Abstract
The conceptual breakthrough that the energy of the Na+ gradient generated by the Na+/K+ ATPase (pump) could be used as the driving force for another membrane transport protein has led to the functional and molecular identification of multiple secondary active transporters. We have organized this chapter to address the expression, function, regulation, and evolutionary importance of the two isoforms of the electroneutral sodium–potassium–chloride cotransporter (NKCC). The combination of basolateral expression of the sodium–potassium pump and NKCC1 in various non-renal epithelial results in salt and water secretion, whereas basolateral expression of the pump with an apical expression of NKCC2 in the thick ascending limb of Henle of the kidney nephron results in salt and water reabsorption. NKCCs are regulated by phosphorylation of specific serine/threonine residues in their cytosolic amino-terminal domains, and the evolutionary conservation of these cotransporters from protists to humans confirms their vital role in cellular and whole-organism physiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Prokaryotic and eukaryotic cells have surrounded themselves with two leaflets of phospholipids forming a membrane or “oil” interface that isolates their biochemical reactions from the extracellular environment. However, complete isolation from the outside environment is incompatible with life, as the uptake of nutrients and elimination of waste are necessary steps of cell metabolism. By insertion of transmembrane proteins, cells have acquired the capability to move water and solutes across this lipid barrier and gained the capacity to modulate the flow of these substrates. Sugars, amino acids, organic compounds, and inorganic ions such as Na+, K+, Cl−, Ca2+, Mg2+, and HCO3 − are able to cross the lipid membrane (in conjunction with water) in and out of cells. In a multicellular organism, depending on its specific function within the organism, a cell will express a certain array of channels, pumps, cotransporters, and exchangers at its plasma membrane, allowing for a defined and regulated movement of solutes.
2.2 Ouabain-Insensitive Cation Pump?
In the early 1960s, physiologists believed that transport of Na+ and K+ across human red blood cell membranes was either energy-dependent, through the ATP-dependent cation (Na+/K+) pump, or a result of passive diffusion in the direction of the ions electrochemical gradient. The Na+/K+ ATPase exchanges K+ outside for Na+ inside and is inhibited by cardiac glycosides (e.g., ouabain). Identification of another transport pathway for Na+ and K+ which was “ouabain-insensitive” but still dependent on external Na+ prompted investigators to search for another type of cation pump (Hoffman and Kregenow 1966). In fact, what they considered to be an active transport mechanism dependent on external Na+ was actually the first evidence of a secondary active transport mechanism (i.e., Na-K-2Cl cotransporter) dependent on the electrochemical gradient of Na+ generated by the “ouabain-sensitive” cation pump. This “sodium-gradient hypothesis” was first proposed by R.K. Crane and represents a conceptual breakthrough in ion transport (Crane 1965).
In Fig. 2.1, the cation exchange activity of the Na+/K+ ATPase maintains the intracellular Na+ concentration significantly lower than the extracellular concentration while simultaneously maintaining the intracellular K+ higher than the extracellular concentration. These concentration gradients provide the energy that secondary active transporters, such as the Na-K-2Cl cotransporter and the Na-glucose cotransporter, use to power the movement of other solutes against their own concentration gradients. In Box 2.1, the individual driving forces acting on Na+, K+, and Cl− across the cell membrane can be added together to determine the net driving force and direction of the Na-K-2Cl cotransporter. Although accepted today, there was considerable resistance to the concept of a membrane transport protein using the downhill concentration gradient of one solute to move another solute uphill against its concentration gradient. Such a mechanism did not conform to the Second Law of Thermodynamics. Today, there are multiple Na+-coupled cotransporters which not only move inorganic ions but also amino acids, neurotransmitters, and even carbohydrates. In the remainder of this chapter, we will focus on a subset of the inorganic ion transporters, the Na+-coupled cation–chloride cotransporters, and their role in epithelial health and disease.
The Na-K-2Cl cotransporter is a secondary active transport mechanism. Schematic representation of a cell with the Na+/K+ ATPase accumulating K+ and extruding Na+, thus creating energy in ionic concentration gradients that can be used by other transport mechanisms. As the very low [Na+] inside cells constitutes a driving force for the inward movement of glucose (even if higher glucose inside), the low [Na+] and [Cl−] inside cells also facilitate the inward movement of ions through the Na-K-2Cl cotransporter
Box 2.1 Calculating the Electrochemical Driving Forces on the Na-K-2Cl Cotransporter
The electrochemical potential gradient for an ion, Δμ ion, is the sum of the chemical and electrical components: Δμ ion = RT ln([ion]i/[ion]o) + ZFEm. For a cotransport system that couples the movement of multiple substrates, the net free energy or overall chemical potential gradient is the sum of the gradient for each substrate: Δμ Na,K,Cl = Δμ Na + Δμ K + 2Δμ Cl (because of two Cl ions transported on NKCC). Note that there is no electrical component as the cotransport of two cations with two anions is electroneutral.
Thus,
We can see that the direction of transport will be dictated by the ratio of the products. For a ratio larger than 1, Δμ Na,K,Cl will be positive, whereas for a ratio lower than 1, Δμ Na,K,Cl will be negative. At 1, the chemical potential gradient will be 0 (since ln(1) = 0), which represents thermodynamic equilibrium. If we consider external and internal ion concentrations of [Na]i = 10 mM, [Cl]i = 30 mM, [K]i = 100 mM, [Na]o = 130 mM, [Cl]o = 110 mM, and [K]o = 3, the ratio favors inward transport by 5:1.
2.3 Electrically Silent Plasma Membrane Cotransporters
In a set of companion papers in 1971, Floyd Kregenow described the two-phase response for duck erythrocytes incubated in nonhemolytic hypotonic and hypertonic media (Kregenow 1971a, b). He found that the instantaneous cell shrinkage induced by hypertonic shock was followed by a slower “volume regulatory phase” in which the cells swell back to their original size through a Na+-dependent gain of K+, Cl−, and water (Kregenow 1971b). The accumulation of K+ against its electrochemical gradient suggested an active transport mechanism; however, blockage of the Na+/K+ ATPase by ouabain had no effect on this volume controlling mechanism. Interestingly, regardless of the severity of the hypertonic challenge, there was no water recovery when external K+ concentration was maintained at normal levels (i.e., 2.8 mM). However, when the external K+ concentration was increased to 15 mM, the rate of cell water recovery was faster the greater the hypertonic challenge (see Fig. 2.2a). Although at first counter-intuitive, if we once again consider the individual driving forces acting on the Na-K-2Cl cotransporter (see Box 2.1), an increase in the external K+ concentration actually reduces the resistance of the outwardly directed K+ gradient on the net activity of the Na-K-2Cl cotransporter. This reduced resistance allows the cotransporter, to work more efficiently (ratio favors inward transport ~26:1) in transporting ions (and cell water) to promote cell volume recovery (see Fig. 2.2b).
The Na-K-2Cl cotransporter participates in cell volume regulation. (a) Data redrawn from Kregenow showing cell water in duck red blood cells recovering after an initial water loss due to the osmotic phase during a hypertonic stimulus (Kregenow 1971a, b). Note that the recovery is larger in cells exposed to larger osmotic shock and it occurs only when the driving force for Na-K-2Cl cotransport is increased by raising external K+. (b) Schematic representation of a cell responding to a hypertonic stimulus. The cell loses water during the osmotic phase, leading to NKCC1 activation, uptake of ions, and obligatory water
Although we have been identifying the Na+-dependent K+ and Cl− transport mechanism described by Kregenow as the Na-K-2Cl cotransporter, it actually took another 6 years before Geck and coworkers proposed the existence of a “secondary-active” cotransport system for Na+, K+, and Cl− in Ehrlich cells which exhibited the same “ouabain-insensitivity” and “volume control” identified by Kregenow. Thermodynamic experiments revealed a tight stoichiometric coupling of 1 Na+, 1 K+, and 2 Cl− ions in every transport cycle (Geck et al. 1980).
In 1994, independent research teams identified cDNA sequences for two Na-K-2Cl cotransporter isoforms. The first isoform, termed NKCC1, isolated from fish (Xu et al. 1994) and mammals (Delpire et al. 1994), appears to be ubiquitously expressed and has been mapped to chromosome 5q23 in humans and chromosome 18 in mice. The second isoform, termed NKCC2, was isolated from the mammalian kidney (Gamba et al. 1994; Payne and Forbush 1994), is expressed exclusively in the apical membrane of the thick ascending limb of Henle, and has been mapped to chromosome 15 in humans and chromosome 2 in mice. In Fig. 2.3, amino acid sequences of the two mouse isoforms have been aligned to illustrate identical (yellow), conserved (blue) and non-conserved (white) regions of the two proteins. The predominance of yellow color throughout the protein illustrates the high (58% overall and 76% in the transmembrane core) degree of amino acid residue similarity between the two cotransporters. Alternating light pink and light blue boxes identify the exons of the cotransporters and the portion of the protein (NH2-terminal, transmembrane, or COOH-terminal) encoded by each exon. There are three distinctive properties that functionally define Na-K-2Cl cotransport: (1) all three transported ions bind the cotransporter on the same side of the membrane; (2) transporter inhibition by loop diuretics (e.g., bumetanide and furosemide); and (3) electrically silent stoichiometry of ion translocation. In Sect. 2.5, we will discuss the functional regulation of these cotransporter proteins and the involvement of conserved kinase-binding sites and serine/threonine phosphorylation sites on the cytosolic NH2-terminal domain.
Schematic representation of NKCC1 and NKCC2 proteins. Alignment of the full-length mouse NKCC1 and NKCC2 amino acid sequences illustrating identical (yellow), conserved (blue), and non-conserved (white) regions of the two proteins are superimposed on the topology of the cotransporters with odd-numbered transmembrane domains in black and even-numbered domains in gray. Also shown in the cytosolic amino-terminal domain are conserved SPAK/OSR1 binding sites (RFxV sequences) and a region of multiple key phosphorylation sites (PO4). The exons of the SLC12A1 (NKCC2) and SLC12A2 (NKCC1) genes are represented in the background by alternate pink (odd) and blue (even) boxes
2.4 NKCC1
The human SLC12A2 gene encodes for a 1205 amino acid protein with 58% similarity with the second kidney-specific human isoform encoded by the SLC12A1 gene (see below). NKCC1 has 12 alpha-helical membrane-spanning regions flanked by large cytosolic NH2- and COOH-terminal domains. There are also several N-linked glycosylation sites on a large extracellular loop between transmembrane domain 7 and 8. RNA and protein expression studies have demonstrated that NKCC1 is expressed in various tissues including eye, stomach, heart, lung, brain, thymus, smooth and skeletal muscle, neurons, testis, colon and red blood cells (Delpire et al. 1994; Payne et al. 1995). This ubiquitous distribution suggests multiple physiological roles beyond salt reabsorption including Cl− and fluid secretion (Cook and Young 1989; Liedkte 1992), acid secretion (Soybel et al. 1995), cell volume homeostasis (Palfrey and O’Donnell 1992), and possibly even cell division and proliferation (Panet et al. 1994, 2000).
2.4.1 Electroneutrality, Stoichiometry, and Kinetic Properties
Geck and coworkers first described the “electrically-silent” nature of NKCC1 transport in 1980 (Geck et al. 1980). In their study, pulse-response experiments revealed a furosemide-sensitive tight coupling of 1 Na+, 1 K+, and 2 Cl− ions. As the two positive charges are nullified by the two negative charges, the cotransport of these ions neither affect the membrane potential nor does the membrane potential affect cotransport activity. In 1998, Lytle and coworkers (Lytle 1998) proposed a model of ordered binding and gliding symmetry where Na+ binds first, followed by Cl−, then K+, and a second Cl− and on the inside, the ions were released in the same order as binding: first on–first off (Fig. 2.4a). Through manipulation of the ionic composition both inside and outside the cell, they found that the tightly coupled ratio of 1 Na+; 1 K+; and 2 Cl− was maintained regardless of the internal and external ion composition. They also observed two partial reaction cycles: Na+/Na+ exchange in cells with high [Na+]i, and K+/K+ exchange in cells with high [K+]i (Lytle 1998). Although their model adequately explained the 1 Na+, 1 K+, 2 Cl− stoichiometry of the cotransporter, their first on-first off gliding symmetry model does not explain why Na+/Na+ exchange would be dependent on external K+ or why K+/K+ exchange would require internal Na+ but not external Na+?
Kinetic Models of NKCC1 cotransport. (a) Model proposed by Lytle et al. (1998). The cotransporter outside loads ions in an ordered fashion with Na+ binding first, followed by Cl, K+, then the second Cl− (steps 1–4). The fully loaded cotransporter then translocates (step 5) and releases ions on the other side on a first-on first-off basis. (b) Model proposed by Delpire and Gagnon (2011) where the cotransporter outside also loads ions in an ordered fashion but with Cl− binding first, followed by Na+, a second Cl−, then K+. After translocation, the release occurs in a more traditional fashion. Binding rate constants for each reaction are indicated by labels above and below the directional arrows. Computer simulations performed by Delpire and Gagnon (2011) provided several sets of rate constants, depending upon the number of cotransporters inserted in the membrane. Note that translocation of the loaded cotransporter is much faster (thicker arrows) than the translocation of the empty cotransporter (thinner arrows). Kinetics allow for some partial reactions to occur (slippage step)
We and others have observed atypical NKCC transport stoichiometries in many cell types which cannot be resolved by first on–first off gliding symmetry (Canessa et al. 1986; Gagnon and Delpire 2010; Hall and Ellory 1985; Lytle 1998; Orlov et al. 1996; Russell 1983). In 2011, we proposed a different model for ion binding in a preferred order based on velocity equations for K+ influx under both rapid equilibrium assumptions and combined equilibrium and steady-state assumptions. Our model has Cl− binding first, followed by a Na+, the second Cl−, and finally a K+ ion (Fig. 2.4b). Upon binding, the fully loaded cotransporter undergoes a conformational change (“translocates”) and releases the ions inside the cell in the reverse order to which they bound (i.e., K+, Cl−, Na+, and Cl−). In rapid equilibrium kinetics, the rate-limiting steps are the translocation steps, with the “fully-loaded” cotransporter translocating much faster than the “empty” cotransporter. In this model, some partial reactions are permitted (i.e., slippage), while preserving transport electroneutrality. Rapid translocation of fully loaded transporter and slippage steps can explain why under certain conditions, hypertonicity, for instance, the transporter unidirectionally moves more K+ than Cl−, and more Cl− than Na+. In these conditions, the preferred mode of transport is K+/K+ exchange, followed by K-Cl cotransport (Na+ is not released), and finally Na-K-2Cl cotransport (Delpire and Gagnon 2011).
In the Lytle study (Lytle 1998), they measured endogenous transport activity in duck red blood cells, whereas in our study (Delpire and Gagnon 2011), we measured transport activity of mouse NKCC1 heterologously overexpressed in Xenopus laevis oocytes. In our study, we altered the tonicity of the extracellular solutions, whereas, the Lytle study replaced transportable cations (Na+ and K+) with less-transportable cations (Li+ and Rb+), respectively. Our study used radiolabeled 22Na+, 86Rb+, and 36Cl− as tracers, whereas, the Haas study used a combination of radiolabeled tracers (22Na+, 86Rb+, and 36Cl−) and ion measurements from dilute perchloric acid extracts via air-acetylene flame spectrophotometry. Any combination of these differences could explain the two divergent models, and more experiments will be necessary to distinguish which better characterizes actual transport activity.
2.4.2 NKCC1 in Cl− Secreting Epithelia
Contrary to the salt reabsorption observed with NKCC2 (see next section), the basolateral expression of NKCC1 in most non-renal epithelial cells results in salt secretion. In Fig. 2.5, a model of a prototypical epithelial cell illustrates how NKCC1 utilizes the Na+ gradient generated by the basolateral expression of the Na+/K+ ATPase to import Na+, K+, and Cl− into the cell. The resulting increase in intracellular [Cl−] above electrochemical equilibrium provides the driving force for Cl− secretion into the lumen through apical Cl− channels. In many epithelia, the apical Cl− channel is the cAMP/Protein Kinase A (PKA)-regulated cystic fibrosis transmembrane conductance regulator (CFTR). CFTR is so named as it is the primary chloride channel mutated in patients with cystic fibrosis. Activation of CFTR by cAMP/PKA stimulation results in Cl− secretion into the lumen thereby depleting the cells of intracellular Cl−, which is then replenished through NKCC1 activity. This generic model is applicable to salivary, sweat, and lacrymal glands, as well as airway, gastric, and intestinal epithelial cells.
Schematic representation of a prototypical Cl− secreting non-renal epithelial cell. In this model, the Na+/K+ ATPase provides the energy for Cl− transport from the basolateral (blood) side to the apical (luminal) side of the epithelium. Cl− is secreted to the lumen at the apical membrane through a Cl− channel (typically CFTR) and the Na-K-2Cl cotransporter on the basolateral membrane constitutes the entry pathway for Cl− into the epithelial cell. Activation of the Cl− channel (e.g., through cAMP) results in a drop in intracellular Cl− which stimulates the NKCC1 function. The Na+/K+ ATPase recycles Na+ ions entering through the cotransporter. Basolateral K+ channels recycle K+ ions entering through the cotransporter and the Na+/K+ ATPase
In 1989, Wiener and colleagues were the first to demonstrate that Cl− secretion in colonic epithelial cells (Wiener and van Os 1989) follows the model of the shark rectal gland (i.e., driven by Na+-dependent Cl− transporter on the basolateral membrane and Cl− channels on the apical membrane) (Silva et al. 1977). Using [3H]-bumetanide binding and loop diuretic-sensitive isotope uptake experiments, Weiner and colleagues showed that a Na-K-Cl cotransporter was present on the basolateral membrane of both surface and crypt cells of the rabbit distal colon epithelium. The physiological relevance of this transporter was highlighted in studies using bumetanide in Ussing chamber preparations. For example, in rat colon, cAMP stimulation by forskolin greatly increased short circuit current due to activation of apical Cl− channels. The addition of bumetanide to the basolateral side completely abolishes this effect, confirming the importance of NKCC1 expression in the secretion process (Cheng 2012). A study in mouse intestine and human intestinal (HT29) cells showed that rosiglitazone and pioglitazone, two PPARγ agonists, significantly reduced forskolin-induced (cAMP) and carbachol-induced (Ca2+) electrogenic Cl− secretion. This decrease was associated with a significant reduction in CFTR, KCNQ1, and NKCC1 expression (Bajwa et al. 2009). Similarly, butyrate decreased NKCC1 expression in human colonic T84 epithelial cells, but not to a degree that affected forskolin or carbachol-mediated Cl− secretion (Resta-Lenert et al. 2001). In contrast to the agents described above that decrease Cl− secretion, there are also many factors that increase Cl− and fluid secretion in the intestine. These include pathogens such as Vibrio cholerae that causes diarrhea predominantly by activating the net secretion of chloride ions by the colon (Das et al. 2018). The mechanism is through internalization of the cholera toxin, stimulation of cAMP production, and activation of CFTR (Das et al. 2018, see also Chap. 2 of Volume 2 of this book). Again, similar studies were performed using other Cl− secreting epithelia, such as salivary, lacrimal, and airway epithelia.
2.4.3 NKCC1 in Kidney
Even though NKCC2 is the renal-specific cotransporter, NKCC1 is also present in several structures within the mammalian kidney. In the mouse, the cotransporter has been detected in smooth muscle cells of the afferent arteriole of the glomerulus, the extraglomerular mesangium, and the inner medullary collecting duct (Kaplan et al. 1996). In the rat, NKCC1 is also expressed in alpha intercalated cells of the outer medullary collecting duct (Ginns et al. 1996). These cells are acid-secreting cells and the cotransporter on the basolateral membrane might provide a pathway for Cl− entry from the blood side, thus helping H+ and Cl− secretion on the apical side. Note that the mouse versus rat difference in expression of the cotransporter in the medulla may reflect significant physiological differences between the two species. In fact, a recent study demonstrated that in middle-aged mice, expression of NKCC1 and reduced age-related hearing loss is dependent upon aldosterone (Halonen et al. 2016). Expression of NKCC1 in renin-producing smooth muscle cells suggests a role for the cotransporter in tubuloglomerular feedback and/or renin release, both sensitive to luminal Cl− concentrations (Kaplan et al. 1996). Basolateral expression of the cotransporter in the collecting duct epithelial cells may serve several roles. Physiological studies have shown that atrial natriuretic peptide (ANP) stimulates upregulation of Na-K-2Cl cotransporter expression and downregulation of the Na+ channel and Na+/K+ ATPase, in effect, altering the inner medullary collecting duct epithelial cells from re-absorbing Na+ to secreting Na+ (Rocha and Kudo 1990; Sonnenberg et al. 1990; Zeidel et al. 1988). The capacity of NH4 + to displace K+ at the binding site within the cotransporter suggests that basolateral expression of NKCC1 in the collecting duct epithelial cells may participate in H+/NH4 + secretion similar to that observed in the stomach (Soybel et al. 1995). Finally, the “volume sensitivity” of the cotransporter initially identified by Kregenow (1971b) in duck red blood cells may have a significant role in collecting duct epithelial cells that are routinely exposed to severe osmotic challenges.
2.4.4 NKCC1 in Other Epithelia
The stria vascularis is a multilayered epithelium of the inner ear extending from Reissner’s membrane to the spiral prominence. This unusual tissue has three primary cell types (marginal, intermediate, and basal cells) as well as intraepithelial capillaries. Basolateral expression of NKCC1 in the marginal cells, which directly face the endolymphatic compartment, does not participate in Cl− secretion like in most other epithelia, but in K+ secretion thereby generating a low-sodium, high-potassium cochlear endolymph (Tasaki and Spiropoulos 1959). On the apical membrane of these cells, a K+ channel (KCNQ1) associated with a beta-subunit (KCNE1) participates in the apical secretion of the cation. Mutations in either of the subunits result in Jervell and Lange-Nielsen syndrome, a type of long QT syndrome, associated with severe, bilateral hearing loss (Tyson et al. 1997). Knockout of the KCNQ1 channel in mice (Casimiro et al. 2001) or of its beta-subunit (Vetter et al. 1996) results in sensorineural deafness. Knockout of the Na-K-2Cl cotransporter in mice similarly results in sensorineural deafness (Delpire et al. 1999; Flagella et al. 1999). This precise localization of the cotransporter in the stria vascularis also explains the ototoxicity caused by the use of large doses of loop diuretics (Ikeda et al. 1997; Rybak 1993).
Another epithelial tissue expressing NKCC1 is the four choroid plexuses of the brain. Interestingly, NKCC1 expression is localized on the apical rather than the basolateral membrane of the choroid plexus (CP). As illustrated in Fig. 2.6, choroid plexus epithelial cells participate in the secretion of cerebrospinal fluid. Na+ movement through the Na+/K+ ATPase generates fluid movement. What is actually secreted in the ventricle is Na+-bicarbonate. On the basolateral side, Na+ enters through Na+-coupled bicarbonate transporters, such as the Na+-dependent Cl−/HCO3 − exchanger (NCBE) or the Na+-HCO3 − cotransporter (NBCn1) (Christensen et al. 2013). The location of NKCC1 on the apical membrane is counter to the movement of Na+ as Na-K-2Cl cotransport is inward. It is also known that choroid plexus epithelial cells can participate in K+ reabsorption. This is an important function, as the cerebrospinal fluid (CSF) K+ content, and consequently, the K+ concentration surrounding neurons need to be tightly regulated. The Na+/K+ ATPase can also serve as the primary mechanism for K+ reabsorption. However, placing the cotransporter alongside the pump has the advantage of uncoupling the pump obligatory exchange of Na+ with K+. Indeed, the Na+ that exits through the pump can now be reclaimed by the cotransporter (see Fig. 2.6).
Na-K-2Cl cotransport in choroid plexus. Picture: Single choroid plexus cell isolated with collagenase to keep apical versus basolateral polarity. After fixation, the cell was permeabilized and exposed to rabbit anti-NKCC1 polyclonal antibody followed by Cy3-conjugated anti-rabbit secondary antibody. Model: In these cuboidal epithelial cells, NKCC1 is located on the apical membrane alongside the Na+/K+ ATPase. The pump provides the energy for the secretion of Na-bicarbonate from the blood side to the luminal (cerebrospinal fluid (CSF)) side. Bicarbonate enters and exits the cell through distinct Na+-coupled bicarbonate transporters. The pump also provides the driving force for K+ reabsorption or movement of K+ from CSF to blood. Expression of NKCC1 on the apical membrane may allow for the uncoupling of K+ reabsorption from Na+ secretion
Several studies were recently published that question the role of NKCC1 in choroid plexus. First, it was shown that following brain hemorrhage, an inflammatory response involving toll-4 receptor activation, leads to the activation of SPAK, stimulation of NKCC1 function, and hypersecretion of CSF (Karimy et al. 2017). Intracerebroventricular (ICV) injection of blood into 8-week-old Wistar rats showed TLR4 signaling activation, increased nuclear translocation of p65 (a subunit of the NFκB–p65 transcription complex), and increased numbers of activated CD68+ choroid plexus myeloid cells. This response could be prevented using an NFκB inhibitor. Some 48 h after ICV blood injection, the rate of CSF production was threefold greater than control and remained stimulated for an additional 7 days. ICV injection of acetazolamide, an inhibitor of carbonic anhydrase activity, did not significantly reduce this CSF hypersecretion. In contrast, the rate of secretion was reduced 80% by ICV injection of bumetanide, implicating the Na-K-2Cl cotransporter. The levels of phospho-NKCC1 and phospho-SPAK, the active forms of the cotransporter and its activating kinase, were increased by 6.8-fold and twofold, respectively. The use of an inhibitor that prevents interaction between the kinase and the cotransporter also reduced CSF secretion. These data clearly indicate that NKCC1 function is key to CSF production. How can an inwardly poised cotransporter expressed on the apical membrane of choroid plexus epithelial cells participate to CSF secretion?
One possibility was offered in a second recent paper that argued for outward water transport associated with outward movement of Na+, K+, and Cl− ions through NKCC1 (Steffensen et al. 2018). They demonstrated that (1) water can move in association with NKCC1 activity even against an osmotic gradient, and (2) that the ion concentrations in the epithelial cells and CSF produced gradients that actually favored the outward instead of inward movement of ions. A third paper, however, challenged this second point, reporting ion concentrations in CP epithelial cells that are more consistent with traditional inward transport (Gregoriades et al. 2019). In a viewpoint published in the same issue of the American Journal of Physiology Cell Physiology, we identified possible weaknesses with each approach, arguing that the measured CP concentrations in the Steffensen paper were possibly affected by their assumptions of extracellular volume contamination. Any small changes in the amount of extracellular volume (rich in NaCl) associated with the tissue will affect the calculated intracellular ion concentrations. We also argued that isolation conditions and incubation of isolated cells in artificial bathing solutions for extended periods of time might have also affected the intracellular ion concentration in the Gregoriades study.
Interestingly, Gregoriades and coworkers, with evidence for inward Na-K-2Cl cotransport, did not argue against the role of NKCC1 in CSF secretion (Gregoriades et al. 2019). They propose that the activity of the cotransporter must be critical in maintaining CP epithelial cell volume and ion homeostasis to support CSF secretion. In agreement with the Karimy study (Karimy et al. 2017), Steffenson and coworkers also demonstrated that the application of bumetanide on the luminal side significantly reduced the level of CSF secretion in both in vitro and in vivo models (Steffensen et al. 2018). Thus, while NKCC1 clearly plays a role in the secretion of cerebrospinal fluid by the choroid plexus, the exact mechanism is still a matter of debate. Additional information can also be found in Chap. 10 of Volume 2 of this book.
2.4.5 NKCC1 in Non-epithelial Cells
Aside from being expressed in epithelial cells, NKCC1 is also found in many other cell types, such as myocytes, vascular smooth muscle cells, and neurons. In muscle, the cotransporter participates in conjunction with the Na+/K+ ATPase in the accumulation of K+. This is a critical function as skeletal muscle cells store 70–75% of the body K+ (Gosmanov et al. 2003). Furthermore, a high K+ concentration is needed for proper contraction, as a significant decrease in plasma K+ (and therefore muscle K+) caused by high doses of bumetanide is known to produce musculoskeletal pain, cramping, and/or muscle weakness (Howard and Dunn 1997; Vaduganathan et al. 2013). As chloride conductances in muscle cells are critical in stabilizing the membrane potential at the resting level, NKCC1 might also be involved in this stabilization by accumulating Cl− above electrochemical potential equilibrium (Aickin et al. 1989). In addition, NKCC1 activity is also critical in helping muscle cells maintain their water content (Gosmanov et al. 2003). In vasculature, NKCC1 is thought to contribute to vasoconstriction (Akar et al. 1999), as vascular smooth muscle tone is reduced in NKCC1 knockout mice (Meyer et al. 2002). In neurons, the cotransporter affects the level of intracellular Cl− that ultimately determines the direction and strength of GABA- and/or glycine-mediated Cl− currents. Thus, the cotransporter is a key determinant of inhibitory synaptic neurotransmission.
2.4.6 NKCC1 in Disease
Considering the wide-expression pattern of NKCC1 and its importance in the control of cell volume, the modulation of inhibitory synaptic transmission, and epithelial transport, it would be reasonable to expect disruptions in NKCC1 expression and/or function to have severe health consequences. Although we know that the multiple NKCC1-deficient models generated are viable, global and tissue-specific NKCC1 knockout mice do exhibit multiple and severe phenotypes that greatly affect their health (Delpire et al. 1999; Dixon et al. 1999; Flagella et al. 1999; Pace et al. 2000). In addition to the inner ear defects already mentioned that affects both balance and hearing, the mice exhibit intestinal obstruction (Flagella et al. 1999; Grubb et al. 2000), deficit in saliva secretion (Evans et al. 2000), decreased pain perception (Laird et al. 2004; Sung et al. 2000), and male infertility (Gagnon and Delpire 2013a; Pace et al. 2000). The defect in intestinal transit is most relevant as the mice transition from milk to solid food. Indeed, many mutant pups are lost during the peri-weaning period, confirming the severe consequence of loss of NKCC1. The observed intestinal dysfunction could be a result of a deficit in fluid secretion, as the transporter is involved in Cl-mediated fluid secretion (Grubb et al. 2000). A second hypothesis is a deficit in peristalsis, as Na-K-2Cl cotransporter is expressed in interstitial cells of Cajal surrounding the myenteric plexus (Wouters et al. 2006; Zhu et al. 2016). Another possibility is a deficit in autonomic control of intestinal function, as the transporter is highly expressed in the fibers that provide sensory feedback to the central nervous system (Alvarez-Leefmans et al. 1988; Sung et al. 2000).
Analysis of genomic databases reveals that, as with all other genes, SLC12A2 is not “immune” from mutations in the human population. The Exome Aggregation Consortium (ExAC) database (http://exac.broadinstitute.org) reports some 280 missense mutations in SLC12A2. It is highly likely that some of them result in loss of function of the mutated allele. It is also possible that a 50% reduction in NKCC1 expression results in no pathophysiological effect. However, combined with another mutation or particular circumstance, a reduction in NKCC1 expression might facilitate the development of severe health issues.
In 2010, the whole-genome exome sequencing of an 8-year-old female with multiple health issues identified an 11 bp deletion in exon 22 of SLC12A2 resulting in a frameshift mutation in NKCC1 and premature truncation of the translated protein. At the time, the patient suffered from orthostatic hypotension, autonomic bladder dysfunction, small intestine dysmotility, dietary intolerance, decreased energy, and seizure episodes (Delpire et al. 2016). Now 16, she recently experienced a complete loss of intestinal and bladder function. In addition, pancreatic, thyroid, and parathyroid gland deficiencies are affecting her endocrine system. A credible explanation for the failure of all these systems is some type of dysautonomia or dysregulation of the autonomic nervous system.
Development of a mouse model replicating this 11 bp deletion resulted in no overt phenotype (Koumangoye et al. 2018). However, the assessment of any deficits in sensory feedback in this mouse still needs to be undertaken. One interesting aspect of the mutant transporter in epithelial cells is its mistrafficking away from the basolateral membrane due to the truncation of a portion of the carboxyl-terminal tail. We showed that in Madin–Darby Canine Kidney (MDCK) cells, the transporter is not trapped in the endoplasmic reticulum, but accumulates at the apical pole, both at the apical membrane co-localizing with Gp135 (podocalyxin) and in subapical vesicles (Koumangoye et al. 2018). The subapical vesicles were identified as Rab5-positive apical early endosomes, indicating retrieval of transporters units from the apical membrane (Koumangoye et al. 2019). Due to dimerization, some wild-type monomers are also found at the apical membrane. Serial deletions and mutagenesis of the C-terminal tail of NKCC1 identified a di-leucine motif responsible for this effect (Koumangoye et al. 2019). Certainly, either deletion of this motif or mutation of the two leucine residues to alanine residues resulted in behavior similar to that of the patient NKCC1 mutant transporter. Immunohistochemistry analysis of NKCC1 in the NKCC1 mutant mouse confirms the mislocalization of the transporter in the salivary gland and colonic epithelia (Koumangoye et al. 2018). Interestingly, some of the transporters appear to still be expressed on the basolateral membrane, most likely enough to sustain saliva secretion.
A 5-year-old boy with sensorineural hearing loss, developmental delays, and a variety of other clinical presentations was shown to have a homozygous 22 kb deletion in SLC12A2 resulting in exons 2–7 being absent and a premature truncation codon in exon 8. In this condition, if the RNA were stable, the gene would only produce a fragment (89%) of the cysotolic N-terminal tail of the cotransporter. Analysis of the patient’s fibroblasts demonstrated a complete absence of NKCC1 protein expression (Macnamara et al. 2019). Table 2.1 illustrates striking similarities (i.e., gastrointestinal deficits, lack or reduced saliva and tear secretion, low blood pressure) between these two human cases of NKCC1 disruption with the known phenotypes of the NKCC1 knockout mouse.
2.5 NKCC2
In man, 99.8% of the filtered Na+ is reabsorbed, with 15–25% of this reabsorption occurring in the thick ascending limb of Henle (TALH). The SLC12A1 gene encodes for an 1100 amino acid protein that RNA and protein expression studies have demonstrated is kidney-specific, localizes to the TALH epithelial apical membrane, and serves to re-absorb sodium and chloride from the tubule lumen. However, as salt is reabsorbed along the TALH, the luminal concentration of Na+ and Cl− decreases approximately fivefold from medulla to cortex. Interestingly, alternative splicing of the exons encoding for transmembrane domain 2 has resulted in three variants of NKCC2. Variant F which has the lowest binding affinity (Km(Na+) = 66.72 ± 5.8 and a Km(Cl−) = 111.3 ± 13.4) is expressed in the medullar TALH where the Na+ concentration is the highest. The mid- (variant A) and high-affinity (variant B) cotransporters are expressed in the cortical TALH where the Na+ concentration is lowest. Variant A has a Km(Na+) = 16.45 ± 1.9 and a Km(Cl−) = 44.65 ± 3.87) and Variant B has a Km(Na+) = 20.65 ± 2.4 and a Km(Cl−) = 8.95 ± 1.3) (Giménez et al. 2002). This expression pattern results in maximal reabsorption of luminal salt and water (see Fig. 2.7a). Similar to NKCC1, the driving force for apical NKCC2 cotransport activity is the basolateral expression of the Na+/K+ pump. However, now combined with Cl− channels on the basolateral membrane, apical NKCC2 serves to absorb Na+ and Cl− into the blood. The K+ ions transported by NKCC2 are recycled back to the lumen through ROMK, an apical K+ channel (see Fig. 2.7b).
NKCC2 function in the kidney. (a) Schematic representation of the thick ascending limb of Henle (TALH) showing its location in cortex and medulla. In the deep medulla, the osmolarity is elevated whereas, in the superficial cortex, the osmolarity is equivalent to blood. Therefore, there is a gradient of Na+ in the TALH from the inner medulla to the cortex. Represented are three variants of the Na-K-2Cl cotransporter, NKCC2. In the medulla where the Na+ concentration is high, the low (variant F) and mid (variant A) affinity cotransporters are expressed, whereas, in the cortex where the Na+ is low, the high-affinity cotransporter (variant B) is expressed. (b) Schematic representation of a TALH epithelial cell with expression of NKCC2 and a K+ channel (ROMK) at the urine or luminal membrane, the Na+/K+ ATPase, and Cl− channels (CLCKA & B) at the blood or serosal membrane
Along with NKCC2 in the TALH, Na+ reabsorption in the distal kidney tubule is accomplished by the thiazide-sensitive Na-Cl cotransporter (NCC) and the amiloride-sensitive epithelial Na+ channel (ENaC). Each of these transport mechanisms is a major target for pharmacological modification. Proper water and salt retention involves these transport mechanisms along with activation of the renin-angiotensin-aldosterone system, a secondary target for anti-hypertensive drugs. In Fig. 2.8, the inhibitory actions of furosemide, thiazide, and amiloride determine the amount of fluid reabsorbed into the blood and ultimately blood pressure. The juxtaglomerular cells in the macula densa (MD) match glomerular filtration rate (GFR) with tubular reabsorption through tubuloglomerular feedback (TGF) mechanism. Renin release into the blood from the MD converts angiotensinogen to angiotensin I (Ang I) which is then converted to angiotensin II (Ang II) by angiotensin-converting-enzyme (ACE). Ang II increases the blood pressure through several mechanisms: (1) increase in sympathetic activity of the heart; (2) stimulation of aldosterone release from the adrenal gland which increases tubular Na+, Cl− reabsorption, K+ excretion and water retention; (3) arteriolar vasoconstriction; and (4) stimulation of anti-diuretic hormone release from the pituitary which increases water absorption in the collecting duct. Beta-blockers acting on adrenergic receptors, ACE inhibitors preventing the conversion of Ang I to Ang II, spironolactone acting on mineralocorticoid (aldosterone) receptor and loop diuretics are used to regulate salt and water reabsorption in the kidney tubule (Fig. 2.8).
Schematic representation of major targets of anti-hypertensive drugs. The Na+ reabsorptive transport mechanisms (NKCC2, NCC, and ENaC) in kidney tubules constitute major drug targets. The renin–angiotensin–aldosterone system, which affects both blood vessel contraction and Na+ transport, constitutes a second site of drug targets. Both angiotensin-converting enzyme (ACE) and aldosterone receptors are targets of anti-hypertensive drugs. By acting on adrenergic receptors of heart and blood vessels, beta-blockers decrease blood flow and contractility of vessels. There are two major sources of catecholamines: the adrenal gland, and postganglion sympathetic fibers. The flow of the urine, which is regulated by the glomerular filtration rate (GFR) and renin–angiotensin-mediated tubuloglomerular feedback (TGF) mechanism, also affects the rate of Na+ reabsorption. BO blood pressure; MD macula densa; GFR glomerular filtration rate; TGF tubuloglomerular feedback; ACE angiotensin-converting enzyme; Ang angiotensin; NE norepinephrine; βAR beta-adrenergic receptors
2.5.1 NKCC2 in Intestine
Euryhaline fish are capable of living in fresh, brackish, and even saltwater. In freshwater, these fish are hyposmotically challenged producing tremendous amounts of dilute urine to eliminate excess water. Conversely, in seawater, these fish are hyperosmotically challenged and must drink and absorb water to prevent dehydration (Lin et al. 2001; Seale et al. 2014). Although NKCC2 expression is considered kidney-specific in man, real-time PCR demonstrates that the cotransporter (variant B) is present in the intestine of the Mozambique tilapia and the Japanese seawater eel (Ando et al. 2014; Hiroi et al. 2008). Since only mucosal application of bumetanide on seawater eel intestine inhibited ion transport, expression of the variant B isoform of the cotransporter is likely located on the apical membrane. As such, similar to the mammalian kidney, NKCC2 variant B most likely serves as a key mechanism for water absorption and prevention of dehydration.
2.5.2 NKCC2 in Disease
Human mutations in NKCC2 which disrupt cotransporter function are responsible for Type I Bartter’s syndrome, a rare inherited disorder characterized by prenatal onset, low potassium levels (hypokalemic), low blood pressure (hypotension), increased blood pH (alkalosis), excessive urination (polyuria), and excessive amounts of calcium in the urine (hypercalciuria) (Bartter et al. 1962). More than 70 mutations in the SLC12A1 gene have been identified in patients suffering from Type I Bartter’s syndrome (Simon et al. 1996). Both homozygous mutations (same mutation on both alleles) and compound heterozygous mutations (each allele has a different mutation) will cause this salt-wasting disorder to become clinically relevant. Symptoms of Type I Bartter’s syndrome often appear before birth; however, patients can also manifest the disorder later in life as a result of residual NKCC2 function (Pressler et al. 2006).
2.6 NKCC Activity Is Regulated by Phosphorylation
The activity of the Na-K-2Cl cotransporter is increased by phosphorylation and decreased by dephosphorylation. Consensus exists as to the major phosphorylation event affecting the intrinsic transport activity of carriers already inserted in the plasma membrane. However, this consensus does not rule out the possibility that other phosphorylation events can affect the forward trafficking or retrograde recycling of transporters from the plasma membrane.
Original evidence that phosphorylation activates Na-K-Cl cotransport comes from studies performed by John Russell in 1988 in squid giant axon (Altamirano et al. 1988). The study showed that ATP depletion causes a marked decrease in Na-K-Cl cotransport activity. While this decrease could have been due to the collapse of the Na+ gradient generated by the ATP-driven Na+/K+ ATPase, the fact that it was diminished by the addition of protein phosphatase inhibitors (such as orthovanadate or fluoride) indicated that instead, NKCC phosphorylation was likely affected by ATP depletion. This was later confirmed in studies performed in the shark rectal gland by Chris Lytle and Biff Forbush (Lytle and Forbush 1992a, b). They demonstrated that a specific residue in the cytosolic NH2-terminal tail of the cotransporter acquired a phosphate residue upon cotransporter activation. In fact, we know now that multiple neighboring threonine and serine residues are phosphorylated upon cotransporter activation (Fig. 2.9). How phosphorylation of the NH2-terminus leads to conformational changes in the protein core and consequently increased cotransport activity is currently unknown. Moreover, whether specificity exists in the multiple phosphorylation sites that were identified is also unknown.
Phosphorylation “hot spot” in Na+-dependent cation–chloride cotransporters. Depicted is an amino acid alignment of human and mouse sequences for NCC, NKCC1, and NKCC2 of a small portion of the cytosolic NH2-terminal tail of the cotransporters. Note the presence of five conserved serine/threonine residues. Highlighted by boxes are phosphorylation sites identified by mass spectrometry in two studies (a) (Darman and Forbush 2002) and (b) (Vitari et al. 2006). Horizontal bar delineates the peptide epitope of a phosphospecific antibody (anti-phospho NKCC1 R5 antibody) created by the Forbush Laboratory (Flemmer et al. 2002)
Identification of the kinases involved in the activation of the cotransporter was made 10 years later (Dowd and Forbush 2003; Piechotta et al. 2003; Piechotta et al. 2002). There are still many details that are not resolved, mostly concerning the precise role of each kinase identified as regulators of the Na-K-2Cl cotransporter. We will start by addressing the current consensus. Upstream of the phosphorylation sites, in both NKCC1 and NKCC2 reside a short sequence that constitutes a binding site for serine/threonine kinases. The minimal amino acid sequence is an arginine residue, followed by a phenylalanine, any residue, then a valine or isoleucine (RFxV/I). This site is found once in the cytosolic amino-terminal domain of NKCC2 and twice in the amino-terminal domain of NKCC1. The kinases that bind to the RFxV/I site are members of a relatively large family of serine-threonine kinases: the mammalian Ste20p-like kinase family (Delpire 2009). SPAK (STE20/SPS1-related Proline/Alanine-rich Kinase, also known as PASK and STK39) and OSR1 (Oxidative stress response 1, also known as OxSR1) are the two members regulating the cotransporters. OSR1 is the original kinase, as it is found from protist to human, whereas SPAK originated from gene duplication late during vertebrate evolution (Gagnon and Delpire 2012). The two kinases possess an NH2-terminal catalytic domain, followed by a COOH-terminal regulatory domain, whose last 90 residues form a protein fold that accommodates or binds RFxV/I peptides (Austin et al. 2014; Villa et al. 2007). Thus, constitutively active SPAK and OSR1 when overexpressed in heterologous expression systems, like Xenopus laevis oocytes, significantly activate both Na-K-2Cl cotransporters. Interestingly, when overexpressed as native kinases in oocytes, they fail to activate the cotransporters and they themselves require activation by an upstream WNK kinase. The C-terminal domain of WNK kinases also contains RFxV/I sequences which makes them binding partners of SPAK/OSR1, thus facilitating their activation by WNK kinases (Fig. 2.10). A recent study (Piala et al. 2014) has indicated that WNK kinases also possess a portion of the binding domain of SPAK, which means that this upstream kinase can also bind to the cotransporters (Fig. 2.10). Figure 2.10 also highlights an adaptor protein (Cab39) which has been shown to facilitate Ste20 and WNK kinase activation, as well as the serum kinase SGK1 as a possible upstream regulator of WNK kinases. Finally, recent structural studies have uncovered the presence of a Cl− ion within the crystal structure of the WNK1 catalytic domain, indicating a possible role for the anion in modulating kinase function (Piala et al. 2014).
Illustration of phosphoregulation of NKCC2 by WNK-SPAK pathway. Depiction of the signaling cascade leading to NKCC activation with two members of the Ste20p-like family of protein kinases (SPAK and OSR1) anchoring to the cytosolic NH2-terminal tail and phosphorylating the cotransporter. Upstream of the Ste20p kinases are WNK kinases that can, through a similar binding mechanism (RFxV/I motif), anchor and activate SPAK and OSR1. In addition, an adaptor protein (Cab39) is depicted as a facilitator of kinase activation. Upstream regulatory mechanisms of WNK kinases, such as the serum glucocorticoid regulated kinase (SGK) and intracellular Cl−, are also shown. WNK kinase also possesses a portion of the binding domain of SPAK, which means that this upstream kinase can bind directly to the cotransporter. Also depicted is the PKC-induced clathrin-dependent internalization of the cotransporter
As kinases that directly bind and phosphorylate NKCC1 and NKCC2, SPAK and OSR1 are likely to be essential components of cotransporter function. Thus, disruption of their expression should have significant physiological consequences. Global knockout mice were generated for both kinases. While the complete absence of OSR1 results in embryonic lethality, the absence of SPAK results in a viable mouse with no overt phenotype (Delpire and Gagnon 2008). However, upon closer analysis, SPAK knockout mice demonstrate a renal phenotype that resembles the loss of NCC function in the distal convoluted tubule: salt-sensitive hypotension accompanied by electrolyte imbalance, such as hypokalemia, hypomagnesemia, and hypercalciuria (Grimm et al. 2012; McCormick et al. 2011; Yang et al. 2010). In contrast, the targeted deletion of OSR1 in the kidney led to a viable mouse with a phenotype resembling, in this case, the loss of NKCC2 function. Indeed, the mice displayed impaired Na+ reabsorption in the TALH on a low-Na+ diet with a significant reduction in NKCC2 phosphorylation, and a blunted response to furosemide (Lin et al. 2011). Thus, whereas these two kinases fulfill the same function in heterologous expression systems, they seem to have distinct functions in the kidney: OSR1 mainly regulating NKCC2, and SPAK mostly regulating NCC. In agreement with SPAK mostly regulating NCC, genetic variations in the human SPAK gene have been linked to hypertension (Wang et al. 2009). In other tissues, such as primary afferent sensory neurons, the two kinases seem to be complementing each other, as disruption of one kinase reduces NKCC1 function by half (Geng et al. 2009).
Mutations in WNK1 and WNK4 have been identified in the human population causing a disorder called Gordon syndrome or pseudohypoaldosteronism type II (PHAII). Features of the disorder are opposite to Gittleman’s, indicating an increase instead of a decrease in NCC function. It is now recognized that these mutations prevent degradation of WNK1 or WNK4 by the cullin-3/Kelch-like 3 ubiquitinylation-mediated protein degradation pathway. In fact, mutations in both proteins also result in Gordon syndrome (Boyden et al. 2012). The consequence of all these mutations is an increased abundance of WNK proteins, leading to increased function of the Na-Cl cotransporter, and increased distal Na+ reabsorption. This phenotype has been recapitulated in the mouse (Yang et al. 2007). Furthermore, disruption of the WNK4 gene in mice results in a mild Gitelman phenotype, consistent with WNK4 being a positive modulator of NCC function (Castañeda-Bueno et al. 2012; Ohta et al. 2008). Much less effort has been placed on determining whether manipulation of the WNK kinases also affects the function of the Na-K-2Cl cotransporters.
Aside from the major phosphorylation event that leads to stimulation of the cotransporters, there is evidence that Protein Kinase C (PKC) mediates the internalization of NKCC1 (Mykoniatis et al. 2010; Tang et al. 2010) (Fig. 2.10). Using a green fluorescent protein-tagged NKCC1 cotransporter expressed in MDCK cells, it was shown that phorbol ester activated PKC leads to internalization of NKCC1 through a clathrin-dependent endocytic pathway (Mykoniatis et al. 2010). Using small hairpin RNA (shRNA) delivered with adenovirus, PKCδ and PKCε were identified as the isotypes modulating cell surface expression of the cotransporter (Tang et al. 2010).
As discussed, in Sect. 2.4.2, activation of PKA through cAMP also leads to an increase in NKCC1 stimulation. However, the effect is indirect and mediated by the drop in the intracellular Cl− concentration that follows the cAMP-induced opening of CFTR (Fig. 2.5). This indirect stimulation not only pertains to the cAMP stimulation of colonic NKCC1 in mammals but also to the cAMP/PKA mediated stimulation of NKCC1 in the shark rectal gland (Lytle and Forbush 1992a, b).
2.7 Gene Structure, Cotransporter Family, and Super Family
We mentioned in Sect. 2.3 that human NKCC1 and NKCC2 are the products of two distinct genes located on chromosomes 5 and 15, respectively. Analysis of animal genomes revealed that the SLC12A2 gene originated first since NKCC1 is found in bacteria and protists (e.g., NCBI accession numbers: WP_008693040 and EFW46279), whereas SLC12A1 originated from gene duplication 400–500 million years ago in early vertebrate evolution, as NKCC2 is found in cartilaginous fish (NCBI accession number: AAM749868). Examination of their genetic structure revealed that despite this very significant length of time since duplication, the exons have been well-conserved (Fig. 2.11). Indeed, 21 out of 27 exons are conserved (including 2 exons which have a single codon added). Therefore, there are only 6 exons which are somewhat different between the two genes: exons 1 and 2 for SLC12A1 (NKCC2) are different from exon 1 in SLC12A1 (NKCC1). Despite exon 3 of SLC12A1 and exon 2 of SLC12A2 starting differently, they end up with conserved sequences. Another difference can be seen in exons 21 and 22 for SLC12A1 which corresponds to exons 20, 21, 22 in SLC12A2 with very little conservation, if we exclude the terminal part of exon 22. Finally, exon 27 encodes the end of the open reading frame of both cotransporters, followed by gene-specific 3’untranslated region. This high degree of overall exon (Fig. 2.11) and protein (Fig. 2.3) conservation reflects the evolutionary pressure that was imposed to keep intact the vital function of the two cotransporters. In the end, there are very few functional differences between the two cotransporters, their strict stoichiometry with three (even four if we include the second Cl−) distinct ions require tertiary, secondary, and primary structure integrity. To this note, we can add the surprising finding that while sequences of the 5′ end of the N-terminal tails are highly divergent, the cotransporters have managed to retain a SPAK/OSR1 binding motif.
Alignment of the human SLC12A1 and SLC12A2 genes. The gene structure with all 27 exons spanning 100 kb genomic DNA is displayed. The vast majority of exons are conserved with 21 out of 27 exons having identical sizes and coding the same protein fragments. Exons 1–3 which encode the NH2-terminal tails of both cotransporters show the lowest degree of conservation. The 3′-untranslated region of the transcript, encoded by exon 27, is typically gene-specific, differs significantly between the two genes. Exon 5 in the SLC12A1 gene is triplicated to give rise to the three splice variants: NKCC2F, NKCC2A, and NKCC2B. A short 48 bp alternatively spliced cassette in exon 21 is unique to the SLC12A2 gene. Large genomic regions present between exons are depicted by a gray background
Very little is known about transcriptional promoters for the two genes. While the promoter of SLC12A2 NKCC1 contains features of housekeeping genes with a TATA-less promoter and several activator protein-2 (AP-2) and Specificity Protein 1 (SPS1) putative binding sites, the promoter of SLC12A1/NKCC2 only contains a TATA-box, and seems to be under the control of Hepatocyte Nuclear Factor (Igarashi et al. 1996). There is no indication that either transcript can be generated from alternative promoters. In contrast, as already mentioned, NKCC2 exists in three variants, due to alternative splicing of exon 5, whereas NKCC1 exists in two variants one with and one without exon 21 (Randall et al. 1997). Exon 5, as shown in Fig. 2.3, encodes for the second transmembrane domain and the sequence variations that affect the cotransporters binding affinity for ions (Giménez et al. 2002).
As mentioned earlier, the membrane targeting of the two NKCC isoforms is directly related to their different physiological roles. In an elegant set of experiments, Biff Forbush and coworkers generated chimeras of the two isoforms to identify what motifs within the two proteins mediate their basolateral (NKCC1) versus apical (NKCC2) membrane expression (Carmosino et al. 2008). Transposition of the cytosolic amino-terminus of NKCC1 onto NKCC2 did not alter the apical distribution of the chimeric protein, whereas replacement of the cytosolic carboxy-terminus of NKCC2 onto NKCC1 altered the membrane targeting of the chimeric protein from basolateral to apical. Through a series of chimeras where regions of the carboxy-terminus of NKCC1 was replaced with corresponding regions of NKCC2, they identified a basolateral sorting motif within the carboxy-terminus of NKCC1 which is encoded by exon 21 that contains a di-leucine motif (Carmosino et al. 2008). Di-leucine motifs have been shown to mediate endocytosis and basolateral sorting of membrane receptors in epithelial cells (Hunziker and Fumey 1994). Both the alternative NKCC1 isoform that lacks exon 21 and the apically targeted NKCC2 lack this di-leucine motif. A recent paper, however, challenged the role of exon 21 in membrane targeting. Removal of exon 21 was indeed not sufficient to affect basolateral targeting of NKCC1 in MDCK cells (Koumangoye et al. 2019). In contrast, a far downstream C-terminal di-leucine motif was shown essential to basolateral membrane expression of NKCC1. Whether the alternatively spliced NKCC1 isoform, abundantly expressed in the brain, is localized to the apical membrane in choroid plexus epithelial cells due to the absence of this di-leucine motif is still unknown. However, the absence of this motif does explain the localization of NKCC2 on the apical membrane of the thick ascending limb of Henle (Carmosino et al. 2008).
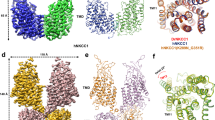
The two Na-K-2Cl cotransporters are also closely related to the thiazide-sensitive Na-Cl cotransporter (NCC). Together, these cotransporters constitute the Na+ transporting branch of the cation–chloride cotransporter family and share high homology to four Na+-independent K-Cl cotransporters (KCC1-KCC4; (Gagnon and Delpire 2013a). All these transporters belong to the family of SLC12A cotransporters which itself shares ancestry with many other families of transport proteins which share the basic structure of an inverted repeat of five transmembrane domains (TM1–5 and TM6–10), followed by two additional transmembrane α-helical segments located either at their N- and/or C-termini (Wong et al. 2012). Because of high conservation at the structure level, the three-dimensional structural resolution of related transporters (e.g., a bacterium sodium-galactose symporter PBD#: 3dh4 and a bacterium glutamate/GABA antiporter PDB#: 4dji) allows for modeling of NKCC proteins. Using the Phyre2 protein fold recognition server (www.sbg.bio.ic.ac.uk/phyre2/), we drew a model of the first 10 transmembrane domains of NKCC1 (Fig. 2.12). We can see that specific helices run parallel to each other (TM1 and 6 in blue, TM2 and 7 in red, TM3 and 8 in green, TM4 and 9 in white). Considering the distance that separates LeuTAa, a bacterial homolog of a neurotransmitter sodium symporter, it is extraordinary that four out of six residues in TM9 and that seven out of ten residues in TM10, shown to promote LeuTAa dimerization (Yamashita et al. 2005), are conserved in NKCC1. In a 2012 study, Biff Forbush and coworkers identified several pore-lining residues within TM3 (green helix in Fig. 2.12) as functionally important in ion coordination (Tyr-383, Ala-379, Ala-375, Asn-376, Ile-368, and Gly-369), loop diuretic binding (Phe-372 and Ile-371), and complete bumetanide-insensitivity (Met-382) (Somasekharan et al. 2012). Conserved identity within the transmembrane domains of the cation–chloride cotransporter family suggests that future homology studies may help understand which pore-lining residues define the specific characteristics of ion transport in both the Na+-dependent and Na+-independent cotransporters.
Three-dimensional model of the transmembrane core of NKCC1. Three-dimensional resolution of two related transporter structures: a bacterium sodium-galactose symporter (PDB#: 3dh4) and a bacterium glutamate/GABA antiporter (PDB#: 4dji) were used to model the transmembrane core of NKCC1. Anti-parallel transmembrane helices from the first and second halves of the transmembrane core are identically colored. The structure is rotated 180 degrees (right) to visualize the helices visible from the back. The model was created using the Phyre2 software (see text)
2.8 Summary
Over the past 50 years, physiologists have used functional, biochemical, and molecular biological experiments to go from the initial Na+/K+ Pump II hypothesis to the conceptual breakthrough of secondary active transport where the energy of the Na+ gradient generated by the Na+/K+ ATPase is used by the Na-K-2Cl cotransporter in multiple organ systems. Expression of the older of the two isoforms, NKCC1, in various tissues suggests multiple physiological roles including Cl− and fluid secretion, acid secretion, cell volume homeostasis, and possibly cell division and proliferation. The kidney-specific expression of three variants of NKCC2 to optimize salt reabsorption demonstrates its vital role in renal homeostasis and regulation of systemic blood pressure. The evolutionary conservation of these cotransporters from protists to humans confirms their vital role in cellular and whole-organism physiology.
References
Aickin CC, Betz WJ, Harris GL (1989) Intracellular chloride and the mechanism for its accumulation in rat lumbrical muscle. J Physiol 411:437–455
Akar F, Skinner E, Klein JD, Jena M, Paul RJ, O’Neill WC (1999) Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl− cotransporter in rat aorta. Am J Phys 276:C1383–C1390
Altamirano AA, Breitwieser GE, Russel JM (1988) Vanadate and fluoride effects on Na-K-Cl cotransport in squid giant axon. Am J Phys 254:C582–C586
Alvarez-Leefmans FJ, Gamiño SM, Giraldez F, Nogueron I (1988) Intracellular chloride regulation in amphibian dorsal root ganglion neurons studied with ionselective microelectrodes. J Physiol Lond 406:225–246
Ando M, Wong MK, Takei Y (2014) Mechanisms of guanylin action on water and ion absorption at different regions of seawater eel intestine. Am J Physiol Regul Integr Comp Physiol 307:R653–R663
Austin T, Nannemann DP, Deluca SL, Meiler J, Delpire E (2014) In silico analysis and experimental verification of OSR1 kinase - peptide interaction. J Struct Biol 187:58–65
Bajwa PJ, Lee JW, Straus DS, Lytle C (2009) Activation of PPARγ by rosiglitazone attenuates intestinal Cl− secretion. Am J Physiol Gastrointest Liver Physiol 297:G82–G97
Bartter FC, Pronove P, Gill JRJ, Maccardle RC (1962) Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33:811–828
Boyden LM et al (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102
Canessa M, Brugnara C, Cusi D, Tosteson DC (1986) Modes of operation and variable stoichiometry of the furosemide-sensitive Na and K fluxes in human red cells. J Gen Physiol 87:113–142
Carmosino M, Giménez I, Caplan M, Forbush B (2008) Exon loss accounts for differential sorting of Na-K-Cl Cotransporters in polarized epithelial cells. Mol Biol Cell 19:4341–4351
Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K (2001) Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen syndrome. Proc Natl Acad Sci USA 98:2526–2531
Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G (2012) Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109:7929–7934
Cheng SX (2012) Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gasterointest Liver Physiol 303:G60–G70
Christensen HL, Nguyen AT, Pedersen FD, Damkier HH (2013) Na(+) dependent acid-base transporters in the choroid plexus; insights from slc4 and slc9 gene deletion studies. Front Physiol 4:1–10
Cook DI, Young JA (1989) Fluid and electrolyte secretion by salivary glands. In: Handbook of physiology. The gastrointestinal system. Salivary, pancreatic, gastric and hepatobiliary secretion. American Physiological Society, Bethesda, MD, pp 1–23
Crane RK (1965) Na+-dependent transport in the intestine and other animal tissues. Fed Proc 24:1000–1006
Darman RB, Forbush B (2002) A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem 277:37542–37550
Das S, Jayaratne R, Barrett KE (2018) The role of ion transporters in the pathophysiology of infectious diarrhea. Cell Mol Gastroenterol Hepatol 6:33–45
Delpire E (2009) The mammalian family of Sterile20p-like protein kinases. Pflügers Arch 458:953–967
Delpire E, Gagnon KB (2008) SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409:321–331
Delpire E, Gagnon KB (2011) Kinetics of hyperosmotically-stimulated Na-K-2Cl cotransporter in Xenopus laevis oocytes. Am J Physiol Cell Physiol 301:C1074–C1085
Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR (1994) Molecular cloning and chromosome localization of a putative basolateral Na-K-2Cl cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem 269:25677–25683
Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22:192–195
Delpire E, Wolfe L, Flores B, Koumangoye R, Schornak CC, Omer S, Pusey B, Lau C, Markello T, Adams DR (2016) A patient with multisystem dysfunction carries a truncation mutation in human SLC12A2, the gene encoding the Na-K-2Cl cotransporter, NKCC1. Cold Spring Harb Mol Case Studies 2:a001289
Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP (1999) Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet 8:1579–1584
Dowd BF, Forbush B (2003) PASK (Proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278:27347–27353
Evans RL, Park K, Turner RJ, Watson GE, Nguyen H-V, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275:26720–26726
Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE (1999) Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274:26946–26955
Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B (2002) Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277:37551–37558
Gagnon KB, Delpire E (2010) Molecular determinants of hyperosmotically activated NKCC1-mediated K+/K+ exchange. J Physiol Lond 588:3385–3396
Gagnon KB, Delpire E (2012) Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiol Rev 92:1577–1617
Gagnon KB, Delpire E (2013a) Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically-engineered mouse knockouts. Am J Physiol Cell Physiol 304:C693–C714
Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee W-S, Hediger M, Hebert SC (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269:17713–17722
Geck P, Pietrzyk C, Burckhardt B-C, Pfeiffer B, Heinz E (1980) Electrically silent cotransport of Na+ , K+ and Cl− in Ehrlich cells. Biochim Biophys Acta 600:432–447
Geng Y, Hoke A, Delpire E (2009) The Ste20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem 284:14020–14028
Giménez I, Isenring P, Forbush B (2002) Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277:8767–8770
Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB (1996) Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7:2533–2542
Gosmanov AR, Lindinger MI, Thomason DB (2003) Riding the tides: K+ concentration and volume regulation by muscle Na+-K+-2Cl− cotransport activity. News Physiol Sci 18:196–200
Gregoriades JMC, Madaris A, Francisco J, Alvarez FJ, Alvarez-Leefmans FJ (2019) Genetic and pharmacologic inactivation of apical NKCC1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00026.2018
Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA (2012) SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287:37673–37690
Grubb BR, Lee E, Pace AJ, Koller BH, Boucher RC (2000) Intestinal ion transport in NKCC1-deficient mice. Am J Physiol Gastrointest Liver Physiol 279:G707–G718
Hall AC, Ellory JC (1985) Measurement and stoichiometry of bumetanide-sensitive (2Na:1K:3Cl) cotransport in ferret red cells. J Membrane Biol 85:205–213
Halonen J, Hinton AS, Frisina RD, Ding B, Zhu X, Walton JP (2016) Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear Res 336:63–71
Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T (2008) Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211:2584–2599
Hoffman JF, Kregenow FM (1966) The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci 137:566–576
Howard PA, Dunn MI (1997) Severe musculoskeletal symptoms during continuous infusion of bumetanide. Chest 111:359–364
Hunziker W, Fumey C (1994) A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J 13:2963–2969
Igarashi P, Whyte DA, Li K, Nagami GT (1996) Cloning and kidney cell-specific activity of the promoter of the murine renal Na-K-Cl cotransporter gene. J Biol Chem 271:9666–9674
Ikeda K, Oshima T, Hidaka H, Takasaka T (1997) Molecular and clinical implications of loop diuretic ototoxicity. Hearing Res 107:1–8
Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E (1996) Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal IMCD, the glomerular and extraglomerular mesangium and the glomerular afferent arteriole. J Clin Invest 98:723–730
Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, Vera A, DiLuna ML, Delpire E, Alper SL, Gunel M, Gerzanich V, Medzhitov R, Simard JM, Kahle KT (2017) Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med 23:997–1003
Koumangoye R, Omer S, Delpire E (2018) Mistargeting of a truncated Na-K-2Cl cotransporter in epithelial cells. Am J Physiol Cell Physiol 315:C258–C276
Koumangoye R, Omer S, Delpire E (2019) A dileucine motif in the C-terminal domain of NKCC1 targets the cotransporter to the plasma membrane. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00023.2019
Kregenow FM (1971a) The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol 58:372–395
Kregenow FM (1971b) The response of duck erythrocytes to hypertonic media. Further evidence for a volume-controlling mechanism. J Gen Physiol 58:396–411
Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F (2004) Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci Lett 361:200–203
Liedkte CM (1992) Electrolyte transport in the epithelium of pulmonary segments of normal and cystic fibrosis lung. FASEB J 6:3076–3084
Lin LY, Weng CF, Hwang PP (2001) Regulation of drinking rate in euryhaline tilapia larvae (Oreochromis mossambicus) during salinity challenges. Physiol Biochem Zool 74:171–177
Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS (2011) Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci USA 108:17538–17543
Lytle C (1998) A volume-sensitive protein kinase regulates the Na-K-2Cl cotransporter in duck red blood cells. Am J Physiol Cell Physiol 274:C1002–C1010
Lytle C, Forbush BI (1992a) Na-K-Cl cotransport in the shark rectal gland. II. Regulation in isolated tubules. Am J Physiol Cell Physiol 262:C1009–C10117
Lytle C, Forbush BI (1992b) The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem 267:25438–25443
Lytle C, McManus TJ, Haas M (1998) A model of Na-K-2Cl cotransport based on ordered ion binding and glide symmetry. Am J Phys 274:C299–C309
Macnamara EF, Koehler AE, D’Souza P, Estwick T, Lee P, Vezina G, Network MUD, Fauni H, Braddock SR, Torti E, Holt JM, Sharma P, Malicdan MCV, Tifft CJ (2019) Kilquist syndrome: a novel syndromic hearing loss disorder caused by homozygous deletion of SLC12A2. Hum Mut 40(5):532–538
McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH (2011) A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14:352–364
Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE (2002) Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(−) cotransporter. Am J Physiol Heart Circ Physiol 283:H1846–H1855
Mykoniatis A, Shen L, Fedor-Chaiken M, Tang J, Tang X, Worrell RT, Delpire E, Turner JR, Matlin KS, Bouyer P, Matthews JB (2010) Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am J Physiol Cell Physiol 298:C85–C97
Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S (2008) Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet 18:3978–3986
Orlov SN, Tremblay J, Hamet P (1996) Bumetanide-sensitive ion fluxes in vascular smooth muscle cells: lack of functional Na+, K+, 2 Cl− cotransport. J Membr Biol 153:125–135
Pace AJ, Lee E, Athirakul K, Coffman TM, O’Brien DA, Koller BH (2000) Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl− cotransporter. J Clin Invest 105:441–450
Palfrey HC, O’Donnell ME (1992) Characteristics and regulation of the Na/K/2Cl cotransporter. Cell Physiol Biochem 2:293–307
Panet R, Markus M, Atlan H (1994) Bumetanide and furosemide inhibited vascular endothelial cell proliferation. J Cell Physiol 158:121–127
Panet R, Marcus M, Atlan H (2000) Overexpression of the Na+/K+/Cl− cotransporter gene induces cell proliferation and phenotypic transformation in mouse fibroblasts. J Cell Physiol 182:109–118
Payne JA, Forbush BI (1994) Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci USA 91:4544–4548
Payne JA, Xu J-C, Haas M, Lytle CY, Ward D, Forbush BI (1995) Primary structure, functional expression, and chromosome localization of the bumetanide sensitive Na-K-Cl cotransporter in human colon. J Biol Chem 270:17977–17985
Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ (2014) Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7:ra41
Piechotta K, Lu J, Delpire E (2002) Cation-chloride cotransporters interact with the stress-related kinases SPAK and OSR1. J Biol Chem 277:50812–50819
Piechotta K, Garbarini NJ, England R, Delpire E (2003) Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem 278:52848–52856
Pressler CA, Heinzinger J, Jeck N, Waldegger P, Pechmann U, Reinalter S, Konrad M, Beetz R, Seyberth HW, Waldegger S (2006) Late-onset manifestation of antenatal Bartter syndrome as a result of residual function of the mutated renal Na+-K+-2Cl− co-transporter. J Am Soc Nephrol 17:2136–2142
Randall J, Thorne T, Delpire E (1997) Partial cloning and characterization of Slc12a2: the gene encoding the secretory Na+-K+-2Cl− cotransporter. Am J Physiol Cell Physiol 273:C1267–C1277
Resta-Lenert S, Truong F, Barrett KE, Eckmann L (2001) Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281:C1837–C1849
Rocha AS, Kudo LH (1990) Atrial peptide and cGMP effects on NaCl transport in inner medullary collecting duct. Am J Physiol (Renal Fluid Electrolyte Physiol) 259:F258–F268
Russell JM (1983) Cation-coupled chloride influx in squid axon. Role of potassium and stoichiometry of the transport process. J Gen Physiol 81:909–925
Rybak LP (1993) Ototoxicity of loop diuretics. Otolaryngol Clin N Am 26:829–844
Seale AP, Stagg JJ, Yamaguchi Y, Breves JP, Soma S, Watanabe S, Kaneko T, Cnaani A, Harpaz S, Lerner DT, Grau EG (2014) Effects of salinity and prolactin on gene transcript levels of ion transporters, ion pumps and prolactin receptors in Mozambique tilapia intestine. Gen Comp Endocrinol 206:146–154
Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH (1977) Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol (Renal Fluid Electrolyte Physiol) 233:F298–F306
Simon DB, Karet FE, Rodriquez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP (1996) Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14:152–156
Somasekharan S, Tanis J, Forbush B (2012) Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1). J Biol Chem 287:17308–17317
Sonnenberg H, Honrath U, Wilson DR (1990) In vivo microperfusion of inner medullary collecting duct in rats: effect of amiloride and ANF. Am J Physiol (Renal Fluid Electrolyte Physiol) 259:F222–F226
Soybel DI, Gullans SR, Maxwell F, Delpire E (1995) Role of basolateral Na-K-Cl cotransport in HCl secretion by amphibian gastric mucosa. Am J Physiol Cell Physiol 269:C242–C249
Steffensen AB, Oernbo EK, Stoica A, Gerkau NJ, Barbuskaite D, Tritsaris K, Rose CR, MacAulay N (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat Commun 9:2167
Sung K-W, Kirby M, McDonald MP, Lovinger DM, Delpire E (2000) Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci 20:7531–7538
Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB (2010) Activated PKCδ and PKCε inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl− cotransporter NKCC1. J Biol Chem 285:34072–34085
Tasaki I, Spiropoulos CS (1959) Stria vascularis as source of endocochlear potential. J Neurophysiol 22:149–155
Tyson J, Tranebjaerg, L., Bellman, S., Wren, C., Taylor, J. F. N., Bathen, J., Aslaksen, B., Sorland, S. J., Lund, O., Malcolm, S., Pembrey, M., Bhattacharya, S., Bitner-Glindzicz, M. (1997) IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet 61:2179–2185
Vaduganathan M, Allegretti AS, Manchette AM, Patel SS, Olson KR, Bazari H (2013) Intravenous moderate-dose bumetanide continuous infusion and severe musculoskeletal pain. Int J Cardiol 168:e29–e31
Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17:1251–1264
Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DMF (2007) Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep 8:839–845
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397:223–231
Wang Y et al (2009) Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA 106:226–231
Wiener H, van Os CH (1989) Rabbit distal colon epithelium: II. Characterization of (Na+,K+,Cl-)-cotransport and [3H]-bumetanide binding. J Membr Biol 110:163–174
Wong FH, Chen JS, Reddy V, Day JL, Shlykov MA, Wakabayashi ST, Saier MHJ (2012) The amino acid-polyamine-organocation superfamily. J Mol Microbiol Biotechnol 22:105–113
Wouters M, De Laet A, Ver Donck L, Delpire E, van Bogaert PP, Timmermans JP, de Kerchove d’Exaerde A, Smans K, Vanderwinden JM (2006) Subtractive hybridization unravels a role for the ion co-transporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol 290:G1219–G1227
Xu J-C, Lytle C, Zhu TT, Payne JA, Benz EJ, Forbush BI (1994) Molecular cloning and functional expression of the bumetanide-sensitive Na-K-2Cl cotransporter. Proc Natl Acad Sci USA 91:2201–2205
Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437:215–223
Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S (2007) Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5:331–344
Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH (2010) SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21:1868–1877
Zeidel ML, Kikeri D, Silva P, Burrowes M, Brenner BM (1988) Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Invest 82:1067–1074
Zhu MH, Sung TS, Kurahashi M, O'Kane LE, O’Driscoll K, Koh SD, Sanders KM (2016) Na+-K+-Cl− cotransporter (NKCC) maintains the chloride gradient to sustain pacemaker activity in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 311:G1037–G1046
Selected Readings
Gagnon KB, Delpire E (2013b) Physiology of Slc12 transporters: lessons from inherited human genetic mutations and genetically-engineered mouse knockouts. Am J Physiol Cell Physiol 304:C693–C714
Gamba G (2009) The sodium-dependent chloride cotransporters. In: Alvarez-Leefmans FJ, Delpire E (eds) Physiology and pathology of chloride transporters and channels in the nervous system. Academic Press (Elsevier), London, pp 307–332
Gamba G, Garbarini N, Delpire E (2009) Regulation of cation-chloride cotransporters. In: Alvarez-Leefmans FJ, Delpire E (eds) Physiology and pathology of chloride transporters and channels in the nervous system. Academic Press (Elsevier), London, pp 357–382
Russell JM (2000) Sodium-potassium-chloride cotransport. Physiol Rev 80:212–276
Russell JM (2009) Sodium-coupled chloride cotransporters: Discovery and newly emerging concepts. In: Alvarez-Leefmans FJ, Delpire E (eds) Physiology and pathology of chloride transporters and channels in the nervous system. Academic Press (Elsevier), London, pp 17–26
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The American Physiological Society
About this chapter
Cite this chapter
Delpire, E., Gagnon, K.B. (2020). Na+-K+-2Cl− Cotransporter. In: Hamilton, K.L., Devor, D.C. (eds) Studies of Epithelial Transporters and Ion Channels. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-55454-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-55454-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55453-8
Online ISBN: 978-3-030-55454-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)