Abstract
Checking vehicles and engines for compliance with the declared environmental classes in operating conditions is a very time- and cost-inefficient process, because it requires a test bench with rollers and additional equipment for tests on the standard driving cycles. Taking into account this fact, the present paper proposes a simplified method for providing performance tests of the catalytic converter with the assessment of the impact of certain modes and some operational factors on the emissions of hydrocarbons and carbon monoxide. The efficiency of the converter was estimated by comparing the experimental data on the emissions of named toxic components in the exhaust system before and after the converter. Bench tests were carried out in stationary conditions on the engines, mounted on fully serviceable cars using the diagnostic complex equipped with a multi-component gas analyzer and software. To test the efficiency of the converter, cold start and warm-up modes were selected and investigated, as well as several modes close to those typical for testing engines and automobiles in urban areas. As operational factors, malfunctions that often occur during the operation of an engine and significantly increase the amount of these harmful emissions were investigated. The analysis of test results according to the proposed method allows determining the efficiency level of a converter for reducing hydrocarbons and carbon monoxide contents in exhaust gases during engine operation. Recommendations for improving the converter’s efficiency are proposed for the automobile engine to comply with respective emission standards.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Spark-ignition engine
- Exhaust gas
- Toxic components
- Catalytic converter
- Cold start
- Operational factors
- Oxygen sensor
1 Introduction
Currently, the problem of further pollution of large cities with harmful car emissions remains relevant [1, 2]. The widespread use of catalytic converters has proven to be an effective method for reducing the toxicity of exhaust gases throughout the engine life cycle [3, 4]. For the purpose of testing for compliance with the declared emission standards, the cars are subject to test procedures including the so-called standard driving cycle. The European Driving Cycle (NMEDC) is adopted in most European countries including Russia and is supposed to simulate traffic conditions in the city and partially the extra-urban mode [5, 6]. However, there are a number of factors related to the engine operating conditions during testing and to malfunctions that arise during the operation of the vehicle, the impact of which may impede the ultimate fulfillment of existing standards for the maximum emissions of hydrocarbons (CH) and carbon monoxide (CO) for the car according to the declared environmental class.
The most ecologically adverse operating modes of IC engines are a cold start, warm-up, and repeated low-gear acceleration/braking [7, 8]. Forced operation on the rich fuel mixture at cold start and warm-up conditions inhibit the reactions of oxidation in a catalytic converter, thus leading to a drastic increase of CO and CH emissions. It should be noted that intensive oxidation of toxic components in a standard converter starts at 300 °C, despite the search for new ways of oxidizing these components at lower temperatures [9,10,11]. While the closed circuit of the electronic control unit (ECU), which corrects the air–fuel ratio using feedback from the O2 sensor, is activated only at 350 °C [12, 13]. It means that at the cold start it takes some time to warm-up the catalytic converter and O2 sensor to working temperatures at which the converter effectively neutralizes harmful components of the exhaust gases. Since the test procedure for compliance of a car to standards higher than Euro-3 starts with the selection of the exhaust gases immediately after the cold start, any delay in the work of the catalytic converter results in high concentrations of CO and CH in exhaust gases. It has also been reported that at transient operation modes of the engine (i.e. rapid acceleration or rapid braking) during the NMDEC maximums of CO and CH emissions are much higher than at steady-state operation mode [14]. Furthermore, the catalytic converter degrades with time because of thermal aging and chemical poisoning [15, 16].
The factors mentioned above tell us that widely adopted in diagnostics method of checking the catalytic converter’s operability by the difference of sinusoidal readings of two O2 sensors cannot guarantee the fulfillment of the declared environmental class standards. During the start, idle and acceleration the air–fuel ratio deviates from stoichiometric to rich so that throughout the several consequent working cycles voltage signals of O2 sensor reach their maximums then return to their initial values. The duration of these transient processes depends on the enrichment ratio determined by the ECU. At such operation modes, it is almost impossible to track the catalyzer efficiency by the comparison of readings of two O2 sensors. The only way to quantitatively assess the reduction of the CO and CH concentration in the exhaust gases is to provide gas analysis.
During the exploitation period of a car, it is of particular interest to evaluate the performance of the converter in case of emerging engine malfunctions. Among the malfunctions that most affect the amount of harmful emissions are misfires in cylinders that are caused by problems with the ignition system, fuel system, or the O2 sensor. It is known that these malfunctions are the main source of CH emission growth and, to a lesser extent, to CO emission growth [17]. Even the short-time exposure of the catalytic converter to operational malfunctions listed can lead to the situation when declared emission standard is exceeded.
For performing a standard driving cycle test of a car and its engine a test bench is required including rollers and special equipment. In the conditions of continuous operation of a car, the implementation of such a test procedure seems to be quite costly and laborious. Given this circumstance, this paper presents a simplified method for assessing the impact of individual modes and the aforementioned operational factors on the amount of CH and CO emissions in order to determine the capabilities of a standard converter to minimize these emissions.
2 Description of the Test Method
Efficiency of the catalytic converter was assessed by comparing the experimental data on CH and CO emissions in the exhaust system before and after the converter. Such a method is quite widespread to obtain information on the effect of various materials and structural features of catalytic converters on the amount of toxic emissions [18,19,20,21,22]. The subject of the present research is the determination of the effect of various operating conditions and engine malfunctions on the efficiency of reducing CH and CO emissions in the exhaust gas by the converter.
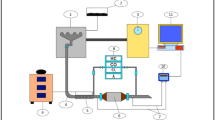
To obtain experimental data in stationary conditions fully serviceable Chevrolet Cruze and Lada Granta automobiles were used, equipped with 4-cylinder engines with a working volume of 1600 cm3 with multipoint fuel injection into the intake port and standard three-component converters. The condition of the converters was previously checked on running engines by comparing the voltage signals coming from the primary and secondary O2 sensors using the ESI[tronic] 2.0 diagnostic software. Comparative tests were carried out using the Bosch FSA-740 diagnostic system, equipped with the BEA-050 gas analyzer with embedded ESA (Emission-System-Analysis) software, which allows to quickly record the values of mentioned toxic components of hydrocarbons CH (in ppm units) and carbon monoxide CO (in % by volume), free oxygen O2 and carbon dioxide CO2 (in % by volume), and also to calculate the air–fuel ratio of the mixture by gas analysis. These values were recorded using photo and video fixation, a fragment of which is shown in Fig. 1.
The ESI[tronic] 2.0 software also allows instantaneous registration of the values of working fluids temperature, crankshaft rotation speed (n), ignition timing, and other engine parameters, as well as to monitor voltage levels on both O2 sensors. The test procedure was divided into two cycles. On the first cycle, tests were carried out under the conditions of cold start and engine warm-up, and on the second cycle, tests were carried out on the fully warmed up engine at idle and transient modes with low revs (n).
The tests on the first cycle were carried out in a box at an initial temperature of 15 °C and consisted of three stages. The first stage was performed under normal conditions without simulating any malfunctions in the engine control system (ECS) with exhaust gas extraction from the exhaust pipe. At the second stage, the control O2 sensor was turned off and the engine run in “open-loop mode” without feedback from the O2 sensor to the ECU simulating a malfunction in the wiring connecting the O2 sensor to the ECU. At the third stage, a fitting was installed instead of the O2 sensor and exhaust gas was selected from the exhaust system before the converter. For achieving the identical test conditions, each stage was carried out on different days, so that the engine could completely cool down (to 15 °C). The duration of each test stage was 300 s and was controlled using a stopwatch. The measurements were carried out after 10 s then 30 s and then every 30 s from the engine start. The coolant temperatures were in the range from 15 °C before starting up to 70–75 °C at the end of the test. With the engine warming up the value of n gradually decreased from 1100 to 800 rpm and the ignition timing was within the limits set by the ECS.
On the second test cycle, four modes of normal engine operation and four modes of operation with imitation of common defects that occur during operation were selected to assess the influence of other previously mentioned factors. The first four modes included idling at constant values of n = 1000 and 3000 rpm, acceleration from 1000 to 3000 rpm with 20% throttle, and braking mode from 3000 to 1000 rpm. The choice of such test modes is due to the attempt to approximately reproduce in stationary conditions the unsteady modes of engine operation according to the European driving cycle on first and second gears. The remaining four modes were carried out only at steady-state idling modes with the same values of n, but with short-term shutdowns of one cylinder in two different ways. First, one nozzle was turned off, simulating a malfunction in the fuel supply system, and then, the cylinder was turned off by installing a deliberately defective spark plug, simulating a malfunction in the ignition system.
3 Results and Discussion
The most important results for the first cycle Chevrolet Cruze engine test are presented in Fig. 2 as curves of CH and CO emissions versus time of engine operation from start.
CH and CO concentrations versus operating time (Chevrolet Cruze engine, cold start): CH (1) and CO (1)—values at the first stage of testing under normal conditions; CH (2) and CO (2)—values at the second stage without O2 sensor; CH (3) and CO (3)—values at the third stage without O2 sensor and with exhaust gas selection before the converter
Analysis of the data demonstrates that the concentration of CO and CH in first 10–30 s after the engine start is quite high independently of the gas selection method. However, at the first stage of testing under normal conditions of exhaust gas selection, the concentration of CH and CO is somewhat lower than the content of the same components before the converter. This fact means that reactions of oxidation in the converter initiated shortly after starting a cold engine. The process of elimination of these components went much faster at about 60 s after the start, which was due to the beginning of the active signaling from the control O2 sensor (according to the ESI [tronic] 2.0 program) and a gradual transition to the stoichiometric mixture. Subsequently, the CH values gradually decreased, but remained somewhat excessive (25–90 ppm), despite the converter reaching an operating temperature of about 550 °C. It should be noted that throughout the test stage, the O2 concentration in the exhaust gas was close to zero (not shown in Fig. 2) and it was not enough for the complete oxidation of CH.
The second stage of testing demonstrates the significant raise of CH concentration after the converter in the absence of a control voltage from the O2 sensor. This can be attributed to the constant supply of a richer mixture and low concentration of O2 in the exhaust. At the third stage of the test with the selection of the exhaust gas before the converter, the content of CH and CO increased even more. However, comparing the test results from the second and third stages, it can be seen that even in the absence of control voltage from the O2 sensor, the converter is able to significantly reduce emissions of CH and CO at the engine warm-up.
Using the same methodology to assess the CO and CH correlations with time at start-up and warm-up conditions, by analogy with the tests of the Chevrolet Cruze engine, the engine of the Lada Granta car was tested, including the first and second stages. The dependency graphs shown in Fig. 3, were supplemented with data of O2 concentration in the exhaust gas.
Comparison of the test results in Figs. 2 and 3 shows that the behavior of experimental curves for CH and CO during start-up and subsequent warm-up of the tested engines is approximately the same. The difference in absolute values can be explained by different approaches to mixture formation, embedded in the ECU software. While the Chevrolet Cruze engine with a mixture of λ ≤ 1 demonstrates an O2 deficiency and there is some reserve for reducing the CH emission even under normal conditions, the Lada Granta engine with λ > 1 has a slight excess of O2 and, accordingly, the CH concentration is lower. However, it should be noted that too lean mixture at the considered modes can cause misfires and skipping work cycles, which in turn will lead to an increase in CH. In addition, excessive depletion of the mixture is often accompanied by jerks and other disturbances when driving a car with an unheated engine.
Test results for the second cycle are presented in Fig. 4, where the values of concentration of individual components in the exhaust gas and the approximate composition of mixture λ are shown, calculated according to the gas analysis data at the operating modes listed above. In transient conditions, the values of all components are fixed at the time of maximum values of CH and CO.
Values of the concentration of individual components in the exhaust gases and mixture composition λ at various engine operating modes (Chevrolet Cruze engine): 1—idle at n = 1000 rpm; 2—idle at n = 3000 rpm; 3—acceleration from n = 1000 rpm to n = 3000 rpm; 4—braking from n = 3000 rpm to n = 1000 rpm; 5—idle at n = 1000 rpm with the nozzle turned off; 6—idle at n = 3000 rpm with the nozzle turned off; 7—idle at n = 1000 rpm with a faulty spark plug; 8—idle at n = 3000 rpm with a faulty spark plug
To evaluate the efficiency of the converter at various operating modes, including modes with malfunctions, the exhaust gas was selected in different locations. At the first stage of testing, the results of which are presented in the upper part of Fig. 4, exhaust gas selection was carried out in the usual way from a standard exhaust system. At the second stage, the results of which are presented in the lower part of Fig. 4, the exhaust gas was taken from the fitting installed before the converter at the control O2 sensor mounting point. In this case, the control O2 sensor was disconnected and, therefore, the engine control system worked as an open circuit.
The results shown in Fig. 4 demonstrate that when exhaust gas is taken from a standard exhaust system and a three-component catalytic converter is functioning, the emissions of CH and CO at steady-state are close to zero. There is a certain surge in the values of these components at the transition mode (mode 3), due to the short-term enrichment of the mixture. Nevertheless, the CH emissions during the considered transitional modes after the converter are reduced by an order of magnitude compared to emissions before the converter.
Turning off one cylinder by switching off the nozzle (modes 5 and 6) does not cause a noticeable increase of CH and CO emissions compared with emissions of a serviceable engine during exhaust gas extraction from a regular exhaust system due to the excess O2 in the exhaust gas. In this case, short-term shutdown of a cylinder does not endanger the normal operation of the converter.
A different picture is present if the cylinder is turned off due to the absence of a spark and with a working nozzle (modes 7 and 8). Such a malfunction immediately increases the emission of CH by many times in comparison with the emissions of a serviceable engine. Although in this case, the CH emission (exhaust gas extraction after the converter) is still an order of magnitude lower than before the converter, which demonstrates its efficiency. However, even short-term work with such a defect is dangerous, since a significant part of fuel burns out within the converter, heating it to unacceptable temperatures and destroying its structure. The above example shows that even random misfires caused by defects in the ignition system are extremely undesirable due to a sharp increase in CH emissions and risk of converter failure. Therefore, in most modern cars the control system immediately turns off the nozzle in a cylinder should the misfires occur.
4 Conclusions
A general quantitative assessment of the influence of individual operational modes and factors on CH and CO emissions with the selection of exhaust gases before and after the catalytic converter is given.
Under operating conditions of cold start and warm-up conventional serviceable converters are able to reduce by several times the concentration of CH and CO in the exhaust gas, provided that the control O2 sensor is immediately turned on. The efficiency of reduction depends on the activation time of the control O2 sensor and the amount of residual O2 in the exhaust gas. The lack of O2 concentration in the exhaust gas can be compensated for by the mixture depletion with the adjustment of the ECU.
With the engine fully warmed up and a working control circuit from the O2 sensor the standard converter effectively reduces the emissions of CH and CO to the minimum values for all tested modes with low mixture flow rates. Of the operational defects considered, skipping of operating cycles caused by malfunctions in the ignition system is the malfunction that most affects the emissions of CH. At the same time, a serviceable converter is capable of short-term reduction of emissions of CH in the exhaust gases by an order of magnitude. Turning off the fuel supply in a faulty cylinder allows us to temporarily eliminate the influence of the aforementioned defects on CH emissions and maintain the operability of the converter.
The practical importance of the proposed method for assessment of the converter’s efficiency consists in the possibility of using the test results to determine the reserves for reducing toxic components in the exhaust gases in cases of exceeding the established standards.
References
Lozhkin VN, Lozhkina OV (2015) Estimation of road transport related air pollution in Saint Petersburg using European and Russian calculation models. Transp Res Part D 36:178–189. https://doi.org/10.1016/j.trd.2015.02.013
Lozhkin VN, Lozhkina OV, Dobromirov V (2018) A study of air pollution by exhaust gases from cars in well courtyards of Saint Petersburg. In: Thirteenth international conference on organization and traffic safety management in large cities (SPbOTSIC 2018)/Transportation Research Procedia, vol 36, pp 453–458. https://doi.org/10.1016/j.trpro.2018.12.124
Morozov КA (1997) Toxicity of automobile engines. MADI, Moscow
Gorbunov VV, Pastrahaltsev NN (1998) The toxicity of internal combustion engines. Publishing House of the Peoples’ Friendship University of Russia, Moscow
Eggers M, Konig G (2005) Toxicity limits in: control systems for gasoline engines. 1st edn. Translation from German. Za rulem, Moscow, pp 363–382
Kulchitsky AR (2011) Toxicity of piston ICE. Experimental assessment of ecological level of engines. Publ. Vlad State University, Vladivostok
Chen H, Koo K, Rieck J et al (2014) Gasoline cold start concept technology for low temperature emission control. SAE Int J Fuels Lubrp 480–488. https://doi.org/10.4271/2014-01-1509
McAtee C, McCullough G, Douglas R (2019) Performance and characteristics of platinum, palladium/rhodium automotive catalysts when subjected to ethanol, acetaldehyde and a synthetic E85 exhaust gas mixture. Proc Inst Mech Eng Part D: J Automob Eng 226(11):1536–1546. https://doi.org/10.1177/0954407012446911
Pardiwala Julie M, Femina P, Sanjay P (2011) Review paper on catalytic converter for automotive exhaust emission. In: International conference on current trends in technology, NUiCONE, pp 1–6
Dorit A, Elyse D (2013) Improving the effectiveness of catalytic converters via reduction of cold start emissions. Univ Pittsbg, Swanson Sch Eng. https://doi.org/10.1088/1757-899X/197/1/012026
Gusakov SV, Sharipov AZ, Menshikh AA (2011) Improvement of ecological parameters of the automobile engine with spark ignition in period heating after cold start-up. Bull Peoples Friendsh Univ Russ Ser: Eng Res 3:60–67
Leshchenko VP (2003) Oxygen sensors. Legion-Avtodata, Moscow
Erokhov VI (2013) Designing and calculation of the motor vehicles control system oxygen sensor. Altern Fuel Veh 6(36):17–26
Srinivasa Chalapathi K, Murthy Bhavanarayana C, Pkumar SB (2014) Development of automobile catalytic converter during last four decades. Int J Res Appl Sci Eng Techn 2:321–333
Wang D, An H, Gong J, Currier N, Yezerets (2019) A Diagnostics of field-aged three-way converter (TWC) on stoichiometric natural gas engines. SAE Technical Papers. https://doi.org/10.4271/2019-01-0998
Karuppusamy P, Senthil R (2013) Design analysis of flow characteristics of catalytic converter and effects of back pressure on engine performance. Int. J. of Res. in Eng. & Adv. Technol 1:1–6
Prashant K (2016) Review paper on catalytic converter for automotive exhaust emission. Int. J. of Sc. and Res, p 30–33
Makwana Narendrasinh R, Amin Chirag M, Dabhi Shyam K (2013) Development and performance analysis of nickel based catalytic converter. Int J Adv Eng Technol 4:10–13
Promit C, Srisha D (2014) An innovative approach for emission control using copper plate catalytic converter. Int J Adv Sci Eng Technol 3:19–23
Venkatesan SP, Uday DS, Hemant BK, Kumar GL, Kumar KP (2017) Engine emission reduction by copper oxide catalytic converter. IOP Conf Ser: Mater Sci Eng 197(1)
Ivanov AK, Galyshev YV (2018) Performance of car petrol engine converter as a function of operational factors. Dvigatelestroeniye 1:16–19
Khaibulov RR, Ivanov AK (2016) Evaluation of the performance of a three-component converter of a gasoline engine in start-up and warm-up modes. Week of science SPbPU. IE and TS Part 1:246–248
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Ivanov, A.K., Abyzov, O.V., Galyshev, Y.V. (2021). Method for Assessing Performance of Catalytic Converter of Spark-Ignition Car Engine in Operating Conditions. In: Radionov, A.A., Gasiyarov, V.R. (eds) Proceedings of the 6th International Conference on Industrial Engineering (ICIE 2020). ICIE 2021. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-54814-8_104
Download citation
DOI: https://doi.org/10.1007/978-3-030-54814-8_104
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54813-1
Online ISBN: 978-3-030-54814-8
eBook Packages: EngineeringEngineering (R0)