Abstract
Novel minimally -invasive techniques, such as video-assisted thoracic surgery (VATS) or robotic surgery, have expanded the indications of surgical access to the mediastinum. Robotic surgery, in particular, is very well suited for complex mediastinal surgery. In selected cases of deep intrathoracic goiters where a simple cervical incision is not enough, we advocate consideration for a minimally invasive robotic approach either standalone or in combination with a cervical approach to avoid morbidity of a median sternotomy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The normal thyroid gland is a butterfly-shaped organ composed of two lobes (right and left) connected via the isthmus. A third and variable lobe called the pyramidal lobe can be seen arising from the isthmus at its superior aspect. The normal anatomical location of the thyroid is in the anterior neck starting from the thyroid cartilage and extending down into the fifth or sixth tracheal ring. A normal thyroid gland usually weighs approximately 18–60 grams in the adult.
The blood supply to the thyroid comes from the superior and inferior thyroid arteries, branches of the external carotid, and of the thyrocervical trunk, respectively. A not constant artery, the thyroid ima from the brachiocephalic trunk can also be present. Venous drainage is similar to its arterial supply, utilizing the superior and inferior thyroid veins draining into the internal jugular and the left brachiocephalic veins, respectively.
Important parasympathetic nerves supply innervation to the thyroid , mainly the superior laryngeal nerve and the recurrent laryngeal nerve. The recurrent laryngeal nerves are often intimately associated with the thyroid gland, coursing deep to the gland within the tracheoesophageal groove.
The term goiter, comes from the French (goitre) and Latin (guttur) which mean throat. A goiter is any enlargement of the thyroid gland. Many etiologies are known to cause goiter and are not the focus of this chapter.
Traditionally, thyroid operations are done with a cervical collar incision as initially described by Kocher and Halsted in the early 1900s. New minimally invasive techniques exist to perform cervical thyroidectomies including minimally invasive video-assisted thyroidectomies (MIVAT) and transaxillary robotic approaches [1]. Both of these approaches address resection of thyroid glands that are limited to the neck or minimally enter the thoracic cavity, but true intrathoracic goiters are very difficult to resect via these techniques.
Intrathoracic goiters are rare, less than 6% of all mediastinal masses in adults are of thyroid origin [2]. The first anatomical description of an intrathoracic goiter was by Haller in 1749 [3], since then, different terminology has been used including the terms retrosternal, mediastinal, or subclavicular. The definition for intrathoracic goiter is a thyroid mass with 50% or more of it located below the thoracic inlet [4]. The vast majority of these cases are a result of direct caudal growth of cervical goiters into the superior mediastinum.
Intrathoracic goiters can be divided into primary and secondary. True primary intrathoracic goiters (also called aberrant or ectopic) are very rare with an incidence of less than 1% of all mediastinal masses [2, 5]. They are characterized by a blood supply from intrathoracic vessels rather than cervical ones. Frequently the cervical component of the thyroid gland is normal or absent. Secondary intrathoracic goiters are much more common; they arise in the normal cervical position and, due to their growth, descend into the mediastinum, as it is the path of least resistance for the gland. The vast majority of intrathoracic goiters are found in the anterior mediastinum (85–90%) with the remainder (10–15%) in the posterior mediastinum [6, 7]. Rios et al. analyzed their experience with intrathoracic goiters and in a bivariate analysis found it to occur more often in older males, in longer standing goiters [8].
2 Clinical Findings
Symptoms usually present late in the evolution of this problem and up to 30% of patients remain asymptomatic [9,10,11]. The most frequent symptoms observed due to an intrathoracic goiter are dyspnea, cough, hoarseness, stridor, and shortness of breath secondary to distortion and direct compression of the trachea. Dysphagia, a sensation of choking from esophageal compression and occasionally swelling of the head and neck when superior vena cava compression is present is less common. Up to 50% of patients can present with symptoms related to hyperthyroidism [12, 13]. A palpable thyroid mass is found in the majority of patients, nevertheless the absence of a palpable mass does not rule out a totally intrathoracic goiter. In multiple reported series, up to 35% of intrathoracic goiters did not have a palpable cervical component [6, 14, 15].
3 Preoperative Testing
The work up for patients with intrathoracic goiters includes a simple PA and Lateral Chest X-Ray which usually show a mediastinal mass with potential tracheal deviation, but up to 30% of chest roentograms are normal [16]. This examination needs to be followed by a computerized tomography (CT) of the chest with contrast, which provides detailed information about the intrathoracic mass and its anatomic relations with other vital intrathoracic structures. While some advocate MRI, its use adds minimal additional benefit and significant unnecessary cost.
Ultrasound, thyroid scintigraphy and fine needle aspiration (FNA) are not necessary in the preoperative evaluation for intrathoracic goiters. Basic thyroid function test should be performed and correction of any significant abnormalities corrected prior to surgery.
If the patient has hoarseness or voice changes, the vocal cords should be inspected with either direct or indirect laryngoscopy prior to any surgical intervention to address possible vocal cord paresis or paralysis. Loss of function of both vocal cords can lead to an emergent airway issue in the immediate post-operative period, which should be avoided at all costs.
4 Treatment—Indications for Surgery
Medical treatment for patients with elevated TSH or deficiency in thyroxin production with suppressive medications has shown poor results for patients with thyroid goiters. Radioactive iodine is traditionally used in patients with multinodular toxic goiter, but some reports exist of its use in non-toxic goiters. They have demonstrated goiter size reduction up to 40%, albeit this did not correlate with symptom resolution. Furthermore, radioactive iodine has the potential side effects of thyroiditis and post treatment gland swelling with risks of worsening airway compression. This modality of treatment should only be used in very high risk operative patients who would not tolerate surgery.
The preferred treatment for intrathoracic goiter is surgery. Potential malignant degeneration of the intrathoracic goiters has been described [17]. The mere size of the gland can cause significant compression of the airways, esophagus, or mediastinal vessels causing serious morbidity. The discovery on an intrathoracic goiter is criteria enough to merit surgical resection.
5 Surgical Technique
All procedures are performed under general anesthesia using a double-lumen endotracheal tube to achieve single lung ventilation. Either a left or right thoracic approach is appropriate depending on the location of the intrathoracic goiter. The patient is positioned supine with a small tilt (bump) up in the operative side. The ipsilateral shoulder is allowed to drop to minimize collision with the robotic arm. Three of the robotic arms are typically used but the fourth arm can be used if necessary. A single 8 or 12 mm port for the camera and two 8 mm instrument ports are placed. The camera port is positioned in the fourth intercostal space in the anterior axillary line. One 8 mm port is placed along the inframammary crease in the third intercostal space and anterior to the mid axillary line and the other 8 mm port is placed in the fifth intercostal space in the mid clavicular line. The robot is docked facing the patient from the contralateral side.
An additional 5 mm port can be placed for the assistant to help with retraction and suction. C02 insufflation to 8–10 mm Hg is used to assist with the retraction and dissection and helps in collapsing the lung. Careful attention to the patient’s hemodynamics during insufflation is required since the rapid compression of the lung can lead to significant, but transient, hypotension.
Upon chest entry, the mediastinum is thoroughly inspected. Dissection is performed using either the vessel sealer device or monopolar cautery (spatula or hook). The fenestrated bipolar cautery in the opposite arm allows for retraction as well as dissection near vital structures, including the phrenic nerve. .
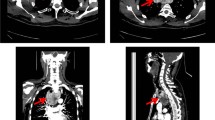
Dissection should start with the opening of the mediastinal pleura along the phrenic nerve to completely identify and dissect it free. Attention is placed to avoid any thermal injury to the phrenic nerve resulting in diaphragmatic paralysis. The most inferior aspect of the goiter is then identified, and the contralateral pleura is encountered. Sometimes the contralateral pleural space needs to be entered and one must be cognizant of the phrenic nerve when doing the dissection in the opposite chest. Identification of the innominate vein, as well as the internal mammary vessels, allows for safe dissection of any portion of the goiter that enters the thoracic inlet. With very large intrathoracic goiters (Fig. 34.1), visualization may be difficult due to the sheer bulk of the gland. Placing the partially dissected gland in the contralateral chest as you complete the dissection aids not only your visualization but also supplies counter-traction during the final stages of the resection. The specimen can be removed through the axillary incision after placing a larger port or extending the incision slightly. A single 28-Fr chest tube is placed through the lowest trocar site at the conclusion of the case. In some cases, a combined cervical and thoracic approach is mandatory if there is a large cervical component to the goiter as well. In these cases, the gland can be delivered via the neck incision.
6 Tips and Pitfalls
An experienced anesthesiologist is vital to a successful resection, as some patients may have significant tracheal compression. During induction, the airway compression may become critical and the ability to ventilate is lost. Also, a double-lumen endotracheal tube may not be adequate and a bronchial blocker will be required to achieve single lung ventilation. It is also critical to recognize that tracheomalacia may result from prolonged compression of the airway by the goiter, specifically around the thoracic inlet, though its incidence is low (0.001–1.5%) [18]. Therefore, extubating the patient needs to be done in a controlled environment, by an experienced anesthesiologist to avoid airway complications.
Placing the robotic ports too high in the chest cavity can lead to difficulty with the most inferior dissection of the gland. Furthermore, this limits the utility of the endowrist, creating an instrument that is similar to Tyrannosaurus Rex arms—essentially useless. For patients with short thoracic cavities, moving the ports an interspace inferiorly will avoid this problem.
7 Comments
While the majority of intrathoracic goiters can be removed through a cervical incision , there are some that require direct access to the mediastinum for removal either with a full sternotomy, partial sternotomy, hemiclamshell, or thoracotomy. New minimally invasive techniques have been described to remove intrathoracic goiters [7, 19, 20].
Cho et al. reported on 70 patients with intrathoracic goiters where a cervical incision was used even for large goiters down to the aortic arch [21]. Similarly Judd from the Mayo Clinic advocated a collar incision even in large goiters [22]. In a series of 170 patients with substernal goiters, Erbil et al. was required to perform partial sternotomy in only 7% of patients [6]. A number of surgeons diverge from this approach arguing that it can lead to uncontrollable hemorrhage, injury to the recurrent laryngeal nerves, and incomplete removal of the goiter [23, 24].
Various techniques to assist in the removal of intrathoracic goiters through a cervical incision have been devised. Katlic in 1985 reported about a special mediastinal spoon devised by Kocher to assist delivery of the goiter into the cervical incision [14]. Other published on using a Foley balloon catheter to help deliver the goiter into the neck [25], while others morcellate the specimen and remove it in fragments—a technique not recommended as this can spread potential malignant cells, cause more bleeding, and leave thyroid tissue behind. .
Minimally invasive techniques are used more often for removal of mediastinal masses. Specifically both VATS and robotic-assisted techniques have been described for thymomas, intrathoracic goiters, and intrathoracic parathyroid adenomas [7, 19, 20, 26,27,28,29,30]. Combined cervico-mediastinal approaches have also been described utilizing a traditional Kocher cervical incision and a robotic approach for the mediastinum [7], this approach is particularly useful when a large intrathoracic goiter would mandate the need for an extended chest incision to remove the specimen after all the dissection is completed. This way, the specimen can be removed through the much better tolerated cervical incision although without the cosmetic benefit.
The advent of robotic surgery has unveiled a new era of minimally invasive surgery overcoming some of the shortcoming of VATS technology. The Da Vinci™ system has added a new technical dimension to minimally invasive surgery. The movement of the instruments in robotic surgery is more precise and controlled, sometimes even more than in open surgery. The camera provides a magnified, three dimensional image and the operative field remains stable and easily adjusted, regardless of the assistant. These features allow the robotic arms access to small spaces where precise dissection can be safely achieved.
8 Conclusion
Intrathoracic goiters are rare, only compromising approximately 6% of all mediastinal masses. Their mere presence is enough to merit surgical removal as they have potential for malignant transformation and are associated with high morbidity from airway, esophageal, and mediastinal compression.
Even though the majority of intrathoracic goiters can be removed through a cervical incision, for the selected patient, a robotic approach can be a safe, minimally invasive alternative to the standard median sternotomy or thoracotomy with much less morbidity, quicker recovery, and early return to activities.
It is essential for the surgeon performing this procedure to have enough robotic experience before attempting complex robotic procedures.
References
Lewis CM, Chung WY, Holsinger FC. Feasibility and surgical approach of transaxillary robotic thyroidectomy without CO(2) insufflation. Head Neck. 2010;32(1):121–6.
Creswell LL, Wells SA. Mediastinal masses originating in the neck. Chest Surg Clin N Am. 1992;2:23–55.
Haller A. Disputationes Anatomica Selectae. Vandenhoeck. 1749;96.
Katlic MR, Grillo HC, Wang CA. Substernal goiterAnalysis of 80 patients from Massachusetts General Hospital. Am J Surg. 1985;149(2):283–7.
Grondin SC, Buenaventura P, Luketich JD. Thoracoscopic resection of an ectopic intrathoracic goiter. Ann Thorac Surg. 2001;71(5):1697–8.
Erbil Y, et al. Surgical management of substernal goiters: clinical experience of 170 cases. Surg Today. 2004;34(9):732–6.
Podgaetz E, Gharagozloo F, Najam F, Sadeghi N, Margolis M, Tempesta B. A novel robot-assisted technique for excision of a posterior mediastinal thyroid goiter. Innovations. 2009;4(4):225–8.
Rios A, et al. Toxic intrathoracic goiter. Clinical profile and surgical morbidity in an endocrine surgery unit. Endocrinol Nutr. 2010;57(5):196–202.
Sianesi, M., et al. [Cervico-mediastinal goiter]. Chir Ital. 2002;54(1):15–8.
Rodriguez JM, et al. Substernal goiter: clinical experience of 72 cases. Ann Otol Rhinol Laryngol. 1999;108(5):501–4.
Hedayati N, McHenry CR. The clinical presentation and operative management of nodular and diffuse substernal thyroid disease. Am Surg. 2002;68(3):245–51; discussion 251–2
Torre G, et al. Surgical management of substernal goiter: analysis of 237 patients. Am Surg. 1995;61(9):826–31.
Madjar S, Weissberg D. Retrosternal goiter. Chest. 1995;108(1):78–82.
Katlic MR, Wang CA, Grillo HC. Substernal goiter. Ann Thorac Surg. 1985;39(4):391–9.
Sanders LE, et al. Mediastinal goiters. The need for an aggressive approach. Arch Surg. 1992;127(5):609–13.
Wright CD, Mathisen DJ. Mediastinal tumors: diagnosis and treatment. World J Surg. 2001;25(2):204–9.
Rautu F. A case of malignant degeneration of an aberrant intrathoracic goiter. Rev Med Chir Soc Med Nat Iasi. 1983;87(3):493–4.
Geelhoed GW. Tracheomalacia from compressing goiter: management after thyroidectomy. Surgery. 1988;104(6):1100–8.
Al-Mufarrej F, et al. Novel thoracoscopic approach to posterior mediastinal goiters: report of two cases. J Cardiothorac Surg. 2008;3:55.
Bodner J, et al. Robotic resection of an ectopic goiter in the mediastinum. Surg Laparosc Endosc Percutan Tech. 2005;15(4):249–51.
Cho HT, Cohen JP, Som ML. Management of substernal and intrathoracic goiters. Otolaryngol Head Neck Surg. 1986;94(3):282–7.
Judd ES, Beahrs OH, Bowes DE. A consideration of the proper surgical approach for substernal goiter. Surg Gynecol Obstet. 1960;110:90–8.
Ehrenhaft JL, Buckwalter JA. Mediastinal tumors of thyroid origin. AMA Arch Surg. 1955;71(3):347–56.
Van Schil P, et al. Primary intrathoracic goitre. Acta Chir Belg. 1989;89(4):206–8.
Pandya S, Sanders LE. Use of a Foley catheter in the removal of a substernal goiter. Am J Surg. 1998;175(2):155–7.
Augustin F, Schmid T, Bodner J. The robotic approach for mediastinal lesions. Int J Med Robot. 2006;2(3):262–70.
Freeman RK, et al. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. Ann Thorac Surg. 2011;92(3):1018–22; discussion 1022–3
Harvey A, et al. Robotic thoracoscopic mediastinal parathyroidectomy for persistent hyperparathyroidism: case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2011;21(1):e24–7.
Profanter C, et al. Robot-assisted mediastinal parathyroidectomy. Surg Endosc. 2004;18(5):868–70.
Ruckert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg. 2011;141(3):673–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Podgaetz, E., Schwartz, G., Tapias, L., Mason, D.P. (2021). The Robotic Approach to Intrathoracic Goiters. In: Gharagozloo, F., Patel, V.R., Giulianotti, P.C., Poston, R., Gruessner, R., Meyer, M. (eds) Robotic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-53594-0_34
Download citation
DOI: https://doi.org/10.1007/978-3-030-53594-0_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53593-3
Online ISBN: 978-3-030-53594-0
eBook Packages: MedicineMedicine (R0)