Abstract
Bats serve as hosts to many lineages of arthropods, of which the blood-sucking bat flies (Nycteribiidae and Streblidae) are the most conspicuous. Bat flies can in turn be parasitized by Laboulbeniales fungi, which are biotrophs of arthropods. This is a second level of parasitism, hyperparasitism, a severely understudied phenomenon. Four genera of Laboulbeniales are known to occur on bat flies, Arthrorhynchus on Nycteribiidae in the Eastern Hemisphere, Dimeromyces on Old World Streblidae, Gloeandromyces on New World Streblidae, and Nycteromyces on Streblidae in both hemispheres. In this chapter, we introduce the different partners of the tripartite interaction and discuss their species diversity, ecology, and patterns of specificity. We cover parasite prevalence of Laboulbeniales fungi on bat flies, climatic effects on parasitism of bat flies, and coevolutionary patterns. One of the most important questions in this tripartite system is whether habitat has an influence on parasitism of bat flies by Laboulbeniales fungi. We hypothesize that habitat disturbance causes parasite prevalence to increase, in line with the “dilution effect.” This can only be resolved based on large, non-biased datasets. To obtain these, we stress the importance of multitrophic field expeditions and international collaborations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Parasites and Parasites of Parasites
Of the traditional categories of ecological relationships, parasitism is arguably the most common in nature. Price (1980) began his Evolutionary Biology of Parasites by arguing that “[it] has not been generally realized that the most extraordinary adaptive radiations on the earth have been among parasitic organisms.” A decade later, Windsor (1990, 1995) made a case to give equal rights to parasites, as they may equal in number free-living species (Price 1980; Windsor 1998). Indeed, interactions among trophic levels may be an important driver of microevolutionary processes ultimately leading to reproductive isolation and thus speciation. Although parasites maintain the stability, integrity, and structure of ecosystems and are important contributors to ecosystem functioning (Brooks and Hoberg 2001; Hudson 2005; Frainer et al. 2018), studies on species diversity that include parasites are rare (Wibbelt et al. 2009; Carlson et al. 2020).
Taking it one step further, hyperparasitism (parasitism of other parasites) is also thought to be a common phenomenon (Parratt and Laine 2016). When we suggest that parasites are a legitimate part of the earth’s biodiversity and important components of ecosystems, this applies to hyperparasites as well; all organisms are almost sure to pick up a parasite during their lifetime, even parasites. Hyperparasitism is relatively common. For example: entire aggregations of myialgine mites can be attached to and feed on the hemolymph of bloodsucking hippoboscoid flies (Goater et al. 2018), parasitic wasps can be parasitized by other wasps (van Nouhuys et al. 2016), and ectoparasitic bat flies are prone to carrying ectoparasitic fungi (Haelewaters et al. 2018b). Although common, hyperparasites are often overlooked. As a result, virtually nothing is known regarding functional roles and key ecological and physiological interactions between hosts and their (hyper)parasites.
2 The Vampire’s Vampire
As a remarkably successful mammalian radiation, bats (Order Chiroptera) have become hosts to numerous groups of parasites and pathogens. Their ecological abundance and sometimes dense roosting aggregations in combination with high roost fidelity create conditions favorable for transmission of symbionts. There are nearly a million described insect species on Earth (Grimaldi and Engel 2005) and many vertebrates are infested by parasites. However, true ectoparasites—blood feeders that spend most of their life-span on the host—are reported in only four orders (Diptera, Hemiptera, Phthiraptera, and Siphonaptera) and all but the Phthiraptera contain clades that have radiated on bats. Bat flies, with about 570 nominal species, have far surpassed the bat fleas (Ischnopsyllidae, 122 species), and bat bugs (Polyctenidae, 32 species) in species richness.
Bat flies have traditionally been divided into two families (Streblidae and Nycteribiidae) and together with tse tse (Glossinidae) and keds/louse flies (Hippoboscidae) form the superfamily Hippoboscoidea (Petersen et al. 2007). The Hippoboscoidea, as well as the bat flies (Streblidae+Nycteribiidae), have generally been accepted as monophyletic (Dittmar et al. 2006; Petersen et al. 2007). Moreover, there is support for a monophyletic Nycteribiidae, but not for the family Streblidae as currently comprised (Dittmar et al. 2015). All bat flies are obligate blood feeders and they are found only in association with bats. The streblids reach their zenith of diversity in the New World tropics, particularly in association with the Phyllostomidae. For example, about 80% of the described genera and 70% of the described species of Streblidae are known from the tropics and subtropics of South and Central America, including tropical portions of Mexico (Dick and Patterson 2006).
2.1 Nycteribiidae
The Nycteribiidae family is represented by 276 recognized species, arranged into 11 genera and three subfamilies. These flies are often referred to as “spider flies” due to the dorsal attachment of the legs, giving them a superficial “spider-like” appearance (Fig. 21.2a). The subfamilies and genera are largely similar in overall morphology, and appear to vary more along a gradient of size rather than shape. All nycteribiid species are entirely wingless, yet still possess halteres. Their global distribution is largely tropical and subtropical, but nearly 80% of nycteribiid species are limited to the Eastern Hemisphere. In the Western Hemisphere, nycteribiids mainly parasitize species of the Vespertilionidae, but also the Thyropteridae and one genus of Phyllostomidae (Gardnerycteris).
Dorsal habitus drawings of six species of bat flies, depicting some of the morphological diversity present in the group. (a) Nycteribiidae: Phthiridium biarticulatum (Hermann), female, modified from Theodor (1967). (b)–(f) Streblidae, (b) Ascodipteron africanum Jobling, male, modified from Jobling (1940). (c) Neotrichobius stenopterus Wenzel and Aitken, female, from Wenzel et al. (1966). (d) Metelasmus pseudopterus Coquillett, male, modified from Jobling (1936). (e) Speiseria ambigua Kessell, female, modified from Jobling (1939). (f) Anatrichobius scorzai Wenzel, female, from Wenzel et al. (1966)
2.2 Streblidae
Streblidae is represented by 240 recognized species, arranged into 33 genera and five subfamilies. Similar to nycteribiids, this family possesses much size variation, ranging from the tiny Mastoptera minuta (total length 0.5 mm) to the large Joblingia schmidti (total length 5.5 mm). However, within this family there exists much shape variation (Figs. 21.2b–f), including laterally-compressed “flea-like” forms (e.g., Nycterophiliinae), dorso-ventrally compressed forms (e.g., Streblinae), dealate and endoparasitic forms (e.g., Ascodipterinae), forms with extremely elongated legs (e.g., some Trichobiinae), and also flies that possess the typical muscoid form (e.g., some Trichobiinae). The streblids are far more diverse in the tropics and subtropics of the Western Hemisphere, which possesses 67% of the species diversity. There, the Streblidae have diversified extensively with phyllostomid and mormoopid bats, but also parasitize members of the Emballonuridae, Furipteridae, Molossidae, Natalidae, Noctilionidae, and Vespertilionidae.
2.3 Host Specificity
Host specificity is one of the most intriguing properties to emerge from host-parasite associations. It is a measure of the degree to which a parasite species occurs on a single host species. Traditionally, these associations have been categorized as monoxenous (one host species), stenoxenous (a few closely related host species) or polyxenous (many host species) (Wenzel et al. 1966). Historically, bat flies were largely viewed as not particularly host specific, owing to the fact that bat species often share roosting environments, and that records of many bat fly species were known from a variety of host bat species (Dick and Dittmar 2014). However, carefully controlled collection techniques have made it clear that many early records were attributable to human error such as sampling contamination, and a new consensus has emerged that bat flies are remarkably host specific, given their size, mobility, life cycle, and the multi-species roosting associations of their hosts (Dick 2007; Dick and Patterson 2007). Exceptional cases are known, however, where a single bat fly species may parasitize several well-demarcated host species yet show no population structuring, as appears to be the case with the nycteribiid Cyclopodia horsfieldi on three species of Pteropus bats in Asia (Olival et al. 2013). In other cases, it is quite possible that less-specific bat fly species may represent unrecognized species complexes (cryptic species) mirroring species complexes that are recognized in bats, e.g., in the genus Sturnira (Velazco and Patterson 2013, 2014). We note that such host-specific, near-cryptic segregation has also been detected in Laboulbeniales fungal ectoparasites of certain insects (Haelewaters et al. 2018a). The degree of specificity and the dynamics driving it is important, as it informs the potential for flies to encounter novel hosts in the environment (e.g., in roosts), to potentially spread hyperparasites such as Laboulbeniales to novel host species, or to move pathogens from host to host, including potentially to humans.
3 Ectoparasitic Fungi on Arthropods
There is little scientific consensus in the field of mycology, but for the acknowledgement that it will take many, many years to describe the vast diversity that lies in the Kingdom Fungi. Currently, 135,000 species are accepted (Hibbett et al. 2016), but estimates range from 1.5 to six million species of fungi. The number of fungal parasites is particularly underestimated. Focusing on insect-specific fungi, only 1.5% is estimated to be currently known (Mueller and Schmit 2007). These include necrotrophic and biotrophic parasites (Benjamin et al. 2004). Whereas necrotrophs kill their hosts and then use dead host cells as a source for nutrition, biotrophic parasites require a living host. A third type, “hemibiotrophy,” involves an initial biotrophic phase followed by a switch to necrosis (De Silva et al. 2016). An example is Magnaporthe grisea, the causal agent of blast diseases in agriculturally important crops.
One group of fungal biotrophic parasites are the Laboulbeniales (Ascomycota, Laboulbeniomycetes). They live as external parasites on arthropod hosts. Laboulbeniales fungi are microscopic in size, have peculiar morphology and complicated taxonomy, and are vastly understudied—even neglected—by the mycological community. The name Laboulbeniales honors the French entomologist Joseph A. Laboulbène, who was one of the first to observe these fungi back in the 1840s. Another French entomologist, Auguste Rouget, independently from Laboulbène, made observations of what he thought were antennal segments of a Brachinus ground beetle. Only later did he recognize them as living organisms (Rouget 1850). The earliest account of Laboulbeniales in the literature dates from 1849. An anonymous summary of a meeting of the Wissenschaftsfreunde mentioned that Ferdinand J. Schmidt had found clusters of bristles on Nebria “stentzii,” which he had identified as parasitic plants. Mayr (1853) thought the hairlike structures on Nebria beetles were outgrowths of the insect integument, but he described differences in the structures on younger and older host specimens. It was Robin (1852, 1853) who recognized these organisms as fungi. A few years later, two species of bat fly-associated Laboulbeniales were described as acanthocephalan worms (Kolenati 1857).
Laboulbeniales, colloquially dubbed beetle hangers by Mordecai C. Cooke in his book Vegetable wasps and plant worms: a popular history of entomogenous fungi, or fungi parasitic upon insects, are one of three orders in the class Laboulbeniomycetes, the others being Herpomycetales and Pyxidiophorales (Haelewaters et al. 2019). All three orders comprise fungi that are obligately associated with arthropods either as biotrophs (Herpomycetales, Laboulbeniales) or for dispersal (Pyxidiophorales). What sets the Laboulbeniales apart is their diversity, with 2325 known species and many more awaiting discovery and description. On the other hand, the orders Herpomycetales and Pyxidiophorales together include fewer than 50 accepted species. Laboulbeniales require a single host for successful development. A two-celled ascospore adheres to the new host and either penetrates the cuticle making contact with the body cavity for nutrition and support or remains superficially attached without penetration (Tragust et al. 2016). Subsequent divisions of the ascospore lead to a three-dimensional, multicellular unit of determinate growth, or a thallus. This sets the group apart from other fungi, which usually form hyphae and are recognized by unlimited growth.
The host range of Laboulbeniales as a group includes three subphyla of arthropods: Chelicerata, Myriapoda, and (mainly) Hexapoda. About 80% of described species have a beetle host (Coleoptera); other hosts are mites (Acari), harvestmen (Opiliones) (Chelicerata), millipedes (Diplopoda) (Myriapoda), cockroaches and termites (Blattodea), earwigs (Dermaptera), flies (Diptera), true bugs (Hemiptera), ants (Hymenoptera), crickets and allies (Orthoptera), lice (Psocodea), and thrips (Thysanoptera) (Hexapoda). Despite this wide host distribution, most Laboulbeniales show strict host specificity (De Kesel 1996; Haelewaters et al. 2018a). Others are “habitat specific”; they have multiple hosts in phylogenetically unrelated groups that occur in the same micro-habitat, such as ant nests and subterranean caves (De Kesel and Haelewaters 2014). There are two other types of specificity; some taxa are restricted to a specific position of the host integument (= position specificity) or to a given host sex (= sex-of-host specificity). An extreme example is Chitonomyces unciger, which only occurs on the claw of the left posterior leg of male Laccophilus maculosus beetles. There are opposing views as to the taxonomic significance of morphological variability in thalli among host species, between sexes of the hosts, and among locations on a given host. Different morphologies relating to the different types of specificity are treated as distinct species by some researchers, or as morphotypes (formae) of the same biological species by others. However, DNA-based studies at the species level have shown that morphology alone may be a poor means to understand the diversity of Laboulbeniales.
The small community of researchers studying Laboulbeniales primarily focuses on taxonomy (description of species). In recent years, however, several papers have resolved species-level taxonomic problems and clarified phylogenetic relationships among the order. Studies of the Laboulbeniales have long been challenging for multiple reasons. Thalli are microscopic in size, which requires micro-manipulation techniques and specific tools. Thalli are also long-lasting and so must absorb impacts and friction during their entire existence on a given host. This requires tough and resilient cells, which are difficult to break open. Hosts can carry different species of Laboulbeniales, but they can also carry multiple morphological forms (morphotypes) of the same species as well as multiple morphotypes of different species. Given this, DNA extractions ideally should be performed of single thalli. Many species are heavily pigmented with melanin in their cell walls, which interferes with molecular protocols to amplify regions of interest. Finally, contrary to the majority of fungi, researchers have not been able to grow Laboulbeniales in culture.
The relationships of the order Laboulbeniales to other members of the class Laboulbeniomycetes are far from established, with several lineages underrepresented in terms of taxa and sequence data. In addition, its intra-ordinal relationships are completely unresolved. Two major ordinal classifications have been proposed, one by Roland Thaxter in 1896, which he updated in 1908, and the other by Isabelle I. Tavares in 1985. Both are entirely based on morphology. The only criterion for grouping taxa in Thaxter’s system (1896) was the formation of spermatia. He separated the then “family Laboulbeniaceae” in two “groups,” the Exogenae and Endogenae. The Exogenae included genera with species that produce spermatia (gametes) on the appendages. The Endogenae, on the other hand, comprised taxa in which spermatia are formed inside of specialized organs, antheridia. This group included two “orders” depending on the way spermatia are discharged. In many genera, multiple simple antheridia are formed; these are individual cells, usually with a slender neck functioning as a discharge tube. In other genera, compound antheridia are produced: antheridial cells are arranged such that spermatia are released into a chamber with one common exit.
In Monoicomyces, the compound antheridia are distally rounded with an indistinguishable pore, whereas the compound antheridia of Peyritschiella have an elongated neck. Recent preliminary phylogenetic reconstructions of the Laboulbeniales (e.g., Goldmann and Weir 2018) show that compound antheridia originated more than once. Joseph H. Faull had pointed this out in 1911, but it took until Tavares for a new classification scheme to be introduced. Tavares (1985) used perithecial development and wall structure as well as antheridial characters in her classification. She divided the order into two suborders, three families, six subfamilies, as well as many tribes and subtribes. Some features that were considered by Tavares are phylogenetically informative, such as the number of perithecial wall cells, which seems to be undergoing a progressive reduction through evolutionary time. However, quite a number of higher taxa introduced by Tavares are polyphyletic, meaning that the taxa are placed in these unnatural groups that have derived from different common ancestors. For example, the Stigmatomycetinae tribe consists of 40 genera, but recent studies do not include half of these genera and they belong to multiple, unrelated clades (Goldmann and Weir 2018, Haelewaters et al. 2018c). In conclusion, the phylogeny of the Laboulbeniales order is in complete disarray. More taxa need to be sampled and more sequence data are needed in order to resolve this.
4 Bats, Bat Flies, and Laboulbeniales Fungi
Jonathan Swift, when writing his 1733 poem about multitrophic interactions—The vermin only teaze and pinch/Their foes superior by an inch/So, naturalists observe, a flea/Has smaller fleas that on him prey;/And these have smaller still to bite ‘em,/And so proceed ad infinitum—might not have realized that organisms involved in such interactions probably outnumber free-living organisms. Although understudied, we know of a number of hyperparasites specific to bats. This knowledge has resulted from comprehensive studies, sometimes triggered by accident during fieldwork. The bats–bat flies–Laboulbeniales project that is the focus of this chapter began with a single bat fly collected as bycatch by colleague and collaborator Jasmin J. Camacho. She had collected it along with other materials and preserved it in ethanol because she had remembered that her friend “did something with insects and fungi.” It would take a couple of months until that friend (DH) showed some interest in that bat fly. The first attempt to identify the fungus was a failure and it has been a steep learning curve since that time. But now, just 4–5 years later, that small project has led us to investigate 12000 bat flies in total with other collaborators in the United States, Panama, Germany, Hungary, and Switzerland.
The addition of a second level of parasitism to the study of bats is relatively new. Bats serve as host for all kinds of ectoparasites, including flies (Diptera), true bugs (Hemiptera), fleas (Siphonaptera), ticks and mites (Acari), and earwigs (Dermaptera). Some of those can be parasitized by other pathogens or parasites. For example, bat flies appear to be vectors for Bartonella bacteria, which are causal agents for zoonotic diseases in mammals (including humans) (Morse et al. 2012b). Also several lineages of bacterial endosymbionts are associated with bat flies. These associations are ancient in evolutionary time but due to a lack of integrative studies, we know little about the nature of these relationships (Morse et al. 2012a). When parasites (bat flies) serve as hosts to other parasites (Laboulbeniales fungi), we can see the bat itself as a microhabitat. A microhabitat can be defined as a small, localized environment within a larger ecosystem. For example, standing tree remnants and fallen logs are important microhabitats that serve as nutrient and energy resources and provide protection for invertebrates, amphibians, small mammals, plants, and fungi. In the bat microhabitat, options exist for host shifts of Laboulbeniales between bat flies. This is where things become interesting from an evolutionary point of view. Divergent natural selection among populations of Laboulbeniales fungi that are now exploiting different bat flies may ultimately lead to reproductive isolation and the formation of new species (Mayr 1942; Dobzhansky 1951; Schluter 2000).
4.1 Laboulbeniales on Bat Flies
Around 10% of Laboulbeniales species parasitize flies. Laboulbeniales that are associated with flies belong to eight genera: Arthrorhynchus, Dimeromyces, Gloeandromyces, Ilytheomyces, Laboulbenia, Nycteromyces, Rhizomyces, and Stigmatomyces. The eponymous genus Laboulbenia is by far the largest genus with hundreds of species, of which only 24 species are described from flies. The second-largest genus in the order is Stigmatomyces, with 171 described species that are all described from flies, although none from bat flies. Thus far, four genera have been reported from bat flies: Arthrorhynchus, Dimeromyces, Gloeandromyces, and Nycteromyces. Arthrorhynchus, Gloeandromyces, and Nycteromyces are specific to bat flies, whereas Dimeromyces has a wide host distribution.
4.1.1 Arthrorhynchus
Arthrorhynchus (Figs. 21.2 and 21.3a) is restricted to Eastern Hemisphere bat flies of Nycteribiidae. Four species are currently known, but this number is expected to increase in coming years. As an interesting fait divers, the first two species in the genus were described in the nineteenth century as acanthocephalan worms (Kolenati 1857). Peyritsch (1871) described a species Laboulbenia nycteribiae and considered both of Kolenati’s species as synonyms of the new taxon. Later, realizing his species was not actually a representative of the genus Laboulbenia, he established a new genus to accommodate this species: Helminthophana nycteribiae (Peyritsch 1873). Thaxter (1896) followed Peyritsch’s opinion but later retained the genus Arthrorhynchus and described two additional species so that there were three species in the genus: Arthrorhynchus cyclopodiae, A. eucampsipodae, and A. nycteribiae (Thaxter 1901). A fourth species, A. acrandros, was described by Aldo Merola (1952).
Thalli of bat fly-associated Laboulbeniales: (a) Arthrorhynchus nycteribiae (D. Haelew. 1015c, Felsőtárkány, Hungary); (b) Gloeandromyces streblae forma sigmomorphus (D. Haelew. 1099b, Gamboa, Panama); and (c) Nycteromyces streblidinus, a female thallus (D. Haelew. 1012a, Michoacan, Mexico). Scale bars: a = 100 μm and b–c = 50 μm
It is fair to say that the taxonomic history of the genus has been complicated. In addition, the current taxonomic status of these four species is unclear because of the lack of sequence data. Since their description, no-one has ever really done any work in this genus, except for Meredith Blackwell. At the 1979 Annual Meeting of the Mycological Society of America (Stillwater, OK), she reported on host associations, intraspecific morphological plasticity, and the description of developmental stages of thalli. Blackwell had screened 2517 nycteribiid bat flies for presence of Laboulbeniales fungi and observed thalli on 56 bat flies (= parasite prevalence of 2.2%). These results were later published in the journal Mycologia (Blackwell 1980a, b). It took almost four decades until this genus was dusted off again, with the publication of a paper in Parasites and Vectors. In this paper, Haelewaters et al. (2017a) examined 1494 bat flies and found 45 infected ones (= prevalence of 3.0%). The authors built a host-parasite-parasite network, discussed distributional and host ranges, and reported that Arthrorhynchus spp. may have a preference for female over male bat flies.
Celebrating the 40th birthday of the first-ever talk on Arthrorhynchus, Tamara Szentiványi and colleagues (2019) presented a poster at the International Bat Research Conference in Phuket, Thailand about the current conceptions of host specificity, species-level diversity, and geographic distribution. Preliminary molecular data show that Arthrorhynchus eucampsipodae and probably also A. nycteribiae are complexes of multiple species, which are segregated by host fly species. It is too early to make taxonomic decisions, but this is not a stand-alone case in the Laboulbeniales. Hesperomyces virescens is a taxon associated with over 30 species of ladybirds (Coleoptera, Coccinellidae). Using an integrative approach—combining morphometric, molecular phylogenetic, and ecological data—we found that H. virescens consists of different species, each adapted to an individual ladybird host (Haelewaters et al. 2018a). Discovering the same pattern of speciation in another genus of Laboulbeniales gives us insight to the untold diversity in this order. Current estimates predict up to 75,000 species in the order (Weir and Hammond 1997) but even this number does not incorporate the idea of species complexes.
4.1.2 Dimeromyces
Dimeromyces is one of the largest genera in the order, with about 115 described species, of which only two are known from bat flies (Rossi et al. 2016). The genus is dioecious, which means that (male) antheridia and (female) perithecia are housed on separate individuals. Species of Dimeromyces parasitize mites (Acari), termites (Blattodea), beetles of many families (Coleoptera), earwigs (Dermaptera), flies (Diptera), ants (Hymenoptera), crickets (Orthoptera), and thrips (Thysonaptera). Only recently, two species of Dimeromyces were described from bat flies (Dogonniuck et al. 2019). These are Dimeromyces capensis on Brachytarsina africana [as Nycteribosca] from South Africa, and D. streblidarum on Brachytarsina amboinensis [as Nycteribosca] from the Philippines. The two new species form a blackened foot, which is the (single) point of attachment to the host. The presence of a simple foot, however, is not a generic character, and this was already observed by Thaxter (1908).
Several species of Dimeromyces from earwigs and flies carry a haustorium. A haustorium is a simple or branched rhizoidal apparatus that penetrates the host’s integument to provide added stability and to increase surface area for nutrient uptake. Haustoria make contact with the body cavity (haemocoel) and draw nutrients from it. There had been a long-running debate whether all species of Laboulbeniales produce haustoria—simple and minute or well-developed—until Tragust et al. (2016), using light and electron microscopy, found no evidence for any penetration in four species of Laboulbeniales. Some hypothesize that the presence of a haustorium may trigger certain defense mechanisms of the host, which, in turn, requires physiological adjustments of the fungus (Haelewaters and De Kesel 2017, 2020). All this may facilitate specialization and reproductive isolation (speciation). Currently insufficient data are available to test this hypothesis across the order. As is the case for Dimeromyces, several other genera include species with a simple foot as well as species with a haustorium, including Gloeandromyces and Nycteromyces.
4.1.3 Gloeandromyces
Gloeandromyces was described by Thaxter (1931) to accommodate two species he had earlier reported as Stigmatomyces (Thaxter 1917). The fan-like organization of the appendage in Gloeandromyces is different from Stigmatomyces, and the gelatinous disorganization of the appendage structure in mature thalli is often indecipherable. After description, both species had not been found until a century later, when Haelewaters et al. (2017b) re-discovered them in Central America. A third species was described in the same paper, from Trichobius dugesioides bat flies in Gamboa: Gloeandromyces pageanus, named after long-time Panamanian collaborator Rachel A. Page. Most recently, a fourth species was described, Gloeandromyces dickii, from Trichobius joblingi in Nicaragua and Panama. The fungus was originally found on bat flies that were part of a loan of 7792 specimens kindly provided by CWD, and so was named in his honor.
Based on sequence data for the large subunit ribosomal DNA, Haelewaters and Pfister (2019) pointed out that G. pageanus is a conglomerate of three morphotypes or formae in two clades. The first clade, with G. pageanus f. pageanus, only occurs on T. dugesioides. This morphotype always seems to occur on the dorsal part of the thorax. The second clade is most-often observed on T. jobilingi and consists of two morphotypes, one of which is specific to the base of the wings (G. pageanus f. alarum) and the other does not show any positional preference the host (G. pageanus f. polymorphus). In other words, in G. pageanus, two mechanisms seem to drive diversity: host specialization and phenotypic plasticity leading to position-induced morphological alterations. Also in G. streblae, the same two mechanisms are observed; G. streblae forms two clades segregated by host, and one morphotype is recognized at the last segment of the abdomen (G. streblae f. sigmomorphus, Fig. 21.4b). In both G. pageanus and G. streblae, even though there is divergence of hosts, no speciation has occurred. This may be a case of either incipient or ephemeral speciation (Rosenblum et al. 2012).
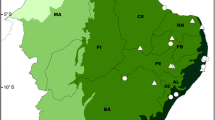
An overview of the multitrophic associations between families of bats, subfamilies of bat flies, and genera/species of Laboulbeniales fungi, presenting the geographic distributions of the taxonomic groups as currently understood. Illustrations by Damond Kyllo. Updated and modified from Haelewaters et al. (2018b)
4.1.4 Nycteromyces
Nycteromyces is a small genus with only two described species, both occurring on Streblidae. Nycteromyces streblidinus (Fig. 21.4c) was described by Thaxter (1917) on a Strebla wiedemanni (Streblinae), but since then it has only been found on species of Metelasmus (Streblinae), Aspidoptera, Eldunnia, Megistopoda, Speiseria, and Trichobius (Trichobiinae) (Haelewaters et al. 2018b; Dogonniuck et al. 2019, unpublished data). The second species, Nycteromyces orientalis, was described recently from Brachytarsina spp. [as Nycteribosca] (Brachytarsininae) (Dogonniuck et al. 2019). Whereas N. streblidinus is limited to New World streblids, N. orientalis is associated with an Old World streblid genus. From a biogeographical point of view, this poses an intriguing question: Does Nycteromyces have a Gondwanan origin? Southern Hemisphere biota have been shaped by the fragmentation of the supercontinent Gondwana, which caused the isolation and diversification of ancestral Gondwanan taxa on each respective landmass. Nycteromyces orientalis is very different from N. streblidinus, with a series of cells that give rise to multiple perithecia (Dogonniuck et al. 2019), whereas in N. streblidinus only a single perithecium is produced (Fig. 21.4c). However, this would be less surprising given a potential Gondwanan origin. Similarly, in another group of fungi (genus Amanita), researchers have identified taxa in southern South America to be grouped with relatives from Australia in a clade dating back to 34.5 million years ago, which fits with the timing of the fragmentation of South American, Australian, and Antarctic Plates (Truong et al. 2017).
4.2 Parasite Prevalences
Based on the study of 2517 nycteribiid bat flies, which were screened for the presence of Laboulbeniales fungi, a parasite prevalence of 2.2% was found (Blackwell 1980b). More recently, Haelewaters et al. (2017a) screened 1494 nycteribiid bat flies, and found 45 specimens to be infected with either Arthrorhynchus eucampsipodae or A. nycteribiae (3%). During a seven-night expedition in a Panamanian cloud forest (Walker et al. 2018), 227 bats were captured, resulting in 437 bat flies (436 streblids + 1 nycteribiid) of which 30 streblids carried thalli of Laboulbeniales (7%). Szentiványi et al. (2018) captured 270 Miniopterus schreibersii bats across Europe, resulting in 667 nycteribiid bat flies of which 60 were infected (9%). And finally, a comprehensive study of 7949 bat flies from both the New World and Old World resulted in a prevalence of only 4.6% (Haelewaters et al. 2018c).
These low percentages have been explained by life history. Ascospore transmission between bat flies likely occurs only on the bat through direct contact (De Kesel 1995). Based on the Smithsonian Venezuelan Survey collections, of 79 bat species that were captured five or more times and infested with bat flies, 7395 individual bats yielded 36,631 flies with an overall mean intensity of 4.95 streblid flies per bat host (Patterson et al. 2007). From 1594 bats of 28 species captured in central Europe, Haelewaters et al. (2017a) collected 1494 nycteribiid bat flies, with an average number of 1.79 flies per individual bat host. The mean intensity of bat fly parasitism is highly variable and dependent on myriad factors including bat host sex, species, roost type, and parasite life history, but in general it seems that the number of times a Laboulbeniales-infected bat fly comes into contact with another bat fly of the same species (or of a species that serves as a host to that Laboulbeniales species) is very low.
4.3 Effect of Climate
The presence and parasite “load” of Laboulbeniales infections can be influenced by biotic factors such as host age, sex, and aggregation behavior (Nalepa and Weir 2007; Báthori et al. 2018). But how abiotic factors affect the temporal and spatial distribution of these fungi is unexplored, except for one recent study by Szentiványi et al. (2019). These authors found a higher likelihood of presence of Arthrorhynchus on bat flies in habitats with low annual mean temperature and humidity. One of the factors that may play a role is the temperature-dependency of the immune response of arthropods; higher temperatures contribute to disease resistance in insects. In general, our knowledge on how climatic elements might alter host behavior or ectoparasite transmission, presence, and prevalence is still very limited.
4.4 Cospeciation Patterns
Coevolutionary studies can shed light on specific instances of host shifting and cospeciation. The application of molecular phylogenetic methods with various symbiotic associations has revealed patterns of congruence between the individual partners. These patterns can resolve questions regarding whether symbionts have diversified in parallel (cospeciation or coevolution) or reveal instances of host shifting over their evolutionary lineages. Such studies can also be applied to multitrophic systems. For example, the symbiosis between fungus-growing ants, the fungi they cultivate for nutrition, and the microfungal parasites of the ants’ fungus gardens has a coevolutionary history dating back tens of millions of years. At the deepest nodes, the phylogenies of these three partners are in perfect congruence, which implies the symbiosis results from a tripartite coevolution (Currie et al. 2003).
A preliminary coevolutionary study of Laboulbeniales fungi and their bat fly hosts resulted in congruence of the basal-most Old World clades (Haelewaters et al. 2018c). Bat roosting behavior may explain some of the other patterns that were observed. However, a major issue in the accurate interpretation of coevolutionary patterns is that the taxonomy of bat fly-associated Laboulbeniales fungi has not yet been resolved. For example, Nycteromyces streblidinus has bat fly hosts in seven genera. If this species turns out to be a complex of multiple species segregated by host, then the fungus phylogeny will look very different (multiple nodes) compared to our current understanding of N. streblidinus as a single species (a single node). In the case of multiple Nycteromyces species within N. streblidinus (sensu lato), different conclusions will need to be drawn from a coevolutionary study. The same is the case for Arthrorhynchus spp., which we now consider to be species complexes (Szentiványi et al. 2019).
5 Synergistic Interactions Leading to Uncharted Collaborations
It has been hypothesized that the majority of known species on earth exhibit characteristics of parasitism broadly defined. Conversely, parasite species necessarily associate with host species. Hence, it is not an overstatement that nearly all living beings are part of one or more host-parasite associations. Much of our understanding of the diversity, ecology, and evolution of parasitism was built upon a foundation of natural history collections, assembled by field biologists practicing traditional, often taxon-specific studies such as mammalogy, ornithology, entomology, and mycology. For example, parasites of mammals are routinely collected by mammalogists while conversely, mammals are collected by parasitologists in order to obtain their parasites. The synergy between mammalogists and parasitologists is rich and longstanding, if not assumed. For simplicity, we refer to a parasitologist as anyone studying parasitic organisms, whether fungi, bacteria, protozoan, or metazoan. Synergism between mammalogists and parasitologists may be nearly as old as those fields of inquiry. Correspondence between Charles R. Darwin and Henry Denny dating back to January 1865 focused on lice and various aspects of their host associations, specificity, as well as speciation and species boundaries (Darwin 1865).
5.1 Early Expeditions
With respect to bats and bat flies of the Western Hemisphere, specimens collected during early zoological expeditions were examined by numerous taxonomic specialists of mammals and parasites alike. The book Ectoparasites of Panama (Wenzel and Tipton 1966a) was a seminal and systemic work on various ectoparasitic groups collected from Panamanian mammals. This effort resulted from close collaborations between federal agencies in Panama (Gorgas Memorial Laboratory) and the United States (NIH Middle America Research Unit, the US Army, and the Smithsonian Institution). During the course of this highly collaborative study, more than 360 species of ectoparasites in over 120 genera were collected, of which 15 genera and 115 species were new to science. Moreover, the bats captured and surveyed for ectoparasites yielded around 12,000 specimens of streblid bat flies, with 44 species described as new. The mammalogical aspects of the survey were overseen by Charles O. Handley, Jr. of the Smithsonian Institution.
Another massive and collaborative effort was undertaken about a decade later in Venezuela, from 1965 to 1968, with the focus on mammal–parasite–habitat relationships. This was the Smithsonian Venezuelan Project, again with the mammalogical aspects overseen by Handley. This survey sampled bat flies and other ectoparasites from more than 6800 bats of 95 species, yielding over 36,000 specimens of streblids of 115 species and 22 genera. Two genera and 45 species were new to science (Wenzel 1976). These two massive collections alone produced nearly 50,000 specimens of streblid bat flies representing at least 40% of known collections for this group.
5.2 Recent Expeditions
Our current work in Panama, which has been a collaborative effort between researchers from Harvard University (USA), the Smithsonian Tropical Research Institute (Panama), the Universidad Autónoma de Chiriquí (Panama), and the University of Ulm (Germany), focuses particularly on the tripartite interactions. A three-month field trip capturing bats in Gamboa and at Soberanía National Park in the Canal Zone, in Chilibre, and at Chucantí Nature Reserve, resulted in 634 bats, of which 367 carried bat flies. Overall, our fieldwork in 2015–2020 has thus far resulted in the study of 4279 bat flies, of which 228 carried Laboulbeniales fungi (5.3%). Two new species and four morphotypes of Gloeandromyces have been described, and at least two more species await description. A one-month field trip in Cusuco National Park, Honduras in 2019 led by Operation Wallacea yielded 601 bats, of which 258 carried ectoparasites (bat flies, mites, and ticks). The study of these ectoparasites is still in progress.
5.3 Scientific Attention for Bats and Bat Flies Through Time
All of this fieldwork and related systematic activities have fueled the publication of catalogues and keys to regional bat fly faunas (Wenzel et al. 1966; Theodor 1967; Wenzel 1976; Guerrero 1993; Graciolli and de Carvalho 2001; Dick and Miller 2010). Keys, descriptions, diagnoses, and an increasing number of reference specimens have greatly simplified problems of identification, stimulating both systematic and ecological studies on the bat flies and additional investigations of their parasitic relationships. Moreover, the monumental and pioneering work of Wenzel et al. (1966), Theodor (1967), and Wenzel (1976) paved the way for population-level studies of bat flies and their host bats by researchers and their students alike.
For example, at an early NASBR meeting (then called the Second Southwestern Symposium on Bat Research) in November 1971, William L. Overal gave an oral presentation entitled “Host relationships of the batfly, Megistopoda aranae, a parasite of Artibeus jamaicensis in Panama.” Later, Overal received his Ph.D. degree based on the study of North American Trichobius bat flies (Overal 1980a). His dissertation committee consisted of George Byers (Chair), Charles Michener, and Robert Beer. Overal acknowledged having received invaluable assistance by Rupert Wenzel as well as other well-known bat biologists such as Merlin Tuttle and Thomas Kunz. Overal (1980b) subsequently published his study on the life cycle of Megistopoda.
Illustrating the growing trajectory of work on bat flies, a Web of Science search on 22 Aug 2019 produced 272 unique references (one article was not dated) published between 1901 and 2019 that used the terms “Streblidae” (196 hits), “Nycteribiidae” (175 hits), or both terms (99 hits). The resulting graph (Fig. 21.5) shows a recent exponential increase in scientific attention to a crucial link in this tripartite system, and bodes well for future understanding of both host-parasite interactions in which it is involved. This remarkable increase in attention is also obvious from talks and posters presented at scientific meetings—NASBR and other—between 1971 and 2019 (Supplementary File 21.1).
Total numbers of publications per decade citing Streblidae and/or Nycteribiidae. Inset: Bats and their ectoparasites. (a) Several streblid bat flies crawling through the fur of a Pteronotus mesoamericanus, Parque Nacional Soberanía, Panama. (b) A Rhinolophus bat with a large nycteribiid bat fly (Penicillidia fulvida), coastal Kenya
6 Future Research Directions
In a remarkable chapter entitled Some relationships between mammal hosts and their ectoparasites, Wenzel and Tipton (1966b) described many patterns of parasite-host associations and posed numerous outstanding questions that have motivated decades of inquiry into the ecology and evolution of parasite host associations. The phenomena highlighted and discussed in that chapter included host specificity, coexistence and competitive displacement, as well as altitudinal zonation and zoogeographic relationships between parasites and hosts. Many of the broad questions posted in this seminal chapter are still being addressed five decades later. Similarly, many of these same broad questions are also posed in the tripartite system of bats, bat flies, and Laboulbeniales fungi.
One of the most important questions that we aim to address concerns the effect of habitat on parasitism of bat flies by ectoparasitic fungi (Haelewaters et al. 2018b, Haelewaters and Martin 2019). We hypothesize that habitat disturbance causes parasite prevalence to increase, in line with the dilution effect (Fahrig 2003). The main idea is that healthy ecosystems reduce the average risk of disease, and habitat loss results in an elevated risk of wildlife diseases through a decline in overall biodiversity. However, parasitic reactions to habitat alterations depend on the parasite and its associated host. Given the low parasite prevalence encountered for Laboulbeniales on bat flies, we stress the need for large, non-biased datasets resulting from focused multitrophic fieldwork. We call for global collaborations with bat scientists and organizations. The aim is to keep building on our dataset of currently 11936 bat flies with associated metadata from the Western and Eastern Hemispheres to (1) define ecological and life history traits that are correlated with parasitism of bats by bat flies and of bat flies by Laboulbeniales fungi, and (2) fully understand (co)evolutionary relationships through the generation of phylogenetic and phylogenomic-scale data.
References
Báthori F, Pfliegler WP, Radai Z, Tartally A (2018) Host age determines parasite load of Laboulbeniales fungi infecting ants: implications for host-parasite relationship and fungal life history. Mycoscience 59(2):166–171
Benjamin RK, Blackwell M, Chapella I, Humber RA, Jones KG, Klepzig KA, Lichtwardt RW, Malloch D, Noda H, Roeper RA, Spatafora JW, Weir A (2004) The search for diversity of insects and other arthropod associated fungi. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, New York, pp 395–433
Blackwell M (1980a) Developmental morphology and taxonomic characters of Arthrorhynchus nycteribiae and A. eucampsipodae (Laboulbeniomycetes). Mycologia 72(1):159–168
Blackwell M (1980b) Incidence, host specificity, distribution, and morphological variation in Arthrorhynchus nycteribiae and A. eucampsipodae (Laboulbeniomycetes). Mycologia 72(1):143–158
Brooks DR, Hoberg EP (2001) Parasite systematics in the 21st century: opportunities and obstacles. Trends Parasitol 17(6):273–275
Carlson CJ, Hopkins S, Bell KC, Doña J, Godfrey SS, Kwak ML, Lafferty KD, Moir ML, Speer KA, Strona G, Torchin M (2020) A global parasite conservation plan. Biol Conserv 250:108596
Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, Sung GH, Spatafora JW, Straus NA (2003) Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299(5605):386–388
Darwin C (1865) Correspondence of Charles Darwin
De Kesel A (1995) Relative importance of direct and indirect infection in the transmission of Laboulbenia slackensis (Ascomycetes, Laboulbeniales). Bel J Bot 128(2):124–130
De Kesel A (1996) Host specificity and habitat preference of Laboulbenia slackensis. Mycologia 88:565–573
De Kesel A, Haelewaters D (2014) Laboulbenia slackensis and L. littoralis sp. nov. (Ascomycota, Laboulbeniales), two sibling species as a result of ecological speciation. Mycologia 106:407–414
De Silva NI, Lumyong S, Hyde KD, Bulgakov T, Phillips AJL, Yan JY (2016) Mycosphere essays 9: defining biotrophs and hemibiotrophs. Mycosphere 7(5):545–559
Dick CW (2007) High host specificity of obligate ectoparasites. Ecol Entomol 32:446–450
Dick CW, Dittmar K (2014) Parasitic bat flies (Diptera: Streblidae and Nycteribiidae): host specificity and potential as vectors. In: Klimpel S, Melhorn H (eds) Parasitology research monographs (Vol. 5): bats (Chiroptera) as vectors of diseases and parasites. Springer, Berlin Heidelberg, pp 131–155
Dick CW, Miller JA (2010) Streblidae. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado M (eds) Manual of central American diptera (Vol II). National Research Council Press, Ottawa, pp 1249–1260
Dick CW, Patterson BD (2006) Bat flies: obligate ectoparasites of bats. In: Morand S, Krasnov BR, Poulin R (eds) Micromammals and macroparasites. Springer, Tokyo, pp 179–194
Dick CW, Patterson BD (2007) Against all odds: explaining high host specificity in dispersal-prone parasites. Int J Parasitol 37:871–876
Dittmar K, Porter ML, Murray S, Whiting MF (2006) Molecular phylogenetic analysis of nycteribiid and streblid bat flies (Diptera: Brachycera, Calyptratae): implications for host associations and phylogeographic origins. Mol Phylogenet Evol 38:155–170
Dittmar K, Morse SF, Dick CW, Patterson BD (2015) Bat fly evolution from the eocene to the present (Hippoboscoidea, Streblidae and Nycteribiidae). In: Morand S (ed) Parasite diversity and diversification: evolutionary ecology meets phylogenetics. Cambridge University Press, Cambridge, pp 246–264
Dobzhansky T (1951) Genetics and the origin of species. Columbia University Press, New York
Dogonniuck AE, Squires TJ, Weir A (2019) Studies on Dimorphomyceteae I. new species of Nycteromyces and Dimeromyces (Laboulbeniales) on bat flies (Streblidae). Mycologia 111(1):118–126
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34(1):487–515
Frainer A, McKie BG, Amundsen PA, Knudsen R, Lafferty KD (2018) Parasitism and the biodiversity-functioning relationship. Trends Ecol Evol 33(4):260–268
Goater CP, Dyck J, Proctor H, Floate KD (2018) Hyperparasitism of an avian ectoparasitic hippoboscid fly, Ornithomya anchineuria, by the mite, Myialges cf. borealis, in Alberta, Canada. J Parasitol 104(2):111–117
Goldmann L, Weir A (2018) Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biol 122(2–3):87–100
Graciolli G, de Carvalho CJB (2001) Moscas ectoparasitas (Diptera, Hippoboscoidea) de morcegos (Mammalia, Chiroptera) do Estado do Paraná. II. Streblidae. Chave pictórica para gêneros e espécies. Rev Bras Zool 18:907–960
Grimaldi D, Engel MS (2005) Evolution of the insects. Cambridge University Press, Cambridge
Guerrero R (1993) Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo. I. Clave para los géneros y Nycterophiliinae. Acta Biol Venez 14:61–75
Haelewaters D, De Kesel A (2017) De schimmel Hesperomyces virescens, een natuurlijke vijand van lieveheersbeestjes. Entomol Ber 77(3):106–118
Haelewaters D, De Kesel A (2020) Checklist of thallus-forming Laboulbeniomycetes from Belgium and the Netherlands, including Hesperomyces halyziae and Laboulbenia quarantenae spp. nov. MycoKeys 71:23–86
Haelewaters D, Martin TE (2019) Bat fly-associated fungi: current developments and a call for global collaborations. Paper presented at the 49th North American Symposium on Bat Research, Kalamazoo, 23–26 October 2019
Haelewaters D, Pfister DH (2019) Morphological species of Gloeandromyces (Ascomycota, Laboulbeniales) evaluated using single-locus species delimitation methods. Fungal Syst Evol 3:19–33
Haelewaters D, Pfliegler WP, Szentiványi T, Földvári M, Sándor AD, Barti L, Camacho JJ, Gort G, Estók P, Hiller T, Dick CW, Pfister DH (2017a) Parasites of parasites of bats: Laboulbeniales (fungi: Ascomycota) on bat flies (Diptera: Nycteribiidae) in Central Europe. Parasit Vectors 10(1):96
Haelewaters D, Verhaeghen SJC, Ríos Gonzáles TA, Bernal Vega JA, Villarreal Saucedo RV (2017b) New and interesting Laboulbeniales from Panama and neighboring areas. Nova Hedwigia 105(3–4):267–299
Haelewaters D, De Kesel A, Pfister DH (2018a) Integrative taxonomy reveals hidden species within a common fungal parasite of ladybirds. Sci Rep 8:15966
Haelewaters D, Hiller T, Dick CW (2018b) Bats, bat flies, and fungi: a case of hyperparasitism. Trends Parasitol 34(9):784–799
Haelewaters D, Page RA, Pfister DH (2018c) Laboulbeniales hyperparasites (fungi, Ascomycota) of bat flies: independent origins and host associations. Ecol Evol 8(16):8396–8418
Haelewaters D, Pfliegler WP, Gorczak M, Pfister DH (2019) Birth of an order: comprehensive molecular phylogenetic study excludes Herpomyces (fungi, Laboulbeniomycetes) from Laboulbeniales. Mol Phylogenet Evol 133:286–301
Hibbett D, Abarenkov K, Kõljalg U, Öpik M, Chai B, Cole J, Wang Q, Crous P, Robert V, Helgason T, Herr JR, Kirk P, Lueschow S, O’Donnell K, Nilsson RH, Oono R, Schoch C, Smyth C, Walker DM, Porras-Alfaro A, Taylor JW, Geiser DM (2016) Sequence-based classification and identification of fungi. Mycologia 108(6):1049–1068
Hudson P (2005) Introduction—parasites, diversity, and the ecosystem. In: Guegan JF, Renaud F, Guegan JF (eds) Thomas F. Oxford University press, Parasitism and ecosystems, pp 1–13
Jobling B (1936) A revision of the subfamilies of the Streblidae and the genera of the subfamily Streblinae (Diptera Acalypterae) including a redescription of Metelasmus pseudopterus Coquillett and a description of two new species from Africa. Parasitology 28(3):355–380
Jobling B (1939) On some American genera of the Streblidae and their species, with the description of a new species of Trichobius (Diptera: Acalypterae). Parasitology 31(4):486–497
Jobling B (1940) Description of the young female and of the male of Ascodipteron africanum Jobling (Diptera: Streblidae). Parasitology 32(4):399–400
Kolenati FA (1857) Epizoa der Nycteribien. Wien Entomol Monatsschr 1:66–69
Mayr E (1942) Systematics and the origin of species. Columbia University Press, New York
Mayr G (1853) Abnorme Haargebilde an Nebrien und einige Pflanzen Krains. Verh Zool-Bot Ges Wien 2:75–77
Merola A (1952) Interessante ritrovamento di labulbeniologia cavernicola: Arthrorhynchus acrandros n. sp. (con considerazioni sul gen. Arthrorhynchus). Boll Soc Nat Napoli 60:1–30
Morse SF, Dick CW, Patterson BD, Dittmar K (2012a) Some like it hot: evolution and ecology of novel endosymbionts in bat flies of cave-roosting bats (Hippoboscoidea, Nycterophiliinae). Appl Environ Microbiol 78:8639–8649
Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, Dittmar K (2012b) Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infect Genet Evol 12:1717–1723
Mueller GM, Schmit JP (2007) Fungal biodiversity: what do we know? What can we predict? Biodivers Conserv 16:1–5
Nalepa CA, Weir A (2007) Infection of Harmonia axyridis (Coleoptera: Coccinellidae) by Hesperomyces virescens (ascomycetes: Laboulbeniales): role of mating status and aggregation behavior. J Invert Pathol 94(3):196–203
Olival KJ, Dick CW, Simmons NB, Morales JC, Melnick DJ, Dittmar K, Perkins SL, Daszak P, DeSalle R (2013) Lack of population genetic structure and host specificity in the bat fly, Cyclopodia horsfieldi, across species of Pteropus bats in Southeast Asia. Parasit Vectors 6:231
Overal WL (1980a) Biology and behavior of North American Trichobius bat flies (Diptera: Streblidae). PhD dissertation, University of Kansas, Lawrence
Overal WL (1980b) Host-relations of the batfly Megistopoda aranea (Diptera: Streblidae) in Panama. Univ Kansas Sci Bull 52:1–20
Parratt SR, Laine AL (2016) The role of hyperparasitism in microbial pathogen ecology and evolution. ISME J 10:1815–1822
Patterson BD, Dick CW, Dittmar K (2007) Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). J Trop Ecol 23:177–189
Petersen FT, Meier R, Kutty SN, Wiegmann BM (2007) The phylogeny and evolution of host choice in the Hippoboscoidea (Diptera) as reconstructed using four molecular markers. Mol Phylogenet Evol 45:111–122
Peyritsch J (1871) Über einige Pilze aus der Familie der Laboulbenien. Sitzungsber Kaiserl Akad Wiss Math-naturwiss Cl 64:441–458
Peyritsch J (1873) Beiträge zur Kenntniss der Laboulbenien. Sitzungsber Kaiserl Akad Wiss Math-naturwiss Cl 68:227–254
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton
Robin CP (1852) Végétaux parasites sur une insecte du genre Brachynus. C R Séances Soc Biol 4:11
Robin CP (1853) Histoire naturelle des végétaux parasites qui croissent sur l’homme et sur les animaux vivants. J.-B. Baillière, Paris
Rosenblum EB, Sarver BA, Brown JW, Des Roches S, Hardwick KM, Hether TD, Eastman JM, Pennell MW, Harmon LJ (2012) Goldilocks meets Santa Rosalia: an ephemeral speciation model explains patterns of diversification across time scales. Evol Biol 39(2):255–261
Rossi W, Máca J, Preisler J (2016) A new parasitic fungus on the cleptoparasite of bees Braula coeca (Insecta, Diptera): Dimeromyces braulae (Ascomycota, Laboulbeniales). Nova Hedwigia 102(1–2):271–274
Rouget A (1850) Notice sur une production parasite observée sur le Brachinus crepitans. Ann Soc Entomol Fr 8:21–24
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Szentivanyi T, Haelewaters D, Pfliegler WP, Clément L, Christe P, Glaizot O (2018) Laboulbeniales (fungi: Ascomycota) infection of bat flies (Diptera: Nycteribiidae) from Miniopterus schreibersii across Europe. Parasit Vectors 11(1):395
Szentiványi T, Pfliegler WP, Sándor AD Christe P, Glaizot O, Blackwell M, Haelewaters D (2019) Distribution, host specificity, and variation in Arthrorhynchus nycteribiae and A. eucampsipodae (Laboulbeniomycetes), an update. Poster presented at the 18th International Bat Research Conference, Phuket, Thailand, 28 July–1 August 2019
Tavares II (1985) Laboulbeniales (Fungi, Ascomycetes). Mycol Mem 9:1–627
Thaxter R (1896) Contribution towards a monograph of the Laboulbeniaceae. Mem Am Acad Arts Sci 12:187–429
Thaxter R (1901) Preliminary diagnosis of new species of Laboulbeniaceae. III Proc Am Acad Arts Sci 36(23):397–414
Thaxter R (1908) Contribution toward a monograph of the Laboulbeniaceae. Part II. Mem Am Acad Arts Sci 13:217–469. Plates XXVIII–LXXI
Thaxter R (1917) New Laboulbeniales, chiefly dipterophilous American species. Proc Am Acad Arts Sci 52(10):649–721
Thaxter R (1931) Contribution toward a monograph of the Laboulbeniaceae V. Mem Am Acad Arts Sci 16:1–435. Plates I–LX
Theodor O (1967) An illustrated catalogue of the Rothschild collection of Nycteribiidae. Trustees of the British Natural History Museum, London
Tragust S, Tartally A, Espadaler X, Billen J (2016) Histopathology of Laboulbeniales (Ascomycota: Laboulbeniales): ectoparasitic fungi on ants (hymenoptera: Formicidae). Myrmecol News 23:81–89
Truong C, Sánchez-Ramírez S, Kuhar F, Kaplan Z, Smith ME (2017) The Gondwanan connection–southern temperate Amanita lineages and the description of the first sequestrate species from the Americas. Fungal Biol 121(8):638–651
van Nouhuys S, Kohonen M, Duplouy A (2016) Wolbachia increases the susceptibility of a parasitoid wasp to hyperparasitism. J Exp Biol 219(19):2984–2990
Velazco PM, Patterson BD (2013) Diversification of the yellow-shouldered bats, genus Sturnira (Chiroptera, Phyllostomidae), in the New World tropics. Mol Phylogenet Evol 68:683–698
Velazco PM, Patterson BD (2014) Two new species of yellow-shouldered bats, genus Sturnira Gray, 1842 (Chiroptera, Phyllostomidae) from Costa Rica, Panama and western Ecuador. ZooKeys 402:43–66
Walker MJ, Dorrestein A, Camacho JJ, Meckler LA, Silas KA, Hiller T, Haelewaters D (2018) A tripartite survey of hyperparasitic fungi associated with ectoparasitic flies on bats (Mammalia: Chiroptera) in a neotropical cloud forest in Panama. Parasite 25:19
Weir A, Hammond PM (1997) Laboulbeniales on beetles: host utilization patterns and species richness of the parasites. Biodivers Conserv 6:701–719
Wenzel RL (1976) The streblid batflies of Venezuela (Diptera: Streblidae). Brigham Young Univ Sci Bull 20:1–177
Wenzel RL, Tipton VJ (1966a) Ectoparasites of Panama. Field Museum of Natural History, Chicago
Wenzel RL, Tipton VJ (1966b) Some relationships between mammal hosts and their ectoparasites. In: Wenzel RL, Tipton VJ (eds) Ectoparasites of Panama. Field Museum of Natural History, Chicago, pp 677–723
Wenzel RL, Tipton VJ, Kiewlicz A (1966) The streblid batflies of Panama (Diptera: Calypterae: Streblidae). In: Wenzel RL, Tipton VJ (eds) Ectoparasites of Panama. Field Museum of Natural History, Chicago, pp 405–675
Wibbelt G, Speck S, Field G (2009) Methods for assessing diseases in bats. In: Kunz THP (ed) Ecological and behavioral methods for the study of bats. John Hopkins University Press, Baltimore, pp 775–794
Windsor DA (1990) Heavenly hosts. Nature 348:104
Windsor DA (1995) Equal rights for parasites. Conserv Biol 9:1–2
Windsor DA (1998) Most of the species on Earth are parasites. Int J Parasitol 28:1939–1941
Acknowledgements
DH received support from the following sources: David Rockefeller Center for Latin American Studies (2015/2017 Summer and 2015 Term-Time Research Travel Grants), Harvard University Herbaria (2017 Fernald Fund), Mycological Society of America (2016 Graduate Fellowship, 2017 Robert W. Lichtwardt Student Research Award, 2018 Forest Fungal Ecology Postdoctoral Research Award), and Smithsonian Tropical Research Institute (2016 Short-Term Research Fellowship). CWD was supported in part by a WKU RCAP (18-8001) and the Robinson Professorship. KPCP thanks Juan A. Bernal Vega, Tina Antje Hofmann, Angelica M. Rodríguez Castillo, and Rosa V. Villarreal Saucedo for support with her thesis project “Diversidad de Laboulbeniales (Fungi: Ascomycota) en moscas ectoparásitas (Insecta: Diptera) de murciélagos en dos bosques de tierras bajas, Panamá.”
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
File 21.1
(DOCX 16 kb)
Rights and permissions
Copyright information
© 2021 North American Society for Bat Research (NASBR)
About this chapter
Cite this chapter
Haelewaters, D., Dick, C.W., Cocherán Pittí, K.P., Dittmar, K., Patterson, B.D. (2021). Bats, Bat Flies, and Fungi: Exploring Uncharted Waters. In: Lim, B.K., et al. 50 Years of Bat Research. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-54727-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-54727-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54726-4
Online ISBN: 978-3-030-54727-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)