Abstract
The thiazide-sensitive NaCl cotransporter (NCC, SLC12A3) is a member of the solute carrier 12 (SLC12) family of electroneutral cotransporters. NCC is expressed in the distal convoluted tubule (DCT) of the kidney, where it mediates the transport of sodium with chloride in 1:1 stoichiometry. NCC is responsible for reabsorbing approximately 5–10% of sodium from the glomerular filtrate. NCC plays a key role in determining the blood pressure set point and potassium balance. NCC is the major molecular target of thiazide-type diuretics, drugs commonly used as first-line agents in the treatment of essential hypertension and edema. Since its cloning decades ago, much has been learned about the physiological transport properties of NCC, its regulation, and its connections to human disease. In this chapter, I provide an overview of the biology of this important cotransporter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
The thiazide-sensitive Na-Cl cotransporter (NCC, SLC12A3) is a member of the SLC12 family of cation–chloride cotransporters. It is the primary mediator of sodium transport in the distal convoluted tubule (DCT) of the kidney, a short but important nephron segment positioned between the Loop of Henle and collecting duct that regulates blood pressure and electrolyte balance. This chapter provides a summary of our current knowledge of NCC, extending from early studies of its transport characteristics and cloning, to recent work on its physiologic regulation in vivo, and its role in human disease.
3.2 Early Studies and Cloning of NCC
The first studies of thiazide-sensitive salt transport in the kidney were conducted in the 1950s. These studies were motivated by the search for new diuretic drugs that lacked the toxic effects of organic mercurial diuretics such as Mersalyl (Freeman et al. 1962). Proximal tubule carbonic anhydrase inhibitors, such as the sulfonamide acetazolamide, were among the first nontoxic diuretics identified, although it was immediately recognized that their ability to stimulate Na and water excretion waned over repeated uses. In addition, the diuretic effects of acetazolamide are almost always accompanied by clinically significant metabolic acidosis (Novello and Sprague 1957). In an effort to develop new sulfonamides with enhanced effects on sodium chloride excretion, investigators at Merck synthesized the first thiazide diuretic, chlorothiazide (Diuril), in 1957 (Novello and Sprague 1957). Although chlorothiazide inhibited carbonic anhydrase to some degree, it also stimulated chloride excretion in a manner similar to mercurial compounds, which act more distally. In the 1960s, experiments by Orloff and others suggested that chlorothiazide blocks NaCl reabsorption in the distal nephron (Earley and Orloff 1964). In the mid-1970s, Kunau et al. narrowed the site of action to the distal convolution (Kunau 1974), studies that were later verified by Constanzo et al. (Costanzo 1988; Costanzo and Windhager 1978). Ellison et al. established that thiazide diuretics block a coupled sodium chloride cotransport pathway in the DCT (Ellison et al. 1987). These important papers established that the natriuretic effects of thiazides were predominantly due to inhibition of a distal nephron salt cotransporter, not proximal tubule carbonic anhydrase.
Initial efforts to identify the molecular identity of the “thiazide receptor” from kidneys using an expression cloning approach were unsuccessful, possibly because the DCT is the shortest tubular segment of the nephron and NCC, therefore, comprises only a tiny fraction of total mRNA from a kidney extract. Thus, a radically different strategy had to be employed. In the 1980s, studies by Stokes (1984) and Renfro (1977) identified a salt cotransporter in the bladder of the winter flounder (Pseudopleuronectes americanus) whose activity was sensitive to thiazides. The transport properties were thought to be identical to the NCC in human DCT. Thus, in 1993, Gamba and Hebert astutely used mRNA from flounder bladder as starting material to derive the first NCC cDNA (Baggio et al. 1993). The flounder sequence was then used as a template to isolate mammalian NCC cDNAs via homology-based strategies (Gamba et al. 1994).
3.3 Primary Structure and Molecular Architecture
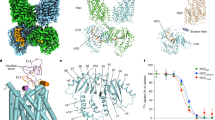
Analysis of the primary structure of NCC suggested a complex topology consisting of 12 transmembrane domains (TMs) flanked by large intracellular amino- and carboxy-termini (Baggio et al. 1993). It is believed that the N-terminal intracellular domain is unstructured, while the C-terminus adopts a complex and compact fold. A large extracellular loop containing N-glycosylation sites was predicted to reside between TMs 7 and 8 (Fig. 3.1). In mammals, this extracellular loop contains two N-glycosylation sites, whereas, in teleosts, three sites are present (Gamba 2005). Only one of these asparagines, Asn-403 in flounder, is conserved among all NCCs. This general topology is consistent with that of the two other electroneutral sodium-coupled cotransporters within the SLC12 family, the bumetanide-sensitive Na+-K+-2Cl− cotransporters NKCC1 (SLC12A2) and NKCC2 (SLC12A1).
Two-dimensional topology diagram of the thiazide-sensitive NaCl cotransporter (NCC), based on the 5 + 5 transmembrane inverted repeat common to members of the APC superfamily, and the solved Cryo-EM structure of NKCC1. The inverted “pseudosymmetric” folds are indicated with triangles. NCC has two large intracellular termini and a sizeable extracellular loop that contains N-glycosylation sites. The N-terminus is a regulatory domain that undergoes phosphorylation, while the structured C-terminus is important for homodimerization
Initial clues into the transmembrane topology of NCC were extrapolated from the solved crystal structures of other sodium-coupled secondary transporters, such as the bacterial sodium-coupled leucine transporter LeuT (Krishnamurthy and Gouaux 2012). This topology was recently further supported when the structure of Danio rerio NKCC1 (DrNKCC1) was solved in a partially inward open configuration by cryo-electron microscopy (Chew et al. 2019). Based on the fold of this cotransporter and other members of the amino acid-polyamine-cation (APC) transporter superfamily (of which the SLC12 cotransporters are a part of), the first ten TMs organize into two internally folded domains (Krishnamurthy et al. 2009). The two domains each consist of five helices that are oriented perpendicular to the membrane in opposite directions to each other in a “pseudosymmetric” manner (Fig. 3.1). The DrNKCC1 fold suggests that the sites for sodium and chloride binding and translocation in NCC are located near TMs 1 and 6. Discussed in further detail below, phosphorylation of the intracellular amino terminus increases NCC activity. Since this intracellular domain is immediately contiguous with TM1, it is conceivable that phosphorylation of the NCC N-terminus may provoke structural alterations in the positioning of transmembrane domains within the solvent-accessible vestibule, thereby enhancing the efficiency of sodium chloride cotransport.
Previous studies by de Jong et al. indicate that NCC assembles into homodimers prior to plasma membrane delivery (de Jong et al. 2003). Like many proteins (Zerangue et al. 1999), macromolecular NCC assembly takes place in the ER, since high mannose immature ER-localized glycoforms of NCC readily form dimers (unpublished observations). Protein interaction studies suggest that the large and highly folded C-terminal domain (CTD) is essential for homodimer formation. These studies have been confirmed by the recently reported crystal structure of DrNKCC1 (Chew et al. 2019) as well as the intracellular C-terminus of MaCCC, a bacterial cation–chloride cotransporter whose C-terminus shares some homology to NCC (Warmuth et al. 2009). The resolved structure of the CTD of one DrNKCC1 subunit interacts with the transmembrane domain (TMD) of the opposing subunit in a domain-swap configuration (Chew et al. 2019). The connections between the TMDs and CTDs are mediated by a flexible helix of ~10 amino acids in length (termed the “scissor helix”). This structure wraps around a scissor helix from its associated dimeric partner, resulting in the domain-swap orientation. Conformational variations noted by cryo-electron microscopy revealed that the structure is therefore likely highly dynamic, undergoing swiveling and rocking motions that alter the orientations between the two swapped domains.
3.4 NCC Transport Characteristics
The initial functional characterization of flounder NCC confirmed that ion influx is saturable, consistent with a carrier-mediated mechanism. According to this study and others, the Km values for Na+ and Cl− are approximately 30 and 15 in flounder NCC, respectively (Gamba et al. 1993; Vazquez et al. 2002). Mammalian (rat) NCC, in contrast, exhibits a much higher affinity for ions with Km values for Na+ and Cl− of 6 and 0.3, respectively (Vazquez et al. 2002). The mammalian NCC appears to be much more thiazide-sensitive, with an IC50 for metolazone of 0.3 μM, compared to the flounder NCC IC50 of 4.0 μM (Moreno et al. 2006; Vazquez et al. 2002).
Over a decade ago, studies carried out with NCC-NKCC2 chimeras revealed that, as expected, the hydrophobic 12 transmembrane domain region of NCC confers ion translocation and diuretic sensitivity (Tovar-Palacio et al. 2004). This is consistent with more recent structural information obtained from the solved structures of DrNKCC1 and other APC superfamily members (Chew et al. 2019; Krishnamurthy and Gouaux 2012; Krishnamurthy et al. 2009; Singh et al. 2008). Moreno et al. (2006) took advantage of the functional differences between rat and flounder NCC to define sites of ion translocation and diuretic binding. Using several rat-flounder chimeric constructs, they concluded that transmembrane domains 1–7 contained residues that alter chloride affinity. This was recently supported by the solved structure of NKCC1, in which TMs 1, 3, 6, and 8 form the solvent-accessible vestibule for ion translocation (Chew et al. 2019). Within this vestibule, the binding site for sodium is identical to the classical “Na2” site contained in other APC transporters. Within human NCC, two adjacent serine residues at positions 467 and 468 appear to be critical. Molecular dynamics simulations of NKCC1 also indicate that Cl− diffuses between TM1s and 6, indicating that the vestibule is important for the translocation of both ions. These studies also suggested that transport of Na+ and Cl− are likely cooperative as residency of Na+ in the Na2 binding site enhances Cl− residence time, and vice versa.
Moreno et al. also reported that swapping transmembrane domains 8–12 between species altered the thiazide binding affinity (Moreno et al. 2006). This indicates that the binding sites for chloride and thiazide diuretics are different. Further analysis of these transmembrane domains revealed that substituting residues within TM11 was sufficient to confer altered sensitivity to metolazone (Castaneda-Bueno et al. 2010). Mutating serine 575 in the rat cotransporter to the corresponding residue in flounder (a cysteine) was sufficient to mediate this effect. Because only cysteine substitution at this site was capable of altering thiazide sensitivity, it is possible that the flounder cotransporter contains an extra intramembranous disulfide bond that alters the NCC fold. Although it is unclear if rat serine 575 participates in a local binding site for metolazone, these findings implicate TM11 as an important structural component of NCC that defines its thiazide-sensitivity.
Mammalian NCC undergoes N-linked glycosylation on two asparagines harbored within the third extracellular loop, located between TM5 and TM6 (Hoover et al. 2003). N-glycosylation at these sites may play an important role in thiazide and chloride binding, since mutation of the glycosylation site asparagines to glutamine decreased the rat NCC Km for chloride binding and IC50 for thiazide inhibition substantially, without affecting its affinity for sodium (Hoover et al. 2003). As mentioned above, the binding sites of chloride and thiazide diuretics are different (Moreno et al. 2006). Therefore, these findings suggest that N-linked glycosylation alters NCC conformation at multiple sites. One interpretation of the data is that glycosylation may introduce broad changes into the NCC tertiary structure, allowing it to achieve a mature conformation that can mediate thiazide-sensitive salt transport. This would be consistent with the well-appreciated role of N-glycosylation in the global folding of other polytopic membrane proteins, such as the cystic fibrosis transmembrane conductance regulator (CFTR) (Glozman et al. 2009).
3.5 NCC Biogenesis
3.5.1 NCC Processing in the ER
Given the complex topology of NCC, it must undergo considerable processing within the biosynthetic pathway before it can achieve a proper fold. Current data suggest that, similar to other polytopic membrane proteins (Vembar and Brodsky 2008), this process is slow and/or inefficient. This is sensed by protein quality control mechanisms, which target suboptimally folded NCC conformers for ER-associated degradation (ERAD). Once the NCC polypeptide becomes integrated into the plasma membrane, molecular chaperones facilitate its proper folding. Heterologous expression studies in yeast and in mammalian cells have identified a host of cytoplasmic chaperone proteins are responsible for this process (Fig. 3.2). These molecular chaperones are exclusively located within the cytoplasm. ER luminal chaperones, in contrast, appear to be less important (Needham et al. 2011). The cytoplasmic Hsp70/Hsp40 chaperone complex participates in one of the earliest quality control steps during NCC processing in the ER (Needham et al. 2011). Within this complex, Hsp70 is critically important, while Hsp40 is bound to Hsp70 and NCC but is dispensable for NCC quality control. If Hsp70 is able to fold NCC into a stable intermediate conformation, it will then be transferred from Hsp70 to Hsp90 via the Hsp70/Hsp90 organizer cochaperone (HOP/Stip1) (Donnelly et al. 2013) (Fig. 3.2). Hsp90 will then engage NCC and attempt to fold it further. If the cotransporter is interacting with either of these chaperones and becomes terminally misfolded, it will be targeted for ubiquitylation and ERAD (Donnelly et al. 2013). Thus far, three E3 ubiquitin ligases have been identified that can mediate this process. Based on studies of NCC ERAD in yeast, suboptimal folding and aggregation of transmembrane domains are likely a common mishap, resulting in targeted degradation by HRD1 (Needham et al. 2011). Misfolded cytoplasmic regions of NCC, on the other hand, can be recognized by the E3 ligase Doa10/TEB4 (Needham et al. 2011), or by the C-terminus of Hsp70 interacting protein (CHIP/Stub1) (Donnelly et al. 2013) (Fig. 3.2). Following ubiquitylation, NCC is then extracted from the ER membrane and extruded into the cytosol by the AAA+ ATPase Cdc48 and degraded by the 26S proteasome (Needham et al. 2011). In each case, interaction with Hsp70 or Hsp90 appears to be a prerequisite prior to ubiquitylation and ERAD (Needham et al. 2011; Donnelly et al. 2013).
Early steps in NCC biogenesis. NCC is recognized at an early stage of its biogenesis by an Hsp70/Hsp40 complex. Hsp70 recognizes misfolded regions of the cotransporter and attempts to fold it into a stable conformation. NCC will then be transferred from Hsp70 to Hsp90 via HOP. Cotransporters that achieve a stable Hsp90-bound conformational intermediate will then advance further along the biosynthetic pathway and out of the ER. At various stages of this pathway, the E3 ubiquitin ligases HRD, TEB4, and CHIP can ubiquitylate misfolded NCC conformers, targeting them for ER-associated proteasomal degradation (ERAD)
As NCC is being folded in the ER, it undergoes glycosylation on two asparagines harbored within its third extracellular loop (Hoover et al. 2003). Like other transmembrane glycoproteins such as CFTR (Ward and Kopito 1994), the N-linked sugars attached to NCC are processed in the ER into a high-mannosylated form. Upon transit to the Golgi, the mannoses are cleaved into mature shorter glycan chains. Proper glycosylation is essential for NCC to exit the biosynthetic pathway and reach the plasma membrane (Hoover et al. 2003).
3.5.2 Post-ER Processing and Endosomal Storage of NCC
Little is known about how later steps of NCC maturation are carried out from within the Golgi. Presumably, most NCC will be processed in the Golgi cisternae when it is already in its homodimeric form, as oligomerization events commonly take place in the ER (Geva and Schuldiner 2014). As it matures, the dimeric NCC passes through the Golgi cisternae, is packaged into vesicles at the trans-Golgi network (TGN), and is sent to the apical plasma membrane via an endosomal system. In some cases, however, a subpopulation of cotransporters can be sorted directly from the TGN to the lysosome for degradation. Protein interaction studies suggest that this process may require the adaptor protein complex AP-3 (Subramanya et al. 2009), or the lysosomal sorting receptor sortilin (Zhou et al. 2009). In heterologous expression systems, this alternative TGN-to-lysosome transport pathway appears to be greatly enhanced in cells overexpressing With-No-Lysine kinase 4 (WNK4, discussed in detail below).
Ultrastructural studies performed in rodent kidneys commonly demonstrate that NCC exists in apical and subapical populations in DCT cells (Lee et al. 2009; Sandberg et al. 2006). The subapical population resides within endosomes, which can be rapidly mobilized to the surface by hormones such as vasopressin and angiotensin II (Pedersen et al. 2010; Mutig et al. 2010; Sandberg et al. 2007). Conversely, physiological states such as acute hypertension can cause the NCC to quickly retract from the plasma membrane into endosomes and lysosomes (Lee et al. 2009). This process is linked to site-specific ubiquitylation of the cotransporter at the plasma membrane at several lysine residues localized within the cytoplasmic NCC CTD (Rosenbaek et al. 2017).
3.6 NCC Activity at the Plasma Membrane
3.6.1 NCC Phosphorylation
In order for NCC to be active, it must first be “switched on” by phosphorylation (Pacheco-Alvarez et al. 2006). These phosphoactivation events specifically occur on serines and threonines present in the intracellular NCC amino terminus. Figure 3.3a illustrates sites of phosphorylation that have been identified, either through phosphoproteomic studies or through analysis of homologous phosphorylation sites experimentally identified on other sodium-coupled cotransporters that are members of the SLC12 family, such as NKCC1 (Darman and Forbush 2002). To date, seven phosphoacceptor residues have been identified. According to the human NCC numeration, these amino acids correspond to threonines 46, 50, 55, and 60, and serines 73, 91, and 126 (Richardson et al. 2008; Rosenbaek et al. 2012). Phosphorylation at each of these sites enhances NCC activity, although some evidence suggests that threonine 60 may have a unique and dominant role in dictating NCC-mediated sodium transport (Pacheco-Alvarez et al. 2006) (Fig. 3.3a). Since all of these phosphosites are harbored within the NCC amino terminus, they are located immediately proximal to the first transmembrane domain. Thus, phosphorylation of the NCC N-terminus may somehow alter the positioning or structure of residues related to TM 1. Since structural data from other members of the APC superfamily indicate that TMs 1 and 6 help form the Na-Cl translocation pathway (Chew et al. 2019; Krishnamurthy et al. 2009), phosphorylation may enhance the efficiency of Na-Cl transport by altering the structure of the transporter vestibule.
Control of NCC activity by phosphorylation. (a) A regulatory locus of phosphorylation consistent of a cluster of serines and threonines is contained within the cytoplasmic amino terminus of NCC. The amino terminus is contiguous with the TM1, which likely forms a sodium binding site with TM6. A binding motif for the serine/threonine kinases SPAK and OSR1 is located at the extreme end of the N-terminus. SPAK/OSR1 phosphorylation sites are shown in pink. Threonine 60 is critical for NCC activity. Other known phosphoacceptor residues are shown in gray. The kinases that phosphorylate these residues are unknown, but some of them (including serine 73) are phosphorylated in concert with SPAK/OSR1 activation. This suggests that other kinases may cooperate with SPAK/OSR1 to activate NCC. (b) Current model for SPAK/OSR1 activation. SPAK and OSR1 are activated by WNK kinases and phosphorylate NCC at the plasma membrane. The WNKs are a convergent regulatory point for upstream stimuli, including low intracellular chloride levels and hormones that stimulate renal salt reabsorption. Phosphorylation increases NCC stability at the surface. Inactive nonphosphorylated forms of NCC do not transport NaCl and are ubiquitylated and endocytosed more rapidly from the plasma membrane
Recent data also indicate a relationship between NCC phosphorylation status and expression at the plasma membrane. Immunolocalization and ultrastructural studies using phosphospecific antibodies to one or more of the aforementioned NCC phosphorylation sites indicate that phosphorylated NCC is tightly restricted to the DCT apical membrane, whereas the unphosphorylated form appears to be more diffusely spread in both plasma membrane and intracellular compartments (Yang et al. 2007a; Mutig et al. 2010; Sandberg et al. 2006; Lee et al. 2009). This suggests that phosphorylation enhances NCC accumulation at the plasma membrane. Consistent with this, phosphorylated forms of NCC are not internalized via clathrin-mediated endocytosis as rapidly as unphosphorylated forms of the cotransporter, and exhibit decreased ubiquitylation (Rosenbaek et al. 2014). Although the mechanism by which the phosphorylated form of NCC is relatively spared from endocytosis is unclear, it seems to be related to a change in its membrane complex properties. Blue native PAGE electrophoresis (BN-PAGE) studies suggest that upon phosphorylation, plasma membrane NCC shifts from a 400 kDa complex to heavier complexes of ~700 kDa in molecular mass (Lee et al. 2013). Included in the 700 kDa complex is γ-adducin, a membrane cytoskeletal protein that stimulates NCC (Lee et al. 2013; Dimke et al. 2011). Other data suggest that plasma membrane-localized NCC may be associated with other membrane proteins in heteromeric complexes, such as the epithelial sodium channel ENaC (Mistry et al. 2016). The incorporation of phosphorylated NCC into high molecular weight complexes at the plasma membrane may prevent the cotransporter from being incorporated into clathrin-coated vesicles.
3.6.2 The WNK-SPAK/OSR1 Signaling Pathway
3.6.2.1 SPAK and OSR1
Two structurally homologous serine–threonine kinases directly phosphorylate the NCC amino terminus. These kinases are the Ste20-like proline-alanine rich kinase (SPAK, encoded by the gene STK39), and oxidative stress-responsive kinase 1 (OSR1, encoded by OXSR1) (Richardson et al. 2008). The relationship between SPAK, OSR1, and sodium-coupled cation–chloride cotransporters was discovered initially in yeast two-hybrid studies of the secretory Na+-K+-2Cl− cotransporter NKCC1 (SLC12A2) (Piechotta et al. 2003). These studies identified SPAK as a binding partner with the NKCC1 amino terminus. The findings were then extended to OSR1, whose amino acid sequence is 67% identical to SPAK (Delpire and Gagnon 2006). Both SPAK and OSR1 bind to the NKCC1 N-terminus via an [R/K]FX[V/I] motif (where X can be any amino acid residue) (Piechotta et al. 2003). Later, these motifs were identified on NCC and the renal thick ascending limb Na+-K+-2Cl− cotransporter NKCC2 (SLC12A1) and were confirmed to be SPAK and OSR1 binding sites on those cotransporters as well (Gagnon et al. 2006; Richardson et al. 2008; Villa et al. 2007). Consistent with a broad role for SPAK and OSR1 in stimulating the activity of SLC12 cotransporters in the kidney, SPAK and OSR1 are highly expressed in the thick ascending limb of the Loop of Henle (TAL) and DCT (McCormick et al. 2011). Moreover, SPAK mutant knock-in mice that express a catalytically inactive form of SPAK that cannot be phosphorylated by upstream signals in its kinase activation loop (i.e., “T-loop”), exhibit decreased salt reabsorption and NKCC2 and NCC phosphorylation in both the TAL and DCT (Rafiqi et al. 2010). Presumably, this inactive form of SPAK acts as a dominant-negative, preventing the association of native SPAK and/or OSR1 with the N-terminal binding sites in NKCC2 and NCC. Many of the identified phosphorylation sites on NCC are SPAK/OSR1 phosphoacceptor residues. These sites correspond to amino acids 46, 50, 55, and 60 of the human NCC N-terminus (Richardson et al. 2008). Serine 73 may also be directly phosphorylated by SPAK, as SPAK KO mice exhibit decreased phosphorylation at this residue (Yang et al. 2010), this site was not modified in in vitro studies. Likewise, phosphorylation of serine 91 is diminished in cells lacking SPAK activity, although this residue was not a direct SPAK/OSR1 phosphorylation site. Thus, some of the phosphoacceptor residues in the NCC N-terminus may be targeted by other kinases that cooperate with SPAK and OSR1 to stimulate salt transport in the DCT.
Studies in SPAK and OSR1 knockout animals illustrate the importance of these kinases in renal salt transport. Although both of SPAK and OSR1 can activate NCC, their genetic ablation in the kidney results in disparate renal salt-wasting phenotypes. Mice deficient in SPAK exhibit salt wasting similar to a mild form of Gitelman syndrome (Yang et al. 2010; McCormick et al. 2011), suggesting that this kinase stimulates salt reabsorption in the DCT. These mice exhibit decreased NCC phosphorylation, but their mild salt wasting is due to the fact that they exhibit a dramatic increase in NKCC2 activity. In contrast, OSR1 knockout mice exhibit a more Bartter-like phenotype, due to a strong decrease in NKCC2 activity in the TAL (Lin et al. 2011). NCC activity is relatively unaffected.
Current evidence suggests that these different phenotypes are due to discrepancies in the expression and activity of SPAK and OSR1 in the TAL and DCT. In the TAL, SPAK activity appears to be low, due to the expression of short forms of the kinase (McCormick et al. 2011; Grimm et al. 2012). These short forms of SPAK are exclusively expressed in the TAL and are either truncated transcripts or proteolytic fragments that lack kinase activity (McCormick et al. 2008; Markadieu et al. 2014). These kinase-deficient species can suppress the kinase activity of full-length SPAK and OSR1, functioning in a dominant-interfering manner. Genetic deletion of SPAK eliminates the expression of both its long and short forms in the TAL; this effectively releases OSR1 from inhibition in the TAL, causing it to hyperphosphorylate NKCC2. Since only the full-length form of SPAK is expressed in the DCT, SPAK KO mice exhibit a net decrease in NCC phosphorylation, resulting in the Gitelman-like phenotype. In the absence of SPAK, OSR1 redistributes into an intracellular location, collecting in large puncta (McCormick et al. 2011; Grimm et al. 2012; Terker et al. 2015). These puncta, termed “WNK bodies,” contain upregulated components of the WNK-SPAK/OSR1 pathway and are further discussed below (see section on WNK1) (Boyd-Shiwarski et al. 2018). More work is needed to further elucidate the relationship between SPAK, OSR1, and cation–chloride cotransporters in the kidney, but the current body of work suggests that SPAK is the dominant activator of NCC in the DCT, while OSR1 serves as the main activator of NKCC2 in the TAL.
3.6.2.2 WNK Kinases
In order for SPAK and OSR1 to phosphorylate and activate the sodium-coupled cation–chloride cotransporters, they must first be in an active state. SPAK and OSR1 activation occurs through phosphorylation of key residues in their kinase domains (Vitari et al. 2005, 2006; Thastrup et al. 2012). A family of proteins called With-No-Lysine (amino acid = [K]; WNK) kinases mediates this process. The mammalian WNK family consists of four genes, which encode large serine–threonine kinases found in many eukaryotes. All four WNK kinases share a similar domain organization, consisting of an N-terminal kinase domain, an autoinhibitory domain that suppresses intrinsic kinase activity and can cross-inhibit other WNKs, 2–3 coiled-coil domains which facilitate oligomerization, and multiple SPAK/OSR1 binding motifs (Richardson et al. 2008). They were named “With-No-Lysine” kinases after bioinformatic analysis of the kinase domain revealed that the catalytic lysine required for ATP binding and phosphoryl transfer is missing from its usual location in β strand 3 of the active site; instead, this lysine is positioned in β strand 2 (Xu et al. 2000; Min et al. 2004). Due to this unusual arrangement of residues within the kinase domain, chloride can bind directly to the catalytic site and block its ability to trigger autophosphorylation and kinase domain activation (Piala et al. 2014). Thus, the unique structure of WNK kinases allows them to act as intracellular chloride sensors.
The WNKs were identified as NCC regulators over a decade ago, through studies of a rare chloride-dependent thiazide-sensitive Mendelian blood pressure disorder called Familial Hyperkalemic Hypertension (FHHt, also known as Pseudohypoaldosteronism type 2 or Gordon Syndrome). In 2001, mutations in two WNK kinases, WNK1 and WNK4, were linked to FHHt (Wilson et al. 2001). Both of these kinases were expressed with NCC in the DCT (Wilson et al. 2001; Rinehart et al. 2005). While important roles for WNK3 have been established in the brain, and some reports indicate that it is also expressed in the kidney, WNK3 KO mice do not exhibit a renal phenotype, suggesting that it not be as important as WNK1 and WNK4 in renal tubular physiology (Mederle et al. 2013). WNK2, is a brain, heart, and intestine-expressed WNK kinase that is excluded from the kidney and therefore does not appear to be a major NCC regulator (Rinehart et al. 2011).
WNK1 is unique from WNK4 and WNK3 in that it contains multiple promoters and undergoes extensive alternative splicing in the kidney (O’Reilly et al. 2003; Delaloy et al. 2003). Thus, its renal expression pattern consists of multiple isoforms with disparate functions. These isoforms can be classified into two major functional groups: Long kinase active isoforms (“L-WNK1”) with intact serine–threonine kinase activity, or short Kidney Specific isoforms that are kinase-defective (“KS-WNK1”). KS-WNK1 isoforms are generated by an alternative promoter located between exons 4 and 5. This promoter is only active in the kidney and drives the high expression of KS-WNK1 in the DCT (O’Reilly et al. 2006; Vidal-Petiot et al. 2012; Liu et al. 2011).
Although mutations in WNK1 and WNK4 were the first to be implicated in the pathogenesis of FHHt, most FHHt affected harbor mutations in two other genes, the E3 ubiquitin ligase Cullin 3 (CUL3) and its adaptor, Kelch-like 3 (KLHL3) (Boyden et al. 2012; Louis-Dit-Picard et al. 2012). The CUL3-KLHL3 complex binds to WNK1 and WNK4 and facilitates their ubiquitylation and degradation (Shibata et al. 2013; Ohta et al. 2013).
3.6.2.2.1 WNK4
The first studies defining relationships between the WNKs and NCC were carried out in the Xenopus laevis oocyte expression system. This in vitro system had been used for the initial expression cloning studies that permitted the isolation of the cDNAs encoding NCC and other members of the cation–chloride cotransporter family. Thus, it was a natural extension to utilize the system to test the effects of WNK kinases on the NCC function. The general approach for these experiments was to coinject in vitro transcribed RNA encoding a WNK kinase along with NCC and measure NCC activity by performing 22Na+ radioisotopic fluxes in the presence or absence of thiazide. These initial experiments indicated that overexpression of WNK4 suppresses NCC activity (Yang et al. 2003; Wilson et al. 2003). Later, this finding was also seen in mammalian overexpression systems and mouse distal convoluted tubule cell lines (Subramanya et al. 2009; Ko et al. 2012; Cai et al. 2006; Zhou et al. 2009). Follow-up studies revealed that this overexpression phenotype is due to the suppression of NCC trafficking from the TGN to the plasma membrane (Golbang et al. 2006; Subramanya et al. 2009). Instead of being trafficked from the TGN to the surface, NCC is routed directly to lysosomes for degradation via an intracellular route. This event was associated with enhanced interaction between NCC and the AP-3 adaptor protein complex, which sorts cargo to the lysosome from the TGN (Subramanya et al. 2009; Simpson et al. 1997; Dell’Angelica et al. 1997). In addition, WNK4 overexpression promotes NCC packaging into vesicles that contain the lysosomal sorting receptor sortilin (Zhou et al. 2009). Although some studies suggested that intact WNK4 kinase activity is required for this inhibitory effect on NCC forward trafficking (Wilson et al. 2003; Golbang et al. 2006; Cai et al. 2006), other studies have argued that the effect is mediated by the WNK4 C-terminus, and therefore, is a kinase-independent process (Yang et al. 2005). The MAP kinase ERK1/2 may mediate the inhibitory effect of WNK4 on NCC, as ERK1/2 activation is associated with decreased NCC plasma membrane expression, and WNK4 overexpression can activate the ERK1/2 signaling pathway (Zhou et al. 2012; Ko et al. 2007). However, these studies remain largely correlative to date, and conflicting experiments have implicated ERK1/2 as a stimulator of NCC endocytosis, a trafficking operation that is not affected by WNK4 overexpression (Golbang et al. 2006; Subramanya et al. 2009).
Although numerous laboratories have independently corroborated this “inhibitory” effect of WNK4 (Subramanya et al. 2009; Yang et al. 2003, 2005; Golbang et al. 2006; Wilson et al. 2003; Cai et al. 2006; Ko et al. 2012), caution should be exercised when interpreting these experiments. Importantly, nearly all of the in vitro studies looking at this aspect of WNK4-NCC regulation have been performed in heterologous expression systems. Overexpression of a WNK kinase can cause aberrant sequestration and mislocalization of other components of the signaling pathway, including other WNK kinases or SPAK and OSR1. Thus, the inhibitory phenotype seen in in vitro systems may not have an in vivo correlate. Indeed, transgenic mice overexpressing wild type WNK4 have provided conflicting results; one of the mouse models exhibited strong inhibition of NCC expression and DCT atrophy, while the other mouse actually exhibited increased NCC activity due to overstimulation of SPAK and OSR1 (Wakabayashi et al. 2013; Lalioti et al. 2006).
Consistent with this second mouse model, a number of studies have shown that WNK4 can activate NCC via SPAK/OSR1 (Vitari et al. 2005; Yang et al. 2007a). In contrast to the inhibitory effect of WNK4 described above, there is a general consensus that the stimulatory effect requires direct phosphorylation of SPAK/OSR1 by WNK4. This, in turn, results in downstream enhanced SPAK/OSR1 mediated phosphorylation of NCC. Moreover, several hormones that activate salt transport via NCC (discussed in detail below) appear to trigger NCC phosphorylation by stimulating WNK4-mediated activation of SPAK/OSR1. Thus, many experts in the field view the stimulatory effect of WNK4 on NCC phosphorylation status as its primary and more physiologically relevant mode of operation (Takahashi et al. 2014; Richardson et al. 2008).
In summary, current data on the role of WNK4 in NCC regulation provide evidence on both sides of the fence: some studies support its role as an inhibitor of NCC trafficking to the plasma membrane, while others have shown that it can stimulate NCC activity via SPAK- and OSR1-mediated phosphorylation. So, how does one reconcile these two radically different effects into a unifying model? One view is that the kinase-inactive form of WNK4 may act as an inhibitor at baseline, holding NCC in an intracellular compartment via a mechanism that does not require intact kinase activity (Subramanya and Ellison 2014; McCormick et al. 2008) (Fig. 3.4a). Upon exposure to physiological factors that stimulate salt reabsorption in the DCT, WNK4 is converted into an active state. This results in WNK4 kinase domain activation and downstream SPAK and OSR1 phosphorylation, and loss of the inhibitory effect (Fig. 3.4b). This would increase NCC phosphorylation and the number of active NCC cotransporters expressed at the plasma membrane due to enhanced forward trafficking. Studies utilizing RNA interference in DCT-derived cell lines that endogenously express SPAK, WNK4, and NCC provide evidence in support of such a model (Ko et al. 2010, 2012).
WNK kinases. A model for WNK-mediated regulation of SPAK/OSR1 and NCC. (a) In the baseline unstimulated state, WNK4 may act as an inhibitor, suppressing NCC forward trafficking from endosomes derived from the biosynthetic pathway. WNK4, WNK3, and full-length WNK1 (L-WNK1) are all expressed in the DCT. The kinase-defective short isoform of WNK1, KS-WNK1, suppresses the kinase activity of WNK4, WNK3, and L-WNK1 at baseline. (b) When the DCT is challenged with a physiological stimulus, the kinase activity of WNK4, WNK3, and L-WNK1 increases relative to KS-WNK1. This converts these kinases into activators of SPAK and OSR1, which triggers NCC phosphorylation
3.6.2.2.2 WNK1
Unlike WNK4, early studies with L-WNK1 in the Xenopus oocyte expression system were carried out with the original L-WNK1 cDNA, which was derived from rat brain (Xu et al. 2000). Studies with this cDNA revealed no effect of L-WNK1 on NCC activity or surface expression when it was coexpressed with the cotransporter in oocytes. However, when WNK4, L-WNK1, and NCC were co-expressed together, L-WNK1 associated with WNK4 and reversed its inhibitory effect on NCC transport back to baseline levels (Yang et al. 2003). This effect was not seen when either the kinase defective KS-WNK1 isoform or a kinase-dead mutant of L-WNK1 was coexpressed with WNK4 and NCC, suggesting that intact L-WNK1 kinase activity is required to suppress WNK4-mediated inhibition (Yang et al. 2005; Subramanya et al. 2006). As expected, like the other WNKs, L-WNK1 can also stimulate SPAK/OSR1 activation by phosphorylating residues within the SPAK and OSR1 kinase domains (Vitari et al. 2005). A more recent study that used human L-WNK1 found a stimulatory effect on NCC in Xenopus oocytes, via SPAK/OSR1 activation (Chavez-Canales et al. 2014). Collectively, these studies indicate that L-WNK1 can activate SPAK and OSR1, and can also override the inhibitory effect of WNK4 commonly seen in heterologous expression systems (Fig. 3.4b).
As mentioned above, DCT-localized KS-WNK1 isoforms do not contain a functional kinase domain. They do, however, contain C-terminal coiled coil domains that are important for interactions with other WNK gene products, including L-WNK1 and WNK4 (Subramanya et al. 2006). Thus, KS-WNK1 appears to function as a scaffold for other WNK kinases. Consistent with this, a recent study showed that KS-WNK1 is required for WNK kinases to organize into large membraneless punctate foci in the DCT. These structures, termed “WNK bodies,” have been visualized in the setting of NCC activation by hypokalemia, aldosterone, and in mouse models of FHHt (Boyd-Shiwarski et al. 2018). They have also been visualized in SPAK and OSR1 knockout mice, where genetic ablation of SPAK and OSR1 would be expected to result in upstream stimulation of WNK kinases to enhance NCC activity. Though the functional role of WNK bodies is being elucidated, this suggests that the formation of these structures is a signature of WNK pathway activation.
Currently, there are conflicting data regarding the role of KS-WNK1 in NCC regulation. KS-WNK1 contains an “autoinhibitory” region that suppresses L-WNK1 kinase activity in vitro (Subramanya et al. 2006), suggesting that it may function as an inhibitory scaffold for WNK kinases. Consistent with this, early studies in Xenopus oocytes suggested that KS-WNK1 blocks the ability of L-WNK1 to activate NCC (Subramanya et al. 2006). These findings were supported by two mouse models of KS-WNK1 inactivation (Hadchouel et al. 2010; Liu et al. 2011). However, more recent studies have argued that KS-WNK1 can function as an NCC activator (Argaiz et al. 2018), a finding that would be consistent with the role of KS-WNK1 in the formation of WNK bodies. Thus, though it is clear that KS-WNK1 is required for WNK body formation in the DCT, its precise role in NCC regulation remains unresolved and an active area of investigation.
3.6.2.2.3 WNK3
Comparative studies of the WNK kinases indicate that WNK3 is a potent NCC activator (Yang et al. 2007a; Rinehart et al. 2005) (Fig. 3.4b). In vitro, WNK3 stimulates NCC transport activity by enhancing its phosphorylation and surface expression (Rinehart et al. 2005; Yang et al. 2007a; Chavez-Canales et al. 2014). This effect can be blocked by KS-WNK1 (Yang et al. 2007a). Immunolocalization studies in kidney have identified WNK3 in several segments of the renal tubule, including the proximal tubule, Loop of Henle, DCT, and collecting duct (Rinehart et al. 2005). Based on the strong stimulatory effects of WNK3 on NCC activity, and its colocalization with NCC in the DCT, some have proposed that WNK3 may be a critically important regulator of thiazide-sensitive NaCl reabsorption. This hypothesis predicts that deleting WNK3 expression from the kidney should cause substantial salt wasting. Surprisingly, however, WNK3 knockout mice do not have an appreciable renal phenotype (Oi et al. 2012; Mederle et al. 2013). Although the reason for this is not entirely clear, compensation by L-WNK1 may play a role, since WNK1 mRNA is increased in WNK3 knockout mice relative to controls (Mederle et al. 2013). These findings suggest a minor role for WNK3 in the control of NCC activity in vivo.
3.6.3 NCC Dephosphorylation by Protein Phosphatases
Although much of the work on NCC phosphorylation has been centered on understanding how protein kinases stimulate NCC activity, a growing body of evidence indicates that protein phosphatases inhibit NCC. Some of these phosphatases likely directly remove phosphate groups from the NCC amino terminus, while others may influence NCC phosphorylation status by inhibiting the WNK-SPAK/OSR1 pathway.
Early studies of the sodium-coupled SLC12 cotransporters indicated that inhibitors of the ubiquitously expressed protein phosphatase 1 (PP1) could suppress NKCC1 activity through dephosphorylation (Darman and Forbush 2002; Lytle and Forbush 1996). Indeed, PP1 also appears to play an important role in NCC dephosphorylation, as one of its canonical inhibitors, PP1 inhibitor 1 (Ppp1r1a) is highly coexpressed with the cotransporter in the DCT (Picard et al. 2014). The deficiency of Ppp1r1a leads to a mild thiazide-sensitive salt-wasting phenotype. This suggests that the absence of Ppp1r1a leads to enhanced PP1 activity and NCC dephosphorylation. In 2010, Glover et al. identified PP4 as another phosphatase that is expressed in the DCT (Glover et al. 2010). The coexpression of PP4 with NCC reduces its phosphorylation at threonine 60 and therefore decreases its transport activity.
Protein phosphatase 3 (also known as calcineurin) was also recently identified as a potential negative regulator of NCC activity. The calcineurin inhibitor tacrolimus (FK506, “Prograf”) commonly causes hypertension, hyperkalemia, hypercalciuria, and metabolic acidosis, despite normal glomerular filtration rate (GFR) (Hoorn et al. 2011). This constellation of findings is similar to the hypertension and hyperkalemia seen in FHHt (Hadchouel et al. 2006; Mayan et al. 2002). In 2012, Hoorn and colleagues showed that calcineurin inhibitors cause hypertension by activating thiazide-sensitive NaCl cotransport in the kidney. The mechanism appears to be directly related to an increase in NCC phosphorylation status. WNK4 abundance and SPAK/OSR1 activation were also increased. These findings suggest that tacrolimus may stimulate NCC activity through an indirect effect, possibly by preventing the dephosphorylation and inactivation of WNK kinases.
3.7 NCC Endocytosis
NCC is removed from the plasma membrane by dynamin- and clathrin-dependent endocytosis (Ko et al. 2010; Rosenbaek et al. 2014). Studies of overexpressed NCC in MDCK renal epithelial cells indicate that the cotransporter can be constitutively endocytosed, with one-third of the total NCC surface pool undergoing internalization within 10 min; this endocytic rate is compatible with other kidney tubule-expressed membrane proteins such as the epithelial sodium channel (ENaC) (Butterworth et al. 2005). Clathrin-dependent endocytic retrieval of NCC is associated with increased cotransporter ubiquitylation and routing to the lysosomal pathway (Rosenbaek et al. 2014). While some groups have reported that endocytosed NCC undergoes polyubiquitylation (Ko et al. 2010), others have reported that the endocytosed ubiquitylated cotransporter migrates at molecular mass similar to monomeric NCC, suggesting oligo- or monoubiquitylation (Rosenbaek et al. 2014). Endocytosis of NCC is dependent on the phosphorylation status of the protein, since phosphorylation at threonines 55, 60, and 73 stabilize NCC surface expression by slowing the rate of endocytosis (Rosenbaek et al. 2014; Yang et al. 2013). This, in turn, is associated with decreased NCC ubiquitylation. Ubiquitylation of the surface NCC pool is mediated by the aldosterone-regulated E3 ubiquitin ligase Nedd4-2 (Ronzaud et al. 2013; Arroyo et al. 2011), though other currently unidentified E3 ubiquitin ligases also probably participate in this process.
Treatment of cultured mDCT cells with the diacylglycerol analog 12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits NCC by stimulating its endocytosis and ubiquitylation (Ko et al. 2010). Although TPA is classically used in the laboratory to activate protein kinase C (PKC), the effect appeared to be unrelated to this kinase, since PKC blockers did not reverse the inhibitory effect of TPA. Instead, the alternative TPA target Ras guanyl-releasing protein 1 (RasGRP1) was the primary mediator of NCC inhibition (Ko et al. 2007). The MAP kinase ERK1/2 may act downstream of RasGRP1 to trigger NCC endocytosis (Ko et al. 2010).
3.8 Mendelian Disorders of NCC Dysfunction
3.8.1 Gitelman Syndrome
In 1996, Simon et al. reported that mutations in the SLC12A3 gene encoding NCC cause Gitelman syndrome, an autosomal recessive salt wasting disorder (Simon et al. 1996). Patients may be hypotensive, but often have normal blood pressures, since the urinary salt wasting is offset by hyperreninemia and upregulation of the renin–angiotensin system. Hypokalemia and hypomagnesemia, however, are always seen in the disorder. Other manifestations include hypocalciuria and metabolic alkalosis. Thus, the overall phenotype of Gitelman syndrome is similar to the constellation of findings seen in patients taking thiazide diuretics.
Since the initial linkage of NCC to Gitelman syndrome, hundreds of disease-causing mutations have been identified. These mutations are generally scattered throughout the NCC protein, with a slight clustering of lesions in the intracellular C-terminus. In vitro analyses indicate that mutations that cause the disorder generally promote NCC misfolding (Kunchaparty et al. 1999). These so-called “Class 2” mutations cause the NCC to be retained in the endoplasmic reticulum and targeted for ERAD (Ellison 2003). Consistent with this finding, a recent study found that Gitelman mutations localized to the NCC C-terminus cause the cotransporter to interact strongly with a molecular chaperone complex containing Hsp70, Hsp40, and the cochaperone/E3 ubiquitin ligase CHIP (Donnelly et al. 2013). On the other hand, some mutations that cause Gitelman syndrome do not affect the biosynthetic processing of NCC; cotransporters with these “Class 1” mutations appear to be processed normally and traffic to the plasma membrane without difficulty (Sabath et al. 2004; Riveira-Munoz et al. 2007; Ellison 2003).
In 2007, a study by Ji et al. suggested that mutations of NCC that are associated with Gitelman syndrome play an important role in determining susceptibility to essential hypertension (Ji et al. 2008). Based on the prevalence of Gitelman Syndrome, about 1% of the general population should be heterozygous for the disorder. On average, individuals in a large study population who were carriers of a loss of function NCC variant had a systolic blood pressure that was 6.3 mmHg lower throughout life, compared with individuals who had two wild type copies of NCC. Thus, the carrier state for Gitelman syndrome conferred lifelong protection from the development of hypertension (Acuna et al. 2011; Subramanya and Welling 2011). As the cost of next-generation sequencing technology continues to drop, one could imagine that screening for these mutations in normotensive individuals could provide information about the risk of developing hypertension over time.
3.8.2 Familial Hyperkalemic Hypertension
Familial Hyperkalemic Hypertension (FHHt) is a rare Mendelian syndrome featuring the unusual triad of chloride-dependent hypertension, severe hyperkalemia, and normal glomerular filtration rate. The disease was initially described by Paver and Pauline in the 1960s (Paver and Pauline 1964), and later by Gordon (Gordon 1986). For years, the disease was known as “Gordon Syndrome” or “Pseudohypoaldosteronism Type II,” although more recently, some have elected to rename the syndrome “FHHt” (Yang et al. 2005; Hadchouel et al. 2006). The chloride-dependent hypertension and hyperkalemia can be cured by low doses of thiazide diuretics (Mayan et al. 2002). Those familiar with the syndrome immediately recognized that its constellation of findings is essentially a “mirror image” of Gitelman syndrome (Hadchouel et al. 2006). A natural conclusion, then, was that gain-of-function mutations of NCC should be linked to the disease. Candidate gene approaches, however, indicated that this was not the case, as targeted sequencing of the SLC12A3 gene revealed no mutations (David Ellison, personal communication).
In 2001, Wilson et al. linked mutations in WNK1 and WNK4 to FHHt (Wilson et al. 2001). Although mutations in WNK kinases were the first to be implicated in the pathogenesis of FHHt, they only account for a minority of families affected by the disorder. As mentioned above, most FHHt affecteds harbor mutations in the genes encoding the E3 ubiquitin ligase CUL3 and its adaptor, KLHL3 (Boyden et al. 2012). These two proteins participate in a protein complex that degrades WNK1 and WNK4. Recent studies from several laboratories show that CUL3 covalently attaches ubiquitin molecules to WNK1 and WNK4, marking them for disposal (Shibata et al. 2013; Ohta et al. 2013). In order to do so, KLHL3 must be present, since it connects CUL3 to the WNKs.
An elaborate interrelationship between WNK1, WNK4, and the KLHL3/CUL3 complex underlies the pathogenesis of FHHt. FHHt-causing mutations in WNK4 are missense mutations that reduce WNK4 binding to KLHL3 (Shibata et al. 2013). Total WNK4 expression is, therefore, increased in patients who have these mutations. Disease-causing WNK4 mutants can phosphorylate SPAK and OSR1, and enhance NCC trafficking to the plasma membrane relative to the wild type protein (Yang et al. 2007b). Thus, increased mutant WNK4 protein expression causes NCC overactivation. FHHt mutations in WNK1 are intron deletions that do not alter the kinase’s protein structure. Instead, these mutations increase total WNK1 mRNA and protein expression (Wilson et al. 2001). The increased protein expression probably overrides ubiquitination and degradation by the KLHL3/CUL3 complex, and the net effect of the mutation is to increase total L-WNK1 kinase activity (Vidal-Petiot et al. 2013). Thus, in FHHt caused by mutations in WNK1, increased L-WNK1 protein expression should suppress the inhibitory effect of WNK4 on NCC traffic while stimulating SPAK and OSR1, causing NCC to be overactive.
FHHt-associated mutations in KLHL3 reduce the binding of the CUL3–KLHL3 complex to WNK1 and WNK4 (Shibata et al. 2013; Ohta et al. 2013; Wakabayashi et al. 2013). Thus, mutations in KLHL3 diminish the amount of CUL3-mediated WNK1 and WNK4 degradation. This increases WNK1 and WNK4 abundance, resulting in enhanced WNK-dependent signaling and NCC activation. Mutations in cullin-3 always cause skipping of an exon, resulting in an in-frame deletion (Boyden et al. 2012; Glover et al. 2014). Recent studies indicate that this specific mutation causes autocatalytic degradation of the KLHL3/CUL3 complex, reducing the number of available circulating E3 ubiquitin ligase complexes, thereby increasing WNK4 and WNK1 abundance (McCormick et al. 2014).
3.9 Physiologic Regulation of NCC
The discovery of the WNK-SPAK/OSR1 signaling pathway and the advent of new tools to study NCC function, including cell lines, genetically modified mice, and phosphospecific antibodies, has led to a number of fascinating insights into the underlying mechanisms that regulate NCC activity. This section summarizes the current understanding of the molecular bases of physiologic NCC regulation.
3.9.1 Regulation by Intracellular Chloride
SLC12 cation–chloride cotransporters are important regulators of intracellular chloride concentration (Gamba 2005). Studies examining the effect of chloride on NKCC1 activity found that intracellular Cl− depletion increases NKCC1 activity (Flatman 2002). The stimulatory effect of chloride depletion on NKCC1 appears to be more than a consequence of generating a simple chemical gradient. Intracellular chloride depletion stimulates phosphorylation of the sodium-coupled SLC12 cotransporters at the same residues that are phosphorylated by SPAK and OSR1 (Richardson et al. 2008). Moreover, as described above, WNK kinases are chloride sensors that are activated by chloride depletion (Piala et al. 2014). Thus, the WNK-SPAK/OSR1 pathway represents an important intracellular signaling mechanism that responds to changes in intracellular Cl− levels to activate NaCl influx via these electroneutral cotransporters. NCC is activated by intracellular chloride depletion in a manner identical to NKCC1 (Pacheco-Alvarez et al. 2006). In Xenopus oocytes and mammalian cells, preincubating cells expressing NCC with chloride-free solutions strongly activates NCC transport activity by stimulating amino-terminal phosphorylation at canonical SPAK/OSR1 target sites (Pacheco-Alvarez et al. 2006; Leaphart et al. 2008). Recently, the chloride sensing function of WNK4 was verified to be critically important in the in vivo regulation of NCC activity in the DCT (Chen et al. 2019).
3.9.2 Regulation by Extracellular Potassium
NCC activity is exquisitely sensitive to changes in the concentration of extracellular K+. Hyperkalemia has been shown to be a potent NCC inhibitor, while hypokalemia activates NCC (Vallon et al. 2009; McDonough and Youn 2013; Rengarajan et al. 2014). Potassium predominantly affects NCC phosphorylation status. Within minutes of ingesting a high potassium load, rodents develop a natriuresis that is accompanied by rapid NCC dephosphorylation at the canonical SPAK/OSR1 phosphorylation sites (Sorensen et al. 2013). Conversely, hypokalemia appears to strongly trigger NCC phosphorylation at those same sites (Vallon et al. 2009). In both cases, the effects of potassium can occur independently of aldosterone action. The inverse relationship between potassium and NCC phosphorylation status is linear within the physiological range of plasma potassium levels (Terker et al. 2016). This indicates the DCT functions as a potassium sensor that controls luminal NaCl concentrations (and downstream Na+-dependent collecting duct potassium secretion) via changes in NCC activity (Ellison and Terker 2015).
Extracellular potassium regulates NCC by altering the basolateral conductance of the DCT (Terker et al. 2015). This affects the chloride-dependent activity of the WNK-SPAK/OSR1 signaling pathway. Consider, for example, the physiological alterations seen during hyperkalemia. A high extracellular K+ concentration in the blood causes the basolateral membrane of the DCT to become depolarized (Brown 1991). This decreased negativity of the cell membrane potential diminishes Cl− exit via the basolateral DCT chloride channel ClC-Kb, raising the intracellular chloride concentration (Zhang et al. 2014). As described above, the corresponding increase in intracellular Cl− suppresses WNK kinase autoactivation (Piala et al. 2014), resulting in reduced downstream SPAK/OSR1 and NCC activity. Supporting this concept, recent studies have shown that knockout mice lacking the major K+ channel mediating basolateral K+ conductance in the DCT, KCNJ10 (Kir4.1), exhibit reduced NCC activity caused by depolarization of the DCT membrane potential and a corresponding increase in intracellular chloride, which inhibits SPAK and OSR1 (Zhang et al. 2014).
3.9.3 Luminal NaCl Delivery
NCC-mediated NaCl reabsorption in the DCT is dependent on the delivered load of NaCl (Khuri et al. 1975). The DCT responds to chronic increases in the delivery of NaCl with an increase in the capacity for NaCl cotransport, as well as marked ultrastructural changes in the DCT cell. These morphologic changes include an increase in the size of DCT cells, increased basolateral membrane surface area, and an increase in DCT mitochondrial mass (Ellison et al. 1989). Accompanying the functional and morphologic changes are an increase in basolateral a Na+/K+-ATPase activity, and an increase in thiazide-binding sites (Chen et al. 1990). It is not clear if these changes are due to transcriptional or post-translational effects on NCC expression, although they do appear to be a consequence of increased sodium entry into the DCT. Inhibition of NaCl entry into DCT cells with chronic thiazide treatment resulted in a loss of cell height, and polarity, and triggered apoptosis (Morsing et al. 1991). The cellular mechanisms whereby NaCl entry affects NCC transport function and DCT morphology are currently not known.
3.9.4 Hormonal Regulation of NCC
3.9.4.1 Angiotensin II
The peptide hormone angiotensin II (AngII) plays a critically important role in blood pressure homeostasis and tubular salt handling. Ang II has dual effects on NCC trafficking and phosphorylation. Infusion of AngII into animals causes the cotransporter to traffic from intracellular vesicles to the plasma membrane, while the treatment of rodents with angiotensin-converting enzyme (ACE) inhibitors redistributes NCC to intracellular vesicles (Sandberg et al. 2007). The surface-expressed cotransporter appears to be highly phosphorylated, based on immunolocalization studies and biochemical inquiry of the aforementioned canonical SPAK/OSR1 phosphorylation sites. The WNK-SPAK/OSR1 pathway mediates these effects, as the stimulatory effects of angiotensin II are associated with SPAK and OSR1 activation (Talati et al. 2010). Moreover, in vitro and in vivo experiments implicate WNK4 as being critically important (Castaneda-Bueno and Gamba 2012; San-Cristobal et al. 2009). Studies in the Xenopus oocyte system suggest that AngII converts WNK4 from its inhibitory state into a SPAK/OSR1-dependent NCC activator, and NCC expression and phosphorylation are unaffected by AngII infusion in WNK4 knockout mice. NCC stimulation could be seen in adrenalectomized animals, suggesting that the AngII effect occurred independently of aldosterone (van der Lubbe et al. 2011).
The classic view of the renin–angiotensin system is that it functions as a whole-body blood pressure and volume control mechanism that acts through the concerted effects of multiple organs (Guyton 1991). Renin released from the kidney triggers the conversion of liver-derived angiotensinogen to angiotensin I, which in turn is converted to AngII by lung-expressed ACE (Atlas 2007). However, it is clear that an intrarenal renin–angiotensin system also exists within the kidney, and that this system is critically important for the effects of AngII on renal hemodynamics (Navar and Rosivall 1984). This intrinsic system has important effects on the activity of NCC since the kidney-specific ablation of intrarenal ACE decreases NCC activity and phosphorylation (Gonzalez-Villalobos et al. 2013).
3.9.4.2 Adrenal Steroids
Current evidence indicates that adrenal steroid hormones stimulate NCC-mediated NaCl cotransport. The presence of both mineralocorticoid and glucocorticoid receptors in the DCT has been demonstrated by immunohistochemistry and by hormone binding experiments (Farman and Bonvalet 1983; Farman et al. 1991). Although mineralocorticoid receptors are expressed throughout the entire DCT, only the “late” portion of this segment (also known as the DCT2) expresses the steroid metabolizing enzyme 11-β-hydroxysteroid dehydrogenase 2 (11-βHSD2) (Velazquez et al. 1998; Bostanjoglo et al. 1998). This enzyme metabolizes cortisol to the inactive metabolite cortisone, thereby preventing circulating glucocorticoids from binding to mineralocorticoid receptors expressed in the DCT2. Because the mineralocorticoid receptors in the DCT2 can only be occupied by aldosterone, the DCT2 is highly sensitive to changes in circulating aldosterone levels.
Early studies by Chen and Fanestil (Chen et al. 1994a), Velazquez et al. (1995), and Kim et al. (1998) collectively demonstrated that aldosterone increases total NCC protein abundance without affects its mRNA expression, indicating that mineralocorticoids stimulate NCC activity through post-translational mechanisms. More recently, McDonough (Sandberg et al. 2006) showed that mineralocorticoid receptor blockade decreases NCC trafficking to the plasma membrane while simultaneously increasing its accumulation in lysosomes. Thus, one way in which aldosterone can increase NCC activity is by enhancing its expression at the plasma membrane. The molecular mechanism likely involves the serum and glucocorticoid regulated kinase, SGK1. The transcription of this serine–threonine kinase is upregulated in response to aldosterone binding to the mineralocorticoid receptor (Pearce 2001). The active form of SGK1 has been reported to regulate NCC trafficking via two mechanisms. First, in in vitro systems, SGK1 can reverse the inhibitory effect of overexpressed WNK4 on NCC delivery to the plasma membrane (Rozansky et al. 2009). Second, SGK1 phosphorylates and inactivates Nedd4-2, an E3 ubiquitin ligase which ubiquitylates and negatively regulates NCC surface expression (Arroyo et al. 2011). Although the first of these two findings has only been shown in the Xenopus oocyte expression system (Rozansky et al. 2009), the in vitro inhibitory effect of Nedd4–2 on NCC has been verified in mouse models (Arroyo et al. 2011; Ronzaud et al. 2013).
Aldosterone also appears to stimulate NCC transport activity by promoting NCC phosphorylation. Chiga et al. (2008) showed that NCC phosphorylation is dynamically regulated by adjustments in dietary NaCl intake and that this effect is at least partially dependent on the mineralocorticoid receptor (MR). Vallon et al. echoed these findings 1 year later (Vallon et al. 2009). In both reports, careful measurements of NCC abundance demonstrated enhanced NCC phosphorylation at all of its canonical SPAK/OSR1 phosphorylation residues. Consistent with this, aldosterone increased SPAK and OSR1 activation in a mineralocorticoid receptor-dependent manner (Chiga et al. 2008). A study by van der Lubbe and colleagues (van der Lubbe et al. 2012) implicated WNK4 as a mediator of the stimulatory effect of aldosterone on SPAK/OSR1 and NCC phosphorylation, though other WNK kinases expressed in the DCT may also be involved. Indeed, the E3 ubiquitin ligase Nedd4-2 binds to and ubiquitylates WNK1, and that this process is blocked by aldosterone, leading to an increase in WNK1 abundance in the DCT, providing a potential mechanism by which mineralocorticoids might augment NCC phosphorylation status (Roy et al. 2015). A more rapid and complex signal for aldosterone action involving cAMP, IP3, GPR30, and EGF Receptor-dependent networks was also recently described (Cheng et al. 2019).
Glucocorticoids such as dexamethasone increase thiazide-sensitive NaCl transport and increase the number of metolazone binding sites in the kidneys of adrenalectomized rodents (Chen et al. 1994a). This strongly suggests that glucocorticoids stimulate NCC-mediated salt transport in the DCT. The DCT1 may be the more relevant site of action of glucocorticoids, since, as mentioned above, the levels of active cortisol in the DCT2 should be relatively low due to its metabolism by 11β-HSD (Bostanjoglo et al. 1998). The WNK-SPAK/OSR1 pathway is expressed in both DCT1 and DCT2; thus, many of the same players involved in mineralocorticoid receptor-dependent regulation of NCC are likely involved. Although NCC overactivity may participate in the salt-sensitive hypertension seen in disorders of cortisol excess such as Cushing’s syndrome, the role of glucocorticoids in the physiological regulation of Na+ transport in the DCT remains understudied.
3.9.4.3 Gonadal Steroids and NCC
Gonadal steroid hormones may also influence NaCl cotransport in the DCT. Chen et al. (1994b) reported gender differences in the density of thiazide binding sites, and in the natriuretic response of thiazides in rodents. Specifically, female rats had higher levels of “thiazide receptors” in the renal cortex than males. The number of thiazide-binding sites decreased following ovariectomy, while levels in males rose following orchiectomy. Consistent with these results, Verlander et al. found that estradiol treatments increased NCC expression in the DCT (Verlander et al. 1998). Collectively, these results are consistent with the view that male sex hormones (e.g., testosterone) may downregulate NCC expression and salt transport, while estrogens increase NCC expression and salt transport in the DCT. The physiological relevance of these findings with respect to human hypertension is currently unclear, as no clear gender difference in the response of humans to thiazide diuretics has been established.
3.9.4.4 Vasopressin
Recent work indicates that vasopressin is a potent NCC activator (Gamba 2010). The vasopressin analog deamino-Cys-1, d-Arg-8 vasopressin (dDAVP) triggers an increase in NCC abundance, trafficking to the plasma membrane, and activation through SPAK/OSR1-mediated phosphorylation of its amino terminus (Saritas et al. 2013; Mutig et al. 2010; Pedersen et al. 2010). The effect probably requires cyclic AMP and protein kinase A (PKA), well-established intermediaries of vasopressin-dependent signaling (Nedvetsky et al. 2009). The effect on NCC abundance may be a Nedd4–2-dependent process, since PKA can phosphorylate and inactivate Nedd4–2 through mechanisms similar to SGK1 (Snyder et al. 2004); however, this hypothesis is yet to be tested.
3.9.4.5 Insulin
One of the first studies suggesting a potential link between insulin and NCC was performed by Bickel et al., who noted that insulin-resistant obese Zucker rats retain salt and exhibit increased NCC abundance (Bickel et al. 2001). Subsequent studies confirmed that NCC is hyperphosphorylated in these animals, caused by an increase in total SPAK/OSR1 activity (Komers et al. 2012; (Chavez-Canales et al. 2013). Similar results were noted in another model of type 2 diabetes, the leptin receptor-deficient db/db mouse. Crossing db/db mice with SPAK/OSR1 catalytically inactive “T-loop” knock-in mice that cannot be activated by WNKs, the stimulatory effect of insulin on NCC was completely abrogated (Nishida et al. 2012). This indicates that insulin activates NCC via the WNK-SPAK/OSR1 pathway. Although it seems clear that SPAK/OSR1 is the key downstream mediator of NCC stimulation, there have been conflicting data with regards to which of the upstream WNK kinases mediate the effect. Data from Chavez-Canales et al. (2013) suggest that WNK3 is required for insulin to stimulate NCC activity, while studies by Sohara et al. (2011) and Takahashi et al. (2014) provide evidence that WNK4 is the dominant WNK kinase that mediates the effect. The phosphoinositol 3-kinase/-mTORC2-Akt pathway transduces the upstream signal from insulin receptors to the WNK-SPAK/OSR1 network (Nishida et al. 2012; Chavez-Canales et al. 2013).
3.10 Concluding Remarks
Although interest in the thiazide-sensitive cotransporter had only been modest for years, exciting genetic studies, the discovery of key NCC regulators, and the advent of tools to biochemically monitor NCC activation status have yielded an explosion of new insights into its role in physiology. Furthermore, the recent linkage of KLHL3 and CUL3 to FHHt, as well as the generation of new genetic models to study the WNK-SPAK/OSR1 pathway has opened up new and important questions and avenues of investigation. As the underlying molecular wiring that connects important blood pressure, potassium, and volume control systems to NCC in the DCT is elucidated further, perhaps new targets for the treatment of clinical disorders such as salt-sensitive hypertension, hyperkalemia, edema, and nephrolithiasis will be established.
References
Acuna R, Martinez-de-la-Maza L, Ponce-Coria J, Vazquez N, Ortal-Vite P, Pacheco-Alvarez D, Bobadilla NA, Gamba G (2011) Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J Hypertens 29(3):475–483. https://doi.org/10.1097/HJH.0b013e328341d0fd
Argaiz ER, Chavez-Canales M, Ostrosky-Frid M, Rodriguez-Gama A, Vazquez N, Gonzalez-Rodriguez X, Garcia-Valdes J, Hadchouel J, Ellison D, Gamba G (2018) Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC. Am J Physiol Renal Physiol 315(3):F734–F745. https://doi.org/10.1152/ajprenal.00145.2018
Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, Ruffieux-Daidie D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O (2011) Nedd4-2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22(9):1707–1719. https://doi.org/10.1681/ASN.2011020132
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. JMCP 13(8 Suppl B):9–20
Baggio B, Bordin L, Clari G, Gambaro G, Moret V (1993) Functional correlation between the Ser/Thr-phosphorylation of band-3 and band-3-mediated transmembrane anion transport in human erythrocytes. Biochim Biophys Acta 1148(1):157–160
Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA (2001) Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 281(4):F639–F648
Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH (1998) 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9(8):1347–1358
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482(7383):98–102. https://doi.org/10.1038/nature10814
Boyd-Shiwarski CR, Shiwarski DJ, Roy A, Namboodiri HN, Nkashama LJ, Xie J, McClain KL, Marciszyn A, Kleyman TR, Tan RJ, Stolz DB, Puthenveedu MA, Huang CL, Subramanya AR (2018) Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent. Mol Biol Cell 29(4):499–509. https://doi.org/10.1091/mbc.E17-08-0529
Brown RH Jr (1991) Molecular basis for hyperkalemic periodic paralysis. Int J Neurol 25-26:89–96
Butterworth MB, Edinger RS, Johnson JP, Frizzell RA (2005) Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125(1):81–101. https://doi.org/10.1085/jgp.200409124
Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB (2006) WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69(12):2162–2170
Castaneda-Bueno M, Gamba G (2012) Mechanisms of sodium-chloride cotransporter modulation by angiotensin II. Curr Opin Nephrol Hypertens 21(5):516–522. https://doi.org/10.1097/MNH.0b013e32835571a4
Castaneda-Bueno M, Vazquez N, Bustos-Jaimes I, Hernandez D, Rodriguez-Lobato E, Pacheco-Alvarez D, Carino-Cortes R, Moreno E, Bobadilla NA, Gamba G (2010) A single residue in transmembrane domain 11 defines the different affinity for thiazides between the mammalian and flounder NaCl transporters. Am J Physiol Renal Physiol 299(5):F1111–F1119. https://doi.org/10.1152/ajprenal.00412.2010
Chavez-Canales M, Arroyo JP, Ko B, Vazquez N, Bautista R, Castaneda-Bueno M, Bobadilla NA, Hoover RS, Gamba G (2013) Insulin increases the functional activity of the renal NaCl cotransporter. J Hypertens 31(2):303–311. https://doi.org/10.1097/HJH.0b013e32835bbb83
Chavez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castaneda-Bueno M, Vazquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J (2014) WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension 64(5):1047–1053. https://doi.org/10.1161/HYPERTENSIONAHA.114.04036
Chen ZF, Vaughn DA, Beaumont K, Fanestil DD (1990) Effects of diuretic treatment and of dietary sodium on renal binding of 3H-metolazone. J Am Soc Nephrol 1(1):91–98
Chen Z, Vaughn DA, Blakely P, Fanestil DD (1994a) Adrenocortical steroids increase renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5(6):1361–1368
Chen Z, Vaughn DA, Fanestil DD (1994b) Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5(4):1112–1119
Chen JC, Lo YF, Lin YW, Lin SH, Huang CL, Cheng CJ (2019) WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci USA 116(10):4502–4507. https://doi.org/10.1073/pnas.1817220116
Cheng L, Poulsen SB, Wu Q, Esteva-Font C, Olesen ETB, Peng L, Olde B, Leeb-Lundberg LMF, Pisitkun T, Rieg T, Dimke H, Fenton RA (2019) Rapid aldosterone-mediated signaling in the DCT increases activity of the thiazide-sensitive NaCl cotransporter. J Am Soc Nephrol 30(8):1454–1470. https://doi.org/10.1681/ASN.2018101025
Chew TA, Orlando BJ, Zhang J, Latorraca NR, Wang A, Hollingsworth SA, Chen DH, Dror RO, Liao M, Feng L (2019) Structure and mechanism of the cation-chloride cotransporter NKCC1. Nature 572(7770):488–492. https://doi.org/10.1038/s41586-019-1438-2
Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S (2008) Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74(11):1403–1409. https://doi.org/10.1038/ki.2008.451
Costanzo LS (1988) Mechanism of action of thiazide diuretics. Semin Nephrol 8(3):234–241
Costanzo LS, Windhager EE (1978) Calcium and sodium transport by the distal convoluted tubule of the rat. Am J Phys 235(5):F492–F506
Darman RB, Forbush B (2002) A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem 277(40):37542–37550. https://doi.org/10.1074/jbc.M206293200
de Jong JC, Willems PH, Mooren FJ, van den Heuvel LP, Knoers NV, Bindels RJ (2003) The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J Biol Chem 278(27):24302–24307. https://doi.org/10.1074/jbc.M303101200
Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X (2003) Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23(24):9208–9221
Dell’Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS (1997) AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J 16(5):917–928
Delpire E, Gagnon KB (2006) SPAK and OSR1, key kinases involved in the regulation of chloride transport. Acta Physiol (Oxf) 187(1–2):103–113
Dimke H, San-Cristobal P, de Graaf M, Lenders JW, Deinum J, Hoenderop JG, Bindels RJ (2011) γ-Adducin stimulates the thiazide-sensitive NaCl cotransporter. J Am Soc Nephrol 22(3):508–517. https://doi.org/10.1681/ASN.2010060606
Donnelly BF, Needham PG, Snyder AC, Roy A, Khadem S, Brodsky JL, Subramanya AR (2013) Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis. J Biol Chem 288(18):13124–13135. https://doi.org/10.1074/jbc.M113.455394
Earley LE, Orloff J (1964) Thiazide diuretics. Annu Rev Med 15:149. https://doi.org/10.1146/Annurev.Me.15.020164.001053
Ellison DH (2003) The thiazide-sensitive Na-Cl cotransporter and human disease: reemergence of an old player. J Am Soc Nephrol 14(2):538–540
Ellison DH, Terker AS (2015) Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc 126:46–55
Ellison DH, Velazquez H, Wright FS (1987) Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Phys 253(3 Pt 2):F546–F554
Ellison DH, Velazquez H, Wright FS (1989) Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 83(1):113–126. https://doi.org/10.1172/JCI113847
Farman N, Bonvalet JP (1983) Aldosterone binding in isolated tubules. III Autoradiography along the rat nephron. Am J Physiol 245(5 Pt 1):F606–F614
Farman N, Oblin ME, Lombes M, Delahaye F, Westphal HM, Bonvalet JP, Gasc JM (1991) Immunolocalization of gluco- and mineralocorticoid receptors in rabbit kidney. Am J Phys 260(2 Pt 1):C226–C233
Flatman PW (2002) Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta 1566(1–2):140–151
Freeman RB, Maher JF, Schreiner GE, Mostofi FK (1962) Renal tubular necrosis due to nephrotoxicity of organic mercurial diuretics. Ann Intern Med 57:34–43
Gagnon KB, England R, Delpire E (2006) Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol 26(2):689–698
Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85(2):423–493. https://doi.org/10.1152/physrev.00011.2004
Gamba G (2010) Vasopressin regulates the renal Na+-Cl− cotransporter. Am J Physiol Renal Physiol 298(3):F500–F501. https://doi.org/10.1152/ajprenal.00723.2009
Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC (1993) Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA 90(7):2749–2753
Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269(26):17713–17722
Geva Y, Schuldiner M (2014) The back and forth of cargo exit from the endoplasmic reticulum. Curr Biol 24(3):R130–R136. https://doi.org/10.1016/j.cub.2013.12.008
Glover M, Mercier Zuber A, Figg N, O’Shaughnessy KM (2010) The activity of the thiazide-sensitive Na(+)-Cl(−) cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88(10):986–995. https://doi.org/10.1139/y10-080
Glover M, Ware JS, Henry A, Wolley M, Walsh R, Wain LV, Xu S, Van’t Hoff WG, Tobin MD, Hall IP, Cook S, Gordon RD, Stowasser M, O'Shaughnessy KM (2014) Detection of mutations in KLHL3 and CUL3 in families with FHHt (familial hyperkalaemic hypertension or Gordon’s syndrome). Clin Sci (Lond) 126(10):721–726. https://doi.org/10.1042/CS20130326
Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL (2009) N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol 184(6):847–862. https://doi.org/10.1083/jcb.200808124
Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O’Shaughnessy KM (2006) Regulation of the expression of the Na/Cl cotransporter (NCCT) by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00468.2005
Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA (2013) The absence of intrarenal ACE protects against hypertension. J Clin Invest 123(5):2011–2023. https://doi.org/10.1172/JCI65460
Gordon RD (1986) Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 8(2):93–102
Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA (2012) SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287(45):37673–37690. https://doi.org/10.1074/jbc.M112.402800
Guyton AC (1991) Blood pressure control—special role of the kidneys and body fluids. Science 252(5014):1813–1816
Hadchouel J, Delaloy C, Faure S, Achard JM, Jeunemaitre X (2006) Familial hyperkalemic hypertension. J Am Soc Nephrol 17(1):208–217. https://doi.org/10.1681/ASN.2005030314
Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X (2010) Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci USA 107(42):18109–18114. https://doi.org/10.1073/pnas.1006128107
Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH (2011) The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17(10):1304–1309. https://doi.org/10.1038/nm.2497
Hoover RS, Poch E, Monroy A, Vazquez N, Nishio T, Gamba G, Hebert SC (2003) N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+):Cl(−) cotransporter. J Am Soc Nephrol 14(2):271–282
Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP (2008) Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40(5):592–599
Khuri RN, Strieder N, Wiederholt M, Giebisch G (1975) Effects of graded solute diuresis on renal tubular sodium transport in the rat. Am J Phys 228(4):1262–1268
Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA (1998) The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95(24):14552–14557
Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS (2007) Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104(50):20120–20125. https://doi.org/10.1073/pnas.0709506104
Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS (2010) RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299(2):F300–F309. https://doi.org/10.1152/ajprenal.00441.2009
Ko B, Mistry AC, Hanson L, Mallick R, Cooke LL, Hack BK, Cunningham P, Hoover RS (2012) A new model of the distal convoluted tubule. Am J Physiol Renal Physiol 303(5):F700–F710. https://doi.org/10.1152/ajprenal.00139.2012
Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH (2012) Enhanced phosphorylation of Na(+)-Cl− co-transporter in experimental metabolic syndrome: role of insulin. Clin Sci (Lond) 123(11):635–647. https://doi.org/10.1042/CS20120003
Krishnamurthy H, Gouaux E (2012) X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481(7382):469–474. https://doi.org/10.1038/nature10737
Krishnamurthy H, Piscitelli CL, Gouaux E (2009) Unlocking the molecular secrets of sodium-coupled transporters. Nature 459(7245):347–355. https://doi.org/10.1038/nature08143
Kunau R (1974) Nature of Chloruretic effect of thiazide diuretics. Kidney Int 6(6):A62–A63
Kunchaparty S, Palcso M, Berkman J, Velazquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH (1999) Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am J Phys 277(4 Pt 2):F643–F649
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP (2006) Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38(10):1124–1132. https://doi.org/10.1038/ng1877
Leaphart CL, Dai S, Gribar SC, Richardson W, Ozolek J, Shi XH, Bruns JR, Branca M, Li J, Weisz OA, Sodhi C, Hackam DJ (2008) Interferon-gamma inhibits enterocyte migration by reversibly displacing connexin43 from lipid rafts. Am J Physiol Gastrointest Liver Physiol 295(3):G559–G569. https://doi.org/10.1152/ajpgi.90320.2008
Lee DH, Riquier AD, Yang LE, Leong PK, Maunsbach AB, McDonough AA (2009) Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol 296(4):F810–F818. https://doi.org/10.1152/ajprenal.90606.2008
Lee DH, Maunsbach AB, Riquier-Brison AD, Nguyen MT, Fenton RA, Bachmann S, Yu AS, McDonough AA (2013) Effects of ACE inhibition and ANG II stimulation on renal Na-Cl cotransporter distribution, phosphorylation, and membrane complex properties. Am J Physiol Cell Physiol 304(2):C147–C163. https://doi.org/10.1152/ajpcell.00287.2012
Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS (2011) Impaired phosphorylation of Na(+)-K(+)-2Cl(−) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci USA 108(42):17538–17543. https://doi.org/10.1073/pnas.1107452108
Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL (2011) Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20(5):855–866. https://doi.org/10.1093/hmg/ddq525
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood P, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X (2012) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44(4):456–460. https://doi.org/10.1038/ng.2218
Lytle C, Forbush B (1996) Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Phys 270(2 Pt 1):C437–C448
Markadieu N, Rios K, Spiller BW, McDonald WH, Welling PA, Delpire E (2014) Short forms of SPAK in the kidney are created by Dnpep-mediated proteolytic cleavage. J Biol Chem. https://doi.org/10.1074/jbc.M114.604009
Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z (2002) Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87(7):3248–3254
McCormick JA, Yang CL, Ellison DH (2008) WNK kinases and renal sodium transport in health and disease: an integrated view. Hypertension 51(3):588–596. https://doi.org/10.1161/HYPERTENSIONAHA.107.103788
McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH (2011) A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14(3):352–364. https://doi.org/10.1016/j.cmet.2011.07.009
McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH (2014) Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124(11):4723–4736. https://doi.org/10.1172/JCI76126
McDonough AA, Youn JH (2013) Need to quickly excrete K+? Turn off NCC. Kidney Int 83(5):779–782. https://doi.org/10.1038/ki.2012.468
Mederle K, Mutig K, Paliege A, Carota I, Bachmann S, Castrop H, Oppermann M (2013) Loss of WNK3 is compensated for by the WNK1/SPAK axis in the kidney of the mouse. Am J Physiol Renal Physiol 304(9):F1198–F1209. https://doi.org/10.1152/ajprenal.00288.2012
Min X, Lee BH, Cobb MH, Goldsmith EJ (2004) Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure 12(7):1303–1311. https://doi.org/10.1016/j.str.2004.04.014
Mistry AC, Wynne BM, Yu L, Tomilin V, Yue Q, Zhou Y, Al-Khalili O, Mallick R, Cai H, Alli AA, Ko B, Mattheyses A, Bao HF, Pochynyuk O, Theilig F, Eaton DC, Hoover RS (2016) The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem J 473(19):3237–3252. https://doi.org/10.1042/BCJ20160312
Moreno E, Cristobal PS, Rivera M, Vazquez N, Bobadilla NA, Gamba G (2006) Affinity-defining domains in the Na-Cl cotransporter: a different location for Cl− and thiazide binding. J Biol Chem 281(25):17266–17275. https://doi.org/10.1074/jbc.M602614200
Morsing P, Velazquez H, Wright FS, Ellison DH (1991) Adaptation of distal convoluted tubule of rats. II Effects of chronic thiazide infusion. Am J Physiol 261(1 Pt 2):F137–F143
Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Bohlick A, Bleich M, Shan Q, Bachmann S (2010) Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298(3):F502–F509. https://doi.org/10.1152/ajprenal.00476.2009
Navar LG, Rosivall L (1984) Contribution of the renin-angiotensin system to the control of intrarenal hemodynamics. Kidney Int 25(6):857–868
Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E (2009) Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 190:133–157. https://doi.org/10.1007/978-3-540-79885-9_6
Needham PG, Mikoluk K, Dhakarwal P, Khadem S, Snyder AC, Subramanya AR, Brodsky JL (2011) The thiazide-sensitive NaCl cotransporter is targeted for chaperone-dependent endoplasmic reticulum-associated degradation. J Biol Chem 286(51):43611–43621. https://doi.org/10.1074/jbc.M111.288928
Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T, Sasaki S, Uchida S (2012) Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 60(4):981–990. https://doi.org/10.1161/HYPERTENSIONAHA.112.201509
Novello FC, Sprague JM (1957) Benzothiadiazine dioxides as novel diuretics. J Am Chem Soc 79(8):2028–2029. https://doi.org/10.1021/Ja01565a079
O’Reilly M, Marshall E, Speirs HJ, Brown RW (2003) WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14(10):2447–2456
O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW (2006) Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17(9):2402–2413
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T (2013) The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451(1):111–122. https://doi.org/10.1042/BJ20121903
Oi K, Sohara E, Rai T, Misawa M, Chiga M, Alessi DR, Sasaki S, Uchida S (2012) A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC co-transporters in vivo. Biol Open 1(2):120–127. https://doi.org/10.1242/bio.2011048
Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G (2006) The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281(39):28755–28763. https://doi.org/10.1074/jbc.M603773200
Paver WK, Pauline GJ (1964) Hypertension and hyperpotassaemia without renal disease in a young male. Med J Aust 2:305–306
Pearce D (2001) The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol Metab 12(8):341–347
Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA (2010) Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78(2):160–169. https://doi.org/10.1038/ki.2010.130
Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ (2014) Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7(324):ra41. https://doi.org/10.1126/scisignal.2005050
Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J (2014) Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25(3):511–522. https://doi.org/10.1681/ASN.2012121202
Piechotta K, Garbarini N, England R, Delpire E (2003) Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem 278(52):52848–52856
Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR (2010) Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2(2):63–75. https://doi.org/10.1002/emmm.200900058
Renfro JL (1977) Interdependence of active Na+ and Cl− transport by the isolated urinary bladder of the teleost, Pseudopleuronectes americanus. J Exp Zool 199(3):383–390. https://doi.org/10.1002/jez.1401990311
Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA (2014) Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306(9):F1059–F1068. https://doi.org/10.1152/ajprenal.00015.2014
Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR (2008) Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121(Pt 5):675–684. https://doi.org/10.1242/jcs.025312
Rinehart J, Kahle KT, de Los HP, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP (2005) WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 102(46):16777–16782. https://doi.org/10.1073/pnas.0508303102
Rinehart J, Vazquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP (2011) WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem 286(34):30171–30180. https://doi.org/10.1074/jbc.M111.222893
Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, Devuyst O, Belgian Network for Study of Gitelman S (2007) Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 18(4):1271–1283. https://doi.org/10.1681/ASN.2006101095
Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O (2013) Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123(2):657–665. https://doi.org/10.1172/JCI61110
Rosenbaek LL, Assentoft M, Pedersen NB, MacAulay N, Fenton RA (2012) Characterization of a novel phosphorylation site in the sodium-chloride cotransporter, NCC. J Physiol 590(Pt 23):6121–6139. https://doi.org/10.1113/jphysiol.2012.240986
Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA (2014) Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289(19):13347–13361. https://doi.org/10.1074/jbc.M113.543710
Rosenbaek LL, Rizzo F, Wu Q, Rojas-Vega L, Gamba G, MacAulay N, Staub O, Fenton RA (2017) The thiazide sensitive sodium chloride co-transporter NCC is modulated by site-specific ubiquitylation. Sci Rep 7(1):12981. https://doi.org/10.1038/s41598-017-12819-0
Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YP, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR (2015) Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125(9):3433–3448. https://doi.org/10.1172/JCI75245
Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH (2009) Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119(9):2601–2612. https://doi.org/10.1172/JCI38323
Sabath E, Meade P, Berkman J, de los Heros P, Moreno E, Bobadilla NA, Vazquez N, Ellison DH, Gamba G (2004) Pathophysiology of functional mutations of the thiazide-sensitive Na-Cl cotransporter in Gitelman disease. Am J Physiol Renal Physiol 287(2):F195–F203. https://doi.org/10.1152/ajprenal.00044.2004
San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G (2009) Angiotensin II signaling increases activity of the renal Na-cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106(11):4384–4389. https://doi.org/10.1073/pnas.0813238106
Sandberg MB, Maunsbach AB, McDonough AA (2006) Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291(2):F503–F508. https://doi.org/10.1152/ajprenal.00482.2005
Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB (2007) ANG II provokes acute trafficking of distal tubule Na+-Cl(−) cotransporter to apical membrane. Am J Physiol Renal Physiol 293(3):F662–F669. https://doi.org/10.1152/ajprenal.00064.2007
Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demaretz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K (2013) SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24(3):407–418. https://doi.org/10.1681/ASN.2012040404
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP (2013) Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA 110(19):7838–7843. https://doi.org/10.1073/pnas.1304592110
Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP (1996) Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12(1):24–30
Simpson F, Peden AA, Christopoulou L, Robinson MS (1997) Characterization of the adaptor-related protein complex, AP-3. J Cell Biol 137(4):835–845
Singh SK, Piscitelli CL, Yamashita A, Gouaux E (2008) A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322(5908):1655–1661. https://doi.org/10.1126/science.1166777
Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC (2004) cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279(44):45753–45758. https://doi.org/10.1074/jbc.M407858200
Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S (2011) Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One 6(8):e24277. https://doi.org/10.1371/journal.pone.0024277
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J (2013) Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83(5):811–824. https://doi.org/10.1038/ki.2013.14
Stokes JB (1984) Sodium chloride absorption by the urinary bladder of the winter flounder. A thiazide-sensitive, electrically neutral transport system. J Clin Invest 74(1):7–16. https://doi.org/10.1172/JCI111420
Subramanya AR, Ellison DH (2014) Distal convoluted tubule. Clin J Am Soc Nephrol 9(12):2147–2163. https://doi.org/10.2215/CJN.05920613
Subramanya AR, Welling PA (2011) Toward an understanding of hypertension resistance. Am J Physiol Renal Physiol 300(4):F838–F839. https://doi.org/10.1152/ajprenal.00078.2011
Subramanya AR, Yang CL, Zhu X, Ellison DH (2006) Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290(3):F619–F624. https://doi.org/10.1152/ajprenal.00280.2005
Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA (2009) WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284(27):18471–18480. https://doi.org/10.1074/jbc.M109.008185
Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S (2014) WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34(3). https://doi.org/10.1042/BSR20140047
Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S (2010) Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Commun 393(4):844–848. https://doi.org/10.1016/j.bbrc.2010.02.096
Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH (2015) Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21(1):39–50. https://doi.org/10.1016/j.cmet.2014.12.006
Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH (2016) Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89(1):127–134. https://doi.org/10.1038/ki.2015.289
Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR (2012) SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J 441(1):325–337. https://doi.org/10.1042/BJ20111879
Tovar-Palacio C, Bobadilla NA, Cortes P, Plata C, de los Heros P, Vazquez N, Gamba G (2004) Ion and diuretic specificity of chimeric proteins between apical Na(+)-K(+)-2Cl(−) and Na(+)-Cl(−) cotransporters. Am J Physiol Renal Physiol 287(3):F570–F577. https://doi.org/10.1152/ajprenal.00124.2004
Vallon V, Schroth J, Lang F, Kuhl D, Uchida S (2009) Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297(3):F704–F712. https://doi.org/10.1152/ajprenal.00030.2009
van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ (2011) Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79(1):66–76. https://doi.org/10.1038/ki.2010.290
van der Lubbe N, Lim CH, Meima ME, van Veghel R, Rosenbaek LL, Mutig K, Danser AH, Fenton RA, Zietse R, Hoorn EJ (2012) Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflugers Arch 463(6):853–863. https://doi.org/10.1007/s00424-012-1104-0
Vazquez N, Monroy A, Dorantes E, Munoz-Clares RA, Gamba G (2002) Functional differences between flounder and rat thiazide-sensitive Na-Cl cotransporter. Am J Physiol Renal Physiol 282(4):F599–F607. https://doi.org/10.1152/ajprenal.00284.2001
Velazquez H, Bernstein PL, Bartiss A, Kunchaparty S, Ellison DH (1995) Thiazide-sensitive Nacl transport capacity of rat renal distal tubule is enhanced by adrenal-steroids. FASEB J 9(3):A586–A586
Velazquez H, Naray-Fejes-Toth A, Silva T, Andujar E, Reilly RF, Desir GV, Ellison DH (1998) Rabbit distal convoluted tubule coexpresses NaCl cotransporter and 11 beta-hydroxysteroid dehydrogenase II mRNA. Kidney Int 54(2):464–472. https://doi.org/10.1046/j.1523-1755.1998.00036.x
Vembar SS, Brodsky JL (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9(12):944–957. https://doi.org/10.1038/nrm2546
Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC (1998) Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101(8):1661–1669. https://doi.org/10.1172/JCI601
Vidal-Petiot E, Cheval L, Faugeroux J, Malard T, Doucet A, Jeunemaitre X, Hadchouel J (2012) A new methodology for quantification of alternatively spliced exons reveals a highly tissue-specific expression pattern of WNK1 isoforms. PLoS One 7(5):e37751. https://doi.org/10.1371/journal.pone.0037751
Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J (2013) WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci USA 110(35):14366–14371. https://doi.org/10.1073/pnas.1304230110
Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DM (2007) Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep 8(9):839–845. https://doi.org/10.1038/sj.embor.7401048
Vitari AC, Deak M, Morrice NA, Alessi DR (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391(Pt 1):17–24. https://doi.org/10.1042/BJ20051180
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397(1):223–231. https://doi.org/10.1042/BJ20060220
Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S (2013) Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 3(3):858–868. https://doi.org/10.1016/j.celrep.2013.02.024
Ward CL, Kopito RR (1994) Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem 269(41):25710–25718
Warmuth S, Zimmermann I, Dutzler R (2009) X-ray structure of the C-terminal domain of a prokaryotic cation-chloride cotransporter. Structure 17(4):538–546. https://doi.org/10.1016/j.str.2009.02.009
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293(5532):1107–1112. https://doi.org/10.1126/science.1062844
Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP (2003) Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100(2):680–684. https://doi.org/10.1073/pnas.242735399
Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275(22):16795–16801
Yang CL, Angell J, Mitchell R, Ellison DH (2003) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111(7):1039–1045. https://doi.org/10.1172/JCI17443
Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH (2005) Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest 115(5):1379–1387. https://doi.org/10.1172/JCI22452
Yang CL, Zhu X, Ellison DH (2007a) The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117(11):3403–3411. https://doi.org/10.1172/JCI32033
Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S (2007b) Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5(5):331–344. https://doi.org/10.1016/j.cmet.2007.03.009
Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH (2010) SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21(11):1868–1877. https://doi.org/10.1681/ASN.2009121295
Yang SS, Fang YW, Tseng MH, Chu PY, Yu IS, Wu HC, Lin SW, Chau T, Uchida S, Sasaki S, Lin YF, Sytwu HK, Lin SH (2013) Phosphorylation regulates NCC stability and transporter activity in vivo. J Am Soc Nephrol 24(10):1587–1597. https://doi.org/10.1681/ASN.2012070742
Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22(3):537–548
Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH (2014) KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111(32):11864–11869. https://doi.org/10.1073/pnas.1411705111
Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H (2009) WNK4 enhances the degradation of NCC through a Sortilin-mediated lysosomal pathway. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2008121275
Zhou B, Wang D, Feng X, Zhang Y, Wang Y, Zhuang J, Zhang X, Chen G, Delpire E, Gu D, Cai H (2012) WNK4 inhibits NCC protein expression through MAPK ERK1/2 signaling pathway. Am J Physiol Renal Physiol 302(5):F533–F539. https://doi.org/10.1152/ajprenal.00032.2011
Acknowledgments
This work was supported by grants from the NIH (R01-DK098145 and R01-DK119252), and by the VA Pittsburgh Healthcare System Research Service.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The American Physiological Society
About this chapter
Cite this chapter
Subramanya, A.R. (2020). Thiazide-Sensitive NaCl Cotransporter. In: Hamilton, K.L., Devor, D.C. (eds) Studies of Epithelial Transporters and Ion Channels. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-55454-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-55454-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55453-8
Online ISBN: 978-3-030-55454-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)