yearly in US healthcare costs.
From what has been described above, CLI is a progressive devastating illness with significant disability, poor quality of life, and morbidity and mortality that exceed cancer. The economic toll is enormous, and the number of cases is increasing dramatically. There is an unmet, immediate need for the development of new therapies to stabilize or reverse the disease course, especially in “no option” patients.
This chapter describes new innovative approaches to treat CLI patients who failed or are not eligible for revascularization procedures and/or those suffering from occlusive disease in medium and small vessels, too numerous and small to revascularize, and suffering from multiple comorbidities. It will review several gene and cell therapy approaches and present preliminary first-in-human (FIH) results of a novel treatment that combines immune cell therapy and a stepwise activation and differentiation of stem/progenitor cells (SPC). Utilizing this innovative technology, peripheral blood cells from a standard blood draw (with no pretreatment or mobilization) can be transformed, within a day, into a cellular therapeutic product code-named BGC101, composed of early endothelial progenitor cells (EPCs), SPCs, alternatively activated pro-tolerogenic and pro-angiogenic dendritic cells (DCs), and T helper cells. [67]. As will be described in detail below, this treatment has shown promising therapeutic effects in patients with otherwise untreatable CLI after a single treatment. The first cohort results have met the expected safety and efficacy primary endpoints. BGC101 has been found to be safe with 6-month amputation-free survival (AFS) in all patients. Additional beneficial effects were observed on increased leg blood flow, wound healing, walking ability, reduction of pain, decreased usage of narcotic medications, and improved quality of life (QoL).
12.2 Review of Gene and Cell Therapy Investigations
12.2.1 Gene Therapy
Gene therapies using naked/plasmid-encoding angiogenic factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), hypoxia inducible factor (HIF), and developmentally regulated endothelial locus (Del-1 and DELTA 1) aimed at promoting neovascularization were highly promising in animal models, but were not effective in inducing functionally significant angiogenesis in clinical trials. Evidence accumulated from 22 phase 1 and phase 2 studies in 2008 supports the safety of these approaches in humans and also provides indications of bioactivity in patients with these dreaded conditions. Even so, true breakthroughs have been elusive [21, 46, 86]. An important example is the report by Powell et al. who tested the safety and bioactivity of HGF plasmid injection for CLI. In this randomized double-blind, placebo-controlled, dose-escalating, multi-center HGF-STAT trial, 104 patients with rest pain or tissue loss due to severe lower extremity ischemia were assigned to receive injections of placebo or 1 of 3 dosing regimens of HGF plasmid into the ischemic leg muscle. A unique, prespecified analysis plan allowed the investigators to identify an increase in TcPO2 in the high-dose group that was not present in other treatment groups, thus providing objective evidence for bioactivity. However, other end points, such as amputation rate, wound healing, and ankle/brachial or toe/brachial index, did not reveal differences between treatment groups [69]. Despite advances in the understanding of the diseases and the gene delivery tools, growth factor therapy results have been inconclusive. New modalities using gene therapy with biomaterials and cell-mediated delivery are very promising, but at this stage they are still in the preclinical research stage [92]
12.2.2 Stem/Progenitor Cells (SPC) Therapy for PAD and CLI
Adult bone marrow-derived cells (BM) that contains hematopoietic SPC (HSPC) and mesenchymal stem cells (MSC) has been widely used for numerous clinical applications. For more than 50 years HSPCs, the oldest form of therapeutic adult stem cells, have been administered in over 50,000 implantations, providing physicians with a thorough understanding of their utility, mainly for replacing blood and immune cells. More than 29,000 autologous transplants performed thus far have proven that they significantly lower the risk of infection due to the rapid recovery of immune function and the avoidance of rejection and graft-versus-host disease (GVHD) . These advantages have established autologous HSPC transplants as a standard second-line treatment for various malignant conditions, enabling the use of more intense chemotherapy [17, 35]. In addition, autologous HSPC infusion was also found to be safe and has been used to treat approximately 900 patients with autoimmune diseases, leading to sustained remissions in about 30% [63]. SPCs are capable of self-renewal and differentiation into organ-specific cell types as well as having paracrine effects via the release of pro-angiogenic growth factors/cytokines. A combination of cellular activities that contribute to the effects of SPCs transplantation include: the cells’ vasculogenic properties; paracrine effect resulting from secretion of multiple growth factors; and secretion of exosomes containing proteins, ribonucleic acids (RNAs), and microRNAs which stimulate both receptor-mediated and genetic mechanisms [32]. Cellular therapies also provide a treatment solution that addresses multiple aspects of CLI, including reduction of inflammation, tissue remodeling, and increased perfusion [9]. Since regenerative medicine treatment began in 1997, the feasibility and safety of BM-derived SPC has been established in over 3000 patients with refractory angina, ischemic cardiomyopathy, and chronic end-stage heart failure [33, 11]. In these studies, HSPCs and MSCs from BM aspirates and mobilized BM, both with and without ex vivo culturing steps, all showed high safety profiles, regardless of the administration method (intramuscular injections (IM), intravenous infusion (IV), or via angiography).
Progress has also been achieved in establishing therapeutic protocols for treating a variety of conditions, such as critical limb ischemia, acute myocardial ischemia, and infarction by using SPC. A variety of allogeneic and autologous tissues have been suggested as SPC sources, such as BM, peripheral blood mobilized cells, and mesenchymal organs . Overall, studies applying cells produced in compliance to good manufacturing practice (GMP) and good tissue practice (GTP) show that cell implantation was well tolerated and improved clinical status and survival, while most of the reported adverse effects stemmed from pre-procedural treatments connected to acquiring cells for the treatment [28, 33, 35].
12.2.3 Results of Stem/Progenitor Cell (SPC)-Based Therapy in PAD and CLI
Results of non-controlled as well as randomized controlled trials (RCT) applying SPC-based therapy in PAD and CLI are summarized in Table 12.3. One of the most promising and innovative treatments for PAD and CLI is the use of SPC that promotes small-to-medium sized blood vessel neovascularization and supports tissue reperfusion in a more physiological way with potentially high effectiveness. More than 70 studies have demonstrated the safety and clinical benefits of autologous BM mononuclear cells (BM-MNC), BM-MSC, G-CSF-mobilized peripheral blood-derived mononuclear cells (PB-MNC), or EPCs for patients with CLI. BM aspiration necessitates systemic/epidural or local anesthesia and aspiration of large amounts of marrow (300–500 mL) that many CLI patients cannot tolerate [52]. For those who undergo the procedure, the most frequent adverse reaction was local pain, responsive to non-steroidal anti-inflammatory drugs [28].
12.2.3.1 BM Mononuclear Cells (BM-MNC)
In the first published study, patients with chronic CLI conditions that were not amenable to revascularization received BM concentrate or peripheral blood mononuclear cells (PBMC) implantation. Cell implantation induced no local inflammatory reaction or edema of the gastrocnemius up to 72 hours after injection. Concentrations of serum creatine phosphokinase (CRP) increased after implantation (maximum after 1 day) and reverted to baseline within 7 days. 25 patients were treated with BM-MNC (1.6 ± 0.6 × 10e9 cells) and injected at 40 points into the gastrocnemius of the more ischemic limb (ankle-brachial index; ABI <0.6). 20 patients were in the control treatment, 4 obtained saline solution, and 16 patients were treated with PBMC (1.5 ± 0.6 × 10e9 cells) injected into the contralateral leg. The cell injection procedure was safe. 2 patients out of 47 died from myocardial infarction judged as unrelated to treatment, and no other treatment-related adverse events (AE) were reported. These results reflect a mortality rate of 4.3% compared to the 20% mortality expected based on historical data with the existing best practice methods [21, 23, 76]. PBMC control group showed moderate effects on stabilizing and improving blood flow and pain. A significant improvement in the BM-MNC group compared with the PBMC or saline was observed in ABI, transcutaneous oxygen pressure (TcPO2), rest pain, and the pain-free walking distance at 4 and 24 weeks [83].
Miyamoto et al. administrated autologous BM-MNC and investigated their safety and efficacy in recovering refractory chronic PAD of limbs and hands. No serious adverse events were reported, and the treatment was effective in relieving severe pain of PAD, especially for Buerger’s disease. The maximum pain level before implantation was 66.5 ± 5.0 (VAS 0–100), and it decreased to 12.1 ± 2.2 after implantation (p < 0.001). Rest pain in legs and fingers was resolved in 11 of 12 cases (92%). Pain-free walking time on a treadmill improved significantly (140 ± 53 seconds before implantation to 451 ± 74 seconds after implantation, p = 0.034). Resting ABI in legs implanted with BM mononuclear cells also improved (0.65 ± 0.08 before implantation vs. 0.73 ± 0.07 after implantation, p = 0.055). Significant perfusion improvement was demonstrated by 99mTc-tetrofosmin perfusion scintigraphy [57].
12.2.3.2 Mobilized BM (PB-MNC) Versus BM Mononuclear Cells (BM-MNC)
Several studies with growth factor mobilization of BM (also referred to as PB-MNC) were performed with safety and efficacy results similar to those of the BM-MNC trials. Overall, reported AE stemmed from pre-procedural treatments with G-CSF. AE included flu-like symptoms, myalgia, fever, and bone pain. A smaller number of AE included three patients who had to discontinue G-CSF due to chest pain, muscle pain, and anaphylaxis, respectively, one patient with ventricular fibrillation who recovered after cardioversion, and one patient who had minor retinal bleeding [27, 28, 43, 44, 49]. The long-term mortality rate in these studies was 2.8%, with 21 deaths reported between 2 months and 3 years after therapy, as compared to the 20% expected based on historical data. Thus, based on data from 761 patients, the authors concluded that no safety concerns exist with this type of cell therapy [28]. Among these, Huang et al. studied the effect of G-CSF mobilized PB-MNC (3.0 × 10e9 cells) in a RCT design on 28 diabetic patients with CLI. The control group received conventional wound care, and both groups were supplemented with an intravenous injection of prostaglandin E1. The study patients received G-CSF for a total of 5 days before PB-MNC collection. Huang reported improvements in pain-free walking distance, healing of diabetic foot ulcers, and significant increase in ABI (from 0.50 ± 0.21 to 0.63 ± 0.25 (p < 0.001)) and in angiographic scores [40]. Despite the limitations of the lack of compatibility between the studies, the outcome of BM-derived cell therapy (as well as that of Mobilized PB-MNC) on perfusion parameters (ABI, TcPO2) and the clinical course (wound healing, walking distance) remains consistent and positive throughout the different reports. Pooled results show that autologous cell therapy can induce an increase in ABI values between 0.1 and 0.2 points, which is considered a significant clinical outcome [72], and an increase in TcPO2 between 10 and 20 mmHg O2. Depending on baseline values, walking distance can improve about 100 to 200 meters. No serious side effects were reported [28, 41].
Dubsky et al. conducted a comparative study of patients with diabetic foot disease, 28 in the treatment arm and 22 control patients (standard care). 17 were treated by BM-MNC cells and 11 by PBMNC. At 6 months, 10 major amputations occurred in the control group, 2 in the BM-MNC group, and 1 in the PB-MNC group. A beneficial effect of cell therapy was observed with no difference between the two cell treatment groups [27].
12.2.3.3 Intramuscular (IM) Versus Intra-arterial (IA) Administration of BM Cells
Rigato and Fadini reported 4 studies utilizing IA administration including one RCT on 41 advanced CLI patients (Rutherford category 5 and 6) comparing IM (21 patients) and IA (20 patients) and one double-blind RCT on 160 CLI patients (Rutherford category 3–6) comparing 3 repeated treatments (once every 3 weeks) of IA administration of BM-MNC or placebo red blood cells. In both studies there was no difference in outcome between the groups. At 6-month follow-up, Klepanec reported 4 deaths (9.7%) and 10 major amputations (24%) without clearly detailing the group-associated events. Teraa reported 4 deaths (4.9%) and 10 major amputations (18.5%) in the IA BM-MNC group and 5 deaths (6.3%) and 10 major amputations (12.3%) in the IA placebo group. Indeed, in Rigato’s analysis of delivery route, only IM but not IA administration was associated with a significant improvement in amputation rate, amputation-free survival, complete wound healing, ABI, and TcO2, while both IM an IA significantly improved rest pain score [47, 74, 84].
12.2.3.4 BM Mononuclear Cells (BM-MNC) Versus BM Mesenchymal Stem Cells (BM-MSC)
As of 2017 Rigato et al. reported 4 studies utilizing BM-MSC obtained either by BM aspiration or by mobilization. Lu et al. conducted a double-blind RCT study in 41 diabetic patients comparing BM-MNC (20 patients) and BM-MSC (21 patients). Selected BM-MNC were injected immediately, while BM-MSC were first expanded ex vivo for 12–15 days. Saline was used as a control and was injected into the second leg. Lu reported that a BM aspiration volume of 30 ml was sufficient for generation of BM-MSC, while 300–500 ml was needed for preparation of BM-MNC. Cells were administrated intramuscularly (20 injections, 3 cm intervals, 1–1.5 cm in depth, (0.5–1 mL BM-MSCs or BM-MNCs per site). There were no serious adverse events. BM-MSC were superior over BM-MNC in limb perfusion, wound healing, and pain-free walking time, but there was no significant difference between the groups in amputations or pain relief [52].
12.2.3.5 Dose Dependency
Losordo et al. assessed dose dependency in a RCT utilizing two doses of mobilized BM purified CD34 SPC. 28 CLI patients were treated with 1 × 10e5 autologous CD34+ cells/kg (low-dose; 7 patients), 1 × 10e6 (high-dose; 9 patients), or placebo (control; 12 patients). 8 IM injections were administered to the ischemic leg. No adverse safety signal was associated with cell administration. 60 SAEs occurred in 22 subjects during the study. 1 occurred during mobilization before treatment (moderate hypotension which required prolonged hospitalization) and 59 after treatment. The majority of SAEs were considered unrelated to the study with 1 judged as possibly related (severe worsening of CLI in the target leg after injection which required prolonged hospitalization). There were 2 deaths during the study (group was not reported) and 11 major amputations. Major amputation incidence and AFS were significantly lower in the combined cell-treated groups compared with the control group with no significant dose related effect. Most amputations occurred within 6-month post injection, 4 in the control group (33%), 3 in the low-dose group (42%), and 2 in the high-dose group (22%) with 2 amputations between 6 and 12 months in the control group (a total of 50%). No treatment-related differences were found in wound healing, rest pain and QoL, whereas 6-min walking test improved in the cell treated groups. Lack of dose dependency in this preliminary study might be due to the small size of the groups [51].
12.2.3.6 Allogeneic Ex Vivo Expanded Cells
Gupta et al. described a double-blind RCT using allogeneic BM-MSC. Patients graded as Rutherford 4 (5 patients), Rutherford 5 (10 Patients), and Rutherford 6 (5 Patients) received a single treatment of 180–220 × 10e6 BM-MSC (10 patients) or placebo suspension (10 patients) via 40–60 IM injections in the gastrocnemius. Incidence of AEs in the BM-MSC arm was 13 vs. 45 in the placebo arm, and serious SAE including death, infected gangrene, and amputations were similar in both arms (5 in BM-MSC and 4 in the placebo group). Two deaths in the BM-MSC were not attributed by the study DSMB to the cell therapy. Two amputations occurred in each group. Nonetheless, a significant increase in ABI and ankle pressure was seen in BM-MSC arm compared to the placebo group. These results may reflect the inclusion of unsalvageable Rutherford 6 patients as suggested by Benoit et al. in their 2011 report [13, 52]. In a preliminary study, applying allogeneic ex vivo expanded placenta-derived MSC, 4 patients obtained 3 sets of IM once every 4 weeks. Safety results were promising with only one mild transient AE of fever. Ulcer healing, pain, pain-free walking test, and ABI improvement were reported, but no ABI or patient grading were provided. One patient had a major amputation [91].
12.2.3.7 Ex Vivo Activated/Differentiated Cell Products
In a double-blind RCT, Powell et al. tested the safety and efficacy of Ixmyelocel-T, a mixture of ex vivo expanded BM-MSC and alternately activated macrophages, in 86 patients (46 Ixmyelocel-T and 24 placebo). Patients received 20 IM injections and were followed for 12 months. Ixmyelocel-T treatment was well tolerated. The occurrence of adverse events and serious adverse events was similar between the two groups. There were four deaths (8%) in the Ixmyelocel-T group. This represented a decreased death rate compared to the 20% mortality expected based on historical data. However, the placebo group had the same mortality rate of 8%. There were ten major amputations (21%) in the Ixmyelocel-T group and six (25%) in the placebo. In both groups, most of the amputations occurred within 6 months. These results indicate that the therapy did not improve the one-year amputation or mortality rates. Improvement was observed in AFS and time to treatment failure (TTF based on one or more of: major amputation of the injected leg; mortality; doubling of total wound surface; and de novo gangrene) in ixmyelocel-T-treated patients compared with controls. In addition, the treatment effect for both TTF and AFS was even more pronounced in patients who entered the trial with baseline wounds (Powell et al. 2012).
While administration of fresh PBMC was inferior to BM-MNC [83], angiogenic cell precursors (ACP), a product resulted from non-mobilized peripheral blood after 5 days of pro-angiogenic ex vivo activation, showed a high safety profile and an improvement in circulation, ulcer healing, and reduced amputation rate. In the immediate follow-up after the intramuscular injection, patients were hemodynamically stable. There were no abnormalities in hematology, kidney and liver function tests, including serum myoglobin. One patient developed dyspnea that was caused by fluid overload, with immediate response to diuretic therapy. Elevated cardiac enzymes were detected in one patient, even though the patient had no symptoms of angina [60]. Similar results were obtained by Szabó et al. from applying ACP in a larger, controlled study of 20 patients (10 patients treated with ACP and 10 with standard care). The treatment was well-tolerated. At the 3-month follow-up, there were no major amputations and only two minor amputations in the treated group versus six major amputations in the controls and one death due to sepsis. Objective (ABI, TcO2) and subjective quality of life (QoL) improvement were seen in the treated group at 3 months. Post-study evaluation showed that the two-year major amputation free rate was 70% in the treated group versus 30% in the controls. The improvement in other objective and subjective parameters was sustained [81].
12.2.3.8 Summary of Cell-Based Therapies
Typical for early development stages of innovative therapeutic modalities, most studies were conducted on small patient groups ranging from 5 to 25 patients, and only part were RCTs or double-blind RCT trials. By 2015, more than 1000 CLI patients were treated with SPCs. The SPCs were directly obtained from organs, such as the BM or fat tissue or, alternatively, using mobilizing agents to induce massive proliferation of BM cells, followed by cell collection using an aphaeresis unit. Most of the studies were performed with autologous BM-derived cells, administrated locally to CLI patients in one treatment session of intramuscular injections. Overall, these studies showed that the cell implantation was well tolerated, not associated with severe adverse events and that they improved the clinical status of the patients. Safety analysis included the evaluation of death, cancer, unregulated angiogenesis, and procedural adverse events (AEs). The overall AE rate was low (4.2%) [12]. Most of the reported AEs were related to the pre-procedural activities for acquiring cells. Post cell therapy AEs were mainly injection site reactions and musculoskeletal disorders [28, 74].
Moreover, the data are supported by systematic reviews and meta-analyses of open labelled and RCTs. For example, Benoit et al. summarized 45 clinical trials, including 7 RCTs, with 1272 patients who received cell therapy. Efficacy analysis included the clinical endpoints of amputation and death as well as functional and surrogate endpoints. Cell therapy patients had a significantly lower amputation rate than controls (odds ratio 0.36, p = 0.0004). Cell therapy also improved a variety of functional and surrogate outcomes, such ABI, TcPO2, and quality of life (QoL) [12]. More recently, Rigato et al. reported a large meta-analysis on 2332 CLI patients, including19 RCTs, 7 non-randomized, and 41 non-controlled studies [74]. The primary analysis (all randomized controlled trials) showed that cell therapy reduced the risk of amputation by 37% and improved amputation-free survival by 18% and wound healing by 59%, without affecting mortality. Taken together, the data indicate that cell therapy significantly increased ABI, TcPO2, reduced rest pain, and improved QoL. Cell therapy patients did not have a higher mortality rate than controls and demonstrated no increase in cancer incidence. Many of these studies show decreased mortality in comparison to the natural history of 20% mortality and 30% amputations that are expected within 6–12 months from CLI diagnosis [21, 23, 76]. As discussed above, Rigato and Fadini in 2017 showed that cell therapy reduced the risk of amputation and improved amputation-free survival but did not affect mortality. This may reflect the fact that the control group is also receiving better care. Indeed, in a summary of placebo-controlled groups of 11 cell therapy studies reported between 2001 and 2015, the average mortality was 9% (range 0–33%) at 6 months. The rate of major amputations was much closer to the expected value with an average of 28% (range 10–67%) at 6 months. The limitation in death rate comparison may stem from the fact that many studies have a 6-month follow-up, while mortality sometimes occurs at a later time after the end of the study. This is not an issue when amputation is measured, since at least 85% of the major amputations occur with the first 6 months after treatment [13, 27, 36, 51, 65, 66, 81, 84, 50, 70, 90].
12.3 Future Application
The promising results of the studies summarized above encouraged us to develop a new combination of cells for effective neovascularization, reduction of inflammation, and recruitment of additional SPC from endogenous resources. CLI is currently an incurable, life-threatening, and seriously debilitating disease. We therefore developed a patient-friendly method, based on a standard blood draw that is safe, accessible to patients in every clinic, and scalable. The goal beyond limb salvage is to extend lifespan and improve functionality and QoL.
Since the number of EPCs and HSPCs in the blood is relatively low in healthy individuals and even lower in diabetic patients [14, 29], an ex vivo method for the enrichment and augmentation of these specific cells was developed. DCs, originally identified by Steinman et al. in 1973 [78], regulate both innate and adaptive immunological responses by the triggering of antigen-specific T-cell responses [7, 18,19,20, 26, 58, 78, 87]. However, in the presence of anti-inflammatory molecules such as transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF) and interleukin (IL)-10, DCs are alternatively activated in an antigen-independent manner and induce secretion of potent pro-angiogenic factors like vascular endothelial growth factor (VEGF) and nitric oxide, resulting in pro-angiogenic effects [8, 15, 54, 73, 79, 82]. In the presence of pro-angiogenic factors such as ischemia and the presence of VEGF, DCs were shown to contribute to neovascularization [16, 30, 75, 80, 93].
Autologous-enriched endothelial progenitor cells (EnEPC) is a defined cell population generated from a standard blood draw using a novel one-day technology employing alternatively activated DCs to specifically direct potentially therapeutic cell activity in vitro (Fig. 12.3). Previous in vitro and animal studies in the hind limb ischemia model have shown promising results in reversing induced limb ischemia [67, 68]. BGC101 is the first EnEPC-derived advanced therapy medicinal product (ATMP) designed to treat ischemic legs. It is produced in adherence to GMP and GTP.
Blood-derived SPC specifically activated by DCs
Flow chart depicting the generation of enriched EnEPC. BGC101 is a serum-free medium advanced therapy medicinal product (ATMP) designed to treat ischemic legs. It is produced in adherence to good tissue practice (GTP) and good manufacturing practice (GMP). Non-mobilized blood-derived plasmacytoid and myeloid DCs activated with pro-tolerogenic and pro-angiogenic cytokines (such as IL-10, VEGF) are used to specifically direct the in vitro activation of SPCs which were enriched from the same blood sample and co-cultured for 12–18 hours. Harvested EnEPCs were tested for safety (Gram stain, sterility, endotoxin and mycoplasma), identity (EPCs, SPC, DC and T helper cells), and potency (Ac-LDL and Ulex Lectin)
We report here a first-in-human (FIH), pilot clinical study assessing the safety and efficacy of BGC101 in the treatment of PAD with CLI.
12.3.1 Methods and Results
This FIH, non-controlled open-label pilot study assessing the safety and efficacy of BGC101 in the treatment of PAD with CLI was conducted in compliance with good clinical practice (GCP) and was closely reviewed by an independent Data and Safety Monitoring Board (DSMB) and sponsored by BioGenCell Ltd (NIH clinicaltrials.gov Identifier: NCT02805023).
Study Population
Patients with severe disease and with no other treatment option (“no option”) were selected. Patients had characteristics including very low to no blood flow in the legs as measured by ABI (<0.5), toe brachial pressure index (TBI), and ultrasound duplex test and had non-healing ulcers and infections. Between September 2006 and January 2017, six eligible patients underwent blood collection, and five patients were treated with BGC101. One patient withdrew before treatment due to gastrointestinal bleeding unrelated to the study. The five eligible patients, one Rutherford 4 and four Rutherford 5, were treated with a single session of 30 IM injections of BGC101 into the gastrocnemius muscle of the diseased leg, under local anesthesia with lidocaine cream (see Table 12.4 for baseline data; Fig. 12.4a for cell injection, 4b for injected leg 30 minutes after injection). Patients were followed for safety 48 hours and 1 week after cell administration. Further follow-up for safety and efficacy assessment was performed at 1, 3, and 6 months. 4 patients completed the 6-month follow-up period, and one patient withdrew from the study immediately prior to the 3-month follow-up visit. This patient had a computed tomography angiogram (CT angiogram) after therapy that showed improved run-off in the calf vessels. He was advised that he was now eligible again for an interventional procedure and underwent transluminal angioplasty (PTA). The patient was amputation-free several days before the 3-months follow-up (2.9 months). For this patient, documented data from the 1-month follow-up visit was used based on last observation carried forward (LOCF).
Study Investigational Product and Dose
Starting with 250–350 ml of peripheral blood, co-culture of activated DCs for 12–18 hours with SPCs from the same patient sample generated a treatment dose of 91.0 ± 23.4 × 10e6 (range 51.1–178.9 × 10e6) of BGC101 cells with 98.1 ± 0.4% viability. BGC101 comprises 58.1 ± 7.0% EPCs (expressing Ulex-lectin, acetylated low-density lipoprotein (AcLDL) uptake, Tie2, vascular endothelial growth factor receptor 1 and 2, and CD31) and 17.3 ± 4.7% SPCs (expressing CD34 and CD184 as well as plasmacytoid and myeloid DCs (expressing CD304 and CD141) and T helper cells (expressing CD3 and CD4).
Safety
The primary outcome of the study was safety. AEs and SAEs were classified in accordance to International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Medical Dictionary for Regulatory Activities (MedDRA), System Organ Class Preferred Term (SOC) based on Common Terminology Criteria for Adverse Events (CTCAE) [22]. A total of 44 AEs were reported, including 4 SAEs that were all typical of CLI or its underlying disease (i.e., happened or could have happened regardless of the therapy). 10 of the 44 AEs occurred during the screening period of 2 weeks prior to BGC101 administration (an average of 4AEs/patient/month), whereas 34 post-treatment AEs were reported during the 6-months follow-up (an average of 1.1AEs/patient/month). The 10 pretreatment AEs included one SAE of gastrointestinal bleeding after blood collection which was judged by the principal investigator (PI) and DSMB as an unrelated SAE. Of the 34 post-treatment AEs, 25 were defined as unrelated or unlikely related including the 3 SAEs (hospitalization due to foot infection and hypokalemia (same patient) and retroperitoneal hematoma due to angiography). Nine AEs were defined as a) possibly related (6 AEs; 4 recovered spontaneously; 1 recovered with medical treatment; 1 was ongoing with medical treatment at study termination), b) probably related (1 AE, recovered spontaneously), and c) related (2 AEs, recovered spontaneously). Based on review of the AEs and SAEs, the DSMB determined that the treatment protocol including blood collection as well as BGC101 IM administration was well tolerated and the BGC101 therapy was safe. The DSMB thus recommended continuing and expanding the study to a larger patient population with one amendment – shortening the post treatment in-patient follow-up time from 48 to 24 hours and thereby reducing the exposure to hospital risks such as nosocomial infections.
Efficacy
In this study, population prevention of deterioration (i.e., stabilizing the disease) or improvement were considered successful outcomes.
Amputation and Mortality
Primary efficacy endpoints were major amputation rate (below or above the knee) and AFS at 6 months. All four patients completed the study with 6 months AFS (amputation and mortality rate = 0). The one patient who withdrew from the study immediately prior to the 3-month follow-up visit was amputation-free at that time. Secondary objectives endpoints included blood flow, assessed by ankle brachial pressure index (ABI), toe-brachial pressure index (TBI), ulcers number and severity score, walking capability, local pain, pain-control medications, and QoL.
Blood Flow
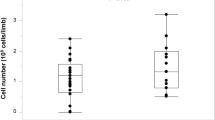
Based on the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) report, a changing ABI is possibly the best individual predictor, because if a patient’s ABI deteriorates, it is most likely to continue to do so in the absence of successful treatment [61]. In addition, to claim cause and effect and attribute the improvement to the treatment, an objective evidence of the ABI or TBI of more than 0.10 is recommended [72]. Three out of the four patients who completed 6-month follow-up had >0.1 (ABI increase ranging from 0.13–0.54). On average ABI increased by from 0.37 ± 0.03 to 0.57 ± 0.13 (Delta of 0.19, 53% improvement). According to [56], patients with ABI <0.4 have severe ischemia grade 3, with ischemic rest pain and increased amputation risk. However, especially in patients with diabetes and wounds complicated by infection, correction of perfusion to 0.4 < ABI <0.8 may speed healing of wounds and leg salvage [56]. In this study, starting from 1 month after treatment the patients’ average blood flow exceeded the level of 0.4, corresponding to moderate-severe arterial disease with possible limb salvage (Fig. 12.5). In one patient, only TBI could be measured and showed an increase from 0.13 to 0.32, which can support wound healing.
Pilot study ABI results
ABI improvement trend is seen at all time points. Starting from 1 month after treatment the patients’ average blood flow exceeded the level of 0.4 which corresponds to ischemic rest pain and risk of limb loss and reached the level of 0.40–0.80, corresponding to moderate-severe arterial disease
Wound Healing
Four out of five patients had one or more wounds in the treated leg as detected in the screening visits. Each ulcer was defined based on its location and specifically traced and ranked by severity damage score (0 = no wound; 1 = limited to skin; 2 = penetrates the subcutaneous layer; 3 = involvement of tendons/fascia/muscle; 4 = bone exposure). A total and average damage score for each time point was calculated by summing the scores for all ulcers per patient and dividing by the number of tested patients. In all patients with wounds, ulcer worsening occurred during the screening period before treatment. In two patients, both the number of ulcers and the damage score increased and in one patient, who entered the study 2 years after amputation of the contralateral leg, the damage score increased dramatically, and a severe infection occurred before treatment. In two patients, deterioration continued after treatment, and in two others both the number and damage score were reduced, some of the wounds healed completely and some improved but were still present at 6 months.
Walking Capability
Four out of five patients had two legs and were potentially capable of walking. At the study initiation, the test chosen to measure walking ability was a 6-minute walking test on a treadmill. However, the patients could not walk unaided on the treadmill or barely walked with a walker. Thus, after amending the protocol, for patients who could not perform the treadmill test, a 6-minute walking test was performed on a flat surface. Distance and time were measured until the patient reported discomfort, for a maximum of 6 minutes.
One patient was not capable of walking at any stage from screening visits until the 1-month follow-up. Since this patient withdrew prior to the 3-month follow-up, this result was included in the study analysis as LOCF. By 6 months, all other patients showed improvement in walking time, distance, and stability that enabled them to go from using a wheelchair or a walker to using a walking stick and take daily walks or to walk more than 6 minutes on the treadmill. Large meaningful improvements from 36 to 115 meters, from 120 to 240 meters, and from 280 to 337.5 meters were observed in the walking distance at baseline and at 6 months in 3 patients. Furthermore, based on the improvement in blood flow and decrease in paresthesia, the amputated patient was allowed to re-use her leg prosthesis.
Pain Assessment
Visual Analogue Scale (VAS) of 1 (no pain) to 10 (worst possible pain) was used to assess the pain level. VAS scores showed moderate pain during the screening period (even though no physical tests or invasive tests were involved in the screening process) . No increase in VAS scale was observed during blood collection (average VAS 5.4 and 3.2 during screening and blood collection, respectively). The acute effect of the cell transplantation by 30 IM injections into the gastrocnemius utilizing local anesthesia with lidocaine cream was assessed using the pain score before and 6, 24, and 48 hours following the injections. The mean VAS score prior to the procedure was 3.9, and 2.2, 2.7, and 3.0 at 6, 24, and 48 hours, respectively, indicating that the blood collection and the IM injection procedures were well tolerated. VAS scale continued at a level of 3.0–3.5 until the end of the follow-up visits at 6 months. A hallmark of CLI is severe rest pain caused by vascular insufficiency. CLI is dominated by pedal pain except in diabetic patients, where superficial pain sensation may be altered and they may experience only deep ischemic pain, such as calf claudication and ischemic rest pain. In most cases, the pedal pain is intolerably severe; it may respond to foot dependency, but otherwise responds only to opiates. This pain not only prevents physical activity, but it also alters the patient’s QoL . In this study, a record of concomitant medications taken by patients was used in addition to other signs to assess the treatment effect on patients’ pain relief and QoL. Medications were scored based on their relative strength as P1 = analgesics (e.g., ibuprofen); P2 = narcotic-like (e.g., Tramadex and Zaldiar), and P3 = narcotics (e.g., percocet, oxycodone, and fentanyl). Medications prescribed specifically for back pain and neuropathy were marked as NP1 and were not included in the assessment of pain severity.
Two patients reported pain or paresthesia relief starting as early as 1 week after treatment. In three patients, a reduction to zero use of P1, P2, and P3 medications between screening and 6 months after treatment was observed.
Quality of Life Assessment
QoL is as an important outcome measure for interventions designed to improve health, well-being, or both. The King’s College Hospital’s Vascular Quality of Life (VascuQol) is a disease-specific QoL Questionnaire for use in lower limb ischemia. It was designed to be an evaluative measure and sensitive to within-patient change [59]. A Hebrew translation of the questionnaire was utilized prior to treatment and 1, 3, and 6 months after the treatment. The questionnaire evaluated physical score, leg disease-related pain, pain not related to the leg, mental score, and patients’ easement of their current condition vs. the last year. A gradual increase in QoL Total score from a base line average of 91.6 ± 11.3 to 135.5 ± 21.2 at 6-month was observed (Fig. 12.6). Improvement in walking capability correlated with a pain relief in these patients that can be seen by their concomitant medication consumption. These trends in reduction of pain should be further assessed as a possible potential early efficacy biomarker.
Summary of this Pilot Study
Five severe CLI “no option” patients were treated in this pilot study. The main aim of the study was to evaluate the safety of the treatment. The study therapy procedures (blood collection and cell transplantation) were well-tolerated and safe. Since all the patients were severe “no option” CLI patients, efficacy endpoints including primary efficacy endpoint of AFS and secondary endpoints of blood flow (assessed by ABI/TBI), ulcers severity, walking capability, local pain, pain-control medications, and QoL were also measured. The primary endpoint of AFS was achieved in all patients that completed the 6-month follow-up period and in one patient who completed 2.9 months before becoming eligible for an interventional treatment option. As of Jun 2020, 3.5–3.8 years after the study, one post study amputation occurred due to sepsis of the foot. The number of tested parameters was different between patients. For example, one patient had no wound and another patient had only one leg, so wound healing and walking capability were not tested in them, respectively. For each patient, at least six of the tested parameters were stabilized or improved and at least three improved. Among the patients who completed the 6-months follow-up, there was a total of 27 improved results out of 31 tested parameters, representing a 77% improvement. Stabilizing the disease status and preventing the disease progression accounted for an additional 12% of the measured parameters. Taken together, in 89% of the tested parameters, BGC101 treatment delayed disease progression and improved the clinical status.
12.4 Conclusions
Despite positive clinical outcomes resulting from better classification of PAD and CLI characteristics (Fontaine, Rutherford, Wifi), more unified treatment protocols and the opening of multidisciplinary centers for treatment of ischemic low extremities and wounds, amputation rate has increased from 19 per 100,000 person/year to 30–50 per 100,000 person/year over the past decades, mainly driven by an increase in the number of diabetics and older patients [1, 3, 21, 34, 39, 61, 62, 89].
In addition to low survival rates, prognosis with respect to limb preservation in CLI patients is poor, particularly in nonrevascularizable, considered as “no-option” CLI patients, where 6-month major amputation rates range from 20% to 30%. Additionally, CLI is associated with a poor quality of life and high treatment costs, especially when amputation is inevitable. New treatment modalities using gene and SPC therapies have been slowly emerging during the last 15 years, but no gene or cell therapy for CLI has yet received a marketing authorization and CLI currently remains a major public health issue [46, 74, 85]. A few meta-analysis reports, with the largest by Rigato et al., in 2017 with data from 2332 patients from 19 RCTs, 7 nonrandomized trials, and 41 non-controlled studies, showed that cell therapy is safe, reduced the risk of amputation by 37%, improved amputation-free survival by 18%, and improved wound healing by 59% without affecting mortality. The latter fact is probably due to the relatively low mortality rate obtained in the placebo groups of the analyzed studies. In addition, cell therapy significantly increased ABI and TCPO2 and reduced rest pain. Thus, they concluded that cell therapy has the potential to modify the natural history of intractable CLI. Considering the severity of a disease burdened by high morbidity and mortality rates, they urged the scientific community to advance cell therapies to market [74]. Based on the summary presented here, ex vivo cultured, differentiated cells are the more promising SPC product. However, the safety issues of source cells collection (allogenic or autologous BM-derived) as well as the complicated and long culture periods required during which patients can further deteriorate make most of these methods unsuitable for mass treatments.
We report here a novel treatment based on a methodology that combines immune DCs specific activation of SPC that leads to differentiated EnEPCs, code-named BGC101, with a short time culture of 12–18 hours. The EnEPC based treatment addresses several biological features of the disease, including long-lasting effect on neovascularization and on chronic inflammation. This new approach is designed to enable mass production of patient friendly, safe personalized products that can be supplied within a day in every clinic and thus open the widespread availability of cell therapies for CLI. Furthermore, in order to address the entire CLI market and the full spectrum of PAD, we aim at developing a fully automated device for production of the EnEPC line of products that can be placed at regional hospitals and laboratories. BGC101 cells were found safe and effective in the FIH pilot study reported here, and further RCT studies on a larger population are planned to evaluate the potential of this new concept. If proven to be safe and effective in future clinical trials, this approach will disrupt current treatment strategies by developing accessible, safe, effective, and user-friendly cell-based treatment platform with the potential to reverse the disease process, save billions to payors, and offer patients the ability to return to their baseline quality of life. Furthermore, we believe that in the future, combining classical improved revascularization with cell therapies that stimulate regeneration and function of the microvasculature will enable a better long-term leg salvage and function. Such an approach may prove fruitful, improve the prognosis, and change the course of severe PAD worldwide.
Abbreviations
- ABI:
-
Ankle-brachial Index
- ACE:
-
Angiotensin-converting enzyme
- AcLDL:
-
Acetylated low-density lipoprotein
- ACP:
-
Angiogenic cell precursor
- AE:
-
Adverse events
- AFS:
-
Amputation-free survival
- ATMP:
-
Advanced therapy medicinal products
- AP:
-
Ankle pressure
- BM:
-
Bone marrow
- CFA:
-
Common femoral artery
- CLI:
-
Critical Limb Ischemia
- CLTI:
-
chronic limb-threatening ischemia
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CPK:
-
Creatine phosphokinase
- CV:
-
Cardiovascular
- DC:
-
Dendritic cell
- Del-1 and DELTA 1:
-
Developmentally regulated endothelial locus
- DSMB:
-
Data and Safety Monitoring Board
- EC:
-
Endothelial cells
- EnEPC:
-
Enriched endothelial progenitor cells
- EPC:
-
Endothelial progenitor cells
- ESC:
-
European Society for Cardiology
- EVT:
-
Endovascular therapy
- FGF :
-
Fibroblast growth factor
- FIH:
-
First in human
- GVHD:
-
Graft-versus-host disease
- GSV:
-
Great saphenous vein
- G-CSF:
-
Granulocyte colony-stimulating factor
- GCP:
-
Good clinical practice
- GMP:
-
Good manufacturing practice
- GTP:
-
Good tissue practice
- Hg:
-
Hemoglobin
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia inducible factor
- HSPC:
-
Hematopoietic stem/progenitor cells
- IA:
-
Intra-arterial
- IC:
-
Intermittent claudication
- ICH:
-
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IL-10:
-
Interleukin-10
- IM:
-
Intramuscular
- IV:
-
Intravenous
- LEAD:
-
Lower extremity arterial disease
- LOCF:
-
Last observation carried forward
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- MI:
-
Myocardial infarction
- MNC:
-
Mononuclear cells
- MSC:
-
Mesenchymal stem cells
- NIH:
-
National Institutes of Health
- NO:
-
Nitric oxide
- PAD:
-
Peripheral artery disease
- PBMC:
-
Peripheral blood mononuclear cell
- PB-MNC:
-
Peripheral blood-derived mononuclear cells
- PI:
-
Principal investigator
- PTA:
-
Percutaneous transluminal angioplasty
- PVR :
-
Pulse volume recording
- QoL:
-
Quality of life
- RCT:
-
Randomized controlled trial
- RNA:
-
Ribonucleic acids
- SAE:
-
Severe adverse effect
- SOC :
-
System organ class
- SPC:
-
Stem/progenitor cells
- TASC:
-
Trans-Atlantic Inter-Society Consensus
- TBI:
-
Toe-brachial index
- TcPO2:
-
Transcutaneous oxygen pressure
- TGF-β:
-
Transforming growth factor beta
- TP:
-
Toe pressure
- TTF:
-
Treatment failure
- Ulex:
-
Plant Ulex europaeus
- VAS:
-
Visual Analogue Scale
- VascuQol:
-
Vascular Quality of Life
- VEGF:
-
Vascular endothelial growth factor
- WBC:
-
White blood cells
- WIfI:
-
Wound, Ischemia, and foot Infection
References
Aboyans V, Ricco J, Bartelink M, Björck M, Brodmann M, Cohnert T, Collet J, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Ross Naylor A, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries “The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS)”. Eur Heart J. 2018;39(9):793–816.
Abualhin M, Sonetto A, Faggioli G, Mirelli M, Freyrie A, Gallitto E, Spath P, Stella A, Gargiulo M. Outcomes of duplex-guided paramalleolar and inframalleolar bypass in patients with critical limb ischemia. Ann Vasc Surg. 2018;53:154–64.
Allie D, Hebert C, Lirtzman M, Wyatt C, Keller V, Khan M, Khan M, Fail P, Vivekananthan K, Mitran E, Allie S, Chaisson G, Stagg S, Allie A, McElderry M, Walker C. Critical limb ischemia: a global epidemic. A critical analysis of current treatment unmasks the clinical and economic costs of CLI. EuroIntervention. 2005;1(1):75–84.
Allie D, Hebert C, Ingraldi A, Patlola R, Walker C. 24-carat gold, 14-carat gold, or platinum standards in the treatment of critical limb ischemia: bypass surgery or endovascular intervention? J Endovasc Ther. 2009;16(1):134–46.
Alonso A, Garcia L. The costs of critical limb ischemia. Endovascular Today. 2011:32–6.
Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18(3):371–80.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52.
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
Bartel R, Booth E, Cramer C, Ledford K, Watling S, Zeigler F. From bench to bedside: review of gene and cell-based therapies and the slow advancement into phase 3 clinical trials, with a focus on Aastrom’s Ixmyelocel-T. Stem Cell Rev Rep. 2013;9:373–83.
Baser O, Verpillat P, Gabriel S, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manag. 2013;10(2):E26–36.
Behfar A, Crespo-Diaz R, Nelson TJ, Terzic A, Gersh BJ. Stem cells: clinical trials results the end of the beginning or the beginning of the end? Cardiovasc Hematol Disord Drug Targets. 2010;10(3):186–201.
Benoit E, O'Donnell TF, Patel AN. Safety and efficacy of autologous cell therapy in critical limb ischemia: a systematic review. Cell Transplant. 2013;22(3):545–62.
Benoit E, O'Donnell TF, Iafrati E, Asher DF, Bandyk J, Hallett W, Lumsden AB, Pearl GJ, Roddy SP, Vijayaraghavan K, Patel AN. The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design. J Transl Med. 2011;9:165.
Berezin AE, Kremzer AA, Martovitskaya YV, Berezina TA, Gromenko EA. Pattern of endothelial progenitor cells and apoptotic endothelial cell-derived microparticles in chronic heart failure patients with preserved and reduced left ventricular ejection fraction. EBioMedicine. 2016;4:86–94. https://doi.org/10.1016/j.ebiom.2016.01.018
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor dec-205 in the steady state leads to antigen presentation on major histocompatibility complex class i products and peripheral cd8+ t cell tolerance. J Exp Med. 2002;196:1627–38.
Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–81.
Canellos GP. CHOP may have been part of the beginning but certainly not the end: issues in risk-related therapy of large-cell lymphoma. J Clin Oncol. 1997;15(5):1713–6.
Caux C, Burdin N, Galibert L, Hermann P, Renard N, Servet-Delprat C, Banchereau J. Functional cd40 on b lymphocytes and dendritic cells. Res Immunol. 1994;145:235–9.
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of cd40 on dendritic cells triggers production of high levels of interleukin-12 and enhances t cell stimulatory capacity: T-t help via apc activation. J Exp Med. 1996;184:747–52.
Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different notch ligands. Blood. 2007;109:507–15.
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, GVG Writing Group, Aboyans V, Aksoy M, Alexandrescu VA, Armstrong D, Azuma N, Belch J, Bergoeing M, Bjorck M, Chakfé N, Cheng S, Dawson J, Debus ES, Dueck A, Duval S, Eckstein HH, Ferraresi R, Gambhir R, Gargiulo M, Geraghty P, Goode S, Gray B, Guo W, Gupta PC, Hinchliffe R, Jetty P, Komori K, Lavery L, Liang W, Lookstein R, Menard M, Misra S, Miyata T, Moneta G, JAM P, Munoz A, Paolini JE, Patel M, Pomposelli F, Powell R, Robless P, Rogers L, Schanzer A, Schneider P, Taylor S, Vega De Ceniga M, Veller M, Vermassen F, Wang J, Wang S. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.
CTCAE (2016). https://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events
Dohmen A, Eder S, Euringer W, Zeller T, Beyersdorf F. Chronic critical limb ischemia. Dtsch Arztebl Int. 2012;109(6):95–101.
Dong Z, Chen B, Fu W, Wang Y, Guo D, Wei Z, Xu X, Mendelsohn FO. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. J Vasc Surg. 2013;58(2):404–411.e403.
Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31:1–296.
Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of il-12 in dendritic cell-induced differentiation of naive b lymphocytes. J Immunol. 1998;161:2223–31.
Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Sixta B, Varga M, Langkramer S, Sykova E, Jude EB. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev. 2013;29(5):369–76.
Fadini G, Agostini PC, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209(1):10–7.
Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24(7):1806–13. https://doi.org/10.1634/stemcells.2005-0440
Fernandez Pujol B, Lucibello FC, Zuzarte M, Lutjens P, Muller R, Havemann K. Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol. 2001;80:99–110.
Fontaine R, Kim M, Kieny R. Surgical treatment of periph- eral circulation disorders. Helv Chir Acta. 1954;21:499–533.
Fujita R, Crist C. Translational control of the myogenic program in developing, regenerating, and diseased skeletal muscle. Curr Top Dev Biol. 2018;126:67–98.
Fuh E, Brinton TJ. Bone marrow stem cells for the treatment of ischemic heart disease: a clinical trial review. J Cardiovasc Transl Res. 2009;2(2):202–18. https://doi.org/10.1007/s12265-009-9095-8.
Gottrup F, Holstein P, Jørgensen B, Lohmann M, Karlsmar T. A new concept of a multidisciplinary wound healing center and a national expert function of wound healing. Arch Surg. 2001;136(7):765–72.
Gratwohl A. Thomas' hematopoietic cell transplantation. Eur J Haematol. 2010;84(1):95.
Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143.
Hardman R, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Interv Radiol. 2014;31(4):378–88.
Hicks CW, Canner JK, Mathioudakis N, Lippincott C, Sherman RL, Abularrage CJ. Incidence and risk factors associated with ulcer recurrence among patients with diabetic foot ulcers treated in a multidisciplinary setting. J Surg Res. 2019;246:243–50.
Hicks C, Canner JK, Mathioudakis N, Lippincott C. Incidence and risk factors associated with ulcer recurrence among patients with diabetic foot ulcers treated in a multidisciplinary. Setting J Surg Res. 2020;246:243–50.
Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28(9):2155–60.
Huang P, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98(6):1335–42.
Jensen S, Vatten LJ, Myhre HO. The prevalence of chronic critical lower limb ischaemia in a population of 20,000 subjects 40–69 years of age. Vasc Endovasc Surg. 2006;32:60–5.
Kawamura A, Horie T, Tsuda I, Ikeda A, Egawa H, Imamura E, Iida J, Sakata H, Tamaki T, Kukita K, Meguro J, Yonekawa M, Kasai M. Prevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantation. Ther Apher Dial. 2005;9(1):59–63.
Kawamura A, Horie T, Tsuda I, Abe Y, Yamada M, Egawa H, Iida J, Sakata H, Onodera K, Tamaki T, Furui H, Kukita K, Meguro J, Yonekawa M, Tanaka S. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J Artif Organs. 2006;9(4):226–33.
Kim SJ, Kim N, Kim EH, Roh YH, Song J, Park KH, Choi YS. Use of regional anesthesia for lower extremity amputation may reduce the need for perioperative vasopressors: a propensity score-matched observational study. Ther Clin Risk Manag. 2019;15:1163–71.
Kitrou P, Katsanos K, Karnabatidis D, Reppas L, Brountzos E, Spiliopoulos S. Current evidence and future perspectives on anti-platelet and statin pharmacotherapy for patients with symptomatic peripheral arterial disease. Curr Vasc Pharmacol. 2017;15(5):430–45.
Klepanec A, Mistrik M, Altaner C, Valachovicova M, Olejarova I, Slysko R, Balazs T, Urlandova T, Hladikova D, Liska B, Tomka J, Vulev I, Madaric J. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012;21(9):1909–18.
Krishna SM, Moxon J, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16:11294–322.
Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53(2):445–53.
Li M, Zhou H, Jin X, Wang M, Zhang S, Xu L. Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: preliminary clinical results. Exp Clin Transplant. 2013;(5):435–9. https://doi.org/10.6002/ect.2012.0129
Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, Carman T, Dohad S, Huang P, Junge CE, Story K, Weistroffer T, Thorne TM, Millay M, Runyon JP, Schainfeld R. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5(6):821–30.
Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36.
Lumsden A, Davies M, Peden E. Medical and endovascular management of critical limb ischemia. J Endovasc Ther. 2009;16(2):II31–62.
Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83.
McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):61–8.
Mills J, Conte M, Armstrong D, Pomposelli F, Schanzer A, Sidawy A, Andros G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). Vasc Surg. 2014;59:220–34.
Miyamoto M, Yasutake M, Takano H, Takagi H, Takagi G, Mizuno H, Kumita S, Takano T. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13(4):429–37.
Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 ifn. J Immunol. 2005;174:1851–61.
Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the vascular quality of life questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33(4):679–87.
Mutirangura P, Ruangsetakit C, Wongwanit C, Chinsakchai K, Porat Y, Belleli A, Czeiger D. Enhancing limb salvage by non-mobilized peripheral blood Angiogenic cell precursors therapy in patients with critical limb ischemia. J Med Assoc Thail. 2009;92(3):320–7.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26(2):81–157.
Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, Schernthaner GH, Stanek A, Fowkes G, Catalano M. Epidemiology of peripheral artery disease in Europe: VAS educational paper. Int Angiol. 2018;37(4):327–34. https://doi.org/10.23736/S0392-9590.18.03996-2.
Perl L, Weissler A, Mekori YA, Mor A. Cellular therapy in 2010: focus on autoimmune and cardiac diseases. Isr Med Assoc J. 2010;12(2):110–5.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9.
Procházka V, Gumulec J, Jalůvka F, Šalounová D, Jonszta T, Czerný D, Krajča J, Urbanec R, Klement P, Martinek J, Klement GL. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19(11):1413–24.
Powell R, Comerota A, Berceli S, Guzman R, Henry T, Tzeng E, Velazquez O, Marston W, Bartel R, Longcore A, Stern T, Watling S. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011;54(4):1032–41.
Porat Y, Assa-Kunik E, Belkin M, Krakovsky M, Lamensdorf I, Duvdevani R, Sivak G, Niven M, Bulvik S. A novel potential therapy for vascular diseases: blood-derived stem/progenitor cells specifically activated by dendritic cells. Diabetes Metab Res Rev. 2014;30(7):623–34.
Porat Y, Abraham E, Karnieli O, Nahum S, Woda J, Zylberberg C. Critical elements in the development of cell therapy potency assays for ischemic conditions. Cytotherapy. 2015;17(7):817–31.
Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118(1):58–65. https://doi.org/10.1161/CIRCULATIONAHA.107.727347
Raval AN, Schmuck EG, Tefera G, Leitzke C, Ark CV, Hei D, Centanni JM, de Silva R, Koch J, Chappell RG, Hematti P. Bilateral administration of autologous CD133+ cells in ambulatory patients with refractory critical limb ischemia: lessons learned from a pilot randomized, double-blind, placebo-controlled trial. Cytotherapy. 2014;16(12):1720–32. https://doi.org/10.1016/j.jcyt.2014.07.011
Raval AD, Vyas A. Trends in healthcare expenditures among individuals with arthritis in the United States from 2008 to 2014. J Rheumatol. 2018;45(5):705–16.
Rutherford R, Baker D, Ernst C, Johnston W, Porter J, Jones S, Darrell N. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38.
Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175(5):2788–92.
Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120(8):1326–40.
Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, van Dorsselaer A, Lotteau V, Rabourdin-Combe C, Rabilloud T, Servet-Delprat C. High expression of antioxidant proteins in dendritic cells: possible implications in atherosclerosis. Mol Cell Proteomics. 2006;5:726–36.
Schanzer A, Conte MS. Critical limb ischemia. Curr Treat Options Cardiovasc Med. 2010;12(3):214–29.
Smith RB, Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. Chapter 13. PMID: 21250079.
Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62.
Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–97.
Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385–92.
Szabo GV, Kovesd Z, Cserepes J, Daroczy J, Belkin M, Acsady G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease-results of the short- and long-term follow-up. Cytotherapy. 2013;15(10):1245–52.
Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through il-10. Blood. 2006;108:1189–97.
Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–35.
Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, Verhaar MC. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015;131(10):851–60.
Teraa M, Conte M, Moll F, Verhaar M. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5(2):2938.
Tongers J, Roncalli JG And Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation 2008;118(1):9–16.
Trinchieri G, Pflanz S, Kastelein RA. The il-12 family of heterodimeric cytokines: new players in the regulation of t cell responses. Immunity. 2003;19:641–4.
Veith FJ, Gupta SK, Wengerter KR, Samson RH, Scher LA, Fell SC, Weiss P, Janko G, Flores SW, Rifkin H, Bernstein G, Haimovici H, Gliedman ML, Sprayregen S. Progress in limb salvage by reconstructive arterial surgery combined with new or improved adjunctive procedures. Ann Surg. 1981;194(4):386–401.
Veith FJ, Gupta SK, Wengerter KR, Goldsmith J, Rivers SP, Bakal CW, Dietzek AM, Cynamon J, Sprayregen S, Gliedman M. Effects of metoprolol on rest and exercise cardiac function and plasma catecholamines in chronic congestive heart failure secondary to ischemic or idiopathic cardiomyopathy. Am J Cardiol. 1990;66(10):843–8.
Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, et al. Intraarterial Administration of Bone Marrow Mononuclear Cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv. 2011;4(1):26–37. https://doi.org/10.1161/CIRCINTERVENTIONS.110.958348
Wang N, Yang BH, Wang G, Gao Y, Cao X, Zhang XF, Yan CC, Lian XT, Liu BH, Ju S. A meta-analysis of the relationship between foot local characteristics and major lower extremity amputation in diabetic foot patients. J Cell Biochem. 2019;120(6):9091–6.
Yu Z, Witmanb N, Wang W, Li D, Yan B, Deng M, Wang X, Wang H, Zhou G, Liu W, Sahara M, Cao Y, Fritsche-Danielsonf R, Zhanga W, Fud W, Chienb K. Cell-mediated delivery of VEGF modified mRNA enhances blood vessel regeneration and ameliorates murine critical limb ischemia. J Control Release. 2019;310:103–14.
Zhang Y, Zhang C. Role of dendritic cells in cardiovascular diseases. World J Cardiol. 2010;2:357–64.
Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Niven, M. et al. (2021). Changing the Course of Peripheral Arterial Disease Using Adult Stem Progenitor Cells. In: Navarro, T.P., Minchillo Lopes, L.L.N., Dardik, A. (eds) Stem Cell Therapy for Vascular Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-56954-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-56954-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56953-2
Online ISBN: 978-3-030-56954-9
eBook Packages: MedicineMedicine (R0)