Abstract
In this chapter, the efficacy of pectin as emulsifier and the structural components that influence its emulsification properties are discussed. The complex molecular structure of pectin makes the assignment of structure vs. function relationships particularly challenging. Nevertheless, around 3% protein with a minimum of 10% degree of acetylation is sufficient for effective arrangement of pectin at the oil-water interface and efficient long-term stabilisation. In addition, side chains present in the rhamnogalacturonan-I regions, provide effective barriers to emulsion coarsening through steric mechanisms whereas the degree of methylesterification appears to have ancillary role. Pectins with intermediate molecular weight (~150,000 g mol−1) are preferred, as smaller chains do not provide successful steric stabilisation whereas the high molecular weight counterparts with high viscosity restrict interfacial accessibility thus impeding fast adsorption of functional groups at the interface. Pectin may be described as a block co-polymer and depending on the botanical source and method of extraction it may be di-block, triblock, or grafted. Accordingly, theories that have been developed for co-polymer adsorption at interfaces may be used to theoretically analyse and treat experimental data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
Food and pharmaceutical industry frequently design their formulations aiming to improve human health (e.g., foods that lower cholesterol), produce products with consumer-tailored specifications (e.g., products for vegetarians) or deliver bioactives to the required site of uptake (e.g., colon). Among other biopolymers, pectin may be also used as a carrier for the protection and targeted delivery of bioactive compounds and for increasing their shelf life and stability (Rehman et al. 2019). The challenges arise from the increasing public interest in the availability of “natural” food ingredients where only naturally available materials such as carbohydrates or proteins should be used in the formulations. In addition, complexities also arise from the gastric environment that usually the product needs to bypass before reaching the desired location in the gastrointestinal tract.
Polysaccharides, in general, are routinely used in food and pharmaceutical industries, mostly as thickeners, dispersion stabilisers or water structuring agents. These functional properties are employed to create structures with reproducible physical properties. In recent years, however, the need to create advanced formulations that bypass gastric environment, delay lipid digestion to prolong satiety, and deliver bioactives in the gastrointestinal tract at the site of interest has boosted research on the fundamental properties of polysaccharides at interfaces (McClements and Jafari 2018; Araiza-Calahorra et al. 2018; Kontogiorgos 2019). The main reason is that polysaccharide-based structures may resist attack from proteases as well as the acidic environment of stomach that frequently impair the performance of protein and surfactant-based formulations (McClements and Gumus 2016). In addition, surface active compounds are used in acidic drinks to emulsify flavour oils, prevent their oxidation and deliver them in a sustained manner (e.g., in the oral cavity) (Matalanis et al. 2011).
Technological performance of polysaccharides as emulsifiers is controlled by their macromolecular properties (e.g., conformation, surface charge density, molecular weight etc.) and intra- and inter-chain interactions that act cooperatively to determine adsorption strength (Kontogiorgos 2019). Pectin is obtained from natural sources using suitable extraction methodologies and may be tailored with chemical or physical modifications to improve the functionality of the extracted material. Depending on the source of extraction (Chap. 4) pectin has the ability to rapidly adsorb at the interface, reduce interfacial tension to facilitate droplet disruption, and impede droplet aggregation. This is typically attributed to the presence of hydrophobic elements in the structure such as proteins, ferulic acids, methyl or acetyl groups (Alba and Kontogiorgos 2017). The objective of this chapter is to identify the role of the surface active functional groups and provide a mechanistic understanding of the phenomenology of pectin adsorption at the oil-water interface.
5.2 Role of Structural Elements on the Interfacial Activity
The emulsifying capacity of pectin is typically associated with the chemical structure of its backbone such as the degree of methylation (DM) and acetylation (DA), the macromolecular characteristics of pectin chains (molecular weight (Mw), branching, hydrodynamic volume etc.) and the presence of functional units such as protein or ferulic acids. The evaluation of the contribution to the emulsification capacity of pectin of each of these structural parameters is still in progress and a matter of debate. However, some general principles may be drawn that may form the basis for further investigations and greater understanding of pectin functionality at the oil-water interface. In this section, we identify the most important structural elements that contribute to its interfacial activity.
5.2.1 The Role of Protein
The protein content in pectin varies depending on the source, isolation conditions and detection methods with higher values typically reported for sugar beet (up to ~9%) and okra (~5%), in contrast to citrus or apple pectin (e.g., ~3% and ~1%, respectively) (Funami et al. 2011; Yapo et al. 2007a; Chen et al. 2016a, 2018; Alba et al. 2015; Schmidt et al. 2015). Proteins are either present as contaminants that are co-extracted during the isolation process or associated with pectin structure through covalent linkages usually attached on the side chains. This association has been also probed by atomic force microscopy describing the protein-pectin complexes as “tadpoles” or as a network of “rods and spheres” (Fishman et al. 2015; Kirby et al. 2008).
Sugar beet pectin stabilised emulsions require about 3% protein for optimum surface activity (Chen et al. 2016a, 2018) whereas enzymic removal of protein results in reduction of interfacial activity and increase of droplet size compared to emulsions fabricated with non-enzymically modified pectin (Funami et al. 2007). The enzymatic treatment also reduces its molecular weight and radius of gyration thus restricting its steric stabilisation efficiency. It has been also shown that adsorbed pectin fractions at the oil-water interface have high protein concentration hinting at the importance of the protein component on emulsion stability (Leroux et al. 2003; Akhtar et al. 2002; Yapo et al. 2007a; Siew and Williams 2008b; Nakamura et al. 2004). Some pectins, as for instance those from pomegranate peel, show limited capacity to lower the surface tension, and its emulsifying properties are mostly attributed to the presence of protein and ester groups (Yang et al. 2018). In contrast, protein-rich and protein-depleted sugar beet pectin fractions have shown a range of emulsion stabilisation properties with protein playing a secondary role (Karnik and Wicker 2018; Chen et al. 2018). Further complications may also arise from the fact that in some pectins covalently-linked ferulic acid-arabinogalactan-protein complex has more notable impact on the interfacial activity and emulsifying capacity than protein alone (Chen et al. 2016b, 2019; Siew and Williams 2008b).
Another school of thought proposes that the accessibility and chemical nature of protein (e.g., amino acid composition and conformation) is more important determinant of emulsification capacity than its overall concentration. For instance, sugar beet pectin fractions with different protein amount ranging between 0.8% and 5.9% result in formation of emulsions of comparable droplet sizes and stability (Williams et al. 2005). In addition, extensin, a hydroxyproline-rich glycoprotein associated with the plant cell walls, was reported to be the main protein-type in pectin isolated from a range of botanical sources (Karnik et al. 2016; Nuñez et al. 2009). However, similar to total protein content, hydroxyproline-rich fractions did not show good emulsifying capacity and could not be directly associated with the emulsifying activity of sugar beet pectin. This is in general agreement with other investigations that have not identified a direct relationship between protein content and emulsifying capacity (Yapo et al. 2007a; Alba et al. 2016) suggesting that protein accessibility to the interface may be hindered by the bulky carbohydrate chains thus restricting interfacial arrangement (Castellani et al. 2010). A mechanistic description of the complex relationships between protein and pectin at the interface is presented in Sect. 6.3 where the different modes of adsorption are detailed.
5.2.2 The Role of Acetyl and Methyl Groups
Acetyl groups, similarly to ferulic groups, enhance interfacial activity of pectin resulting in smaller droplets during emulsification (Akhtar et al. 2002; Dea and Madden 1986; Leroux et al. 2003; Siew and Williams 2008a). Early studies using de-acetylated pectin revealed that the presence of acetyl groups does not contribute to a great extent to emulsification capacity (Leroux et al. 2003). However, the samples had different protein content making difficult to decouple the role of protein and acetyl groups on the overall emulsification performance. For instance, recent studies demonstrate that acetyl groups with a minimum degree of acetylation of ~10% improve considerably the emulsifying properties of pectin, particularly at low protein contents (Chen et al. 2016b; Schmidt et al. 2014).
In addition to the acetyl groups, the presence of methyl groups also contributes to interfacial activity of pectin although the results are sometimes contradicting. Some authors have demonstrated a direct relationship between the DM and emulsifying capacity of citrus pectin with increments of DM from ~70% to ~80% (Schmidt et al. 2014). Interestingly, it has been also shown that increase of DM beyond 80% did not result in further reduction of droplet size due to the self-association of citrus pectin thus restricting the accessibility of hydrophobic groups to the interface. However, recent studies using ultra-high methylated pectin (DM > 90%) of low molecular weight resulted in formation of stable nano-emulsions demonstrating that the importance of methyl group may manifest only at very high degrees of esterification (Hua et al. 2019). Block-wise distribution of carboxylic acid groups at comparable degree of methylation (~63%) showed negligible differences on interfacial tensions of apple pectin also supporting that the overall DM rather than other structural details plays critical role on the interfacial activity (Lutz et al. 2009). In contrast, other authors investigated citrus pectin with DM ranging from 22 to 73% and concluded that the content of methyl esters is of minor importance for the emulsifying properties pectin (Akhtar et al. 2002). The de-methylesterification of sugar beet (Chen et al. 2016b) or citrus pectin (Wan et al. 2019) also resulted in particularly stable dispersions showing that it is possible to create stable emulsions with LM pectin. Other hydrophobic groups may also be attached on the pectin backbone to confer hydrophobicity on the structure. To that end, alkylated citrus pectins with different alkyl chain lengths and degree of alkyl substitution demonstrated improved emulsifying activity, as evidenced by smaller droplet diameters than those stabilised with non-alkylated pectin (Liang et al. 2015).
5.2.3 The Role of Molecular Weight and Side Chains
The accessibility of protein and the other surface active components may be linked to pectin molecular weight although its impact on emulsification is currently inconsistent. Early reports suggested that low molecular weight (e.g., 35–90 × 103 g mol−1) favours emulsifying activity of pectin, possibly due to better accessibility of interfacially active groups. However, pectin fractions of very low molecular weight result in lower interfacial activity and coarser emulsions because of the inability of short chains to provide efficient steric stabilisation (Yapo et al. 2007a, b; Akhtar et al. 2002; Leroux et al. 2003). On the contrary, very low Mw (15,000 g mol−1) but also ultra-high methoxylated pectin spontaneously emulsifies oil (Hua et al. 2019) arguing that the influence of chain size should be viewed in conjunction with its group functionalisation. Similarly, sugar beet pectin of low Mw may form emulsions with smaller droplet diameters than those stabilised with its high Mw counterparts (Williams et al. 2005). However, other studies have not demonstrated a direct relationship between Mw of citrus pectin and its emulsifying capacity, particularly after adjusting the viscosity of emulsions (Schmidt et al. 2014). It has been also shown that reduction of Mw from 76 × 103 to 47 × 103 g mol−1 did not improve emulsifying properties of citrus pectin. In contrast, increase of Mw of sugar beet pectin via cross-linking of ferulic acid groups has shown that emulsions fabricated with cross-linked pectin (Mw ~ 1860 × 103 g mol−1) have smaller droplet mean diameters and improved long term stability compared to those stabilised with non-cross-linked pectin (Mw ~ 780 × 103 g mol−1) (Zhang et al. 2015). The lack of consensus on the impact of molecular weight on the emulsifying capacity of pectin also suggests that the other structural characteristics discussed earlier (acetyl and methyl groups or ferulic acids) cannot be disregarded.
Pectin fractions adsorbed at the oil-water interface are enriched in neutral sugars (e.g., arabinose and galactose) suggesting that RG-I containing pectins could have better emulsifying properties than those with linear backbone (Siew and Williams 2008a). These results were further supported by the enzymatic degradation of sugar beet pectin side chains revealing a reduction in its interfacial and stabilising capacity (Chen et al. 2016b). The impact of side-chains on emulsion-forming properties of sugar beet pectin is attributed to the interfacial activity of protein and presence of ferulic acid that are attached to the side-chains and act as anchors for the attachment of the entire pectin chain. In addition, the presence of neutral sugar side-chains contributes to the long-term emulsion stability due to the formation of thick interfacial layers thus providing effective steric stabilisation that impedes emulsion coarsening (Funami et al. 2011). Results using highly branched okra pectin also confirmed that the prevalence of RG-I segments and the length of their branches influence emulsion stability (Kpodo et al. 2018). It has been also reported that multilayer adsorption of sugar beet pectin at the interface is possible and originates from electrostatic interactions between positively charged protein moieties and the negatively charged galacturonic acid residues (Chee et al. 2008). Generally, emulsions stabilised with pectin are pH- and ionic strength- sensitive and changes in these factors result in alterations in its emulsifying capacity (Table 5.1). At pH values greater than ~3.5 carboxyl groups of pectin are ionised and the biopolymer chains are extended due to the electrostatic repulsions between the carboxylate anions. The number and distribution of negative charges is determined by the degree of methyl esterification and degree of blockiness (DB) of methyl groups. The ionisation of carboxylic groups decreases with pH (pH < pKa) and consequently promotes self-association of the chains. It has been shown that pectin stabilises oil-water interfaces at low pH values, where chains adopt highly compact conformations resulting in the formation of thick interfacial layers thus providing effective steric stabilisation (Alba et al. 2016, 2018; Castellani et al. 2010; Kpodo et al. 2018; Zhao et al. 2018). It becomes evident that modification of conformational characteristics of pectin with the aid of environmental conditions (e.g., pH, ionic strength, type of cation etc.) modulates emulsifying capacity and may enhance steric stabilisation and long-term emulsion stability. From the above discussion it becomes apparent that pectin has multiple elements that cooperatively influence its emulsification properties. This is partially due to the large number of protocols and botanical sources that may be used to extract pectin and control the outcome of the structure. Synthesing information from the discussion that has been presented so far, it emerges that some critical parameters may be successfully manipulated and general guidelines may be drawn in an effort to rationally design pectin-stabilised emulsions with desirable physical properties (Table 5.1).
5.3 Phenomenology of Pectin Adsorption at the Oil-Water Interface
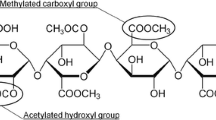
In synthetic polymer chemistry, the result of linking two different monomers to form a polymer chain is termed “copolymer” (Carraher 2014). There are different classes of copolymers depending on the arrangement of monomers along the polymer chain. In alternating copolymers the two monomers are arranged in an alternating fashion whereas in random copolymers are arranged randomly along the polymer chain (Fig. 5.1). A block copolymer contains two or more polymer chains attached at their ends and is termed di-block, tri-block or multi-block for two, three or more than three chains linked together, respectively. Finally, when side chains are attached on the main backbone then it is termed graft copolymer. Pectin consists of more than one sugar monomers thus making it a heteropolysaccharide (Chap. 2). It becomes evident that an idealised pectin structure (Fig. 5.1) may be viewed using synthetic polymer terminology. Specifically, the HG part of the chain may be described as a random or block copolymer depending on the source or post-extraction modifications of pectin. To that end, methyl esterified and non-methyl esterified galacturonic acid residues may arrange randomly or in a blocky pattern along the chain (Voragen et al. 2009; Chan et al. 2017; Mohnen 2008). In addition, the RG-I segment may be described as a graft copolymer due to the presence of lateral arabinose or galactose branches that may also be decorated with covalently linked protein moieties. To further complicate the landscape, the presence of pH-dependant charges due to the presence of carboxylic groups makes pectin a suitable polymer to be described as polyelectrolyte. A crucial distinction between pectin and synthetic copolymers is that in the latter the architecture is controlled by precise polymerisation reactions yielding well-defined chains. Pectin biosynthesis, however, yields complex structures that are further modified during plant maturation or during extraction procedures (Alba and Kontogiorgos 2017).
Idealised description of pectin structure as copolymer. Homogalacturonan (HG) sections may have blockwise or random distribution of esterified groups. The former case may be described as block co-polymer whereas the latter as random co-polymer (grey chains). In pectins where the presence of rhamnogalacturonan segments (RG-I) are prevalent the term graft copolymer may be used as branches made of arabinose, galactose and protein may be present
The aforementioned structure description provides new avenues for greater understanding of pectin interfacial activity, as we may now use well-established theoretical approaches to describe its adsorption at the oil-water interface. Although such a remarkably complex structure is not normally observed in synthetic copolymers, the principles of polymer adsorption at interfaces still apply (Fleer et al. 1998). Adsorption of copolymers at interfaces may be described as the accumulation of chains at the interface that depends strongly on the chemical nature of the chains and the solvent quality. In the case of copolymers, two cases may be distinguished were the aqueous phase is non-selective or selective. In the former case, both blocks (both A and B, Fig. 5.1) are soluble whereas in the latter only one (either A or B) is soluble in the aqueous buffer. The relationship between buffer composition (e.g., pH, ionic strength, type of cation etc.) and pectin will dictate the strength of pectin-buffer interactions, the conformation of the chains, and the amount that is adsorbed thus controlling the overall stability of the dispersion. It starts becoming evident that precise control of buffer composition is one of the first steps towards successful fabrication of pectin-stabilised emulsions.
Block copolymers are adsorbed when one of the blocks has a high affinity for the interface (frequently termed “anchor”) while the other for the continuous phase (“buoy”) (Fleer et al. 1998). Adsorption of copolymers from non-selective solvents occurs into two stages where initially the polymer diffuses to the interface forming a monolayer (Motschmann et al. 1991). It should be mentioned that upon initial adsorption at low interfacial coverage the conformation of buoy blocks is not particularly different than of those in the bulk solution. This may be even more relevant when emulsification proceeds via a covalently-linked protein-assisted mechanisms (see below), as pectin conformation will not be affected by its adsorption at the interface. In the second stage, the adsorbed layer will grow by diffusion of pectin to the surface from the bulk that results in chain overlap and formation of multilayers thus leading to conformational rearrangements. At this stage, chain conformations will be described by the correlation length ξ of the chains. This may also require penetration of chains through the barrier created by those already attached to the droplet (Motschmann et al. 1991). Pectin, depending on the source of extraction, may have multiple segments that are able to adsorb at the oil-water interface, however, adsorption may also take place into two steps (Fig. 5.2a). Initially, the transport of pectin from the bulk to the oil interface is due to diffusion towards the droplets that occurs instantaneously during emulsification. After the initial adsorption, pectin conformation at the interface strongly depends on the pH of the continuous phase and the degree of methylation (Alba et al. 2018) that will in turn determine the ability of pectin to stabilise the dispersion. Specifically, at pH < ~3.5 that is below the dissociation constant of galacturonic acid, pectin conformation is relatively unaffected by the degree of methylation and space occupancy is efficient at these conditions (Fig. 5.2b). In contrast, at pH > ~3.5 chains of low methoxylated pectins attain extended conformation (Fig. 5.2c) but the effect is suppressed with increase of degree of methylation (HM pectin) due to the decrease in charge density and steric hindrance because of the presence of methyl groups (Fig. 5.3d) (Alba et al. 2017, 2018; Cros et al. 1996). This behaviour is preserved in the semi-dilute regime, or in other words, at concentrations where most likely pectin will be used as emulsifier. Consequently, the space filling capacity of pectin in solution, as controlled by pH and degree of methylation as well as branching, has consequences for the thickness of interfacial layer and the effectiveness of steric stabilisation. Indeed these parameters have been extensively investigated revealing that pectin emulsification is responsive to buffer and chain architecture (Schmidt et al. 2015, 2017; Alba et al. 2016; Kpodo et al. 2018; Verkempinck et al. 2018; Hua et al. 2019; Liu et al. 2019; Chen et al. 2016c). This description proposes that the most efficient steric stabilisation capacity would be at acidic environments (Fig. 5.2b) whereas the least efficient with HM pectin at high pH (Fig. 5.2d).
Adsorption of pectin at the oil-water interface takes place into two steps. (a) Initially, pectin diffuses from the bulk to the oil-water interface and occurs instantaneously during emulsification. In the second step, pectin rearranges at the interface depending on the pH of the continuous phase and the degree of methylation (b) At pH < ~3.5 pectin conformation is relatively unaffected by the degree of methylation, and space occupancy is efficient at these conditions (c) At pH > ~3.5 chains of low methoxylated pectins attain extended conformations with space occupancy being less efficient than in (b), (d) Increase of degree of methylation (HM pectin) leads to compact conformations due to the decrease in charge density and steric hindrance because of the presence of methyl groups. Space occupancy is the least efficient compared to (b) and (c)
(a) Four different anchoring mechanisms of pectin at the oil-water interface (see text) (b) interfacial protein localisation in emulsions formed with pectin with substantial amounts of contaminant proteins. Rhodamine B stained protein may be observed forming layers (red layers, right) around Nile red stained oil droplets (green droplets, left)) (c) multilayer formation in pectin-stabilised emulsions forming thin or thick layers depending on pectin architecture and aqueous phase composition (left). Lateral protrusion of the interfacial layer with thickness δ, is responsible for the extent and effectiveness of steric stabilisation (right)
Pectin may anchor at the interface via several mechanisms that act concurrently. The prevalence of one over another depends on the molecular weight and sugar composition of pectin, the strength of interactions between pectin and continuous phase, the chemical properties of interface (e.g., triglyceride or terpene) and the amount of protein present. Specifically, pectin may adsorb unassisted at the interface only with the aid of the hydrophobic groups that are present along the backbone (e.g., methyl, acetyl or ferulic, Fig. 5.3a-I). This mechanism is particularly important in highly methylated (e.g., HM-citrus) or highly acetylated pectin (e.g., from sugar beet or okra). Another dominant mechanism is through anchoring of the chains with the aid of covalently-linked proteins that are found in RG-I units of some pectins (Fig. 5.3a-II). Protein may be also present as contaminant, particularly in pectin isolated in laboratory settings or from novel sources with inherently high protein content (Fig. 5.3a-III). In these cases, contaminant protein may adsorb first with pectin following, resulting in formation of multilayers without pectin having any interaction with the interface. For instance, thick interfacial protein layers may be observed at the interface (Fig. 5.3b) in emulsions formed with pectin containing substantial amounts of contaminant proteins (Alba et al. 2013). Protein-assisted polysaccharide adsorption has been described extensively in the literature either through covalently-linked Maillard conjugates or bilayer formation through intentional protein addition (Dickinson 2008, 2009; Evans et al. 2013; Rodríguez Patino and Pilosof 2011). Despite of these distinct mechanisms of adsorption, multilayer formation would be expected in most cases depending on pectin architecture and solvent composition with some role always given to the protein fraction (Fig. 5.3a-IV). It should be noted that the layer thickness is not necessarily uniform along the interface of the droplet thus resulting in quite complex mixed interfacial layers (Alba et al. 2016) or presence of pectin microgels at the interface (Schmidt et al. 2017) (Fig. 5.3c, left) with intricate interfacial rheology (Sagis and Fischer 2014; Fischer 2013). The thickness, δ, of this layer (Fig. 5.3c, right), is normally responsible for the extent and effectiveness of stabilisation, as it protrudes laterally from the droplets thus conferring stabilisation through steric mechanisms. As a result, some areas in the droplet may be covered by thick multiple mixed layers of pectin and protein whereas other areas may present a thinner interfacial coverage. The latter regions may act as destabilisation centres particularly when adsorption strength is not sufficient and desorption may occur during long term storage. Desorption will expose the oil interface that may lead to coarsening through, for example, coalescence or bridging flocculation.
5.4 Conclusions
The structural components that influence the emulsification properties of pectin and its mechanisms of interfacial arrangement have been discussed with the aim to design pectin that may be used as emulsifier. It is challenging to assign a straightforward structure and function relationships owing to the structural complexities of pectin architecture. It is possible, however, to suggest that in order for pectin to effectively arrange at the interface and to provide efficient emulsification and long-term stabilisation it requires protein content of around 3% with a minimum 10% degree of acetylation. Although higher degree of methylation supports interfacial arrangement it does not seem to be a critical factor. Side chains support steric stabilisation and RG-I rich pectins are generally more efficient emulsifiers. Side chains are important as both protein and ferulic acids are located on the side chains. High molecular weight restricts accessibility of protein at the interface and the particularly high viscosity impedes fast adsorption and reorganisation at the interface. Intermediate molecular weight (~150 × 103 g mol−1) pectins are preferred as lower values do not confer efficient steric stabilisation. In addition, efficient interfacial functionality of pectin requires a certain degree of repetitive structure similar to that of copolymers. Pectin is a typical block co-polymer that depending on the source may be di-block, triblock, or grafted. Accordingly, theories that have been developed for co-polymer adsorption at interfaces are better suited to theoretically analyse and treat experimental data.
References
Akhtar M, Dickinson E, Mazoyer J, Langendorff V (2002) Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll 16(16):249–256
Alba K, Kontogiorgos V (2017) Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll 68:211–218
Alba K, Ritzoulis C, Georgiadis N, Kontogiorgos V (2013) Okra extracts as emulsifiers for acidic emulsions. Food Res Int 54:1730–1737
Alba K, Laws AP, Kontogiorgos V (2015) Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll 43:726–735
Alba K, Sagis LMC, Kontogiorgos V (2016) Engineering of acidic o/w emulsions with pectin. Colloids Surf B Biointerfaces 145:301–308
Alba K, Bingham RJ, Kontogiorgos V (2017) Mesoscopic structure of pectin in solution. Biopolymers 107(6):1–8
Alba K, Bingham RJ, Gunning PA, Wilde PJ, Kontogiorgos V (2018) Pectin conformation in solution. J Phys Chem B122(29):7286–7294
Araiza-Calahorra A, Akhtar M, Sarkar A (2018) Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci Technol 71:155–169
Carraher CE (2014) Carraher’s polymer chemistry. In: Carraher E (ed) Polymer chemistry, 9th edn. CRC Press, Boca Raton
Castellani O, Al-Assaf S, Axelos M, Phillips GO, Anton M (2010) Hydrocolloids with emulsifying capacity. Part 2 – adsorption properties at the n-hexadecane–water interface. Food Hydrocoll 24(2):121–130
Chan SY, Choo WS, Young DJ, Loh XJ (2017) Pectin as a rheology modifier: origin, structure, commercial production and rheology. Carbohydr Polym 161:118–139
Chee KS, Williams PA, Cui SW, Wang Q (2008) Characterization of the surface-active components of sugar beet pectin and the hydrodynamic thickness of the adsorbed pectin layer. J Agric Food Chem 56(17):8111–8120
Chen H, Qiu S, Gan J, Liu Y, Zhu Q, Yin L (2016a) New insights into the functionality of protein to the emulsifying properties of sugar beet pectin. Food Hydrocoll 57:262–270
Chen H-M, Fu X, Luo Z-G (2016b) Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll 54:99–106
Chen HM, Fu X, Luo ZG (2016c) Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll 54:99–106
Chen H, Qiu S, Liu Y, Zhu Q, Yin L (2018) Emulsifying properties and functional compositions of sugar beet pectins extracted under different conditions. J Dispers Sci Technol 39(4):484–490
Chen H, Niu H, Zhang H, Yun Y, Chen W, Zhong Q, Chen W, Fu X (2019) Preparation and properties of ferulic acid-sugar beet pulp pectin ester and its application as a physical and antioxidative stabilizer in a fish oil-water emulsion. Int J Biol Macromol 139:290–297
Cros S, Garnier C, Axelos MAV, Imberty A, Perez S (1996) Solution conformations of pectin polysaccharides: determination of chain characteristics by small angle neutron scattering, viscometry, and molecular modeling. Biopolymers 39(3):339–352
Dea ICM, Madden JK (1986) Acetylated pectic polysaccharides of sugar beet. Food Hydrocoll 1(1):71–88
Dickinson E (2008) Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 4(5):932–942
Dickinson E (2009) Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll 23(6):1473–1482
Evans M, Ratcliffe I, Williams PA (2013) Emulsion stabilisation using polysaccharide-protein complexes. Curr Opin Colloid Interface Sci 18(4):272–282
Fischer P (2013) Rheology of interfacial protein-polysaccharide composites. Eur Phys J Spec Top 222(1):73–81
Fishman ML, Chau HK, Qi PX, Hotchkiss AT, Garcia RA, Cooke PH (2015) Characterization of the global structure of low methoxyl pectin in solution. Food Hydrocoll 46:153–159
Fleer GJ, Cohen-Stuart MA, Scheutjens JMHM, Cosgrove T, Vincent B (1998) Polymers at interfaces. Chapman and Hall, London
Funami T, Zhang G, Hiroe M, Noda S, Nakauma M, Asai I, Cowman MK, Al-Assaf S, Phillips GO (2007) Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll 21(8):1319–1329
Funami T, Nakauma M, Ishihara S, Tanaka R, Inoue T, Phillips GO (2011) Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll 25(2):221–229
Hua X, Ding P, Wang M, Chi K, Yang R, Cao Y (2019) Emulsions prepared by ultrahigh methoxylated pectin through the phase inversion method. Int J Biol Macromol 128:167–175
Karnik D, Wicker L (2018) Emulsion stability of sugar beet pectin fractions obtained by isopropanol fractionation. Food Hydrocoll 74:249–254
Karnik D, Jung J, Hawking S, Wicker L (2016) Sugar beet pectin fractionated using isopropanol differs in galacturonic acid, protein, ferulic acid and surface hydrophobicity. Food Hydrocoll 60:179–185
Kirby A, Macdougall A, Morris V (2008) Atomic force microscopy of tomato and sugar beet pectin molecules. Carbohydr Polym 71(4):640–647
Kontogiorgos V (2019) Polysaccharides at fluid interfaces of food systems. Adv Colloid Interf Sci 270:28–37
Kpodo FM, Agbenorhevi JK, Alba K, Oduro IN, Morris GA, Kontogiorgos V (2018) Structure-function relationships in pectin emulsification. Food Biophys 13:71–79
Leroux J, Langendorff V, Schick G, Vaishnav V, Mazoyer J (2003) Emulsion stabilizing properties of pectin. Food Hydrocoll 17(4):455–462
Liang R-H, Wang L-H, Chen J, Liu W, Liu C-M (2015) Alkylated pectin: synthesis, characterization, viscosity and emulsifying properties. Food Hydrocoll 50:65–73
Liu Z, Pi F, Guo X, Guo X, Yu S (2019) Characterization of the structural and emulsifying properties of sugar beet pectins obtained by sequential extraction. Food Hydrocoll 88:31–42
Lutz R, Aserin A, Wicker L, Garti N (2009) Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocoll 23(3):786–794
Matalanis A, Jones OG, McClements DJ (2011) Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll 25(8):1865–1880
McClements DJ, Gumus CE (2016) Natural emulsifiers — biosurfactants, phospholipids, biopolymers, and colloidal particles: molecular and physicochemical basis of functional performance. Adv Colloid Interf Sci 234:3–26
McClements DJ, Jafari SM (2018) Improving emulsion formation, stability and performance using mixed emulsifiers: a review. Adv Colloid Interf Sci 251:55–79
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11(3):266–277
Motschmann H, Stamm M, Toprakcioglu C (1991) Adsorption kinetics of block copolymers from a good solvent: a two-stage process. Macromolecules 24(12):3681–3688
Nakamura A, Yoshida R, Maeda H, Furuta H, Corredig M (2004) Study of the role of the carbohydrate and protein moieties of soy soluble polysaccharides in their emulsifying properties. J Agric Food Chem 52(17):5506–5512
Nuñez A, Fishman ML, Fortis LL, Cooke PH, Hotchkiss AT (2009) Identification of extensin protein associated with sugar beet pectin. J Agric Food Chem 57(22):10951–10958
Rehman A, Ahmad T, Aadil RM, Spotti MJ, Bakry AM, Khan IM, Zhao L, Riaz T, Tong Q (2019) Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci Technol 90:35–46
Rodríguez Patino JM, Pilosof AMR (2011) Protein–polysaccharide interactions at fluid interfaces. Food Hydrocoll 25(8):1925–1937
Sagis LMC, Fischer P (2014) Nonlinear rheology of complex fluid–fluid interfaces. Curr Opin Colloid Interface Sci 19(6):520–529
Schmidt US, Koch L, Rentschler C, Kurz T, Endreß HU, Schuchmann HP (2014) Effect of molecular weight reduction, acetylation and esterification on the emulsification properties of citrus pectin. Food Biophys 10(2):217–227
Schmidt US, Schmidt K, Kurz T, Endreß HU, Schuchmann HP (2015) Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll 46:59–66
Schmidt US, Schütz L, Schuchmann HP (2017) Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll 62:288–298
Siew CK, Williams PA (2008a) Characterization of the surface-active components of sugar beet pectin and the hydrodynamic thickness of the adsorbed pectin layer. J Agric Food Chem 56:8111–8120
Siew CK, Williams PA (2008b) Role of protein and ferulic acid in the emulsification properties of sugar beet pectin. J Agric Food Chem 56:4164–4171
Verkempinck SHE, Kyomugasho C, Salvia-Trujillo L, Denis S, Bourgeois M, Van Loey AM, Hendrickx ME, Grauwet T (2018) Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll 85:144–157
Voragen AGJ, Coenen G-J, Verhoef RP, Schols HA (2009) Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem 20(2):263
Wan L, Chen Q, Huang M, Liu F, Pan S (2019) Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: a comparison study. Food Hydrocoll 93:146–155
Williams PA, Sayers C, Viebke C, Senan C (2005) Elucidation of the emulsification properties of sugar beet pectin. J Agric Food Chem 53(53):3592–3597
Yang X, Nisar T, Hou Y, Gou X, Sun L, Guo Y (2018) Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll 85:30–38
Yapo BM, Robert C, Etienne I, Wathelet B, Paquot M (2007a) Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem 100(4):1356–1364
Yapo BM, Wathelet B, Paquot M (2007b) Comparison of alcohol precipitation and membrane filtration effects on sugar beet pulp pectin chemical features and surface properties. Food Hydrocoll 21(2):245–255
Zhang L, Shi Z, Shangguan W, Fang Y, Nishinari K, Phillips GO, Jiang F (2015) Emulsification properties of sugar beet pectin after modification with horseradish peroxidase. Food Hydrocoll 43:107–113
Zhao S, Gao W, Tian G, Zhao C, DiMarco-Crook C, Fan B, Li C, Xiao H, Lian Y, Zheng J (2018) Citrus oil emulsions stabilized by citrus pectin: the influence mechanism of citrus variety and acid treatment. J Agric Food Chem 66(49):12978–12988
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alba, K., Kontogiorgos, V. (2020). Emulsification Properties of Pectin. In: Kontogiorgos, V. (eds) Pectin: Technological and Physiological Properties. Springer, Cham. https://doi.org/10.1007/978-3-030-53421-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-53421-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53420-2
Online ISBN: 978-3-030-53421-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)