Abstract
It has been over 50 years since Scrimshaw and colleagues synthesized evidence that the overlap of malnutrition with infectious diseases within the same populations and individuals raised the likelihood that the two conditions interacted. Yet our understanding of causation remains incomplete, despite clear criteria for demonstrating some mechanisms of causality and despite a wealth of experimental and epidemiological studies. In this chapter, we present a framework that conceptualizes the various mechanisms by which host nutritional status might influence infections and/or microbial pathogenesis. The framework considers the impact of nutrition on exposure to the pathogen, the ability of the pathogen to establish in the host, and the ability of the pathogen to proliferate and spread to other hosts. It also considers influences of nutrition on the host responses and experience of infection including immunological responses, disease severity, and the effectiveness of drug interventions. The ways in which infection may change host nutritional status are also outlined. This framework provides a basis for interrogating the literature for observations that may be overlooked and for design of future studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Causality
- Conceptual framework
- Nutrition-infection interactions
- Biomarkers
- Drugs
- Exposure
- Barriers
- Reproduction
- Disease severity
- Immunity
-
Many criteria have been developed for demonstrating causality, but few have been refined for application to the design or analysis of nutrition-infection interactions.
-
A conceptual framework for exploring the impact of host nutritional status on infectious disease needs to consider the impact of nutrition on both the host and the pathogen.
-
Infection alters nutritional status through a variety of mechanisms.
-
Establishing causation in a context of multiple nutrient deficiencies and infections is challenging, especially given the difficulty of directly extrapolating mechanisms identified under controlled experimental conditions to natural populations.
There are varying combinations of infectious diseases and micronutrient and macronutrient deficiencies in the different ecological settings around the world. Even within a community, the combination of infectious agents and nutritional deficiencies varies among individuals. Pathogens can be affected by nutritional status through multiple pathways that may have distinct consequences depending on the host and environment. Host nutritional status can affect success of a pathogen, beginning with exposure to the infectious agent through to the resolution of the infection, either naturally or through response to interventions. Some of the pathways may result from changes in host behavior and others through biochemical and metabolic changes in the host or pathogen. Given the multiple potential pathways, exploring causal relationships between a specific nutritional disorder or combination of disorders and single or multiple infections poses challenges.
To better frame the exploration of these complex relationships, we lay out key definitions, theories of causality, types of causal relationships, and criteria for assessing causal associations between nutrition and infection. To better understand the relationships between nutrition and infectious disease, we follow the classification system used by Scrimshaw et al. [1] of synergism, antagonism, or no effect. These terms are used to reflect the net combination of effects as even within a single nutrient-infection combination, it is conceivable that, at a mechanistic level, there may be a mix of both synergistic and antagonistic relationships. In presenting an overarching conceptual framework of potential pathways of interaction, we seek a unifying construct that helps frame the other chapters in this book and guides conceptualization of future multidisciplinary research into nutrition-infection interactions.
Background: Definitions and Tools for Understanding Relationships Between Nutrition and Infection
Definitions
To set the stage, we clarify the meaning and usage of our central themes: nutritional status and infection.
Nutritional Status
Nutritional status reflects the body’s store of available nutrients and is measured using anthropometry (e.g., height, weight, mid upper arm circumference) and biochemical indicators (e.g., hemoglobin) and concentrations of specific nutrients (e.g., serum retinol) compared against recognized standards or cutoffs. Macronutrients include proteins, lipids (fats), and carbohydrates (sugars), while micronutrients include water-soluble or fat-soluble vitamins, macrominerals, and trace minerals. Nutritional status is an indicator of the balance between nutrient needs and nutrient consumption. Undernutrition includes both micronutrient malnutrition, where intake or absorption of single or multiple micronutrients is inadequate to meet physiological needs for good health, and protein-energy malnutrition, where consumption of macronutrients (protein, carbohydrates, lipids) is insufficient (see Chap. 2); overnutrition often includes both an excess of macronutrients and deficiencies of micronutrients [2].
Infection
This book focuses on infections of relevance to humans caused by viruses, bacteria, protozoans, and helminths. We distinguish between an infection (i.e., the presence of a pathogen) and disease (i.e., signs, symptoms, and pathology [disease progression]). We note that disease severity is a function of pathogen virulence (see Box 1.1), host tolerance, and pathogen load. The chapters address infections that are harmful, but also highlight situations where pathogens may have beneficial effects (e.g., Shea-Donohue et al. [3]), such as within the microbiome [4, 5]. Limited attention is given to infections that cause disease directly within the vector, unless the impact on the vector is also associated with altered impact on human hosts. Both asymptomatic and symptomatic infections are addressed where relevant, particularly as there is a growing awareness of pathological changes that may be happening in the asymptomatic stage of infections, such as in infections with the human immunodeficiency virus (HIV), where asymptomatic infection is associated with a 10% increase in resting energy expenditure [6]. Where helpful in understanding underlying mechanisms, we make reference to studies in livestock as well as rodent models.
Box 1.1 Definition of Terms
-
Susceptibility – the set of complementing genetic or environmental causes sufficient to make a person contract a disease after being exposed to the specific causes [7, 8]
-
Vulnerability – contextual factors that influence the likelihood of exposure of individuals and communities
-
Virulence – the ability of a specific strain of a microorganism to produce disease [9], often related to the rate of replication of the infectious agent or its intrinsic invasive capacity
-
Tolerance – the process whereby the body becomes increasingly resistant through continued exposure [9] or produces less pathology for a given infection load
-
Pathogenicity – the ability of a pathogen to produce disease [9] or symptoms, often as a consequence of an overactive immune or inflammatory response
-
Resistance – the ability of the host to limit infection or disease [10], typically through effective barriers or immune responses that have a genetic component
Theories of Causality
Prior to exploring criteria for assessing causal relationships, it is important to characterize the kind of causality being explored. Historically, understanding causality has been both a philosophical and a natural science pursuit. Aristotle was an influential early proposer of theories of causality, unpacking the theoretical cause of an “object” [11]. More recent representations of causal relationships have reflected on multiple potential meanings of the phrase “A causes B”. Parascandola and Weed concluded that an epidemiological perspective is best served by a counterfactually basedFootnote 1 probabilistic definition of causality that uses probability statistics to assess the impact of a condition when it is present compared to when it is absent [12]. Thygesen and colleagues utilized two theories of causality: regularity and generative. The regularity theory proposes that we can say “A causes B” when we observe a statistical pattern indicating that A is followed by B. The generative theory proposes that we can say “A causes B” when we have identified pathways and biological mechanisms that could lead from A to B [13], independent of epidemiological evidence. In the usage by Thygesen and colleagues, regularity theory is similar to probabilistic causality, while generative theory is focused on biological mechanisms and has no clear counterpart in the Parascandola and Weed framework [13].
From a nutritional epidemiological perspective, both regularity and generative causality can be used as possible pathways to evaluate cause and effect, and their joint use is ideal. Generative causality occurs in nutrition when a specific nutritional deficiency leads to specific physiological conditions in human populations, and we have a mechanistic understanding of the relationship. Vitamin A deficiency causing xerophthalmia (abnormally dry conjunctiva and cornea of the eyes) is a good example. The larger focus of nutritional epidemiology has been on whether deficiencies or excesses of nutrients (such as vitamin A) could affect the acquisition and/or the progression of other diseases, even when mechanisms are not yet well understood. In this second context, causality is understood as either “regular” when the association is solely epidemiological or “generative” [12] when mechanisms are understood.
Criteria for Assessing Relationships Between Infections and Malnutrition
Identifying and characterizing patterns of association and relationships between two events are fundamental to the history of science. In the field of infectious diseases, theories of causality began from detecting the presence of an infectious agent. Koch’s postulates (Table 1.1) were a key conceptual innovation that allowed researchers to demonstrate with scientific precision that a specific agent caused a specific infection. This was revolutionary and led to rapid identification of interventions to reduce infectious diseases such as cholera. With the development of the science of epidemiology over the last century, there has been a growing understanding of causality as more than a single factor leading to a single outcome and an increasing awareness of the importance of nonpathogenic influences on infectious diseases. With this changing understanding, analyses of probabilistic associations and potentially causal relationships rely on much more than Koch’s postulates. In particular, emerging understanding of asymptomatic disease, cofactors in the manifestation of infectious pathology, and other complexities that were not known in Koch’s nineteenth century all inform current understandings of causality.
To give a noninfectious-/non-nutrient-focused example of determining causality, in the 1960s, the growing epidemic of lung cancer motivated scientists to look for causal theories and criteria that were relevant in assessing emerging patterns in medicine and public health. The principal focus was on the role of tobacco in lung cancer, and this contributed to the development of a more probabilistic and epidemiologically focused set of criteria for assessing potentially causal relationships [14]. Bradford Hill (Table 1.1) identified seven criteria, only one of which he considered essential: temporality [15]. Building on the Bradford Hill criteria, and specifically addressing issues that arise in considering causality in the field of nutritional epidemiology, Potischman and Weed [16] focused on a subset of the Bradford Hill criteria (Table 1.1) with the addition of biological plausibility, a criterion reflecting the generative theory of causality of Thygesen [13].
Monteiro and colleagues combined both sets of criteria and added one more: analogies from similar conditions (Table 1.1) [17]. They then used each criterion separately to assess evidence of a causal relationship between a species of malaria, Plasmodium vivax , and subsequent undernutrition [17]. One of the most challenging criteria, temporality, was addressed through multiple cohort studies in P. vivax -endemic areas that assessed nutritional status prior to infection with P. vivax [17]. They concluded that current evidence is consistent with P. vivax contributing to undernutrition in endemic settings, demonstrating the applicability of clearly stated causal analysis to situations of nutritional and infectious disease epidemiology. Their analysis offers a compelling example of using rigorous causal analysis from a breadth of criteria to assess the strength of the evidence for a given relationship.
Types of Relationships
From a theoretical perspective, poor nutritional status could have a direct association with infection in three ways. Poor nutritional status could have a synergistic effect on an infection where it promotes the infection in some way, it could diminish the infection with an antagonistic effect, or it could have no impact on the infection, either because of independence of the two conditions or because the synergistic aspects cancel out the antagonistic dimensions of the association.
Synergistic Relationships Between Nutrition and Infection
A number of nutritional deficiencies can reduce the ability of a host to resist a pathogen, and a number of infections can impair nutritional status. This kind of causality is said to be synergistic in that both the infection and malnutrition exert negative effects on host health. There is an extensive literature documenting synergistic relationships between malnutrition and infections, and the public health significance has become evident [18,19,20]. In the mid-1990s, several researchers developed an approach for estimating population-level effects of malnutrition (low weight-for-age) on risk of infant and child mortality [21, 22]. Pelletier and colleagues estimated population attributable risks from data on mortality rates from specific childhood infections for children with differing nutritional status [22]. Thus, they estimated that 56% of all child deaths from 53 developing countries were due to underlying malnutrition, as malnourished children had a higher mortality rate for diarrhea, malaria, and pneumonia than well-nourished children [21]. This analysis of the synergistic relationship between malnutrition and childhood mortality has clear implications at the population level in terms of the broader clinical and public health efforts needed to address malnutrition in order to reduce childhood mortality.
The impact of undernutrition on transmission of tuberculosis (TB) in the central-eastern Indian states has been explored using modeling [23]. The authors modeled several different scenarios of future undernutrition, based on reductions in undernutrition achieved over the past 20–30 years by countries such as Bangladesh (5.0% annual decrease in the proportion of their population with inadequate caloric intake), Vietnam (8.0% annual decrease), and Ghana (11.7% annual decrease) [23]. The models suggested that a modest improvement in caloric intake (in range of that achieved in Bangladesh) could avert 4.8 million TB cases and 1.6 million TB deaths in Central and Eastern India over 20 years [23]. In this example, the synergistic relationship was inferred by the association between improvements when caloric intake increased and a proportional reduction in TB cases observed across regions, though the ecologic association may or may not hold in real-world circumstances.
Combined diarrhea and malnutrition provide another example of a synergistic relationship. Severely undernourished children (weight for age z score (WAZ) < −3.0) had an odds ratio of 9.5 for mortality from diarrhea when compared with children with a WAZ > −1.0 [24].
Both macronutrient deficiencies such as protein-energy malnutrition and deficiencies of specific micronutrients can lead to increased severity of infections. Vitamin A deficiency increases the severity of measles to the point that vitamin A supplementation is recommended for children with measles [25,26,27]. Iron deficiency can also interact synergistically with infections. For example, progression to gastric cancers was increased if Mongolian gerbils infected with Helicobacter pylori , a bacterial infection that causes stomach ulcers, were fed an iron-depleted diet instead of an iron-replete diet [28, 29].
Antagonistic Relationships Between Nutrition and Infection
An antagonistic relationship is less frequent but can be vitally important. One example is when a nutritional deficiency reduces infection-induced pathology more than it reduces the host immune response; a second example is when an infection reduces severity of malnutrition [1]. One early report of the negative effects of malnutrition on the host being countered by a benefit of reduced infection emerged in a feeding camp in Ethiopia during a 1970s famine in Somalia where iron repletion was used. The odds ratio for positive malaria blood smears was 13.4 among those who received supplemental iron, relative to nomads who were iron deficient and who did not receive iron [30]. The antagonistic relationship between malaria and iron continues to be documented, with a recent longitudinal study showing increased risk of a positive malaria smear among infants in the highest quartile of iron status [31]. In a mouse model of urinary tract infections with uropathogenic Escherichia coli (UPEC), animals on a low-iron diet had a significantly lower bacterial burden [32]. Dietary iron has also been suggested to have an antagonistic relationship in hookworm infections, with both iron deficiency and iron excess reducing hookworm egg output in hamsters and normal levels of iron significantly increasing hookworm egg output and decreasing hamster survival [33]. This latter example emphasizes the nonlinear relationship between iron supplementation and hookworm infection, with antagonistic effects at extremes of the spectrum of iron status and synergistic effects in the normal range of iron status [33]. In an attempt to strengthen the case for causality, recent studies have focused on potential biological mechanisms underlying antagonistic relationships between iron and infections [34].
No Relationship Between Nutrition and Infection
There are some infections where mild to moderate malnutrition does not appear to affect risk of infection or disease, and infection does not appear to affect nutritional status. However, a critical assessment must consider the consistency of conclusions among studies, the number of studies/subjects, and whether a given relationship may hold for more extreme nutritional status, as with anemia/low iron and hookworm [33]. In a review of 13 studies of dengue fever and nutrition, there was no consistent pattern between malnutrition measured solely by anthropometry and the three forms of dengue. Interestingly, however, subgroup analyses suggested that more severe dengue (dengue shock syndrome) might be increased in underweight individuals (i.e., synergistic relationship), whereas less severe dengue may be more prevalent in mildly malnourished individuals. Finding no association between nutritional status and disease severity may indicate that a particular pathogen is not affected by host nutritional state or may reflect a heterogeneity of impacts under differing nutritional conditions and/or study populations [35].
A Conceptual Framework for the Infection-Malnutrition Interface
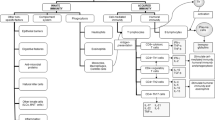
One of the challenges in clarifying the influences of nutritional status on infection is the ongoing host-pathogen “negotiation” during an infection (Fig. 1.1). At each stage of an infection, the host seeks to block the spread of the pathogen, and the pathogen seeks to overcome the host defenses [36]. Building on the stages of an infection, we have developed a framework guided by plausible biological mechanisms that delineates phases during the progression of an infection, to enable a more thorough exploration of the potential pathways of influence of nutritional status on infectious diseases. Six stages are identified, three focused on impacts of nutrition on the pathogen and three focused on impacts on the host. Pathways related to the pathogen include (1) finding the host; (2) establishment, maturation, and survival within the host; and (3) division or reproduction of the pathogen and subsequent release of infectious stages to the environment or to another host. Pathways related to the host include (1) immunological or inflammatory responses that may lead to resolution of infection, (2) progression of disease symptoms that may be due to pathogen-induced damage or immunopathology, and (3) response to treatments (Fig. 1.2).
Classification of host-pathogen interactions. (Reprinted by permission from Springer Nature: Sen et al. [36])
Pathways by which nutritional status may alter (a) pathogen success and (b) host success at all stages of infection, through influences on behavior and immune function. Framework for designing, categorizing, and reporting research questions and evidence at the interface of nutrition and infectious diseases. We note that different research designs and evidence are needed to address the questions in each box
Exposure (Spatial and Temporal Overlap of Host and Pathogen)
Preventing exposure to an infectious pathogen is one of the primary approaches to preventing infections. Examples of individual behavioral interventions include the use of bednets to prevent mosquito bites and malaria infection, washing vegetables thoroughly to remove potential pathogens, or wearing long pants and sleeves to prevent the Borrelia infection leading to Lyme disease. Population-level interventions are exemplified by spraying to kill potential arthropod disease vectors , pasteurization of milk, or fluoridation of a water supply to reduce Streptococcus mutans-mediated dental caries . There are multiple pathways by which nutritional status or dietary intake could affect risk of exposure.
Behavioral Pathways
Both protein-energy malnutrition and iron deficiency can lead to fatigue and limited energy [37], and an early study on human starvation found that reduced mobility compensated for much of the unmet energy needs [38]. Reduced mobility may reduce exposure to outdoor pathogens such as helminths or sylvatic leishmaniasis. In contrast, increased time indoors may increase exposure to airborne respiratory pathogens [39] or exposure to peri-domestic insect vectors that transmit vector-borne diseases such as Chagas disease (see Chap. 6) [40]. A modeling study exploring transmission of mosquito-borne diseases under conditions of varying levels of human mobility found that both low mobility and high mobility populations were predicted to have lower prevalence of infection, with moderately mobile populations having higher rates of transmission [41].
Diet-Related Pathways
Many pathogens are transmitted through food, either because this is a typical route of exposure, or because of accidental contamination. Thus, dietary choices together with decisions about washing or cooking foods can influence exposure to infectious agents, and food choices and styles of food preparation may themselves be influenced by nutritional status. Both zinc and iron deficiency are strongly associated with pica, defined as the craving and consumption of non-food items such as chalk, ice, paint chips, and soil [42, 43]. A study published in 2010 analyzed 88 samples of soils sold for consumption in markets in Africa, Europe, and the United States and identified bacterial and fungal contamination in almost all of the samples, suggesting that consumption of such non-food items could lead to exposure to pathogens [44].
Chemical Cues to the Pathogen
A more hypothetical pathway between nutritional status and exposure is the possibility that a malnourished individual emits volatiles that increase or decrease the attractiveness of the host to particular vectors. Vector biting behavior, including choice of humans over livestock and other nonhuman mammals, is strongly influenced by volatile organic compound scents, and there is increasing evidence that volatiles are influenced by diet and metabolism [45]. Vertebrates are known to rely on chemical cues to detect (and choose) prey and to warn of approaching predators. For example, fish release a chemical alarm signal that warns other fish of the presence of predators [46], and both parasite-infected fish and tadpoles have been shown to release alarm signals [47]. Mosquito vectors detect hosts by recognition of volatile compounds [48, 49], visual cues, CO2 , human odors, body heat, and other nonvolatile compounds (Fig. 1.3) [50].
Graphic illustrating the sensory cues used by mosquito vectors to target human hosts depicting visual cues, CO2 , odors, body heat, and nonvolatiles. (Reprinted with permission from Elsevier: Montell and Zwiebel [50])
While evidence is strong that vectors are influenced by visual and olfactory cues, evidence on the impacts of nutritional status on host odors, CO2 release, and nonvolatiles is still nascent. Restriction of vitamin E intake has been shown to reduce chemical signaling in lizards [51]. If nutritional status affects human emissions in any way, it could alter the ability of parasites or vectors to find a suitable host. Where transmission occurs via active penetration by the pathogen, such as in hookworm, or biting by a vector such as in malaria, volatiles may also attract (or deter) infectious stages and vectors to (from) the host. Malnutrition can also decrease the basal metabolic rate [52], which would lower body heat, thus changing another important vector cue.
Establishment of Infection (Pathogen Successfully Crosses Host Barriers, Establishes at Appropriate Tissue Location, and Matures)
A successful infection requires that infectious agents establish and reach a hospitable final location. This typically involves two steps – (a) breaching protective physical, biological, and chemical barriers, such as epithelial membranes, microbial biofilms, and stomach enzymes, and (b) transitioning (sometimes changing through developmental stages) during migration to a suitable site where replication or reproduction can occur.
Breaching the Barriers
The integrity and health of barriers can be directly (and indirectly) affected by host nutritional status. Humans have three primary barriers – the gut (gastrointestinal), skin (integumentary), and lung (pulmonary) barriers. With the gut barrier, relationships between nutritional status and epithelial integrity have been demonstrated for vitamin A deficiency and protein-energy malnutrition. Vitamin A deficiency leads to increased intestinal permeability in animal and in vitro models [53, 54], and child malnutrition in general has been closely associated with altered structure and function of the intestinal mucosa [55, 56]. A general thinning of the skin with protein-energy malnutrition in a mouse model has been reported [57]. Several studies have identified a role for vitamin D in pulmonary epithelial integrity [58,59,60]. However, research on the impact of nutritional status on barrier functions to pathogens is limited primarily to the gut.
Migration to Target Tissues
As the pathogen migrates through the host, it faces additional physical barriers such as cell walls, blood vessels, and lung tissue, whose integrity and physical or immunological competence may be affected by macro- or micronutrient deficiencies. During migration, pathogens typically undergo morphological, physiological, biochemical, and molecular transitions that have evolved to enable them to exploit their host. Any of these transitions can be sensitive to host nutritional status, as pathogens are dependent on the host for providing essential nutrients. Once the pathogen reaches its destination, it needs to remain in the site and this often involves adherence to target tissues. Nutrients may play a role in this adherence, as with iron in the case of the vaginal pathogen, Trichomonas vaginalis , where iron induces synthesis of key adhesion molecules [61, 62]. Nutrient-dependent mechanisms such as this may be necessary for survival of many pathogens.
Pathogen Proliferation (Pathogen Reproduction and Release of Infectious Stages)
Critical to the ongoing presence of infections in a population is the ability of the pathogen to propagate and then to release infective stages so that the infection can be transmitted to others. Propagation happens in a variety of ways, including viral replication, binary fission, and production of spores or gametes or eggs. As pathogens rely on host nutrients (see Chaps. 4, 5, 6, 7, and 14) [40, 63,64,65,66], deficiencies of essential nutrients can have a direct negative impact on pathogen propagation unless the pathogen is able to outcompete the host for the limited nutrients. Research using an avian model has shown that fleas on nestlings that received dietary supplementation laid more eggs than fleas on unsupplemented nestlings [67].
Once a pathogen has reproduced, it is transmitted to new hosts by direct contact; by release of transmission forms into the environment through bodily fluids, feces, or airborne particles; or by transfer into vectors or intermediate hosts. Host nutritional status may reduce movement of the transmission stage out of the host. For example, if peripheral blood flow is reduced, malaria gametocytes may not be available to mosquitoes when they bite. Host nutritional status may alter the interval between infection and transmission, the duration of release of transmission forms from the host, the longevity of infectious stages outside the host, their infectivity to the next host, or the geographical range over which infection is spread. For example, in a randomized controlled trial (RCT) of adults with moderate selenium deficiency, those who received selenium supplements shed poliovirus in the feces for a shorter period after polio vaccination [68]. This could mean that selenium-deficient individuals might transmit the virus for a longer period of time. If nutritional status alters gut transit time through diarrhea or constipation, infective stages might be released prematurely or, alternatively, might be retained in the gut, thus compromising their viability.
Furthermore, through coevolutionary processes, host nutritional status may change pathogen genetics. This has been demonstrated in an experimental model where repeated passage of coxsackievirus B3 virus through several generations of vitamin E-deficient or selenium-deficient mice led to a viral phenotype with increased virulence [69]. In this case, vitamin E and selenium deficiencies increased the pathogenicity of the virus.
Immune Responses to Natural Infection and to Vaccines Lead to Resolution of Infection
When a host kills or expels an established pathogen, this is referred to as natural recovery. Nutritional deficiencies are well known to impair the ability of the host to respond effectively to an infection, allowing persistence of infections that are usually cleared rapidly (see Chap. 3) [70,71,72,73,74]. Nutritional status is accepted as an important modifier of immune response to vaccines, although the specifics of the impacts are less clear [75]. Studies in the 1980s explored particular dimensions of immune response such as B-cell versus T-cell responses to measles vaccination [76, 77]. Protein-energy malnutrition has been associated with a decreased response to tuberculin sensitivity skin tests following BCG vaccination against tuberculosis in children [78], and a third of protein-deficient pigs that were vaccinated with the attenuated human rotavirus vaccine developed diarrhea after subsequent exposure to rotavirus, but none of the vaccinated protein-sufficient pigs did [79]. In an RCT of newborns in Pakistan, zinc deficiency was shown to reduce the efficacy of oral poliovirus vaccine, but surprisingly zinc supplementation did not improve vaccine efficacy [80]. Demonstration of the causal connection between vaccine efficacy and nutritional status has been limited by the low number of studies, poor quality of data, and heterogeneity in variables across studies that make comparisons very challenging [81]. More recently, it seems the gut microbiome may modulate the interaction between nutritional status and vaccine efficacy, at least for oral vaccines [82]. While there are a number of studies assessing impact of malnutrition on immune function (see Chap. 3), studies are still needed to better unpack how nutritional status affects vaccine efficacy [83, 84].
Disease Severity (Pathogen Leads to Symptoms and/or Immunopathology)
In human populations, infections that are rapidly resolved often go undetected. However, when natural mechanisms of clearance (self-regulation by the infection or immune-mediated processes) are interrupted, infections may become pathogenic, and disease severity may increase. In an observational study of the intestinal protozoan infection Cryptosporidium parvum , the prevalence of clinical complications in Jamaican children was higher in children with low weight-for-height compared to well-nourished children [85]. This situation can be seen as an example where malnutrition may have permitted ongoing parasite replication and in turn increased infection-induced disease severity.
Many pathogens have virulence factors that contribute to their ability to cause disease. Nutrient-dependent virulence factors have been identified in numerous pathogens, such as Entamoeba histolytica [86], T. vaginalis [61], and H. pylori [28]. For example, with host iron deficiency, H. pylori upregulates high iron affinity transporters in order to obtain the iron it needs, and these high affinity transporters are associated with increased pathogenicity [28]. HIV has also been implicated in nutrition-infection pathogenic interactions (see Chap. 9) [87]. Selenium is one micronutrient where deficiency has been linked to poor HIV clinical outcomes, while selenium supplementation has been shown to improve HIV clinical outcomes and to reduce the incidence of TB among HIV-infected persons [88,89,90,91]. Serum retinol has been studied as a risk factor for adverse HIV outcomes, but it is difficult to assess cause and effect, as persons with advancing HIV disease may have altered food intakes and temporality is difficult to determine [92, 93].
The ability of the host to tolerate or limit infection-induced damage can be influenced by nutritional status. Vitamin A/retinoic acid plays a key role in the development of mucosal tolerance through its role in induction of regulatory T cells (Tregs), which are an important downregulator of the immune response. Vitamin A/retinoic acid is an important stimulator of differentiation and proliferation of Tregs, T helper Type 2 (Th2) , T helper 17 (Th17) , and IgA plasma cells [94]. Appetite is another pathway by which nutrition may affect tolerance to a pathogen. A recent analysis of the impact of fasting behaviors on viral and bacterial infections concluded that fasting was protective against disease progression for a bacterial infection, Listeria monocytogenes , in mice, whereas glucose supplementation helped protect mice from influenza virus [95]. With the L. monocytogenes infection, anorexia led to ketogenesis which helped to reduce antibacterial inflammation and release of antibacterial reactive oxygen species. In contrast, with influenza, glucose supplementation helped prevent initiation of stress-mediated apoptosis [95]. Nutritional status and dietary intake are important mediators of disease, through pathways affecting both pathogen virulence and host tolerance.
The severity of many infectious diseases results from an over aggressive immune response that itself causes pathology, as dramatized by the COVID-19 pandemic. In such situations, diet and nutritional status may alter the degree of immunopathology [96,97,98,99]. A recent systematic review of preclinical evidence shows that hepatic granulomas in mice infected with the trematode parasite, Schistosoma mansoni, were fewer in number and/or size in mice fed low-protein or vitamin-restricted diets and in zinc-supplemented mice, but higher in mice fed a high-fat diet (Fig. 1.4) [100].
In vivo preclinical evidence of the impact of different dietary strategies on parasitological, immunological, biochemical, and histopathological parameters in animals infected by Schistosoma mansoni. The main diagonal arrow indicated the primary measure of outcome. Black arrows in each box indicate the effect direction for each accessory outcome. (−) mitigates and (+) stimulates mortality. (?) uncertain impact on parasitemia and mortality (insufficient data). (Used with permission from Cambridge University Press: Marques et al. [100])
Response to Treatment (Pathogen Cleared or Symptoms Reduced)
When an infected individual receives drug treatment, nutritional status may affect the host ability to use the pharmaceuticals effectively. Nutritional status can affect drug distribution, drug absorption, plasma binding of drugs, drug activation, and drug clearance [55, 101,102,103,104,105,106]. Protein-energy malnutrition alters gut integrity and gastric emptying, which can affect drug absorption. Drug activation, the conversion of an inactive form to the active form, and drug clearance are both important steps for a number of drugs. Cytochrome P450 (CYP) enzymes are the dominant pathway by which drugs are metabolized for both activation and clearance [107, 108]. Both protein-calorie malnutrition and iron deficiency have been shown to affect cytochrome P450 3A4 (CYP3A4) activity. Rats on a protein- and calorie-restricted diet had less CYP generally [109, 110] and CYP3A specifically [109]. In addition, rats with protein-calorie malnutrition had decreased hepatic metabolism via CYP3A [111]. The decrease in activation may affect drug dosage, leading to a need for higher doses in malnourished individuals. One study in patients on hemodialysis with low CYP3A4 found an increase in CYP3A4 after receiving intravenous iron supplementation [112]. These examples emphasize the importance of this often-overlooked area. Chapter 13 provides an in-depth review of drug-nutrient interactions relevant to infectious diseases (see Chap. 13) [113].
Pathogen Affects Nutritional Status
In terms of the reverse pathway, namely, the pathogen affecting the host’s nutritional status, multiple biological pathways have been delineated. Persons with infections can lose their appetites and suffer from gastrointestinal disturbances and many other manifestations that compromise optimal nutrition. Pathogens damage host tissues, they may compete with the host for nutrients, and they induce and maintain energy-intensive immune responses [19, 114]. A vigorous immune response can place extraordinary nutritional demands on a host, and even immune surveillance is nutritionally demanding. Persons with chronic or severe infections may become malnourished unless intakes are adjusted to cover increased demand. Hunger and food insecurity contribute to delayed initiation and nonadherence to antiretroviral therapy for HIV in Africa, especially if patients recognize that the medicines stimulate their appetite and food is unavailable [130]. Damage of host tissue in the gut can impair nutrient absorption and lead to malnutrition, as in the case of environmental enteropathy [115, 116]. Damage to host tissue can also lead to increased physiological demand for nutrients to repair the damage, and if the increased demand cannot be met through available diets, the body may draw down other physiological stores, thus limiting physiological functioning [114]. Bartelt and colleagues have developed a model of protein-deficient mice coinfected with Giardia lamblia and enteroaggregative Escherichia coli for exploring impacts of coinfection on malnutrition and vice versa [117]. The authors demonstrated that the combined infection and protein deficiency led to a greater weight loss and alterations in metabolic functioning than the combined infection and an isocaloric complete diet, although both protocols led to weight loss [117].
Infection-induced immune responses can result in release of pyrogens such as prostaglandin E2 that triggers an increased basal metabolic rate and fever. Counter to the old adage “feed a cold and starve a fever,” fever increases the nutrient need of the host. A small study of 12 healthy volunteers demonstrated that fasting facilitated a humoral immune response, whereas eating fostered more of a cell-mediated response [118]. Perhaps infections that are resolved by a humoral response (like many helminth infections) would benefit from fasting, whereas those that are cleared by a cell-mediated response (many bacterial and protozoan infections that induce a fever) would be exacerbated by fasting.
There is a growing awareness of the complex ways that pathogens, diet, and the microbiota of a host interact, and a comprehensive understanding of the impacts of such interactions is still nascent. A recent study highlighting the influences of diet on the microbiome found that two groups of mice with the same initial microbiome composition had different microbial communities 8 weeks after being fed a low-fat and high-plant polysaccharide diet (similar to rural limited resource settings) compared with a high-fat high-sugar diet (Western) [119]. Research on the microbiome as a potential mediator of nutrition-infection interactions is important to consider going forward [120].
Challenges in Investigating Nutrition and Infectious Disease
Once mechanisms by which nutritional status affect infections have been demonstrated, there are several challenges in estimating the impact in human populations: (1) heterogeneity of nutritional status; (2) heterogeneity of infections; (3) a historical research emphasis on the role of infections in causing malnutrition rather than the opposite; and (4) difficulty in extrapolating from controlled lab studies to human populations.
Nutritional status can vary at the level of macronutrient and/or micronutrient concentration within individuals (i.e., high level of one nutrient, low or moderate levels of other nutrients) and also among individuals within communities and between communities. Identifying relatively homogeneous populations that differ in nutritional variables of interest but that are similar for other nutritional variables is challenging. Deficiencies or excesses of macro- or micronutrients seldom occur apart from other nutritional perturbations. Thus measurements of multiple nutrients are essential for accurately characterizing nutritional status, with significant associated clinical or public health research costs [121]. In addition, individual nutritional status is difficult to measure, both at the macro- and micronutrient levels. High-quality biomarkers of nutritional status are expensive to obtain and challenging to interpret, as nutrient levels in bodily fluids can shift as nutrients are shuttled to different locations in the body, despite no changes in overall nutrient status. For example, serum biomarkers of nutritional status are highly variable, depending on multiple factors including time since the most recent meal, infection status, hydration, diurnal variation, varying metabolic demands, and perhaps host genetics [122]. A field study of children in Zambia found alterations in biomarkers of iron and vitamin A that varied by stage of the acute phase response (incubation, early convalescence, convalescence, and healthy) as measured by C-reactive protein and α1-acid glycoprotein (AGP) [123, 124]. Given the multiple potential influences on such biomarkers, identifying optimum cutoffs as indicative of deficiency is challenging.
Similarly, individuals, communities, and regions can vary in terms of the number and variety of infections [125]. In human populations, infections vary in terms of the time since exposure to the pathogen, the exposure dose, or intensity of infection, whether it is an initial infection or a secondary infection with the same agent, and what other pathogens and microbiome the host harbors. Each of these factors can affect immune response and potentially also the interaction between nutrition and infection.
An additional challenge is that a multiplicity of factors can affect nutrition and, similarly, that a large number of factors can affect infection. This complexity makes it challenging to generalize from highly controlled laboratory models to free-living situations (see Box 1.2), even though laboratory models are very helpful in identifying generative causal relationships. The field of ecology is replete with examples where a scientist can demonstrate a clear pattern in the laboratory that is not apparent under more natural conditions. The profile of infection dynamics of an intestinal nematode of mice, Heligmosomoides polygyrus , differed markedly between an inbred susceptible and inbred resistant strain of mouse under controlled laboratory infections. However, when the two strains lived together in a large indoor enclosure where natural transmission occurred, the infection profile was undistinguishable between the two strains [126]. Similarly, resistance of C57BL/6 inbred lab mice to the intestinal nematode, Trichuris muris , was not evident when the mice were moved into a seminatural outdoor enclosure prior to, or after experimental infection, as evidenced by higher number of worms and worm biomass relative to mice infected in the lab [127]. This contextual element for infection extends to nutrition-infection interactions, where laboratory conditions and conclusions may not be generalizable even to natural infections in the mouse. This makes it even harder to extrapolate lab findings to humans.
Box 1.2 Impact of Variations in Pathogen Virulence and Host Susceptibility
Scrimshaw et al. (citing H.S. Schneider, 1950, Strategic concepts in Epidemiology) note that laboratory studies generally use homogeneous animal and disease models, where virulence of the pathogen and susceptibility of the host are known. Schneider used an experimental 3 × 3 study design with three host characteristics (1) inbred resistant, (2) outbred mixed (mix of susceptible and resistant), and (3) inbred susceptible as well as three pathogen characteristics (a) uniformly virulent, (b) mixed virulent and avirulent, and (c) uniformly avirulent, to explore the impact of nutritional status. Schneider found that nutritional status only played a role in the outbred host group that was infected with the mixed virulence pathogen, a situation most likely found in humans. Thus, highly controlled laboratory models may actually underestimate the impact of nutrition in real-world settings [1].
Conclusions
Taken together, this overview highlights the myriad pathways by which host nutritional status may influence all aspects of the host-pathogen interaction. It also highlights the challenges in both design and analysis faced by researchers whose goal is to demonstrate causality, and by inference the challenges faced by clinical and public health sectors charged with preventing and controlling infectious diseases. Through the examples provided in the subsequent chapters of this book, we hope to highlight not only the need for more rigorous application of causality analysis especially during epidemiological research and in systematic reviews and meta-analyses on infection-nutrition interactions but also the importance of laboratory research that identifies plausible mechanisms that may be relevant in human populations. Given the influence of nutritional status on infections and vice versa, effective global control of infectious disease will require addressing the relationships between malnutrition and infections [128], and the conceptual framework proposed in this chapter may be useful in this context.
Notes
- 1.
Counterfactual probabilistic approaches assess the statistical association of presence and absence of a potential risk factor with the condition of concern.
Abbreviations
- AGP:
-
Alpha glycoprotein
- BCG:
-
Bacille Calmette-Guerin
- CYP:
-
Cytochrome P450
- HIV:
-
Human immunodeficiency virus
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- PEM:
-
Protein-energy malnutrition
- RCT:
-
Randomized controlled trial
- TB:
-
Tuberculosis
- Th:
-
T helper
- Treg:
-
Regulatory T cells
- WAZ:
-
Weight-for-age
References
Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329.
Barffour MA, Humphries DL. Core principles: infectious disease risk in relation to macro and micronutrient status. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Shea-Donohue T, Qin B, Smith A. Parasites, nutrition, immune responses and biology of metabolic tissues. Parasite Immunol. 2017;39(5). https://doi.org/10.1111/pim.12422.
Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016;1372(1):53–64.
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179.
Kosmiski L. Energy expenditure in HIV infection. Am J Clin Nutr. 2011;94(6):1677S–82S.
Diderichsen F, Hallqvist J, Whitehead M. Differential vulnerability and susceptibility: how to make use of recent development in our understanding of mediation and interaction to tackle health inequalities. Int J Epidemiol. 2019;48(1):268–74.
Khoury MJ, Flanders WD, Greenland S, Adams MJ. On the measurement of susceptibility in epidemiologic studies. Am J Epidemiol. 1989;129(1):183–90.
Mosby’s Medical Dictionary. 2009. https://medical-dictionary.thefreedictionary.com/susceptibility. Accessed 12 Aug 2019.
McGraw-Hill concise dictionary of modern medicine. New York: McGraw-Hill; 2002.
Falcon A. Aristotle on causality. The Stanford encyclopedia of philosophy. Stanford: The Metaphysics Research Lab, Center for the Study of Language and Information, Stanford University; 2019.
Parascandola M, Weed DL. Causation in epidemiology. J Epidemiol Community Health. 2001;55(12):905–12.
Thygesen LC, Andersen GS, Andersen H. A philosophical analysis of the Hill criteria. J Epidemiol Community Health. 2005;59(6):512–6.
Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–308.
Hill AB. The environment and disease: association or causation? J R Soc Med. 2015;108(1):32–7.
Potischman N, Weed DL. Causal criteria in nutritional epidemiology. Am J Clin Nutr. 1999;69(6):1309S–14S.
Monteiro WM, Alexandre MA, Siqueira A, Melo G, Romero GA, d’Avila E, et al. Could Plasmodium vivax malaria trigger malnutrition? Revisiting the Bradford Hill criteria to assess a causal relationship between two neglected problems. Rev Soc Bras Med Trop. 2016;49(3):274–8.
Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66(2):464S–77S.
Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37(6):386–98. https://doi.org/10.1016/j.it.2016.04.003.
Walson JL, Berkley JA. The impact of malnutrition on childhood infections. Curr Opin Infect Dis. 2018;31(3):231–6.
Pelletier DL, Frongillo EA Jr, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73(4):443–8.
Pelletier DL, Frongillo EA Jr, Schroeder DG, Habicht JP. A methodology for estimating the contribution of malnutrition to child mortality in developing countries. J Nutr. 1994;124(10 Suppl):2106S–22S.
Oxlade O, Huang CC, Murray M. Estimating the impact of reducing under-nutrition on the tuberculosis epidemic in the central eastern states of India: a dynamic modeling study. PLoS ONE. 2015;10(6):e0128187.
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60.
D’Souza RM, D’Souza R. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2002;(1):CD001479.
Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized, placebo-controlled, double-blind trial. Am J Clin Nutr. 1991;54(5):890–5.
Coutsoudis A, Kiepiela P, Coovadia HM, Broughton M. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. Pediatr Infect Dis J. 1992;11(3):203–9.
Haley KP, Gaddy JA. Nutrition and Helicobacter pylori: host diet and nutritional immunity influence bacterial virulence and disease outcome. Gastroenterol Res Pract. 2016;2016:3019362.
Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123(1):479–92.
Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. Br Med J. 1978;2(6145):1113–5.
Moya-Alvarez V, Cottrell G, Ouedraogo S, Accrombessi M, Massougbodgi A, Cot M. High iron levels are associated with increased malaria risk in infants during the first year of life in Benin. Am J Trop Med Hyg. 2017;97(2):497–503.
Bauckman KA, Matsuda R, Higgins CB, DeBosch BJ, Wang C, Mysorekar IU. Dietary restriction of iron availability attenuates UPEC pathogenesis in a mouse model of urinary tract infection. Am J Physiol Renal Physiol. 2019;316(5):F814–22.
Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Dietary iron content mediates hookworm pathogenesis in vivo. Infect Immun. 2006;74(1):289–95.
Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol. 2014;5:125.
Trang NTH, Long NP, Hue TTM, Hung LP, Trung TD, Dinh DN, et al. Association between nutritional status and dengue infection: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:172.
Sen R, Nayak L, De RK. A review on host-pathogen interactions: classification and prediction. Eur J Clin Microbiol Infect Dis. 2016;35(10):1581–99.
Yokoi K, Konomi A. Iron deficiency without anaemia is a potential cause of fatigue: meta-analyses of randomised controlled trials and cross-sectional studies. Br J Nutr. 2017;117(10):1422–31.
Keys A, Brozek J, Henschel A, Mickelson A, Taylor H. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
Sze To GN, Chao CY. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20(1):2–16.
Wiser MF. Nutrition and protozoan pathogens of humans—a primer. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious diseases: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Acevedo MA, Prosper O, Lopiano K, Ruktanonchai N, Caughlin TT, Martcheva M, et al. Spatial heterogeneity, host movement and mosquito-borne disease transmission. PLoS ONE. 2015;10(6):e0127552.
Miao D, Young SL, Golden CD. A meta-analysis of pica and micronutrient status. Am J Hum Biol. 2015;27(1):84–93. https://doi.org/10.1002/ajhb.22598.
Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9(11):1075–80.
Kutalek R, Wewalka G, Gundacker C, Auer H, Wilson J, Haluza D, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104(12):787–95.
Spanel P, Smith D. Volatile compounds in health and disease. Curr Opin Clin Nutr Metab Care. 2011;14(5):455–60.
Brown GE, Elvidge CK, Macnaughton CJ, Ramnarine I, Godin JGJ. Cross-population responses to conspecific chemical alarm cues in wild Trinidadian guppies, Poecilia reticulata: evidence for local conservation of cue production. Can J Zool. 2010;88(2):139–47.
Poulin R, Marcogliese DJ, McLaughlin JD. Skin-penetrating parasites and the release of alarm substances in juvenile rainbow trout. J Fish Biol. 1999;55(1):47–53.
Majeed S, Hill SR, Birgersson G, Ignell R. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R Soc Open Sci. 2016;3(11):160467.
Majeed S, Hill SR, Dekker T, Ignell R. Detection and perception of generic host volatiles by mosquitoes: responses to CO2 constrains host-seeking behaviour. R Soc Open Sci. 2017;4(5):170189.
Montell C, Zwiebel LJ. Mosquito sensory systems. In: Raikhel AS, editor. Advances in insect physiology, vol. 51. London: Elsevier; 2016.
Garcia-Roa R, Saiz J, Gomara B, Lopez P, Martin J. Dietary constraints can preclude the expression of an honest chemical sexual signal. Sci Rep. 2017;7(1):6073.
McCue MD. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(1):1–18.
Li Y, Gao Y, Cui T, Yang T, Liu L, Li T, et al. Retinoic acid facilitates toll-like receptor 4 expression to improve intestinal barrier function through retinoic acid receptor beta. Cell Physiol Biochem. 2017;42(4):1390–406.
de Medeiros PHQS, Pinto DV, de Almeida JZ, Rego JMC, Rodrigues FAP, Lima AAM, et al. Modulation of intestinal immune and barrier functions by vitamin a: implications for current understanding of malnutrition and enteric infections in children. Nutrients. 2018;10(9):1128.
Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS ONE. 2014;9(8):e105017.
Attia S, Feenstra M, Swain N, Cuesta M, Bandsma RHJ. Starved guts: morphologic and functional intestinal changes in malnutrition. J Pediatr Gastroenterol Nutr. 2017;65(5):491–5.
Sugiyama A, Fujita Y, Kobayashi T, Ryu M, Suzuki Y, Masuda A, et al. Effect of protein malnutrition on the skin epidermis of hairless mice. J Vet Med Sci. 2011;73(6):831–5.
Gorman S, Buckley AG, Ling KM, Berry LJ, Fear VS, Stick SM, et al. Vitamin D supplementation of initially vitamin D-deficient mice diminishes lung inflammation with limited effects on pulmonary epithelial integrity. Physiol Rep. 2017;5(15):e13371.
Li W, Dong H, Zhao H, Song J, Tang H, Yao L, et al. 1,25-Dihydroxyvitamin D3 prevents toluene diisocyanate-induced airway epithelial barrier disruption. Int J Mol Med. 2015;36(1):263–70.
Shi YY, Liu TJ, Fu JH, Xu W, Wu LL, Hou AN, et al. Vitamin D/VDR signaling attenuates lipopolysaccharide induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. 2016;13(2):1186–94.
Figueroa-Angulo EE, Rendon-Gandarilla FJ, Puente-Rivera J, Calla-Choque JS, Cardenas-Guerra RE, Ortega-Lopez J, et al. The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect. 2012;14(15):1411–27.
Moreno-Brito V, Yanez-Gomez C, Meza-Cervantez P, Avila-Gonzalez L, Rodriguez MA, Ortega-Lopez J, et al. A Trichomonas vaginalis 120 kDa protein with identity to hydrogenosome pyruvate:ferredoxin oxidoreductase is a surface adhesin induced by iron. Cell Microbiol. 2005;7(2):245–58.
Berkley JA. Bacterial infections and nutrition—a primer. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Green WD, Karlsson EA, Beck MA. Viral infections and nutrition: influenza virus as a case study. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Geary TG, Haque M. Human Helminth infections. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Ezenwa VO. Co-infection and nutrition: integrating ecological and epidemiological perspectives. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Springer; 2020.
Tschirren B, Bischoff LL, Saladin V, Richner H. Host condition and host immunity affect parasite fitness in a bird-ectoparasite system. Funct Ecol. 2007;21(2):372–8.
Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154–62.
Beck MA. Increased virulence of coxsackievirus B3 in mice due to vitamin E or selenium deficiency. J Nutr. 1997;127(5 Suppl):966S–70S.
Stephensen CB. Primer on immune response and interface with malnutrition. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65.
Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203.
Bono MR, Tejon G, Flores-Santibanez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016;8(6):349.
Spinas E, Saggini A, Kritas SK, Cerulli G, Caraffa A, Antinolfi P, et al. Crosstalk between vitamin B and immunity. J Biol Regul Homeost Agents. 2015;29(2):283–8.
Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084–18.
Idris S, El Seed AM. Measles vaccination in severely malnourished Sudanese children. Ann Trop Paediatr. 1983;3(2):63–7.
Powell GM. Response to live attenuated measles vaccine in children with severe kwashiorkor. Ann Trop Paediatr. 1982;2(3):143–5.
Udani PM. BCG vaccination in India and tuberculosis in children: newer facets. Indian J Pediatr. 1994;61(5):451–62.
Miyazaki A, Kandasamy S, Michael H, Langel SN, Paim FC, Chepngeno J, et al. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine. 2018;36(42):6270–81.
Habib MA, Soofi S, Sheraz A, Bhatti ZS, Okayasu H, Zaidi SZ, et al. Zinc supplementation fails to increase the immunogenicity of oral poliovirus vaccine: a randomized controlled trial. Vaccine. 2015;33(6):819–25.
Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139(11):2154S–218S.
Bhattacharjee A, Hand TW. Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin Sci (Lond). 2018;132(11):1169–77.
Smailnejad Ganji K, Mohammadzadeh I, Mohammadnia-Afrouzi M, Ebrahimpour S, Shahbazi M. Factors affecting immune responses to vaccines. Gazz Med Ital. 2018;177(5):219–28.
Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gomara M, Prendergast AJ, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13:97–118.
MacFarlane DE, Horner-Bryce J. Cryptosporidiosis in well nourished and malnourished children. Acta Paediatr. 1987;76(3):474–7.
Gastelum-Martinez A, Leon-Sicairos C, Plata-Guzman L, Soto-Castro L, Leon-Sicairos N, de la Garza M. Iron-modulated virulence factors of Entamoeba histolytica. Future Microbiol. 2018;13:1329–41.
Wanke C, Baum M. Nutrition in HIV and tuberculosis. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Springer; 2020.
Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, et al. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(5):370–4.
Campa A, Baum MK, Bussmann H, Martinez SS, Farahani M, van Widenfelt E, et al. The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr Diet Suppl. 2017;9:37–45.
Campa A, Shor-Posner G, Indacochea F, Zhang G, Lai H, Asthana D, et al. Mortality risk in selenium-deficient HIV-positive children. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(5):508–13.
Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007;167(2):148–54.
Kassu A, Andualem B, Van Nhien N, Nakamori M, Nishikawa T, Yamamoto S, et al. Vitamin A deficiency in patients with diarrhea and HIV infection in Ethiopia. Asia Pac J Clin Nutr. 2007;16(Suppl 1):323–8.
Camp WL, Allen S, Alvarez JO, Jolly PE, Weiss HL, Phillips JF, et al. Serum retinol and HIV-1 RNA viral load in rapid and slow progressors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(4):401–6.
Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac J Allergy Immunol. 2015;33(2):71–89.
Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166(6):1512–25.e12.
Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466.
Ciavarella C, Motta I, Valente S, Pasquinelli G. Pharmacological (or synthetic) and nutritional agonists of PPAR-gamma as candidates for cytokine storm modulation in COVID-19 disease. Molecules. 2020;25(9):2076.
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988.
Cena H, Chieppa M. Coronavirus disease (COVID-19-SARS-CoV-2) and nutrition: is infection in Italy suggesting a connection? Front Immunol. 2020;11:944.
Marques DVB, Felizardo AA, Souza RLM, Pereira AAC, Goncalves RV, Novaes RD. Could diet composition modulate pathological outcomes in schistosomiasis mansoni? A systematic review of in vivo preclinical evidence. Parasitology. 2018;145(9):1127–36.
Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol. 2010;66(10):1025–35.
Murray M. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr Drug Metab. 2006;7(1):67–81.
Murray M, Marden NY, Lee AC. Altered CYP expression and function by dietary factors: potential roles in disease pathogenesis. Drug Metab Rev. 2006;38:19–20.
Mehta S, Nain CK, Sharma B, Mathur VS. Disposition of four drugs in malnourished children. Drug Nutr Interact. 1982;1(3):205–11.
Walker O, Dawodu AH, Salako LA, Alvan G, Johnson AO. Single dose disposition of chloroquine in kwashiorkor and normal children—evidence for decreased absorption in kwashiorkor. Br J Clin Pharmacol. 1987;23(4):467–72.
Boullata JI, Hudson LM. Drug-nutrient interactions: a broad view with implications for practice. J Acad Nutr Diet. 2012;112(4):506–17.
Walter-Sack I, Klotz U. Influence of diet and nutritional status on drug metabolism. Clin Pharmacokinet. 1996;31(1):47–64.
Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73.
Lee PC, Struve MF, Bezerra JA, Duncan B. Effects of protein malnutrition on liver cytochrome P450s. Nutr Res. 1997;17(10):1577–87.
Lee JH, Suh OK, Lee MG. Pharmacokinetic changes in drugs during protein-calorie malnutrition: correlation between drug metabolism and hepatic microsomal cytochrome P450 isozymes. Arch Pharm Res. 2004;27(7):693–712.
Lee YK, Yoon I, Lee MG, Choi YH. Effects of cysteine on the pharmacokinetics of tamoxifen in rats with protein-calorie malnutrition. Xenobiotica. 2012;42(12):1225–34.
Pai AB, Norenberg J, Boyd A, Raj D, Chan LN. Effect of intravenous iron supplementation on hepatic cytochrome P450 3A4 activity in hemodialysis patients: a prospective, open-label study. Clin Ther. 2007;29(12):2699–705.
Boullata JI. Drug-nutrition interactions in infectious diseases. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and infectious disease: shifting the clinical paradigm. Totowa: Humana Press; 2020.
Stephensen CB. Burden of infection on growth failure. J Nutr. 1999;129(2S Suppl):534S–8S.
Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66(9):487–505.
Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18(6):328–36.
Bartelt LA, Bolick DT, Mayneris-Perxachs J, Kolling GL, Medlock GL, Zaenker EI, et al. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog. 2017;13(7):e1006471.
van den Brink GR, van den Boogaardt DE, van Deventer SJ, Peppelenbosch MP. Feed a cold, starve a fever? Clin Diagn Lab Immunol. 2002;9(1):182–3.
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14.
Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73(Suppl 1):32–40.
Gonzalez-Fernandez D, Pons ED, Rueda D, Sinisterra OT, Murillo E, Scott ME, et al. C-reactive protein is differentially modulated by co-existing infections, vitamin deficiencies and maternal factors in pregnant and lactating indigenous Panamanian women. Infect Dis Poverty. 2017;6(1):94.
Gibson R. Principles of nutritional assessment. 2nd ed. Oxford: Oxford University Press; 2005.
Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, et al. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr. 2014;144(6):972–8.
Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr. 2014;5(6):702–11.
Gonzalez-Fernandez D, Koski KG, Sinisterra OT, Del Carmen PE, Murillo E, Scott ME. Interactions among urogenital, intestinal, skin, and oral infections in pregnant and lactating Panamanian Ngabe women: a neglected public health challenge. Am J Trop Med Hyg. 2015;92(6):1100–10.
Scott ME. Heligmosomoides polygyrus (Nematoda): susceptible and resistant strains of mice are indistinguishable following natural infection. Parasitology. 1991;103(Pt 3):429–38.
Leung JM, Budischak SA, Chung The H, Hansen C, Bowcutt R, Neill R, et al. Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biol. 2018;16(3):e2004108.
Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3:17067.
Evans AS. Causation and disease: a chronological journey. The Thomas Parran lecture. Am J Epidemiol. 1978;108(4):249–58.
Chop E, Duggaraju A, Malley A, Burke V, Caldas S, Yeh PT et al. Food insecurity, sexual risk behavior, and adherence to antiretroviral therapy among women living with HIV: A systematic review. Health Care Women Int. 2017; 38(9):927.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Humphries, D.L., Scott, M.E., Vermund, S.H. (2021). Pathways Linking Nutritional Status and Infectious Disease: Causal and Conceptual Frameworks. In: Humphries, D.L., Scott, M.E., Vermund, S.H. (eds) Nutrition and Infectious Diseases . Nutrition and Health. Humana, Cham. https://doi.org/10.1007/978-3-030-56913-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-56913-6_1
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-56912-9
Online ISBN: 978-3-030-56913-6
eBook Packages: MedicineMedicine (R0)