Abstract
Pectin is a highly complex polysaccharide made of three main domains that are covalently linked one to another, homogalacturonan, rhamnogalacturonan I and rhamnogalacturonan II. The dominant feature of pectin consists of a linear chain of α-(1,4)-linked d-galacturonic acid units known as homogalacturonan domain or pectin smooth region. The second fundamental feature of pectin structure is the recurrent presence of rhamnosyl residues. α-(1,4)-Linked d-galacturonopyranosyl units can be interrupted by the insertion of α-(1,2)-linked l-rhamnopyranosyl units giving a type I rhamnogalacturonan to which arabinose- and galactose-containing side-chains are generally attached. Finally, rhamnogalacturonan II consisting of a highly branched homogalacturonan oligosaccharide, is a minor though crucial element of pectin. Although, as illustrated in this chapter, much is known about the structure of the different pectin domains, understanding the intra- and inter-molecular heterogeneity of pectin macromolecules and the way pectin domains are attached to each other is still challenging.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
2.1 Introduction
Pectin is a natural constituent of all terrestrial plants that is particularly abundant—together with hemicelluloses, cellulose, and low amounts of structural proteins—in the primary cell walls of eudicotyledons and non-graminaceous monocotyledons (Carpita and Gibeaut 1993). Once extracted from citrus peel or apple pomace, commercial pectin is widely used as gelling, thickening, stabilising and emulsifying agent in various food products such as jams, acidic milk drinks, ice creams, or salad dressings, and it is well agreed that the fine structure of pectin deeply affects its functionality and applicability (Willats et al. 2006). The existence of a “jelly” in tamarind extract was discovered more than two centuries ago by the French pharmacist and chemist Louis-Nicolas Vauquelin (1790). The word “pectin” from the Greek πηκτός (“pêktós”, which means “thick”) was first used in 1825 by Braconnot, who had resumed Vauquelin’s work (Braconnot 1825a, b). Smolenski in 1923 was the first scientist to describe pectin as a polymer of galacturonic acid (GalA) and Kertesz (1951) defined pectin as a hetero-polysaccharide containing mainly partly methylesterified GalA together with some neutral sugars. In the 80s, the work of de Vries and co-workers was instrumental in showing that neutral sugars were present as side-chains arranged in blocks in so called “hairy regions” while >90% of the GalA residues could be isolated as chains comprising solely GalA (de Vries et al. 1981, 1982, 1983). Nowadays, it is widely accepted that pectin is a heterogonous macromolecule composed of interlinked distinct domains, the relative amount and structure of which vary according to the botanical origin, the organs and cell types considered, the stages of cellular development, and the precise location within the cell wall (Voragen et al. 2009). On top of this biological diversity, pectin structure varies broadly depending on the extraction method used. Different pectin populations that are more or less strongly anchored in the cell wall co-exist and a high number of extraction steps is needed to extract all pectin (Broxterman 2018).
Two main families of pectin structural elements are usually considered: galacturonans and rhamnogalacturonan I (RG-I). Galacturonans are made of a backbone of α-(1,4)-linked d-galacturonic acid (GalA) residues. This galacturonan backbone may be unbranched (homogalacturonan) or decorated with more or less complex side-chains. The backbone of RG-I is made of the diglycosyl repeating unit [2-α-l-Rha-(1,4)-α-d-GalA-(1] (Lau et al. 1985). Rhamnose (Rha) residues are ramified at O-4 (mainly) and O-3 (scarcely) positions with single to polymeric neutral sugar side chains that include arabinose (Ara) and galactose (Gal) residues in various combinations (Lau et al. 1985; Colquhoun et al. 1990). Four main types of polymeric side-chains are usually envisioned, arabinans, galactans, type I arabinogalactans (AG-I) and type II arabinogalactans (AG-II) (Yapo 2011).
As has been briefly described above, pectin is an extremely complex polysaccharide composed of as many as eighteen distinct monosaccharides connected to each other through twenty different linkages. In addition, several of these monosaccharides can be chemically modified by O-ether or O-ester groups. Because of polydispersity and polymolecularity, analyses on whole macromolecules are usually not sufficient to reveal all the structural details of pectin. For structural investigation, pectin is thereby commonly degraded into oligosaccharides by chemicals or enzymes. Enzymatic digestion is usually preferred since it offers additional information thanks to the specificity of cleaving. Degradation enzymes are, however, not available for all linkages. Degradation products are further fractionated usually by chromatographic means. The isolated structural elements thereby recovered are in the analytical range of a broad set of techniques (Schols and Voragen 2002) and very valuable structural information has been obtained by chemical analyses and NMR in the 90s. This information has however been restricted to major oligosaccharides that could be purified in quantities large enough to allow full structural characterisation. In the past fifteen years, significant developments have led to a re-emergence of the use of mass spectrometry (MS) for the structural characterisation of oligosaccharides (Dong et al. 2018). This review includes improvements that have been achieved in separation techniques (ultra-performance liquid chromatography (UPLC), capillary electrophoresis, ion mobility) and developments in activation approaches using electron, cation or photon interactions that are much more efficient than classical fragmentation methods (i.e., low energy collision induced (or activated) dissociation (LE-CI(A)D) for the determination of the fine structure of oligosaccharides. Very recent works have been conducted on instrument development for the structural characterisation of oligosaccharides based on ion activation (Ropartz et al. 2014, 2016, 2017), on the coupling of MS and infrared ion spectroscopy (Schindler et al. 2017; Mucha et al. 2018) and on high resolution ion mobility (Ujma et al. 2019; Ropartz et al. 2019). These approaches allow to shed new light on complex structures and mixtures of oligosaccharides, as can be found in pectin.
2.2 Galacturonans
2.2.1 Homogalacturonans
Homogalacturonan (HG) also known as the “smooth region” of pectin, is a linear homopolymer of α-(1,4)-linked d-galacturonic acid (GalA) residues that can be methyl and acetyl-esterified (Fig. 2.1). It usually accounts for approximately 60% of the total pectin amount (Mohnen 2008; Atmodjo et al. 2013) but there are some exceptions—sugar beet or soybean pectin for instance—in which the relative amount of HG is much lower (Voragen et al. 1995).
Structure of homogalacturonans according to the symbol nomenclature for glycans (SNFG) introduced by Varki et al. (2015). Homogalacturonan is the dominant feature of pectin. It consists of partly methyl-esterified and, in some plant species, partly acetyl-esterified α-(1,4)-linked d-galacturonopyranosyl chains with a degree of polymerisation 100–300. The amount and distribution of methyl- and acetyl-esters onto homogalacturonans is a key factor for pectin functionality
HG have been specifically isolated from several plant sources by exploiting the susceptibility to acid hydrolysis of the different glycosidic linkages (Thibault et al. 1993; Yapo et al. 2007; Ralet and Thibault 2009; Round et al. 2010) or by enzymatic means (Bonnin et al. 2002; Hellin et al. 2005). Depending on the method used for (1) pectin extraction, (2) HG isolation and (3) molar mass determination, the average degree of polymerisation (DP) ranges between 72 and 300. Ralet and Thibault (2009) isolated HG from pectin samples sequentially extracted from different plant sources: pineapple flesh (Ananas comosus, Bromeliaceae), leek (Allium porrum, Alliaceae), cucumber (Cucumis sativus, Cucurbitaceae), sugar beet root (Beta vulgaris, Amaranthaceae), fennel bulb (Foeniculum vulgare, Apiaceae), and lemon albedo (Citrus medica, Rutaceae) and showed that pectin samples encompassed various amounts of HG domains of very similar DP (70–100) and of low polydispersity, confirming the hypothesis of a HG length periodicity (Thibault et al. 1993).
2.2.1.1 Methyl-esterification
In HG, GalA units are usually partially methyl-esterified at C-6 (Fig. 2.1) and not only the degree of methyl esterification (i.e., the number of moles of methanol per 100 moles of GalA), but also the distribution of non-esterified GalA residues on HG segments are key features for pectin functional properties (Willats et al. 2006). Numerous investigations have thereby been devoted to understand the peculiar methyl-ester distribution patterns and their functional implications. The development of methods allowing characterising and quantifying degradation products obtained by treating well-defined pectin samples with HG-degrading enzymes was instrumental in providing such information. The concept of degree of blockiness calculated from the amount of oligogalacturonates released quantified by high-performance anion-exchange chromatography or by capillary electrophoresis, has been developed. This concept allowed differentiating pectin encompassing HG domains exhibiting subtle differences in methylesterification patterns (Guillotin et al. 2005; Daas et al. 2000; Limberg et al. 2000; Ström et al. 2007; Ngouemazong et al. 2011; Ralet et al. 2012). Parallel electrospray ionisation multistage mass spectrometry (ESI-MSn) may be also used to provide information about the location of methyl-esterified GalA residues in oligogalacturonates of DP 3–10 (Körner et al. 1999; van Alebeek et al. 2000; Quéméner et al. 2003; Ralet et al. 2009, 2012). Recent advances in mass spectrometry and separation techniques allowed exploring complex mixtures and characterising oligosaccharides of higher DP (identification up to DP14, structural characterization up to DP9) (Ropartz et al. 2014). Model HGs were degraded by a pectin lyase and digestion products were characterised (Ropartz et al. 2014). The structure of each isomer was stated based on its methylation pattern. These approaches can bring different types of information: (1) the specificity of the enzyme and the tolerance of each subsite to the occurrence of methyl-esters can be stated, and (2) the structure of large oligosaccharides can be revealed, giving insights into parts of the polymers that are recalcitrant to enzymatic digestion.
2.2.1.2 Acetyl-Esterification
In few plant species, GalA residues in HG domains are partially acetyl-esterified at O-2 and/or O-3 (Rombouts and Thibault 1986; Ishii 1997; Needs et al. 1998; Perrone et al. 2002) (Fig. 2.1) and this has a strong negative impact on gelation (Pippen et al. 1950; Kohn and Furda 1968; Kohn and Malovikova 1978; Renard and Jarvis 1999; Oosterveld et al. 2000a; Ralet et al. 2003). The distribution of acetyl groups onto HG segments has been particularly studied in sugar beet. Keenan et al. (1985) showed by nuclear magnetic resonance (NMR) that acetyl groups could be attached on any of the available ring positions (O-2 and O-3) of GalA residues. Ralet et al. (2005, 2008) and Remoroza et al. (2014) used combinations of pectin-degrading enzymes to generate partly methyl esterified and acetyl esterified oligogalacturonates that were further separated by chromatographic means and analysed by ESI-MSn. The large variety of oligogalacturonates that were identified and quantified revealed that (1) the occurrence of O-2 and O-3 acetyl esterification in roughly similar amounts, (2) the absence of 2,3-di-O-acetylation, and (3) the scarcity of GalA residues that are both methyl- and acetyl-esterified (Ralet et al. 2005). Pectins with high degree of methyl esterification (HM) having different patterns of ester distribution could be also discriminated (Remoroza et al. 2014). A blockwise distribution of acetyl groups was evidenced (Ralet et al. 2008) and, in commercially-extracted sugar beet pectin, blocks of (1) non-esterified, (2) partly methyl- and acetyl-esterified, and (3) highly methyl- and acetyl-esterified GalA residues were identified (Remoroza et al. 2014). The balance between intra-chain heterogeneity (i.e., the different blocks that are present within a single macromolecule) and inter-chain heterogeneity (some pectin molecules are very highly acetyl-esterified and others are not) is however very difficult to appraise.
2.2.2 Galacturonans Substituted with More or Less Complex Side-Chains
HG can be more or less heavily substituted at O-2 and/or O-3 by monomers or dimers of apiose or xylose leading to apiogalacturonan (AGA, Fig. 2.2a) or xylogalacturonan (XGA, Fig. 2.2b), respectively. It can also be substituted with complex side-chains to form rhamnogalacturonan II (RG-II, Fig. 2.2c).
Structure of the side-chains of homogalacturonans (SNFG). (a) apiogalacturonan, (b) xylogalacturonan, (c) rhamnogalacturonan-II. More or less complex side-chains can be attached to homogalacturonan. Rhamnogalacturonan-II is a key substituted galacturonan made of a short backbone branched by up to 5 side-chains varying in complexity from single arabinose unit to highly heterogeneous nonasaccharides
2.2.2.1 Apiogalacturonan
Apiogalacturonan (AGA, Fig. 2.2a) is restricted to some aquatic plants, the duckweeds (Lemnoideae) and the marine seagrasses (Zosteraceae) (Hart and Kindel 1970; Ovodov et al. 1971; Longland et al. 1989; Gloaguen et al. 2010; Avci et al. 2018). In AGA, β-d-apiofuranosyl residues are linked to O-2 and/or O-3 of GalA residues as monomers or as the dimer [β-d-Apif-(1,3′)-β-d-Apif-(1)]. The degree of substitution of HG by apiose varies from 25 to 80% (Hart and Kindel 1970; Ovodov et al. 1971). AGA can represent up to 20% of non-cellulosic cell wall polysaccharides in the green fronds of giant duckweeds (Longland et al. 1989).
2.2.2.2 Xylogalacturonan
Xylogalacturonan (XGA, Fig. 2.2b), an HG substituted solely at O-3 by xylose monomers or dimers, has been identified in several plant samples among which duckweeds (Hart and Kindel 1970), apple and watermelon fruits (Schols et al. 1995a; Zandleven et al. 2006; Mort et al. 2008), potato tuber (Zandleven et al. 2006), pea hulls (Le Goff et al. 2001) and Arabidopsis thaliana leaves and stalks (Zandleven et al. 2007). Dimeric side chains of xylose have been shown to contain 1,4-linked xylose residues (Zandleven et al. 2006) but 1,2-linked and 1,3-linked xylose residues have also been identified (Le Goff et al. 2001; Nakamura et al. 2002). Methylesterification of XGA has been reported in apple (Schols et al. 1995a). Both the ratio of Xyl monomers and dimers and the degree of substitution of GalA by Xyl vary according to plant samples. A recent study highlighted that the diversification of the Lemnoideae was accompanied by a reduction in the abundance of cell wall AGA and an increase in XGA (Avci et al. 2018).
2.2.2.3 Rhamnogalacturonan II
Rhamnogalacturonan II (RG-II, Fig. 2.2c) is a far more complex substituted-HG than AGA or XGA since it encompasses thirteen different sugars and twenty one distinct glycosidic linkages arranged as a backbone formed by nine partially methyl-esterified GalA residues (from none to three) (Ishii and Kaneko 1998) substituted by different side chains termed A–F (Ndeh et al. 2017).
Large Side-Chains
Side-chains A and B encompass six to nine sugars and are both bound to the HG backbone through a (2,1) β-d-apiofuranose. Although the structure of RG-II is considered as highly conserved throughout the plant kingdom, some heterogeneity may occur within these side-chains. Side-chain A was first described by Stevenson et al. (1988) in Ficus sycomorus as an oligosaccharide composed of eight different monosaccharides (Fig. 2.2c). The heterogeneity in the structure of side-chain A comes firstly from the α-l-Galp residue that can be replaced by an α-l-Fucp, not only in the Arabidopsis thaliana mutant mur1 (Reuhs et al. 2004), but also in the wild-type plants (Pabst et al. 2013). Additionally, side-chain A contains three uronic acids, one α-d-GalAp, one β-d-GalAp and one β-d-GlcAp, that can be methyl-esterified and/or methyl-etherified (Pabst et al. 2013; Buffetto et al. 2014). Side-chain A is involved in RG-II dimerisation through a boron covalent linkage via a di-ester bond between two β-d-apiofuranose units (O-2 and O-3). The three uronic acids residues are able to chelate cations and methyl-esterification that can occur on the β-d-GlcAp unit may modulate this chelation property. Side-chain B is particularly heterogeneous (Table 2.1).
It was first described in Ficus sycomorus as a heptasaccharide, the main chain being made of a β-d-Apif-(3,1)-β-l-Rhap-(3,1)-α-l-Aceric acid-(2,1)-β-d-Galp-(2,1)-2-O-Me-α-l-Fucp. The Galp residue was substituted by a (4,1)-α-L-Arap unit itself substituted by a (2,1)-α-l-Rhap residue (Spellman et al. 1983). Depending on the botanical origin, the stage of development and the organ considered, the number of constitutive sugar residues may vary. Hexa- to nonasaccharides were observed based on the substitution of the laterally branched α-L-Arap residue (Fig. 2.2). Whitcombe et al. have described an octasaccharide with a (2,1)-β-l-Araf linked to the laterally branched additional α-l-Rhap unit. These authors also described for the first time the occurrence of two acetylation sites on the α-l-Aceric acid and on the 2-O-Me α-d-Fucp. Finally, in 1998, Shin et al. described in Panax ginseng a nonasaccharide resulting from a second α-l-Rhap unit branched at C-3 of the β-l-Arap residue. It is difficult to conclude about the existence of the low DP species in vivo, as α-l-Rhap residues being very labile according to the extraction conditions and the analytical methods used. As for side-chain A, 2-O-Me-α-d-Fucp can be replaced by 2-O-Me-l-Galp in the Arabidopsis thaliana mur1 mutant (Reuhs et al. 2004). In lycophytes, pteridophytes and bryophytes, α-l-Rhap units can be methyl-etherified at O-3 (Matsunaga et al. 2004).
Short Side-Chains
Side chain E is composed of a unique residue of α-l-Araf linked to a d-GalAp at O-3 (Buffetto et al. 2014). This modification is termed side chain F when it occurs on a d-GalA unit that is also substituted by side-chain A. Side-chains C and D are dimers that are branched at O-3 of a d-GalA units. They are composed of a α-3-deoxy-d-manno-2-octulosonic acid (4,1) α-l-Rhap and an α-3-deoxy-d-lyxo-2-heptulosonic acid (5,1) β-l-Araf, respectively. In the fractionation scheme used by Buffetto et al. (2014), these side chains are co-eluted with longer ones (co-elution of A and D and of B and C) (Ropartz 2015).
2.3 Rhamnogalacturonan I
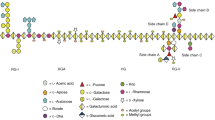
RG-I was pictured as a long sequence of alternating l-Rha and d-GalA residues (Fig. 2.3a), Rha residues being substituted with a variety of l-arabinosyl- and d-galactosyl-containing side-chains (O’Neill et al. 1990, Fig. 2.3b–d). Fucose, glucuronic acid and 4-O-methyl glucuronic acid residues may also be present in small amounts. RG-I usually accounts for 20–35% of the total pectin amount (Mohnen 2008) but can, for certain plant sources such as soybean, make up to 75% of pectic polysaccharides (Voragen et al. 2009).
Structure of Rhamnogalacturonan I (SNFG). (a) Backbone, (b) Arabinans, (c) Type I arabinogalactans, (d) Type II arabinogalactans. Rhamnogalacturonan I is the second major element of pectin. Its backbone is made of strictly alternating galacturonic acid and rhamnose residues, the latter being branched by arabinose- and galactose-containing side-chains of diverse complexity
2.3.1 Rhamnogalacturonan I Backbone
The backbone of RG-I is made of the alternating diglycosyl repeating unit [2-α-l-Rhap-(1,4)-α-d-GalAp-(1] (McNeil et al. 1980, 1984; Lau et al. 1985, Fig. 2.3a). Whole RG-I samples isolated from various plant sources usually exhibit Rha/GalA ratios < 1 (Schols and Voragen 1994; Cornuault et al. 2015; Buffetto et al. 2015). Values very close to 1 have however been reported (Konno et al. 1986; Yapo et al. 2007; Ralet and Thibault 2009) and the series of homologous oligomers recovered after controlled acid hydrolysis of pectin samples from apple, sugar beet and citrus all presented strictly alternating sequences (Renard et al. 1995). The presence of RG-I domains in which single Rha residues alternate with two or three consecutive GalA residues is therefore rather implausible. Unlike HG domains, RG-I backbone seems to exhibit a DP that varies depending on the plant source.
An average DP of up to 300 repeats has been estimated for RG-I isolated from suspension-cultured sycamore cell walls (McNeil et al. 1984). Ralet and Thibault (2009) reported values of 60 Rha-GalA repeats for RG-I isolated from citrus albedo and 120 for RG-I isolated from sugar-beet. Acetylation is much more frequent in RG-I than in HG, and RG-I domains isolated from numerous plant species appeared highly acetylated at O-2 or O-3 of the GalA units (Komalavilas and Mort 1989; Lerouge et al. 1993; Schols and Voragen 1994; Normand et al. 2010; Remoroza et al. 2012). Ishii showed that some GalA residues were 2,3-di-O-acetylated in RG-I oligosaccharides isolated from potato tuber and bamboo shoot cell walls (Ishii 1997). In okra, unusual acetylation at O-3 of Rha residues has been also shown (Sengkhamparn et al. 2009). Finally, to our knowledge, no evidence for methylesterification of the RG-I domain has ever been reported.
2.3.2 Rhamnogalacturonan I Side-Chains
RG-I is also known as the pectin “hairy region” since GalA but mostly Rha residues are substituted by different side-chains varying in their length and composition. GalA residues typically are not substituted and only one study showed that approximately 2% of the GalA residues in the RG-I backbone were substituted at O-3 by single β-d-glucuronic acid residues (Renard et al. 1999). In contrast, 20–80% of the Rha residues are substituted at O-4 with side-chains in which Ara and Gal predominate (Ridley et al. 2001). The proportion of side-chains, their composition, length and degree of branching vary enormously not only depending on plant sources, organs and tissues (Lerouge et al. 1993) but also on developmental stage (Willats et al. 2001) and on the isolation method used (Schols et al. 1995b). For instance, arabinan-rich pectins are particularly abundant in apple, sugar-beet and carrot while type I arabinogalactan-rich pectins are commonly found in citrus, lupin and potato and type II arabinogalactan-rich pectins have been found in ginseng (Sun et al. 2019). Further, galactan and arabinan epitopes can occur in different zones of organs and even at distinct locations within a single developing organ (Willats et al. 2001). Finally, for a given cell wall material, pectins that were more difficult to extract were more substituted with side-chains than the more easily extractable fractions (Schols et al. 1995b). Side-chains consisting in single Gal units have been reported for RG-I oligomers that have been isolated without using any side-chain degrading enzymes (Schols et al. 1995b; Oosterveld et al. 2000b; Sun et al. 2019). In okra, specifically, α-Gal substitutions were identified (Sengkhamparn et al. 2009). Polymeric side-chains commonly consist in arabinans and type I and II (arabino)-galactans.
2.3.2.1 Arabinans
Arabinans are made of a backbone of α-(1,5)-linked l-Araf residues that are substituted at O-2 and/or O-3 with single α-l-Araf units or with short chains of α-(1,3)-linked l-Araf units (Yapo 2011, Fig. 2.3b). Sugar-beet arabinans have been particularly studied over the years. They consist of a long backbone of up to sixty or seventy Ara residues (Oosterveld et al. 2002) one third of which being branched at O-3, mainly with single Araf units (Guillon and Thibault 1989). The presence of minor amounts of double branching at O-2 and O-3 has been further evidenced (Westphal et al. 2010). Occasionally, arabinans substituted with single Galp units or with up to four or five β-(1,4)-linked Galp residues have been isolated in soybean, sugar-beet or potato (Sakamoto and Sakai 1995; Nakamura et al. 2002; Øbro et al. 2004). Arabinans branched to a much lower extent than in sugar-beet have been also observed in ginseng root and in potato tuber (Sun et al. 2019; Øbro et al. 2004).
2.3.2.2 Type I (Arabino)-Galactans
Type I (arabino)-galactans encompass a backbone of β-(1,4)-linked d-Galp residues that may be lowly substituted at O-3 by β-d-Galp or α-l-Araf single units or by short α-(1,5)-linked l-Araf chains (Aspinall et al. 1967; Morita 1965a, b, Fig. 2.3c). Variants of this basic structure have been reported such as (1) termination of the main chain at O-6 with a Galp residue (Lau et al. 1987), (2) termination of the main chain with an Arap residue at O-4 or a Fuc residue at O-2 (Huisman et al. 2001; O’Neill et al. 1990), (3) insertion of a single Araf or a short α-(1,5)-linked l-Araf chain within the β-(1,4)-linked d-Galp main chain (Huisman et al. 2001; Buffetto et al. 2015), and (4) β-(1,3) galactosyl interruption of the main chain (Hinz et al. 2005). An average chain length in the range of 45–50 was estimated in soybean (Huisman et al. 2001).
2.3.2.3 Type II Arabinogalactans
Type II arabinogalactans are highly branched side-chains characterised by a backbone of β-(1,3)-linked d-Galp residues substituted at O-6 with single Galp residues or with short chains of β-(1,6)-linked d-Galp residues, which can in turn be substituted at O-3, O-4 and/or O-6, by Ara-containing chains (Yapo 2011, Fig. 2.3d). Type II arabinogalactans have been recently identified in ginseng (Sun et al. 2019) and it has been shown that the (1,3)-linked galactan backbone was substituted, mainly at O-6 but also at O-4, (1) with short (1,6)-linked galactan chains terminated by single Araf units, and (2) directly with single Araf residues.
2.3.2.4 Ferulic Acid
In species from the Amaranthaceae family such as sugar-beet and spinach RG-I side-chains are esterified by ferulic acid residues (Ishii and Tobita 1993; Colquhoun et al. 1994; Levigne et al. 2004; Quéméner and Ralet 2004). Ferulic acid moieties are mainly ester-linked to O-2 of Ara residues from the α-(1,5)-linked l-Araf arabinan backbone and to O-6 of Gal residues from the β-(1,4)-linked d-Galp type I galactan backbone but a more peripheral location at O-5 of Ara residues from the α-(1,5)-linked l-Araf arabinan backbone has been also identified. Ferulic acid residues can undergo in vivo oxidative coupling reactions to form dehydrodimers, thereby covalently cross-linking the polysaccharides they esterify (Fry 1986). Such cross-links have been identified in sugar-beet pectin (Ralet et al. 2005).
2.3.3 Side-Chain Intra- and Inter-Molecular Distribution
Treatment of “hairy regions” with rhamnogalacturonan hydrolase allowed to evidence that the different types of side-chains were not randomly distributed over the RG-I backbone. Indeed, incubating de-esterified apple or ginseng pectin with rhamnogalacturonan hydrolase released RG-I oligosaccharides based on a GalA2Rha2 and a GalA3Rha3 backbone (Schols and Voragen 1994; Sun et al. 2019). Most of the RG-I oligomers recovered contained no side-chains and single unit Gal side-chains even though single units or short Ara chains were also detected in ginseng (Sun et al. 2019). Since Rha-GalA oligosaccharides substituted solely by Ara residues were identified (O’Neill et al. 1990; Sun et al. 2019), a direct linkage between Ara and Rha is plausible although a single Gal unit or short type I galactan anchor seem to be usually necessary for Ara and arabinan branching onto RG-I backbone (Buffetto et al. 2015). Next to these lowly branched rhamnogalacturonan hydrolase-degradable regions, highly branched arabinan- and type II arabinogalactan-rich regions that are not rhamnogalacturonan hydrolase-degradable have been identified (Schols and Voragen 1994; Sun et al. 2019). It has been hypothesised that these side-chains are flexible and long enough to wrap around the RG-I backbone, hindering enzymatic breakdown of the RG-I backbone by rhamnogalacturonan hydrolase (Willats et al. 2001; Voragen et al. 2009). The intra- and inter-molecular distribution of the different domains (lowly and highly branched) is extremely difficult to characterise and virtually nothing is presently known about the specific location of individual side-chains along the backbone. The co-existence of different “hairstyles” within a single pectin molecule remains speculative (Voragen et al. 2009).
2.4 Connection Between Pectin Domains
It is now fully admitted that pectin is a highly complex macromolecule in which several domains are covalently linked to each other. It is however particularly challenging to accommodate all available structural information into a universal model structure. Currently, two models are under debate (1) the smooth and hairy regions model established by de Vries et al. (1981) and updated by Schols and Voragen (1996) (Fig. 2.4) and (2) the RG-I backbone model proposed by Vincken et al. (2003) (Fig. 2.5).
Pectin smooth and hairy region model. The smooth region consists of homogalacturonan. The hairy region consists of three types of subunits: subunit I made of xylogalacturonan, subunit II made of arabinan- and type II arabinogalactan-rich RG-I and subunit III made of lowly substituted RG-I short stretches. Adapted from Schols and Voragen (1996)
The smooth and hairy regions model proposes that HG domains alternate with “hairy” regions, the latter including three subunits: subunit I: XGA; subunit II: stubs of RG-I backbone substituted by arabinan side-chains (or AG-II, Sun et al. 2019); and subunit III: rather short
RG-I stretches (four or six residues) that are unsubstituted or substituted by Gal single units (Schols and Voragen 1996). In contrast, the RG-I backbone model positions HG and XGA as side-chains of RG-I (Vincken et al. 2003). Coenen et al. (2007) are, according to our knowledge, the only group succeeding in isolating and characterising chimeric oligosaccharides with a HG/XGA segment linked to a RG-I backbone one. A GalA trimer and a Xyl-substituted GalA trimer covalently linked to a short RG-I moiety were identified and H1-NMR unambiguously showed that the GalA trimer was α-(1,2)-linked to the RG-I moiety.
MSn experiments further showed that the RG-I moiety was always located at the reducing end of the chimeric oligosaccharides. These results are undoubtedly in favour of the “smooth and hairy regions” model even though this model does not fulfil all the experimental results obtained so far. In particular, it has been calculated that there are in average seventeen HG domains for one RG-I domain in lemon acid-extracted pectin and eight HG domains for one RG-I domain in sugar beet acid-extracted pectin taking into account (1) the partitioning of GalA between HG and RG-I domains, and (2) the length of these domains (Ralet and Thibault 2009). A similar “excess of GalA” was noticed by Yapo et al. (2007) and by Coenen et al. (2007). It is clear that there is a huge surplus of HG domains to build up a pectin macromolecule following the “smooth and hairy regions” model; the “RG-I backbone” model shall therefore not be excluded irrevocably (Coenen et al. 2007).
2.5 Conclusions
Pectin is a highly complex macromolecule exhibiting a high degree of intra- and inter-molecular heterogeneity. When extracting pectin from a given plant sample, different populations that were located in specific tissues or even in specific spots within a single cell wall are co-extracted and mixed. Studying pectin fine structure implies to be able to master extraction conditions and implement adequate chemical and enzymatic tools to generate oligomers that can be separated and fully analysed. These can further be used to generate probes such as monoclonal antibodies to locate specific motifs in muro. To date, complete deconstruction of pectin into analysable oligomers has not been achieved and non-degradable fractions whose structure is unclear always remain. These fractions, encompassing in particular GalA surplus, should be targeted for getting a better understanding of the architecture of pectin macromolecules.
References
Aspinall GO, Begbie R, Hamilton A et al (1967) Polysaccharides of soy-beans. Part III. Extraction and fractionation of polysaccharides from cotyledon meal. J Chem Soc C 170:1065–1070
Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64:747–779
Avci U, Pena MJ, O’Neill MA (2018) Changes in the abundance of cell wall apiogalacturonan and xylogalacturonan and conservation of rhamnogalacturonan II structure during the diversification of the Lemnoideae. Planta 247:953–971
Bonnin E, Dolo E, Le Goff A et al (2002) Characterisation of pectin subunits released by an optimised combination of enzymes. Carbohydr Res 337:1687–1696
Braconnot H (1825a) Recherches sur un nouvel acide universellement répandu dans tous les végétaux. Ann Chim Phys 28:173–178
Braconnot H (1825b) Nouvelles observations sur l’acide pectique. Ann Chim Phys 30:96–102
Broxterman SE (2018) The architecture of the primary cell walls: the role of pectin reconsidered. PhD thesis, Wageningen University, The Netherland
Buffetto F, Ropartz D, Zhang XJ et al (2014) Recovery and fine structure variability of RGII sub-domains in wine (Vitis vinifera Merlot). Ann Bot 114:1327–1337
Buffetto F, Cornuault V, Ridahl MG et al (2015) The deconstruction of pectic rhamnogalacturonan I unmasks the occurrence of a novel arabinogalactan oligosaccharide epitope. Plant Cell Physiol 56:2181–2196
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Coenen GJ, Bakx EJ, Verhoef RP et al (2007) Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr Polym 70:224–235
Colquhoun IJ, de Ruiter GA, Schols HA et al (1990) Identification by n.m.r. spectroscopy of oligosaccharides obtained by treatment of the hairy regions of apple pectin with rhamnogalacturonase. Carbohydr Res 206:131–144
Colquhoun I, Ralet MC, Thibault JF (1994) Structure identification of feruloylated oligosaccharides from sugar-beet pulp by NMR spectroscopy. Carbohydr Res 263:243–256
Cornuault V, Buffetto F, Rydahl MG et al (2015) Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 242:1321–1334
Daas PJH, Voragen AGJ, Schols HA (2000) Investigation of the galacturonic acid distribution of pectin with enzymes part 2 - characterization of non-esterified galacturonic acid sequences in pectin with endopolygalacturonase. Carbohydr Res 326:120–129
de Vries JA, Voragen AGJ, Rombouts FM et al (1981) Extraction and purification of pectins from alcohol insoluble solids from ripe and unripe apples. Carbohydr Polym 1:117–127
de Vries JA, Rombouts FM, Voragen AGJ et al (1982) Enzymic degradation of apple pectins. Carbohydr Polym 2:25–33
de Vries JA, den Uijl CH, Voragen AGJ et al (1983) Structural features of the neutral sugar side chains of apple pectic substances. Carbohydr Polym 3:193–205
Dong X, Huang YF, Cho BG et al (2018) Advances in mass spectrometry-based glycomics. Electrophoresis 39:3063–3081
Fry SC (1986) Cross linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37:165–186
Gloaguen V, Brudieux V, Closs B et al (2010) Structural characterization and cytotoxic properties of an apiose-rich pectic polysaccharide obtained from the cell wall of the marine phanero- gam Zostera marina. J Nat Prod 73:1087–1092
Glushka JN, Terrell M, York WS et al (2003) Primary structure of the 2-O-methyl-alpha-L-fucose-containing side chain of the pectic polysaccharide, rhamnogalacturonan II. Carbohydr Res 338:341–352
Guillon F, Thibault JF (1989) Methylation analysis and mild acid hydrolysis of the “hairy” fragments of sugar-beet pectins. Carbohydr Res 190:85–96
Guillotin SE, Bakx EJ, Boulenguer P et al (2005) Populations having different GalA blocks characteristics are present in commercial pectins which are chemically similar but have different functionalities. Carbohydr Polym 60:391–398
Hart DA, Kindel PK (1970) Isolation and partial characterization of apiogalacturonans from the cell wall of Lemna minor. Biochem J 116:569–579
Hellin P, Ralet MC, Bonnin E et al (2005) Homogalacturonans from lime pectins exhibit homogeneous charge density and molar mass distributions. Carbohydr Polym 60:307–317
Hinz SWA, Verhoef R, Schols HA et al (2005) Type I arabinogalactan contains β-d-Galp-(1,3)-β-d-Galp structural elements. Carbohydr Res 340:2135–2143
Huisman MMH, Brüll LP, Thomas-Oates JE et al (2001) The occurrence of internal (1,5)-linked arabinofuranose and arabinopyranose residues in arabinogalactan side chains from soybean pectic substances. Carbohydr Res 330:103–114
Ishii T (1997) O-Acetylated oligosaccharides from pectins of potato tuber cell walls. Plant Physiol 113:1265–1272
Ishii T, Kaneko S (1998) Oligosaccharides generated by partial hydrolysis of the borate-rhamnogalacturonan II complex from sugar beet. Phytochemistry 49:1195–1202
Ishii T, Tobita T (1993) Structural characterization of feruloyl oligo- saccharides from spinach-leafs cell walls. Carbohydr Res 248:179–190
Kaneko S, Ishii T, Matsunaga T (1997) A boron-rhamnogalacturonan-II complex from bamboo shoot cell walls. Phytochemistry 44:243–248
Keenan MHJ, Belton PS, Matthew JA et al (1985) A 13C-n.m.r. study of sugar-beet pectin. Carbohydr Res 138:168–170
Kertesz ZI (1951) The pectic substances. Interscience Publishers Inc., New York
Kohn R, Furda I (1968) Binding of calcium ions to acetyl derivatives of pectin. Collect Czechoslov Chem Commun 33:2217–2225
Kohn R, Malovikova A (1978) Dissociation of acetyl derivatives of pectic acid and intramolecular binding of calcium ions to those substances. Collect Czechoslov Chem Commun 43:1709–1719
Komalavilas P, Mort AJ (1989) The acetylation at O-3 of galacturonic acid in the rhamnose-rich portion of pectins. Carbohydr Res 189:261–272
Konno H, Yamasaki Y, Katoh K (1986) Enzymatic degradation of pectic substances and cell walls purified from carrot cell cultures. Phytochemistry 25:623–627
Körner R, Limberg G, Christensen TMIE et al (1999) Sequencing of partially methyl-esterified oligogalacturonates by tandem mass spectrometry and its use to determine pectinase specificities. Anal Chem 71:1421–1427
Lau JM, Mc Neill M, Darvill AG et al (1985) Structure of the backbone of rhamnogalacturonan I, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res 137:111–125
Lau JM, McNeil M, Darvill AG et al (1987) Treatment of rhamnogalacturonan I with lithium in ethylenediamine. Carbohydr Res 168:245–274
Le Goff A, Renard CMGC, Bonnin E et al (2001) Extraction, purification and chemical characterisation of xylogalacturonans from pea hulls. Carbohydr Polym 45:325–334
Lerouge P, O’Neill MA, Darvill AG et al (1993) Structural characterization of endo-glucanase-generated oligoglycosyl side chains of rhamnogalacturonan I. Carbohydr Res 243:359–371
Levigne SV, Ralet MCJ, Quéméner B et al (2004) Isolation from sugar beet cell walls of arabinan oligosaccharides esterified by two ferulic acid monomers. Plant Physiol 134:1173–1180
Limberg G, Körner R, Buchholt HC et al (2000) Analysis of different de-esterification mecha- nisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr Res 327:293–307
Longland JM, Fry SC, Trewavas AJ (1989) Developmental control of apiogalacturonan biosynthesis and UDP-apiose production in a duckweed. Plant Physiol 90:972–978
Matsunaga T, Ishii T, Matsumoto S et al (2004) Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol 134:339–351
McNeil M, Darvill AG, Albersheim P (1980) Structure of plant cell walls. X. Rhamnogalacturonan I, a structurally complex pectic polysaccharide in the walls of suspension-cultured sycamore cells. Plant Physiol 66:1128–1134
McNeil M, Darvill AG, Fry SC et al (1984) Structure and function of the primary cell walls of plants. Annu Rev Biochem 53:625–663
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277
Morita M (1965a) Polysaccharides of soybean seeds. Part 1. Polysaccharide constituents of "hot-water-extract" fraction of soybean seeds and an arabinogalactan as its major component. Agric Biol Chem 29:564–573
Morita M (1965b) Polysaccharides of soybean seeds. Part 2. A methylated arabinogalactan isolated from methylated product of “hot-water-extract” fraction of soybean seed polysaccharides. Agric Biol Chem 29:626–630
Mort A, Zheng Y, Qiu F et al (2008) Structure of xylogalacturonan fragments from watermelon cell-wall pectin. Endopolygalacturonase can accommodate a xylosyl residue on the galacturonic acid just following the hydrolysis site. Carbohydr Res 343:1212–1221
Mucha E, Marianski M, Xu FF et al (2018) Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy. Nat Commun 9:4174
Nakamura A, Furuta H, Maeda H et al (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313
Ndeh D, Rogowski A, Cartmell A et al (2017) Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544:65–70
Needs PW, Rigby NM, Colqhoun IJ et al (1998) Conflicting evidence for non-methyl galacturonoyl esters in Daucus carota. Phytochemistry 48:71–77
Ngouemazong DE, Tengweh FF, Duvetter T et al (2011) Quantifying structural characteristics of partially de-esterified pectins. Food Hydrocoll 25:434–443
Normand J, Ralet MC, Thibault JF (2010) Purification, characterization, and mode of action of a rhamnogalacturonan hydrolase from Irpex lacteus, tolerant to an acetylated substrate. Appl Microbiol Biotechnol 86:577–588
O’Neill M, Albersheim P, Darvill A (1990) The pectic polysaccharides of primary cell walls. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry, Carbohydrates, vol 2. Academic Press, London, pp 415–441
Øbro J, Harholt J, Scheller HV et al (2004) Rhamnogalacturonan I in Solanum tuberosum tubers contains complex arabinogalactans structures. Phytochemistry 65:1429–1438
Oosterveld A, Beldman G, Searle-van Leeuwen MJF et al (2000a) Effect of enzymatic deacetylation on gelation of sugar beet pectin in the presence of calcium. Carbohydr Polym 43:249–256
Oosterveld A, Beldman G, Schols HA et al (2000b) Characterization of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses from sugar beet pulp. Carbohydr Res 328:185–197
Oosterveld A, Beldman G, Voragen AGJ (2002) Enzymatic modification of pectic polysaccharides obtaines from sugar beet pulp. Carbohydr Polym 48:73–81
Ovodov YS, Ovodova RG, Bondarenko OD et al (1971) The pectic substances of Zosteraceae: Part IV. Pectinase digestion of zosterine. Carbohydr Res 18:311–318
Pabst M, Fischl RM, Brecker L et al (2013) Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. Plant J 76:61–72
Perrone P, Hewage CM, Thomson AR et al (2002) Patterns of methyl and O-acetyl esterification in spinach pectins: new complexity. Phytochemistry 60:67–77
Pippen E, McCready RM, Owens HS (1950) Gelation properties of partly acetylated pectins. J Am Chem Soc 72:813–816
Quéméner B, Ralet MC (2004) Evidence for linkage position determination in known feruloylated mono- and disaccharides using electrospray ion trap mass spectrometry. J Mass Spectrom 39:1153–1160
Quéméner B, Désiré C, Lahaye M et al (2003) Structural characterisation by both positive- and negative-ion electrospray mass spectrometry of partially methyl-esterified oligogalacturonides purified by semi-preparative high-performance anion-exchange chromatography. Eur J Mass Spectrom 9:45–60
Ralet MC, Thibault JF (2009) Hydrodynamic properties of isolated pectic domains: a way to figure out pectin macromolecular structure? In: Schols HA, Visser RGF, Voragen AGJ (eds) Pectins and pectinases. Wageningen Academic Publishers, Wageningen, pp 35–48
Ralet MC, Crépeau MJ, Buchholt HC et al (2003) Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochem Eng J 16:191–201
Ralet MC, Cabrera JC, Bonnin E et al (2005) Mapping sugar beet pectin acetylation pattern. Phytochemistry 66:1832–1843
Ralet MC, Crépeau MJ, Bonnin E (2008) Evidence for a blockwise distribution of acetyl groups onto homogalacturonans from a commercial sugar beet (Beta vulgaris) pectin. Phytochemistry 69:1903–1909
Ralet MC, Lerouge P, Quéméner B (2009) Mass spectrometry for pectin structure analysis. Carbohydr Res 344:1798–1807
Ralet MC, Martins W, Tanhatan Nasseri A et al (2012) An innovative enzymatic approach to apprehend the methylesterification pattern of homogalacturonans. Biomacromolecules 13:1615–1624
Remoroza C, Cord-Landwehr S, Leijdekkers AGM et al (2012) Combined HILIC-ELSD/ESI-MSn enables the separation, identification and quantification of sugar beet pectin derived oligomers. Carbohydr Polym 90:41–48
Remoroza C, Buchholt HC, Gruppen H et al (2014) Descriptive parameters for revealing substitution patterns of sugar beet pectins using pectolytic enzymes. Carbohydr Polym 101:1205–1215
Renard C, Jarvis M (1999) Acetylation and methylation of homogalacturonans 2. Effect on ion-binding properties and conformations. Carbohydr Polym 39:209–216
Renard C, Crépeau MJ, Thibault JF (1995) Structure of the repeating units in the rhamnogalacturonic backbone of apple, beet and citrus pectins. Carbohydr Res 275:155–165
Renard C, Crépeau MJ, Thibault JF (1999) Glucuronic acid directly linked to galacturonic acid in the rhamnogalacturonan backbone of beet pectins. Eur J Biochem 266:566–574
Reuhs BL, Glenn J, Stephens SB et al (2004) L-Galactose replaces L-Fucose in the pectic polysaccharide rhamnogalacturonan II synthesized by the L-fucose-deficient mur1 Arabidopsis mutant. Planta 219:147–157
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signalling. Phytochemistry 57:929–967
Rombouts FM, Thibault JF (1986) Enzymatic and chemical degradation and the fine structure of pectins from sugar-beet pulp. Carbohydr Res 154:189–203
Ropartz D (2015) Apport des dernières évolutions en spectrométrie de masse pour l’étude structurale des polysaccharides. Université de Nantes, Nantes
Ropartz D, Lemoine J, Giuliani A et al (2014) Deciphering the structure of isomeric oligosaccharides in a complex mixture by tandem mass spectrometry: Photon activation with vacuum ultra-violet brings unique information and enables definitive structure assignment. Anal Chim Acta 807:84–95
Ropartz D, Li P, Fanuel M et al (2016) Charge transfer dissociation of complex oligosaccharides: comparison with collision-induced dissociation and extreme ultraviolet dissociative photoionization. J Am Soc Mass Spectrom 27:1614–1619
Ropartz D, Li P, Jackson GP et al (2017) Negative polarity helium charge transfer dissociation tandem mass spectrometry: Radical-initiated fragmentation of complex polysulfated anions. Anal Chem 89:3824–3828
Ropartz D, Fanuel M, Ujma J et al (2019) Structure determination of large isomeric oligosaccharides of natural origin through multi-pass and multi-stage cyclic traveling wave ion mobility mass spectrometry. Anal Chem 91:12030–12037
Round AN, Rigby NM, MacDougall AJ et al (2010) A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr Res 345:487–497
Sakamoto T, Sakai T (1995) Analysis of structure of sugar-beet pectin by enzymatic methods. Phytochemistry 39:821–823
Schindler B, Barnes L, Renois G et al (2017) Anomeric memory of the glycosidic bond upon fragmentation and its consequences for carbohydrate sequencing. Nat Commun 8:7
Schols HA, Voragen AGJ (1994) Occurrence of pectic hairy regions in various plant cell wall materials and their degradability by rhamnogalacturonase. Carbohydr Res 256:83–95
Schols HA, Voragen AGJ (1996) Complex pectins: Structure elucidation using enzymes. In: Visser J, Voragen AGJ (eds) Pectins and pectinases. Elsevier, Amsterdam, pp 3–19
Schols HA, Voragen AGJ (2002) The chemical structure of pectins. In: Seymour GB, Knox JP (eds) Pectins and their manipulation. Blackwell Publishing-CRC Press, Oxford, pp 1–29
Schols HA, Bakx EJ, Schipper D et al (1995a) A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr Res 279:265–279
Schols HA, Vierhuis E, Bakx EJ et al (1995b) Different populations of pectic hairy regions occur in apple cell walls. Carbohydr Res 275:343–360
Sengkhamparn N, Verhoef R, Bakx EJ et al (2009) Okra pectin contains an unusual substitution of its rhamnosyl residues with actyl and alpha-linked galactosyl groups. Carbohydr Res 334:1842–1851
Seveno M, Voxeur A, Rihouey C et al (2009) Structural characterisation of the pectic polysaccharide rhamnogalacturonan II using an acidic fingerprinting methodology. Planta 230:947–957
Shin KS, Kiyohara H, Matsumoto T et al (1998) Rhamnogalacturonan II dimers cross-linked by borate diesters from the leaves of Panax ginseng C.A. Meyer are responsible for expression of their IL-6 production enhancing activities. Carbohydr Res 307:97–106
Smolenski K (1923) Pectins. Rocznigi Chemki 3:86–152
Spellman MW, McNeil M, Darvill AG et al (1983) Structure of plant cell-walls.14. Characterization of a structurally complex heptasaccharide isolated from the pectic polysaccharide rhamnogalacturonan-II. Carbohydr Res 122:131–153
Stevenson T, Darvill AG, Albersheim P (1988) Structure of plant-cell walls. 23. Structural features of the plant cell-wall polysaccharide rhamnogalacturonan-II. Carbohydr Res 182:207–226
Ström A, Ribelles P, Lundin L et al (2007) Influence of pectin fine structure on the mechanical properties of calcium-pectin and acid-pectin gels. Biomacromolecules 8:2668–2674
Sun L, Ropartz D, Cui LN et al (2019) Structural characterization of rhamnogalacturonan domains from Panax ginseng C. A. Meyer. Carbohydr Polym 203:119–127
Thibault JF, Renard C, Axelos M et al (1993) Studies of the length of homogalacturonic regions in pectins by acid-hydrolysis. Carbohydr Res 238:271–286
Thomas JR, Darvill AG, Albersheim P (1989) Isolation and structural characterization of the pectic polysaccharide rhamnogalacturonan II from walls of suspension-cultured rice cells. Carbohydr Res 185:261–277
Ujma J, Ropartz D, Giles K et al (2019) Cyclic ion mobility mass spectrometry distinguishes anomers and open-ring forms of pentasaccharides. J Am Soc Mass Spectrom 30:1028–1037
van Alebeek GJWM, Zabotina O, Beldman G et al (2000) Structural analysis of (methyl-esterified) oligogalacturonides using post-source decay matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mass Spectrom 35:831–840
Varki A, Cummings RD, Aebi M et al (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25:1323–1324
Vauquelin LN (1790) Analyse du tamarin. Ann Chim 5:92–106
Vidal S, Doco T, Williams P et al (2000) Structural characterization of the pectic polysaccharide rhamnogalacturonan II: evidence for the backbone location of the aceric acid-containing oligoglycosyl side chain. Carbohydr Res 326:277–294
Vincken JP, Schols HA, Oomen RJFJ et al (2003) If homogalacturonan were a side-chain of rhamnogalacturonan I. Implication for cell wall architecture. Plant Physiol 132:1781–1789
Voragen AGJ, Pilnik W, Thibault JF et al (1995) Pectins. In: Stephen AM (ed) Food polysaccharides and their applications. Marcel Dekker Inc, New York, pp 287–339
Voragen AGJ, Coenen GJ, Verhoef RP et al (2009) Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem 20:263–275
Westphal Y, Kühnel S, de Waard P et al (2010) Branched arabino-oligosaccharides isolated from sugar beet arabinan. Carbohydr Res 345:1180–1189
Whitcombe AJ, Oneill MA, Steffan W, Albersheim P, Darvill AG (1995) Structural characterization of the pectic polysaccharide, rhamnogalacturonan-II. Carbohydr Res 271:15–29
Willats WGT, McCartney L, Mackie W et al (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47:9–27
Willats WGT, Knox JP, Mikkelsen JD (2006) Pectin: new insights into an old polymer are starting to gel. Trends Food Sci Technol 17:97–104
Yapo BM (2011) Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polym Rev 51:391–413
Yapo BM, Lerouge P, Thibault JF et al (2007) Pectins from citrus plant cell walls contain homogalacturonan homogeneous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr Polym 69:426–435
Zandleven JS, Beldman G, Bosveld M et al (2006) Enzymatic degradation studies of xylogalacturonans from apple and potato, using xylogalacturonan hydrolase. Carbohydr Polym 65:495–503
Zandleven J, Sorensen SO, Harholt J et al (2007) Xylogalacturonan exists in cell walls from various tissues of Arabidopsis thaliana. Phytochemistry 68:1219–1226
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ropartz, D., Ralet, MC. (2020). Pectin Structure. In: Kontogiorgos, V. (eds) Pectin: Technological and Physiological Properties. Springer, Cham. https://doi.org/10.1007/978-3-030-53421-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-53421-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53420-2
Online ISBN: 978-3-030-53421-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)