Abstract
Penicillium digitatum and P. expansum are the most devastating pathogens of citrus and pome fruits respectively, causing significant economic losses during postharvest handling worldwide. To obtain new rational and environmentally friendly control alternatives, a better understanding of the fruit-pathogen interaction may be considered as a novel perspective for the control of postharvest diseases. The main objective of our studies was to gain insights into the fruit-pathogen interactions, specifically in oranges and apples defence responses against compatible and non-host pathogens. For such purposes, firstly a deep study of infection capacities of both pathogens in oranges and apples at different conditions was performed. Later, we characterized the effect of wound response in oranges and apples harvested at different maturity stages and temperatures, as well as we identified the possible compounds involved in the wound healing process. In addition, a visualization of lignin, suberin and callose in apple and oranges tissue was conducted using histochemical tests. Finally, in apples, a transcriptomic study in response to compatible and non-host pathogen was conducted; and the expression of several genes involved in the phenylpropanoid pathway were quantified in citrus fruit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- P. expansum
- P. digitatum
- Host-pathogen interaction
- Lignification
- Phenylpropanoid pathway
- Transcriptomics analysis
Introduction
Oranges and apples are some of most produced fruits worldwide. The production of citrus in Mediterranean regions is 25,216 thousand tons in 2016, and Spain is the first producer with 6,882 thousand tons. Specifically, Spain contributed with 3,641 thousand tons of oranges and, therefore, remains the major contribution in Mediterranean Region with around 25% of total production (FAO 2017). In turn, as important apple producer, Spain contributed to the apple worldwide production with 473 thousand tons in 2018 (Prognosfruit 2018).
Quality deterioration of fresh fruits due to fungal pathogens is one of the main economic losses produced in postharvest handling worldwide. The most common cause of citrus and pome fruit decay is the development of rots caused by Penicillium spp. which are one of the most common and destructive genera in postharvest diseases, affecting a wide variety of fruit. In particular, P. digitatum and P. expansum are the most devastating postharvest pathogens of citrus and pome fruits, respectively. Although both are close related species, they show a different range of hosts. Whereas P. digitatum causes only green mould in citrus and could be considered as specialist species, P. expansum has the ability to infect a broader range of hosts (Fig. 2.1). These necrotrophic pathogens need wounds to enter tissues and start the infection process.
The traditional way to control these diseases is the usage of synthetic fungicides. However, there are important problems associated by their use, hence, actual trends are directed to find new rational and environmental friendly control alternatives focused on physical, chemical and biological strategies. These facts justify the need and the interest for more detailed studies of host-pathogen interaction to increase the knowledge on both virulence factors of pathogens and fruit defence mechanisms with the final aim to improve control strategies (Tian et al. 2016).
At this regard, new perspectives to control postharvest diseases are needed and some of them are based on the knowledge of fruit-pathogen interaction. On one hand, the capacity of fungi to adapt to new conditions, overcoming plant defence mechanisms and becoming resistant to fungicides is huge. On the other hand, the defence machinery of the fruit is also complex and unknown, and evolved a multilevel series of structural and biochemical barriers that are both constitutive or preformed and inducible. Both components of host-pathogen interactions should be addressed from different perspectives, pathological, biochemical and molecular studies, to elucidate the factors that can modulate both compatible and non-host interactions.
The main proposal of this chapter is to approach the research line related to control postharvest diseases on pome and citrus fruit from new perspective, mainly focused on fruit-pathogen interactions in apple/oranges- Penicillium spp.
Fruit–Penicillium spp. Interaction
In many instances, the differences in the outcome of a host-pathogen interaction relies on a rapid and efficient deployment of defense responses, which will be modulated depending on whether the host-pathogen interaction involves a compatible or non-host interaction. For that purpose, P. digitatum and P. expansum were defined as compatible pathogens of oranges and apples, respectively, meanwhile P. expansum and P. digitatum were described as non-host pathogens in oranges and apples, respectively (Fig. 2.2).
Fruit defenses are complex and constitute a multilevel series of structural and biochemical barriers that are both constitutive and inducible. Therefore, a multidisciplinary approach including pathological, biochemical and molecular studies were conducted to unravel the underlying mechanisms related to fruit response against both postharvest pathogens. In particular, in this chapter we have focused our research in the following aspects: (1) infection capacity of each pathogen in oranges and apples; (2) characterization of fruit healing process; (3) identification and expression analysis of genes involved in fruit defence mechanisms.
Infection Capacity of Both Pathogens on Apples and Oranges
The infection capacities of P. digitatum on oranges and of P. expansum on apples take into account several key factors such as i) maturity of fruit, ii) pathogen inoculum concentrations and iii) storage temperatures (Fig. 2.3).
Vilanova et al. (2012a, b) clearly defined the compatible and non-host interactions at both studied pathosystems. The main results showed that in compatible interactions the lowest growth rate of both pathogens was observed in the immature harvest fruit stored at both 20 °C and cold temperatures (4 °C and 0 °C for oranges and apples, respectively). In addition to that, the lower growth rate was accentuated at lower concentration of inoculum of pathogens as 104 conidia mL−1. Several authors have observed higher susceptibility of overripe apples compared to immature apples against P. expansum and Botrytis cinerea, which highlight the role of maturity in the susceptibility of fruit to fungal diseases (Torres et al. 2003; Neri et al. 2010; Su et al. 2011). Furthermore, in oranges no differences were observed between the growth rates of P. digitatum in oranges stored at both 20 °C and 4 °C, at the concentration of 107 conidia mL−1 at any maturity stage. This fact could be explained because the inoculum concentration is too high that the maturity states of fruit do not affect the growth rate of this pathogen.
In apples and oranges, the storage temperature influenced the growth rate and visual symptoms of the decay of these pathogens and need shorter incubation times at 20 °C than at cold temperature (Vilanova et al. 2012a, b). These results coincide with those obtained by Baert et al. (2007a, b) in apples inoculated with different strains of P. expansum. Moreover, in in vitro studies in PDA medium, Buron-Moles et al. (2012) showed a decrease in the growth rate of P. digitatum and P. expansum at cold temperature (4 °C and 0 °C, respectively) compared to 25 °C.
Regarding inoculum concentration, in general no significant differences were observed at any concentration of the pathogen tested (from 104 to 107 conidia mL−1). Immature apples inoculated with P. expansum and stored at 20 °C, however, showed a different growth rate dependent on inoculum concentration, in contrast, these differences were not observed in oranges (Vilanova et al. 2012a, b). In a similar work, Morales et al. (2008) observed differences in the growth rate of P. expansum at different inoculum concentrations in apples stored at 20 °C.
On the other hand, considering the non-host interaction, e.g. P. expansum-oranges and P. digitatum-apples, these studies demonstrated that they became compatible interaction depending on the conditions and, therefore, they cannot strictly considered non-host pathogens. Previous studies conducted by Macarisin et al. (2007) have already shown that P. expansum could germinate and develop decay in citrus fruit but only at the point of inoculation. These authors only observed green mould in apples after treatment of wounds with different organic acids (citrus, ascorbic and oxalic) or with the enzyme catalase (CAT) before inoculation, pointing out the role of these substances in the suppression of the production of H2O2. Vilanova et al. (2012b) showed the ability of P. expansum to infect oranges by itself, without any pre-treatment, at 20 °C and 4 °C. However, incidence and severity of these infected oranges were higher at 4 °C than at 20 °C (Fig. 2.4a). This behaviour can be explained because wound healing, as fruit defence mechanisms, is slower at 4 °C than at 20 °C, together with the fact that P. expansum is a pathogen well- adapted to low temperatures. Studies carried out by Ismail and Brown (1975) already pointed out that healing of “Valencia” oranges at 5 °C was slower than at 30 °C. Later, Brown and Barmore (1983) showed that both phenolic and lignification substances could be the responsible of resistance of curing oranges against P. digitatum infection. In turn, the formation of lignin increases at 20 °C more than at 2.5 °C (Mulas et al. 1996).
In contrast, Vilanova et al. (2012a) demonstrated that P. digitatum showed some visual symptoms of decay in apples but only restricted at the site of infection (Fig. 2.4b). Only overmatured apples were decayed by the highest concentration of P. digitatum (107 conidia mL−1), but exceptionally (Fig. 2.5). In addition, Buron-Moles et al. (2012) corroborated these results visualizing the infection process in apples using P. digitatum tagged with a green fluorescent protein (GFP). Likewise, Louw and Korsten (2014) have also revealed that P. digitatum can infect different varieties of apples and pears. In contrast, at 0 °C, no signs of infection by P. digitatum were observed at any studied conditions. The overall results may explain by the ecophysiological difference between P. digitatum and P. expansum (Plaza et al. 2003; Buron-Moles et al. 2012).
A relevant fact in these non-host interactions were detected: when P. expansum and P. digitatum were not able to infect oranges and apples, an important reaction in the skin and pulp was observed (Fig. 2.6). In addition, this reaction increased proportionally to pathogen concentration and decreased as maturity advanced. This reaction could be associated to a rapid localized necrosis of non-host resistance (Mysore and Ryu 2004). Macarisin et al. (2007) in lemons also observed that approximately 4–5 days after inoculation with the non-host pathogen P. expansum appeared the hypersensitive response (HR), with dead cells and lignified tissue surrounding the wounds. HR usually appears in incompatible interactions (Mysore and Ryu 2004) and is recognized by brown necrotric areas resulting from a localized collapse of tissues as well as dead cells at the point of inoculation. In studies in coffee leaves (Silva et al. 2002) and melon (Romero et al. 2008), it was observed rapid HR in incompatible interactions. Whilst this response has been described to prevent the development of biotrophic fungi (Lamb and Dixon 1997; Lu and Higgins 1999), it may not prevent the consequent development of necrotrophic fungi (Mayer et al. 2001). In turn, some authors suggest that it may even stimulate the development of necrotrophic fungi (Govrin and Levine 2000).
In summary, our studies demonstrated that the inoculation of a compatible pathogen (P. digitatum-oranges and P. expansum-apples) at different inoculum concentrations always showed decay development. However, in oranges and apples, the most important differences in rot dynamics among harvests were found at the lowest inoculum concentration analysed (104 conidia mL−1). Surprisingly, depending on the combination of factors (maturity stage, inoculum concentration and storage temperature), the non-host interaction (P. expansum-oranges and P. digitatum-apples) became compatible.
Orange and Apple Defence Mechanisms
Lack of penetration by fungi into non-host tissue can be associated with the deposition of substances or compounds in the host epidermal cell walls. Based on above results, a study of the host defence responses to abiotic (wound) and biotic (pathogen) stresses was conducted following a pathological, biochemical and molecular approach to elucidate the defence reactions underlying in these interaction (Fig. 2.7).
Wound Response Process
P. digitatum and P. expansum are considered as wound fungi, i.e., they need a wound on the fruit epidermis in order to start the infection (Kavanagh and Wood 1967; Spotts et al. 1998). Hence, good handling practices during harvest to avoid wounds in fruit are important to reduce postharvest losses. Beside that, a better knowledge on healing process of apples and oranges is needed.
It is known that different treatments (curing, light UV) or inducing substances increased the resistance of fruit against post-harvest pathogens (Droby et al. 1993; Droby et al. 1999; Ballester et al. 2010, 2011; Shao et al. 2010). Vilanova et al. (2013, 2014a) studied the healing process per se of apples and oranges at different maturity stages when were wounded and infected after different times by P. expansum and P. digitatum. The healing process in both oranges and apples has an important effect limiting infection ability of both host pathogens when the fruit were stored at 20 °C (Fig. 2.8).
In addition, this effect was most outstanding in the immature and mature fruit than in overmatured. Regarding immature and commercial oranges, it was observed a significantly decrease in incidence and severity of wounds inoculated with P. digitatum 7 and 10 d after wounding, while on apples, a similar reductions in incidence and severity of blue mould it was achieved only 3 d after wounding (Vilanova et al. 2013, 2014a). Somehow, the skin of the oranges may be the responsible of this higher susceptibility to rot compared to apples. Brown et al. (1978) showed a reduction in the percentage of infected oranges by P. digitatum 3 d after wounding, whereas Su et al. (2011) and Shao et al. (2010) demonstrated a decrease in incidence of decayed apples by B. cinerea and P. expansum, respectively 96 h after wounding. In pears, Spotts et al. (1998) found greater resistance of fruit to P. expansum infection 2 d after wounding. Likewise, the greatest resistance was in green peppers inoculated with Colletotrichum acutatum only 1 h after wounding (Kim et al. 2008).
The studies on wounding process on oranges and apples demonstrated that it depends on temperature (Vilanova et al. 2013, 2014a). Both temperature and relative humidity are two of the most important factors affecting the process of healing (Brown 1989). The temperature must be high enough to accelerate the development of metabolic reactions involved in the process of healing and, in turn, relative humidity should be sufficient to avoid drying or killing of tissues near damaged cells. Generally, temperatures above 10 °C and relative humidity higher than 85% are necessary to start the healing process in citrus fruit (Brown 1989). Moreover, the incidence and severity of wounded oranges inoculated with P. digitatum and stored at 4 °C for 30 d were lower when healing time increased. However, 45 d after inoculation, in commercial and overmatured oranges, there were no differences in the incidence between the different days of healing (Vilanova et al. 2013). This indicates that low temperatures make the healing process not effective enough to prevent infection with P. digitatum. In apples stored at 0 °C, the healing process was even less effective preventing infections of P. expansum than that observed in oranges. Lakshiminarayana et al. (1987) observed that apples inoculated with P. expansum or B. cinerea at 4 d after wounding and stored at 5 °C presented a substantial resistance to infection, although these authors considered that this healing time was scarce time to modify the cell wall to stop infection.
In contrast to these results, overmatured fruit showed few differences at different healing times, hence, once again it was clearly demonstrated the key role of maturity in susceptibility of oranges and apples to postharvest pathogen infection. Similar results were observed by Su et al. (2011) in overmatured apples inoculated with B. cinerea 96 h after wounding wounds: apples showed a higher incidence and severity of grey mould that immature or commercial apples. However, they did not observe differences in incidence of decay among harvest time when fruits were inoculated just after wounding.
Fruit maturity is a process that involves significant changes in the biochemical level such as alteration of the cell membrane (Cantu et al. 2008a). Consequently, maturity can modify the susceptibility of fruit by decreasing their defence responses and making them more susceptible to pathogen attack. As described above, overripe can trigger that the non-host P. expansum may even infect and develop decay in oranges. Some authors (Stange et al. 2002) defined that citrus skin is an inappropriate and even toxic environment for germination and growth of non-host moulds. In our study related to wound response, however, observed that P. expansum was able to infect and even develop rot at different times of healing in commercial and overmatured oranges at 20 °C, and at any maturity stage at 4 °C (Vilanova et al. 2013). Similar results were also observed in apples at 20 °C; P. digitatum was able to infect overmatured apples at 20 °C, even those inoculated 10 d after wounding, but not at 0 °C.
Cell Wall Reinforcement
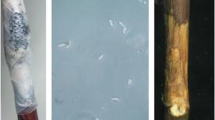
Wound healing process could be associated with a limited accumulation of phenolics and lignin-like compounds in cell wall thickening around wounds and, hence, be an effective protection against pathogen infections. In our studies, fruits inoculated with the non-host pathogen (oranges inoculated with P. expansum and apples inoculated with P. digitatum) showed a visible reaction around wounds when pathogen was not able to infect them (Fig. 2.6). To identify the substances involved in this reaction, a more in–depth study was conducted. Previous authors showed a direct relation of deposition of some substances and orange resistance against fungal infections (Brown and Barmore 1983; Baudoin and Eckert 1985). Therefore, different histochemical tests to detect lignin, suberin and callose and to characterize the accumulation of substances at the site of inoculation were used. Lignin was detected using Maüle and toluidine blue O reactions, suberin by Sudan IV test, and callose by Aniline blue test (Fig. 2.9). Both tests used to detect lignin showed a positive reaction at short response times (24 h and 48 h for apples and oranges, respectively) in both compatible and non-host interactions. These results suggest that lignin production is not exclusive for compatible pathogens. Remarkably, reaction of control fruits (fruits wounded) was not equal on oranges and on apples: wounded apples showed a positive reaction in lignin test at short time, while oranges always showed a negative reaction. Therefore, lignin production seems to be related with abiotic (wounds) and biotic (pathogen) stresses, and appears more important in apples than in oranges.
Images of histochemical tests: (a) Maüle reaction, (b) Blue Toluidine and (c) Sudan IV, in immature apples inoculates inoculated with P. digitatum at the concentration of 107 conidia mL−1 after 7 d at 20 °C and 85% RH. Pictures (a) and (b) show a positive reaction to lignin, and (c) test Sudan IV shows a negative reaction to suberin
Moreover, lignification observed in both oranges and apples inoculated with the non-host pathogen at long-term of response (7 d) was stronger than at 24 h or 48 h. In addition, lignification was apparently more significant in immature fruit than in commercial fruit since non-positive reaction was observed in overmatured fruit.
Neither of the tests used in Vilanova et al. (2012a, b) to detect suberin and callose showed a positive reaction either in oranges or in apples. According to Lai et al. (2003) using nuclear magnetic resonance spectroscopy (NMR spectroscopy) the substances present in curing grapefruit were suberin and not lignin. Regarding the quantification of lignin, Vilanova et al. (2013, 2014a) showed that immature control (mock inoculation at different times after wounding) in oranges and apples increased lignin content along storage period. Su et al. (2011) also observed an increase in lignin content in immature “Gala” apples compared to overmatured ones. Valentines et al. (2005) also found higher lignin content in apples which denoted a major resistant to P. expansum infection. Overall results suggest that lignin has a role in apple defence against P. expansum.
Immature oranges and apples inoculated with the non-host pathogen showed an important increase in lignin content 7 d and 72 h after inoculation, respectively. These results are in agreement with histochemical results obtained by Vilanova et al. (2012a, b), in which the most intense reaction was observed in immature fruits 7 d after inoculation. In the non-host interactions, lignification could begin few days after infection; however, its accumulation was more remarkable at long-term response. In addition, the highest accumulation of lignin was observed in immature fruit that usually are not infected.
Regarding the lignin content in compatible interaction, our studies showed unusually high absorbance values. These values obtained with the acetyl bromine method may be attributed to an increase in polysaccharides degradation and suggests that other factors than solubilisation of lignin were responsible for the increase in absorbance (Hatfield et al. 1999). When necrotrophic pathogens infect fruits, they secrete various cell wall degrading enzymes that increase the polysaccharides (Cantu et al. 2008b). Therefore, the acetyl bromide method applied to macerated tissues could produce erroneous absorbance values.
Molecular Studies in Fruit-Penicillium Interactions
The availability of the complete genome sequence of fruit has greatly aided the development of “omic” approaches such as genomics, transcriptomics and proteomics. Specifically, in Malus x domestica, the information gained from those studies could be particularly useful in the field of postharvest pathology. In our studies, a transcriptomic analysis of apple to understand fruit disease resistance in response to compatible (P. expansum) and non-host (P. digitatum) pathogens was conducted (Vilanova et al. 2014b). Moreover, the expression of several genes involved in the phenylpropanoid pathway were quantified to define their role in citrus fruit response against pathogen and non-host pathogen (Vilanova et al. 2013).
Transcriptomic Approach in Apples
The first transcriptomics study to elucidate the genes involved in the fruit development of “Fuji” apples was conducted by Lee et al. (2007) using a microarray containing 6,253 cDNAs. Few years later, Soria-Guerra et al. (2011) developed a microarray containing approximately 40,000 probes, including positive and negative controls, from 34 libraries of cDNA constructed from vegetative and reproductive apple tissues at different developmental stages, different genotypes and different types of biotic and abiotic stresses. This microarray was used and validated in different studies: in one of them the aim was to characterize the gene expression along fruit development of different varieties of apple (Soria-Guerra et al. 2011); in another one the goal was to study the response of apples after the application of an abscision-promoting treatment (Zhu et al. 2011); in the last ones the objectives were to investigate the response of apples to Erwinia amylovora (Bocsanczy et al. 2009; Sarowar et al. 2011). The microarray was also used in our study (Vilanova et al. 2014b) to perform a global analysis of genes involved in response of immature apples to a compatible pathogen (P. expansum) and a non-host pathogen (P. digitatum) at 20 °C (Fig. 2.10). The results of this study indicated a different pattern of apples in response to P. expansum and P. digitatum infection. Functional annotation based on gene ontology terms (GO) showed that biological processes of apples significantly modified in response to P. expansum were related to defence responses and oxidative stress, meanwhile to P. digitatum were related to phenylpropanoid pathway. Our microarray study identified 3,770 differentially expressed probes that could be used to further study of apple-Penicillium spp.
One of the first defence responses detected in a host-pathogen interaction is the oxidative burst that is characterized by a significant increase in ROS levels (Levine et al. 1994; Low and Merida 1996). Plant nicotinamide adenine dinucleotide phosphate oxidases, also called “Respiratory burst oxidases homologues (Rbo)” was described as an important source of ROS species in majority of plant–pathogen interactions (Torres et al. 2006). ROS metabolism is controlled by Rbos, and other enzymes including superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) (De Gara et al. 2003). SOD activity plays a role in the dismutation of superoxide radicals, while CAT and APX activity contribute to the elimination of hydrogen peroxide (H2O2) (Levine et al. 1994). Our microarray data showed an induction of the expression of the Rbo gene in apples inoculated with P. digitatum compared to apples inoculated with P. expansum. First, this fact pointed out that an increase in ROS content could be related to defence response of apples against P. digitatum. On the other hand, apples inoculated with P. expansum showed a greater increase in the expression of genes encoding ROS-detoxifying enzymes such as SOD, APX and POX (peroxidase) compared to apples inoculated with P. digitatum. Nevertheless, there was no increase in the expression of the Rbo gene when the apples were inoculated by P. expansum, indicating that the highest level of Rbo expression may be earlier than 24 h. Torres et al. (2003) showed that the largest production of H2O2 in apples inoculated with P. expansum awas detected approximately 6 h after inoculation. This ability of P. expansum to prevent the oxidative burst, suppressing the production of H2O2 in apples 24 h after inoculation, may be closely related to its pathogenicity. These results are in agreement to those obtained by Macarisin et al. (2007) in oranges where they observed that P. digitatum (compatible pathogen) suppressed the oxidative burst on host cell, meanwhile P. expansum (non-host pathogen) triggered an accumulation of H2O2. The relationship between ROS levels and detoxifying enzyme levels is considered to be a key factor in determining the H2O2 and O2− tissue-levels (Mittler et al. 1999). Ballester et al. (2006) suggested an imbalance among the activities of enzymes that generate and degrade H2O2 to promote the infection process, as has been reporter for the necrotrophic fungi B. cinerea and Sclerotinia sclerotiorum (Govrin and Levine 2000). Pathogenicity of both moulds seems to be dependent on ROS levels generated during the infection because they may benefit from cell necrosis caused by a hypersensitive response (HR) (Govrin and Levine 2000). Additionally, the bcsod1 gene encoding for a Cu-Zn SOD has been described in B. cinerea as a virulence factor (Rolke et al. 2004). Other study also shows that the virulence factor of Monilinia fructicola MfCUT1 is induced in the presence of ROS generated during infection process (Chiu et al. 2013).
The microarray data also indicated a change in the gene expression of heat shock proteins (HSPs). HSPs are one class of chaperones produced in response not only to high temperatures if not to stress, to exposure to heavy metals, pathogens attack and disturbance in intracellular calcium levels (Vierling 1991). In our study, we observed an induction in the HSP101 gene expression in apples inoculated with P. expansum and in the HSP70 gene expression in apples inoculated with P. expansum and P. digitatum (Vilanova et al. 2014b). These results indicated that HSP101 might be more specific for pathogen compatible although HSP70 may be related to the pathogen attack. Little is known about the role of HSPs in response to pathogens because most of the HSP studies have been focused on plant abiotic stresses. In fruits, for example, the expression HSP has been characterized in response to heat treatments used for control post-harvest diseases (Lauxmann et al. 2012; Pavez et al. 2013). Specifically, Yun et al. (2013) and Pavoncello et al. (2001) observed an induction of genes encoding HSP in heat-treated citrus fruit related with an increase of resistance to P. italicum and P. digitatum.
A differential expression in other genes related to defence-related proteins was observed, as genes encode for pathogenesis-related proteins (PR-proteins), and other related to synthesis of callose (Vilanova et al. 2014b). The PR proteins are divided into 17 families, including glucanases (GLU), chitinases (CHI), taumatins (TAU), peroxidase (POX) and defensins (DEFL) (van Loon et al. 2006; van Loon and van Strien 1999). The results showed that the inoculation of apples with P. expansum induced more PR proteins than P. digitatum, including two CHI, one endoglucanase (EGL), a TAU and a DEFL proteins. The outcomes indicated that induction of genes encoding PR proteins are related to fruit defence against pathogenic fungi. Concerning fruit, there is scarce information on induction of PR genes in response to pathogens. Most of the studies are based on the induction of PR proteins in response to PR proteins biological control and abiotic stress conditions. Ballester et al. (2010) correlated an increase in CHI and GLU enzymes activity with upregulation of genes encoding these enzymes when oranges are firstly cured and after that inoculated with P. digitatum. On the other hand, in oranges, Hershkovitz et al. (2012) detected an induction of the CHI and in apples, Quaglia et al. (2011) detected an induction of the CHI, GLU and TAU when the fruits were treated with biocontrol agents. Buron-Moles et al. (2014) in proteomic studies, revealed a correlation between an increase in the amount of TAU protein and wound response of apples.
An induction of the expression of β-glucosidase13 and β-glucosidase40 genes in response to inoculation by P. digitatum and P. expansum, respectively, was also observed (Vilanova et al. 2014b). Sánchez-Torres and González-Candelas (2003) found that the glycosidase gene was specifically expressed in apple tissue infected by P. expansum. The first evidence of the expression of the β-glucosidase gene in plants was reported by Simmons et al. (2001), that correlated upregulation of the rhm1 corn mutant to resistance to Bipolaris maydis.
Gene Expression of Genes Involved in the Phenylpropanoid Pathway
Because of the involvement of lignin in defence processes of oranges and apples against biotic and abiotic stresses, a gene expression study of several phenylpropanoid pathway-related genes was conducted for apples (Vilanova et al. 2014b) and oranges (Vilanova et al. 2013).
Apples
Microarray study revealed changes in the expression of genes involved in phenylpropanoid pathway, as the phenylalanine ammonium liase (PAL1 and PAL2), caffeic acid O-methyltransferase (COMT) and cinnamoyl-CoA reductase (CCR) genes, in apple inoculated with P. digitatum (non-host pathogen). Transcription levels of some genes (PAL1, PAL2, COMT2 and POX64) were evaluated in apples at immature and commercial harvest, inoculated with P. expansum, P. digitatum and water (control) at 8, 24 and 48 h post inoculation (hpi) using the quantitative PCR (qPCR) technique.
At 24 hpi, higher transcript abundance of PAL1, PAL2 and POX64 genes was observed for both apples inoculated by the non-host pathogen (P. digitatum) than other treatments. In contrast, at 48 hpi the highest increase in gene expression was found in PAL1, PAL2, COMT2 and POX64 in apples inoculated with P. expansum. Ballester et al. (2013) found that some genes related to the phenylpropanoid pathway showed their maximum expression at 48 hpi and decreased at 72 hpi. These variances in the expression along the time may be due to differences in inoculum concentrations. Furthermore, these results are in agreement with that observed in histochemical studies: in a short-term response both fungi showed a positive reaction to lignin, while in a long-term response only a positive reaction was obtained for the non-host pathogen, because the other samples were completely rotten (Vilanova et al. 2012a). Overall results support the hypothesis that apple attempt to defend against both pathogens, although host pathogen is finally able to overcome apple defences and develop infection.
Regarding apple maturity stages, microarray study indicated that PAL1, PAL2 and POX64 genes showed a greater abundance in commercial than in immature apples. Both PAL and POX belong to a family of enzymes that are involved in various biochemical functions in plants as growth, development and senescence. High PAL activity has also been associated with the maturity stage of apples (Wang et al. 2000) and strawberries (Cheng and Breen 1991; Villarreal et al. 2010) due to the accumulation of anthocyanins and other phenolic compounds. An increase in POX activity during ripening has been observed in apples (Torres et al. 2003) and plums (Singh et al. 2012). Therefore, the observed increase in the expression of PAL1, PAL2 and POX64 genes may be more related to fruit ripening rather than defence responses.
Oranges
In previous studies, Ballester et al. (2011, 2013) analysed the gene expression of several genes involved in the phenylpropanoid pathway and the modifications in phenolic compounds associated with citrus resistance against P. digitatum. In Vilanova et al. (2013), some of these genes were analysed in immature oranges infected by P. digitatum but also by P. expansum, because the response of citrus fruit to non-host pathogen remained unexplored.
In oranges, the expression of PAL1, COMT1, POX1, cinnamyl alcohol dehydrogenase (CAD) and sinapyl alcohol dehydrogenase (SAD) genes was analyzed after inoculation with P. digitatum, P. expansum and water (control), 24 and 48 hpi using the semi-quantitative PCR technique (Vilanova et al. 2013). Gene expression analysis showed different behaviour depending on the time after inoculation. At 24 hpi, oranges inoculated with the compatible pathogen (P. digitatum) showed a higher expression of the PAL1 and COMT1 genes compared to oranges inoculated with P. expansum and control, although the POX1 gene showed greater expression in oranges inoculated with both moulds than control. At 48 h, however, oranges inoculated with P. digitatum displayed an important decrease in expression of PAL1, COMT1, POX1 and SAD genes, and oranges inoculated with P. expansum and control the gene expression remained constant. This decrease in expression seems to indicate that the compatible pathogen is able to suppress the gene expression related to the phenylpropanoid pathway and therefore start the infection.
Concluding Remarks
Our studies suggest that maturity stage is an important factor to be considered on pathological studies. Therefore, it would be interesting to continue exploring the relationship between maturity factors and fruit susceptibility in response to pathogens. Besides, the increase of resistance or susceptibility of apples and oranges to host and non-host pathogens depend on it and may vary based on the climacteric or non-climacteric respiration pattern of fruit.
Depending on the combination of certain conditions as maturity stage, inoculum concentration and storage temperature, the non-host pathogen become compatible pathogen in these fruit –pathogen interactions. Furthermore, wounded fruit stored at cold temperatures are, in general, more susceptible to pathogen attack. Lignification is a key factor in fruit defence to both biotic and abiotic stresses together with other multiple reactions. This process was apparently more important in immature than in commercial and over-mature fruit.
Finally, the global study conducted on apples give us an overall picture of metabolic pathways to may activate in response to host and non-host pathogens to continue to work in this research line with the aim of unravelling the fruit defence mechanisms.
Overall knowledge obtained over the time-course of our studies can help us to establish the basis towards the improvement of postharvest control strategies in apples and oranges.
References
Baert K, Devlieghere F, Flyps H, Oosterlinck M, Ahmed MM, Rajković A, Verlinden B, Nicolaï B, Debevere J, De Meulanaer B (2007a) Influence of storage conditions of apples on growth and patulin production by Penicillium expansum. Int J Food Microbiol 119:170–181
Baert K, Valero A, De Meulenaer B, Samapundo S, Ahmed MM, Bo L, Debevere J, Devlieghere F (2007b) Modeling the effect of temperature on the growth rate and lag phase of Penicillium expansum in apples. Int J Food Microbiol 118:139–150
Ballester AR, Lafuente MT, González-Candelas L (2006) Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit-Penicillium digitatum interaction. Postharvest Biol Tec 39:115–124
Ballester AR, Izquierdo A, Lafuente MT, González-Candelas L (2010) Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest Biol Tec 56:31–38
Ballester AR, Lafuente MT, Forment J, Gadea J, De Vos RC, Bovy AG, González-Candelas L (2011) Transcriptomic profiling of citrus fruit peel tissues reveals fundamental effects of phenylpropanoids and ethylene on induced resistance. Mol Plant Pathol 12:879–897
Ballester AR, Lafuente MT, González-Candelas L (2013) Citrus phenylpropanoids and defence against pathogens. Part II: gene expression and metabolite accumulation in the response of fruits to Penicillium digitatum infection. Food Chem 136:285–291
Baudoin A, Eckert JW (1985) Development of resistance against Geotrichum candidum in lemon peel injuries. Phytopathology 75:174–179
Bocsanczy A, Phillips JG, Dardick CD, Korban SS, Bassett CL, Wisniewski ME, Norelli JL (2009) Analysis of apple (Malus) responses to bacterial pathogens using an oligo microarray. Phytopathology 99:S14–S14
Brown GE (1989) Host defenses at the wound site on harvested crops. Phytopathology 79:1381–1384
Brown GE, Barmore CR (1983) Resistance of healed citrus exocarp to penetration by Penicillium digitatum. Phytopathology 73:691–694
Brown GE, Ismai MA, Barmore CR (1978) Lignification of injuries to citrus fruit and susceptibility to green mold. Proc Florida State Hortic Soc 91:124–126
Buron-Moles G, Lopez-Perez M, González-Candelas L, Viñas I, Teixidó N, Usall J, Torres R (2012) Use of GFP-tagged strains of Penicillium digitatum and Penicillium expansum to study host-pathogen interactions in oranges and apples. Int J Food Microbiol 160:162–170
Buron-Moles G, Torres R, Amoako-Andoh F, Viñas I, Teixidó N, Usall J, Keulemans W, Davey MW (2014) Analysis of changes in protein abundance after wounding in ‘golden delicious’ apples. Postharvest Biol Tec 87:51–60
Cantu D, Vicente AR, Greve LC, Dewey FM, Bennett AB, Labavitch JM, Powell AL (2008a) The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc Natl Acad Sci USA 105:859–864
Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL (2008b) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci 13:610–617
Cheng GW, Breen PJ (1991) Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci 116:865–869
Chiu CM, You BJ, Chou CM, Yu PL, Yu FY, Pan SM, Bostock RM, Chung KR, Lee MH (2013) Redox status-mediated regulation of gene expression and virulence in the brown rot pathogen Monilinia fructicola. Plant Pathol 62:809–819
De Gara L, De Pinto MC, Tommasi F (2003) The antioxidant systems vis-a-vis reactive oxygen species during plant-pathogen interaction. Plant Physiol Biochem 41:863–870
Droby S, Chalutz E, Horev B, Cohen L, Gaba V, Wilson CL, Wisniewski M (1993) Factors affecting UV-induced resistance in grapefruit against the green mould decay caused by Penicillium digitatum. Plant Pathol 42:418–424
Droby S, Porat R, Cohen L, Weiss B, Shapiro B, Philosoph-Hadas S, Meir S (1999) Suppressing green mold decay in grapefruit with postharvest jasmonate application. J Am Soc Hortic Sci 124:184–188
European Apple and Pear Crop Forecast. Prognosfruit (August 2018). www.Prognosfruit.eu
Food and Agriculture Organization of the United Nations (FAO) (2017) Citrus fruit, fresh and processed statistical bulletin 2016 – http://www.fao.org/3/a-i8092e.pdf. Accessed 15 Apr 2020
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:51–757
Hatfield RD, Grabber J, Ralph J, Brei K (1999) Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem 47:628–632
Hershkovitz V, Ben-Dayan C, Raphael G, Pasmanik-Chor M, Liu J, Belausov E, Aly R, Wisniewski M, Droby S (2012) Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agent Metschnikowia fructicola. Mol Plant Pathol 13:338–349
Ismail MA, Brown GE (1975) Phenolic content during healing of ‘Valencia’ orange peel under high humidity. J Am Soc Hortic Sci 100:249–251
Kavanagh J, Wood R (1967) Role of wounds in infection of oranges by Penicillium digitatum Sacc. Ann App Biol 60:375–383
Kim SG, Kim YH, Kim HT (2008) Effect of delayed inoculation after wounding on the development of anthracnose disease caused by Colletotrichum acutatum on chilli pepper fruit. Plant Pathol J 24:392–399
Lai S, Lai A, Stange Jr, McCollum TG, Schirra M (2003) Characterization of the wound-induced material in Citrus paradisi fruit peel by carbon-13 CP-MAS solid state NMR spectroscopy. Phytochemistry 63:177–183
Lakshiminarayana S, Sommer NF, Polito V, Fortlage RJ (1987) Development of resistance to infection by Botrytis cinerea and Penicillium expansum in wounds of mature apple fruits. Phytopathology 77:1674–1678
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Lauxmann MA, Brun B, Borsani J, Bustamante CA, Budde CO, Lara MV, Drincovich MF (2012) Transcriptomic profiling during the post-harvest of heat-treated dixiland prunus persica fruits: common and distinct response to heat and cold. PLoS One 7:e51052
Lee YP, Yu GH, Seo YS, Han SE, Choi Y-O, Kim D, Mok I-G, Kim WT, Sung S-K (2007) Microarray analysis of apple gene expression engaged in early fruit development. Plant Cell Rep 26:917–926
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Louw JP, Korsten L (2014) Pathogenic Penicillium spp. on apple and pear. Plant Dis 98:590–598
Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96:533–542
Lu HG, Higgins VJ (1999) The effect of hydrogen peroxide on the viability of tomato cells and of the fungal pathogen Cladosporium fulvum. Physiol Mol Plant Pathol 54:131–143
Macarisin D, Cohen L, Eick A, Rafael G, Belausov E, Wisniewski M, Droby S (2007) Penicillium digitatum suppresses production of hydrogen peroxide in host tissue infection of citrus fruit. Phytopathology 97:1491–1500
Mayer AM, Staples RC, Gil-ad NL (2001) Mechanisms of survival of necrotrophic fungal plant pathogens in hosts expressing the hypersensitive response. Phytochemistry 58:33–41
Mittler R, Lam E, Shulaev V, Cohen M (1999) Signals controlling the expression of cytosolic ascorbate peroxidase during pathogen-induced programmed cell death in tobacco. Plant Mol Biol 39:1025–1035
Morales H, Sanchis V, Coromines J, Ramos AJ, Marín S (2008) Inoculum size and intraspecific interactions affects Penicillium expansum growth and patulin accumulation in apples. Food Microbiol 25:378–385
Mulas M, Lafuente MT, Zacarías L (1996) Lignin and gum deposition in wounded ‘Oroval’ clementines as affected by chilling and peel water content. Postharvest Biol Tec 7:243–251
Mysore KS, Ryu CM (2004) Nonhost resistance: how much do we know? Trends Plant Sci 9:97–104
Neri F, Donati I, Veronesi F, Mazzoni D, Mari M (2010) Evaluation of Penicillium expansum isolates for aggressiveness, growth and patulin accumulation in usual and less common fruit hosts. Int J Food Microbiol 143:109–117
Pavez L, Hödar C, Olivares F, González M, Cambiazo V (2013) Effects of postharvest treatments on gene expression in Prunus persica fruit: normal and altered ripening. Postharvest Biol Tec 75:125–134
Pavoncello D, Lurie S, Droby S, Porat R (2001) A hot water treatment induces resistance to Penicillium digitatum and promotes the accumulation of heat shock and pathogenesis-related proteins in grapefruit flavedo. Physiol Plant 111:17–22
Plaza P, Usall J, Teixidó N, Viñas I (2003) Effect of water activity and temperature on germination and growth of Penicillium digitatum, P. italicum and Geotrichum candidum. J Appl Microbiol 94:549–554
Quaglia M, Ederli L, Pasqualini S, Zazzerini A (2011) Biological control agents and chemical inducers of resistance for postharvest control of Penicillium expansum Link. on apple fruit. Postharvest Biol Tec 59:307–315
Rolke Y, Liu SJ, Quidde T, Williamson B, Schouten A, Weltring KM, Siewers V, Tenberge KB, Tudzynski B, Tudzynski P (2004) Functional analysis of H2O2-generating systems in Botrytis cinerea: the major Cu-Zn-superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol Plant Pathol 5:17–27
Romero D, Rivera ME, Cazorla FM, Codina JC, Fernández-Ortuño D, Torés JA, Pérez-García A, de Vicente A (2008) Comparative histochemical analyses of oxidative burst and cell wall reinforcement in compatible and incompatible melon-powdery mildew (Podosphaera fusca) interactions. J Plant Physiol 165:1895–1905
Sánchez-Torres P, González-Candelas L (2003) Isolation and characterization of genes differentially expressed during the interaction between apple fruit and Penicillium expansum. Mol Plant Pathol 4:447–457
Sarowar S, Zhao Y, Soria-Guerra RE, Ali S, Zheng D, Wang D, Korban SS (2011) Expression profiles of differentially regulated genes during the early stages of apple flower infection with Erwinia amylovora. J Exp Bot 62:4851–4861
Shao X, Tu K, Tu S, Zhao Y (2010) Effects of heat treatment on wound healing in Gala and Red Fuji apple fruits. J Agric Food Chem 58:4303–4309
Silva MC, Nicole M, Guerra-Guimarães L, Rodrigues CJ Jr (2002) Hypersensitive cell death and post-haustorial defence responses arrest the orange rust (Hemileia vastatrix) growth in resistant coffee leaves. Physiol Mol Plant Pathol 60:169–183
Simmons CR, Grant S, Altier DJ, Dowd PF, Crasta O, Folkerts O, Yalpani N (2001) Maize rhm1 resistance to Bipolaris maydis is associated with few differences in pathogenesis-related proteins and global mRNA profiles. Mol Plant-Microbe Interact 14:947–954
Singh SP, Singh Z, Swinny EE (2012) Climacteric level during fruit ripening influences lipid peroxidation and enzymatic and non-enzymatic antioxidative systems in Japanese plums (Prunus salicina Lindell). Postharvest Biol Tec 65:22–32
Soria-Guerra RE, Rosales-Mendoza S, Gasic K, Wisniewski ME, Band M, Korban SS (2011) Gene expression is highly regulated in early developing fruit of apple. Plant Mol Biol Rep 29:885–897
Spotts RA, Sanderson PG, Lennox CL, Sugar D, Cervantes LA (1998) Wounding, wound healing and staining of mature pear fruit. Postharvest Biol Tec 13:27–36
Stange RR, Midland SL, Sims JJ, McCollum TG (2002) Differential effects of citrus peel extracts on growth of Penicillium digitatum, P. italicum, and P. expansum. Physiol Mol Plant Pathol 61:303–311
Su J, Tu K, Cheng L, Tu S, Wang M, Xu H, Zhan G (2011) Wound-induced H2O2 and resistance to Botrytis cinerea decline with the ripening of apple fruit. Postharvest Biol Tec 62:64–70
Tian S, Torres R, Ballester AR, Li B, Vilanova L, González-Candelas L (2016) Molecular aspects in pathogen-fruit interactions: virulence and resistance. Postharvest Biol Tec 122:11–21
Torres R, Valentines MC, Usall J, Viñas I, Larrigaudiere C (2003) Possible involvement of hydrogen peroxide in the development of resistance mechanisms in ‘golden delicious’ apple fruit. Postharvest Biol Tec 27:235–242
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Valentines MC, Vilaplana R, Torres R, Usall J, Larrigaudiere C (2005) Specific roles of enzymatic browning and lignification in apple disease resistance. Postharvest Biol Tec 36:227–234
van Loon LC, van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Vierling E (1991) The roles of heat-shock proteins in plants. Ann Rev Plant Physioly and Plant Mol Biol 42:579–620
Vilanova l, Teixidó N, Torres R, Usall J, Viñas I (2012a) The infection capacity of P. expansum and P. digitatum on apples and histochemical analysis of host response. Int J Food Microbiol 157:360–367
Vilanova l, Viñas I, Torres R, Usall J, Jauset AM, Teixidó N (2012b) Infection capacities in the orange-pathogen relationship: compatible (Penicillium digitatum) and incompatible (Penicillium expansum) interactions. Food Microbiol 29:56–66
Vilanova L, Torres R, Viñas I, González-Candelas L, Usall J, Fiori S, Solsona C, Teixidó N (2013) Wound response in oranges as a resistance mechanism against Penicillium digitatum (pathogen) and P. expansum (non-host pathogen). Postharvest Biol Tech 78:113–122
Vilanova L, Viñas I, Torres R, Usall J, Buron-Moles G, Teixidó N (2014a) Increasing maturity reduces wound response and lignification process against Penicillium expansum (pathogen) and Penicillium digitatum (non-host pathogen) infection in apples. Postharvest Biol Tech 88:54–60
Vilanova L, Wisniewski M, Norelli J, Viñas I, Torres R, Usall J, Phillips J, Droby S, Teixidó N (2014b) Transcriptomic profiling of apple in response to inoculation with a pathogen (Penicillium expansum) and a non-pathogen (Penicillium digitatum). Plant Mol Biol Rep 32:566–583
Villarreal NM, Bustamante CA, Civello PM, Martínez GA (2010) Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J Sci Food Agri 90:683–689
Wang HQ, Arakawa O, Motomura Y (2000) Influence of maturity and bagging on the relationship between anthocyanin accumulation and phenylalanine ammonia-lyase (PAL) activity in ‘Jonathan’ apples. Postharvest Biol Tec 19:123–128
Yun Z, Gao HJ, Liu P, Liu S, Luo T, Jin S, Xu Q, Xu J, Cheng Y, Deng X (2013) Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol 16:13–44
Zhu H, Dardick CD, Beers EP, Callanhan AM, Xia R, Yuan R (2011) Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol 11:138–158
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Torres, R., Vilanova, L., Usall, J., Teixidó, N. (2021). Insights into Fruit Defense Mechanisms Against the Main Postharvest Pathogens of Apples and Oranges. In: Spadaro, D., Droby, S., Gullino, M.L. (eds) Postharvest Pathology. Plant Pathology in the 21st Century, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-56530-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-56530-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56529-9
Online ISBN: 978-3-030-56530-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)