Abstract
High-dose chemotherapy with autologous stem cell transplantation is a standard of care for newly diagnosed multiple myeloma. Even in the era of second- and third-generation novel agents as well as monoclonal antibodies, autologous transplant deepens response and prolongs survival. In this chapter, the authors review the role of stem cell transplant in a changing treatment landscape and summarize current study results on induction therapy and the role of tandem and salvage transplants. Guidance to assess transplant eligibility and practical recommendations for supportive care and maintenance therapy after autologous transplantation is also provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple myeloma
- High-dose chemotherapy
- Melphalan
- Autologous stem cell transplantation
- Induction
- Maintenance
- Tandem transplantation
Introduction
Multiple myeloma (MM) is characterized by the proliferation of monoclonal plasma cells in the bone marrow and usually the presence of a monoclonal protein in the serum and/or urine. Secondary end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone destruction (CRAB criteria) indicates symptomatic disease requiring therapy [1]. Furthermore, the presence of an abnormal serum free light chain ratio (>100, with involved free light chains >100 mg/l), two or more focal lesions in MRI or PET/CT as well as more than 60% monoclonal plasma cells in the bone marrow are myeloma-defining events according to the International Myeloma Working Group guidelines [2]. The introduction of novel agents and monoclonal antibodies revolutionized the treatment of MM in the last years and with every new drug approval, the value of ongoing utilization of autologous stem cell transplantation (ASCT) is questioned. However, recent phase III trials confirmed that combining novel agents with ASCT is associated with longer progression-free survival (PFS) compared to treatment with novel agents alone (Table 18.1) [3,4,5,6]. Although MM is still considered to be an incurable disease, long-lasting remissions over 10 years can be achieved making it difficult to determine if overall survival can serve as a primary endpoint for trials [7]. Furthermore, the outcome varies significantly among newly diagnosed patients based on risk stratification (Table 18.2) [8].
Assessment of Transplant Eligibility
There is no formal age cut-off for transplant eligibility in MM. Most phase III trials of ASCT have enrolled patients with an upper age limit of 65 years but other trials such as BMT CTN 0702 and CALGB 100104 allowed enrollment to 70 years of age. ASCT can be performed safely in older, medically fit patients [9, 10]. Therefore, transplant eligibility should be determined mostly on the basis of comorbidities. Table 18.3 summarizes the recommended assessments prior to ASCT at Roswell Park Comprehensive Cancer Center.
Induction Therapy
The common practice of bortezomib-based induction therapies is supported by large meta-analyses [11]. Recent phase III trials compared different combination partners for bortezomib (Velcade®) during induction therapy before ASCT.
-
1.
The initial EVOLUTION phase I/II study appeared to demonstrate that VCD (bortezomib, cyclophosphamide, and dexamethasone) and VRD had similar outcomes [12].
-
2.
The German GMMG MM5 trial showed that VCD (bortezomib, cyclophosphamide, and dexamethasone) is less toxic than PAd (bortezomib, doxorubicin, and dexamethasone) [13].
-
3.
The French IFM2013-04 trial demonstrated higher rates of high-quality responses for VTD compared to VCD [14].
-
a.
However, VTD was associated with higher rates of neuropathy compared to VCD.
-
b.
Although there has never been a direct prospective, randomized comparison between VTD and VRD (bortezomib, lenalidomide, and dexamethasone), many centers utilize VRD as recently applied in the IFM/DFCI2009 phase III trial [5].
-
a.
-
4.
Currently, second-generation novel agents such as ixazomib (Ninlaro®) (in combination with lenalidomide and dexamethasone [IRD]) [15] and carfilzomib (Kyprolis®) (in combination with lenalidomide/dexamethasone [KRD] or cyclophosphamide/dexamethasone [KCD]) [16] are being tested as induction before ASCT with promising results.
-
5.
The CASSIOPEIA trial investigating VTD with or without daratumumab (Darzalex®) before and after ASCT showed for the first time superiority of an induction regimen incorporating a monoclonal antibody [17].
-
6.
Further results from trials incorporating monoclonal antibodies such as elotuzumab (Empliciti®) and isatuximab (e.g., Clinicaltrials.gov identifier NCT03617731) into induction therapy before ASCT are expected in 2019/2020.
-
7.
Table 18.4 summarizes recent phase II/III trials on induction therapy before ASCT.
Stem Cell Mobilization
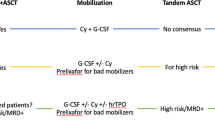
An adequate collection of mobilized peripheral stem cells is a crucial or successful outcome of autoHCT. A dose of >2 × 106 CD34+ cells/kg is considered the minimum target dose to achieve optimal engraftment [18]. The main risk factors for poor mobilization are age >60 years, thrombocytopenia [19], extensive previous treatment with radiotherapy or alkylating agents [18, 20,21,22,23], and prolonged use of lenalidomide [24,25,26,27]. Stem cell mobilization can be performed with growth factors alone, a combination of growth factors with chemotherapy, or with chemokine receptor antagonists (Table 18.5).
High-Dose Therapy
-
1.
Melphalan 200 mg/m2 is considered the standard of care [28] and usually administered intravenously in divided doses on days −3 and −2 or as a single dose on day −2 only before autoHCT.
-
a.
Dose reduction to 100 mg/m2 is associated with an adverse outcome [29].
-
b.
To prevent anticipated toxicities in medically compromised patients (e.g., elderly patients or patients with cardiac disease), the melphalan dosage might be reduced to 140 mg/m2 without apparent loss of efficacy compared to 200 mg/m2 [30].
-
c.
Also in patients with renal insufficiency (RI) and dialysis-dependent renal impairment, melphalan should be reduced accordingly to obtain comparable results to patients with normal/mild RI and potentially achieve dialysis independence [31].
-
a.
-
2.
Tandem transplantation
-
a.
In the past, several studies addressed the question of whether a tandem autoHCT, that is, a second autoHCT usually within 6 months after the first, should be performed [32].
-
b.
In the era of novel agent-based induction and maintenance therapy, conflicting results from two prospective phase III trials have been reported.
-
i.
While the abovementioned EMN02/HO95 phase III trial demonstrated the inferiority of single versus tandem autoHCT [6], especially in patients with the high-risk disease [33], the StaMINA trial showed no significant differences for PFS and overall survival (OS) between single and tandem autoHCT, even in patients with the high-risk disease [34].
-
ii.
In the author’s practice, tandem autoHCT is offered to patients with the suboptimal response after induction therapy, FISH-based high-risk cytogenetics, or those patients not in complete remission after a first autoHCT.
-
i.
-
a.
Supportive Care
-
1.
Patients with newly diagnosed MM are prone to infections due to the impaired humoral and cellular immunity caused by the proliferation of malignant plasma cells and the production of nonfunctional antibodies.
-
2.
Infectious complications are the most common cause of death during the first 3 months of therapy, and one study suggested that antibiotic prophylaxis can reduce febrile episodes and death [35]. Table 18.6 summarizes the recommended prophylaxis.
-
3.
General treatment of infectious complications such as neutropenic fever is discussed separately in this book. Furthermore, vaccinations need to be repeated after autoHCT, and one suggested schedule of administration is summarized in Table 18.7; an alternative schedule of administration is provided in Appendix 9 .
-
4.
Other common side effects of autoHCT for MM are nausea and vomiting as well as gastrointestinal mucositis.
Maintenance Therapy After AutoHCT
Maintenance therapy in MM after autoHCT has been shown to improve OS. The commonly used agent is lenalidomide, whereas new approaches show also improved survival for maintenance therapy with bortezomib and ixazomib [3, 42,43,44,45].
-
1.
Lenalidomide (Revlimid®)
-
a.
Lenalidomide is indicated as standard maintenance therapy after autoHCT in the United States and Europe.
-
b.
4 randomized trials showed significantly improved PFS with lenalidomide maintenance therapy versus placebo or observation [3, 42,43,44,45].
-
c.
Meta-analyses demonstrated improved OS [45].
-
d.
Standard dosing: 10 mg po daily continuous, increase up to 15 mg daily if tolerated [45].
-
e.
Main side effects [46]
-
i.
Hematologic toxicity (neutropenia, anemia, thrombocytopenia)
-
ii.
Increased risk of secondary primary malignancies
-
iii.
Increased risk of venous thromboembolic events (VTE)
-
iv.
Gastrointestinal side effects (esp. diarrhea)
-
v.
Drug rash
-
i.
-
f.
Concurrent medication [47, 48]:
-
i.
If no other risk factors for VTE: aspirin 81 mg/d po.
-
ii.
If other risk factors for VTE: low-molecular-weight heparin or full-dose warfarin.
-
iii.
Oral anticoagulants such as apixaban (Eliquis®) were successfully evaluated for VTE prophylaxis in IMiD-treated patients [49].
-
i.
-
g.
Duration
-
i.
Three out of the four randomized phase III studies involved continuing maintenance treatment until disease progression.
-
ii.
Administration of lenalidomide beyond the achievement of complete remission (CR) is associated with better OS and therefore should be continued until disease progression if toxicities are tolerable [50].
-
i.
-
a.
-
2.
Bortezomib (Velcade®)
-
a.
Bortezomib with induction and maintenance improved PFS compared to vincristine with induction and thalidomide with maintenance [51, 52].
-
b.
Improves outcome in patients with del(17p) [53].
-
c.
Standard dosing: 1.3 mg/m2 sc every 2 weeks [51].
-
d.
Main side effects [54]:
-
i.
Hematologic toxicity (neutropenia, thrombocytopenia)
-
ii.
Peripheral neuropathy
-
iii.
Gastrointestinal side effects
-
i.
-
e.
Concurrent medication:
-
i.
Herpes zoster prophylaxis with low-dose acyclovir [55]
-
i.
-
f.
Duration: In studies discontinuation after 2 years [51]. Based on results from lenalidomide maintenance studies, treatment until progression might prolong survival and should be considered if no severe side effects occur.
-
a.
-
3.
Ixazomib (Ninlaro®)
-
a.
Improved post-autoHCT PFS by 5 months when compared to placebo [70]
-
b.
Standard dosing: 3 mg po every 2 weeks; may increase up to 4 mg if tolerated
-
c.
Main side effects:
-
i.
Hematologic toxicity (thrombocytopenia)
-
ii.
Peripheral neuropathy
-
iii.
Gastrointestinal side effects
-
i.
-
d.
Concurrent medication:
-
i.
Herpes zoster prophylaxis with low-dose acyclovir.
-
i.
-
e.
Duration: In studies, discontinuation after 2 years. Based on results from lenalidomide maintenance studies, treatment until progression might prolong survival and should be considered if no severe side effects occur.
-
a.
Response Criteria
Historically, response criteria were based on the measurement of monoclonal protein in serum and urine as well as bone marrow plasma cell count. Response is categorized in stringent complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), minimal response (MR), stable disease (SD), and progressive disease (PD). Revised criteria include new parameters of minimal residual disease (MRD) measured by flow cytometry or gene sequencing (Table 18.8). Furthermore, sensitive imaging techniques can detect extramedullary residual disease [57].
Salvage AutoHCT
Retrospective analyses demonstrated that salvage autoHCT after re-induction therapy is an option for patients with relapsed disease, particularly those with sustained remission ≥18 months after a first autoHCT procedure [58, 59]. Currently, there are only two published prospective randomized phase III trials comparing salvage autoHCT after novel agent-based re-induction therapy to treatment with a novel agent alone in relapsed MM (Table 18.9) [60, 61]. While the study from the UK showed the superiority of salvage autoHCT over monotherapy with weekly cyclophosphamide, the German study could not show any differences in the intention-to-treat analysis. While major criticism of the study from the UK was the suboptimal control arm with weekly cyclophosphamide, the final analysis of the German study is still pending.
Adoptive Cellular Therapies
-
1.
Allogeneic transplantation (alloHCT)
-
a.
In contrast to autoHCT, alloHCT has the potential to generate an immunologic graft-versus-myeloma (GvM) effect.
-
i.
Studies comparing autoHCT and alloHCT as first-line therapy showed improved long-term OS for patients undergoing alloHCT, while transplant-related mortality (TRM) and toxicity mostly as a consequence of graft-versus-host disease (GvHD) were increased [62,63,64,65].
-
ii.
Whether alloHCTcan overcome high-risk disease features remains controversial since inclusion criteria for high-risk disease varied in the different studies [66,67,68].
-
iii.
As the incidence of TRM is 10–20%, alloHCT in MM should generally be reserved for young patients with primary relapsed/refractory disease, where transplant risk is relatively low (HLA-identical donor, no comorbidities) and no other novel therapy , for example, antibodies or chimeric antigen receptor T-cell is available.
-
i.
-
b.
Studies comparing alloHCT to novel agents such as proteasome inhibitors, immunomodulatory agents, or monoclonal antibodies are lacking.
-
a.
-
2.
Chimeric antigen receptor T (CAR T) cell therapy
-
a.
CAR T cells are genetically engineered T cells utilizing a genetically engineered CAR targeting specific myeloma antigens, of which current studies are mainly directed against B-cell maturation antigen (BCMA).
-
b.
Phase I/II trials are presently investigating safety and efficacy for CAR T cell therapy for myeloma in heavily pretreated patients.
-
c.
Although overall response rates (ORR) up to 100% have been reported and the majority of patients achieved a VGPR or CR, long-term results have not been established to determine the durability of these responses [69].
-
d.
The observed toxicities of this therapy are similar to more established CAR T cell therapies in acute lymphoid leukemia (ALL) and aggressive lymphomas, most frequently grade 1–2 cytokine release syndrome (CRS) and neurotoxicity [69, 70].
-
a.
References
Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385:2197–208.
Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905.
Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Hájek R, Pantani L, et al. Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood. 2017;130:397.
Holstein SA, Suman VJ, McCarthy PL. Should overall survival remain an endpoint for multiple myeloma trials? Curr Hematol Malig Rep. 2019;14:31–8.
Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Merz M, Jansen L, Castro FA, et al. Survival of elderly patients with multiple myeloma-Effect of upfront autologous stem cell transplantation. Eur J Cancer. 2016;62:1–8.
Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31:3279–87.
Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–82.
Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29:1721–9.
Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569–74.
Moreau P, Hulin C, Caillot D, et al. Ixazomib-lenalidomide-dexamethasone (IRd) combination before and after autologous stem cell transplantation (ASCT) followed by ixazomib maintenance in patients with newly diagnosed multiple myeloma (NDMM): a phase 2 study from the Intergroupe Francophone Du MyéLome (IFM). Blood. 2016;128:674.
Gay F. Carfilzomib-lenalidomide-dexamethasone (KRd) induction-autologous transplant (ASCT)-Krd consolidation vs Krd 12 cycles vs carfilzomib-cyclophosphamide-dexamethasone (KCd) induction-ASCT-Kcd consolidation: analysis of the randomized Forte trial in newly diagnosed multiple myeloma (NDMM): ASH; 2018. https://ash.confex.com/ash/2018/webprogram/Paper112093.html. Accessed 18 Feb 2019.
Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Tricot G, Jagannath S, Vesole D, et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85:588–96.
Lacativa CPR, Lacativa PGS, Garnica M, et al. Risk factors for unsuccessful peripheral blood stem cell harvesting using granulocyte-colony stimulating factor mobilization in patients with multiple myeloma. Transfus Apher Sci. 2012;47:331–5.
de la Rubia J, Bladé J, Lahuerta J-J, et al. Effect of chemotherapy with alkylating agents on the yield of CD34+ cells in patients with multiple myeloma. Results of the Spanish Myeloma Group (GEM) Study. Haematologica. 2006;91:621–7.
Musto P, Simeon V, Grossi A, et al. Predicting poor peripheral blood stem cell collection in patients with multiple myeloma receiving pre-transplant induction therapy with novel agents and mobilized with cyclophosphamide plus granulocyte-colony stimulating factor: results from a Gruppo Italiano Malattie Ematologiche dell’Adulto Multiple Myeloma Working Party study. Stem Cell Res Ther. 2015;6:64. https://doi.org/10.1186/s13287-015-0033-1.
Sinha S, Gertz MA, Lacy MQ, et al. Majority of patients receiving initial therapy with lenalidomide-based regimens can be successfully mobilized with appropriate mobilization strategies. Leukemia. 2012;26:1119–22.
Prince HM, Imrie K, Sutherland DR, et al. Peripheral blood progenitor cell collections in multiple myeloma: predictors and management of inadequate collections. Br J Haematol. 1996;93:142–5.
Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295–308.
Giralt S, Stadtmauer EA, Harousseau JL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009;23:1904–12.
Popat U, Saliba R, Thandi R, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718–23.
Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42.
Giralt S. 200 mg/m2 melphalan—the gold standard for multiple myeloma. Nat Rev Clin Oncol. 2010;7:490–1.
Palumbo A, Bringhen S, Bruno B, et al. Melphalan 200 mg/m2 versus melphalan 100 mg/m2 in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. 2010;115:1873–9.
Auner HW, Iacobelli S, Sbianchi G, et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica. 2018;103:514–21.
Mahindra A, Hari P, Fraser R, et al. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone Marrow Transplant. 2017;52:1616–22.
Barlogie B, Attal M, Crowley J, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the Intergroupe Francophone du Myelome, Southwest Oncology Group, and University of Arkansas for Medical Sciences. J Clin Oncol. 2010;28:1209–14.
Cavo M, Gay FM, Patriarca F, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/HO95 study. Blood. 2017;130:401.
Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma. J Clin Oncol. 2019;37(7):589–97.
Drayson MT, Bowcock S, Planche T, et al. Tackling Early Morbidity and Mortality in Myeloma (TEAMM): assessing the benefit of antibiotic prophylaxis and its effect on healthcare associated infections in 977 patients. Blood. 2017;130:903.
Winston DJ, Mullane KM, Cornely OA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:2116–27.
Schmitt T, Goldschmidt H, Neben K, et al. Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. J Clin Oncol. 2014;32:3413–20.
Nooka AK, Johnson HR, Kaufman JL, et al. Pharmacoeconomic analysis of palifermin to prevent mucositis among patients undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:852–7.
Marchesi F, Tendas A, Giannarelli D, et al. Cryotherapy reduces oral mucositis and febrile episodes in myeloma patients treated with high-dose melphalan and autologous stem cell transplant: a prospective, randomized study. Bone Marrow Transplant. 2017;52:154–6.
McQuaker IG, Hunter AE, Pacey S, et al. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J Clin Oncol. 1997;15:451–7.
Martínez-Cibrian N, Magnano L, Gutiérrez-García G, et al. At-home autologous stem cell transplantation in multiple myeloma with and without G-CSF administration: a comparative study. Bone Marrow Transplant. 2016;51:593–5.
McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–81.
Holstein SA, Jung S-H, Richardson PG, et al. Updated analysis of CALGB 100104 (Alliance): a randomised phase III study evaluating lenalidomide vs placebo maintenance after single autologous stem cell transplant for multiple myeloma. Lancet Haematol. 2017;4:e431–42.
Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91.
McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017;77:505–20.
Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23.
Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–9; quiz 1093.
Pegourie B, Karlin L, Benboubker L, et al. Apixaban for the prevention of thromboembolism in immunomodulatory-treated myeloma patients: Myelaxat, a phase 2 pilot study. Am J Hematol. 2019;94:635–40.
Goldschmidt H, Mai EK, Dürig J, et al. Response-adapted lenalidomide maintenance in newly diagnosed, transplant-eligible multiple myeloma: results from the multicenter phase III GMMG-MM5 trial. Blood. 2017;130:400.
Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–55.
Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32:383–90.
Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8.
Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59.
Minarik J, Pika T, Bacovsky J, Langova K, Scudla V. Low-dose acyclovir prophylaxis for bortezomib-induced herpes zoster in multiple myeloma patients. Br J Haematol. 2012;159:111–3.
Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393:253–64.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Sellner L, Heiss C, Benner A, et al. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer. 2013;119:2438–46.
Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015;21:2039–51.
Cook G, Williams C, Brown JM, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:874–85.
Goldschmidt H. Salvage autologous transplant and lenalidomide maintenance versus continuous lenalidomide/dexamethasone for relapsed multiple myeloma: results of the randomized GMMG phase III multicenter trial relapse: ASH; 2018. https://ash.confex.com/ash/2018/webprogram/Paper111203.html. Accessed 19 Feb 2019.
Giaccone L, Storer B, Patriarca F, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011;117:6721–7.
Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20.
Gahrton G, Iacobelli S, Björkstrand B, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–63.
Krishnan A, Pasquini MC, Logan B, et al. Tandem autologous versus single autologous transplantation followed by allogeneic hematopoietic cell transplantation for patients with multiple myeloma: results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 trial. Lancet Oncol. 2011;12:1195–203.
Kröger N, Iacobelli S, Franke G-N, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35:2157–64.
Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–80.
Moreau P, Garban F, Attal M, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008;112:3914–5.
Susanibar Adaniya SP, Cohen AD, Garfall AL. CAR T cell immunotherapy for multiple myeloma: a review of current data and potential clinical applications. Am J Hematol. 2019;94(S1):S28–33. https://doi.org/10.1002/ajh.25428.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
Stroncek DF, Clay ME, Petzoldt ML, et al. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601–10.
Wallis WD, Qazilbash MH. Peripheral blood stem cell mobilization in multiple myeloma: growth factors or chemotherapy? World J Transplant. 2017;7:250–9.
Gertz M, Kumar S, Lacy M, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619–25.
Gettys SC, Gulbis A, Wilhelm K, et al. Modified CVAD and modified CBAD compared to high-dose cyclophosphamide for peripheral blood stem cell mobilization in patients with multiple myeloma. Eur J Haematol. 2017;98:388–92.
Green DJ, Bensinger WI, Holmberg LA, et al. Bendamustine, etoposide and dexamethasone to mobilize peripheral blood hematopoietic stem cells for autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2016;51:1330–6.
Güner ŞI, Yanmaz MT, Selvi A, Usul C. The high effect of chemomobilization with high-dose etopside + granulocyte-colony stimulating factor in autologous hematopoietic peripheral blood stem cell transplantation: a single center experience. Hematol Rep. 2016;8:6319. https://doi.org/10.4081/hr.2016.6319.
Fitoussi O, Perreau V, Boiron JM, et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27:837–42.
Jantunen E, Putkonen M, Nousiainen T, et al. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transplant. 2003;31:347–51.
Corso A, Arcaini L, Caberlon S, et al. A combination of dexamethasone, cyclophosphamide, etoposide, and cisplatin is less toxic and more effective than high-dose cyclophosphamide for peripheral stem cell mobilization in multiple myeloma. Haematologica. 2002;87:1041–5.
Stewart AK, Vescio R, Schiller G, et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771–9.
Uy GL, Costa LJ, Hari PN, et al. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1513–8.
Kim JS, Yoon DH, Park S, et al. Prognostic factors for re-mobilization using plerixafor and granulocyte colony-stimulating factor (G-CSF) in patients with malignant lymphoma or multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy: the Korean multicenter retrospective study. Ann Hematol. 2016;95:603–11.
Tricot G, Cottler-Fox MH, Calandra G. Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transplant. 2010;45:63–8.
Cooper DL, Medoff E, Patel N, et al. Autologous stem cell mobilization in the age of plerixafor. Clin Lymphoma Myeloma Leuk. 2016;16:411–6.
Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1095–22.
Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: Results from the FORTE trial. J Clin Oncol. 2019;37(15_suppl):8002–8002.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Merz, A.M.A., Merz, M., Hillengass, J., Holstein, S.A., McCarthy, P. (2021). Multiple Myeloma. In: Maziarz, R.T., Slater, S.S. (eds) Blood and Marrow Transplant Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-53626-8_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-53626-8_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53625-1
Online ISBN: 978-3-030-53626-8
eBook Packages: MedicineMedicine (R0)