Abstract
For the movement of solutes across epithelial layers to be effective, it must have the potential to be vectorial, which in turn means that epithelial layers must be polarized in the apical-basolateral axis. Of course, this is indeed the case—most ion channels and transporters localize specifically to either the apical or the basal membrane domains. The polarized distribution of membrane components is the result of a finely balanced developmental program, involving an antagonistic relationship between two apical protein complexes, PAR and CRUMBS, and a basolateral protein complex, SCRIBBLE. Their interactions specify the position of the adherens and tight junctions, and thereby the boundary between the apical and basolateral membrane domains. The three complexes also interact to shape the epithelial cell’s microfilament and microtubule cytoskeletons, and in doing so control the polarized delivery of cargo to specific membrane domains. The effects of the complexes on the cytoskeleton are heavily dependent upon the activities of atypical protein kinase C (aPKC), and on small GTPases of the Rho family. In combination these proteins mediate many of the effects of the complexes upon the cytoskeleton, through phosphorylation of cytoskeletal components, or through activation of cytoskeletal remodeling proteins such as N-WASP. Emphasizing the importance of epithelial polarization in pathophysiology, the three components of the SCRIBBLE complex, the Scribble, Dlg, and Lgl proteins, are all known tumor suppressors. Their antagonism of oncogenesis in part reflects the importance of the basal and lateral membranes for the prevention of the epithelial-mesenchymal transition, a common feature of metastasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Epithelial layers play critical roles in every bodily process. Inherent to their usefulness is the capacity to create barriers, either between two bodies of fluids, or between an organism and its external environment. Typically, these barriers separate a protein and polysaccharide-rich fluid, termed extracellular matrix material, from a less-viscous fluid. The less-viscous fluid is commonly contained in a hollow tube or duct, the lumen of which is surrounded by the epithelial layer. The pole of the cell facing the extracellular matrix is termed the basolateral compartment, while the pole facing the lumen of the hollow tube or duct is termed the apical compartment. These apical and basolateral compartments of each epithelial cell are biochemically distinct, the distinctions reflecting the specialized functions of each compartment. Indeed, without the apical-basolateral polarization, epithelial cells could not perform any of their extensive array of functions. For example, without specialization of the apical and basolateral membranes, enterocytes of the gut could not vectorially transport nutrients from the lumen of the gut to the lymph and the portal system blood vessels. Even the simple act of creating a barrier would be compromised by a loss of polarization, as the basement membrane, which is created by an interaction of epithelial cells with underlying stromal cells, is essential for mechanical strength of epithelial structures (Morrissey and Sherwood 2015).
The recognized major apical-basolateral polarity boundary of epithelial cells is a junctional complex that includes the principle barrier to paracellular flow between cells of the epithelial layer. In mammals, this complex is formed from apically-located tight junctions and the adherens junctions. The adherens junctions, formed principally from the intercellular interactions of cell adhesion molecules, is positioned immediately basal to the tight junctions (see Fig. 3.1). In Drosophila (Drosophila melanogaster), the arrangement is slightly different, with adherens junctions positioned apically to the septate junctions, the functional equivalent of the tight junction (St Johnston and Ahringer 2010a). In both deuterostomes and protostomes, the boundary is associated with an underlying cortical actomyosin band. The boundary effectively separates apical and basolateral membrane components, although the mechanism of separation is not clear (St Johnston and Ahringer 2010a). Thus, specific delivery of cargo to the membrane on one side of the boundary by the cell’s transport vesicle apparatus can affect the biochemical composition of that membrane compartment, and thereby engender it with specific functions. This mechanism applies equally to conferring specific functions to either the apical or the basolateral compartments of the cell’s membrane.
Despite the enormous importance of apical-basolateral polarization for epithelial function, the molecular programs controlling the formation of the polarization, and stabilizing the polarization through the life of the cell, have only been revealed over the last 30 years. In fact, the major regulators of the program were not initially revealed in studies of epithelia, but rather in studies of other examples of cellular polarization. In particular, it was the use of a powerful genetic model of cellular polarization, the polarization of the Caenorhabditis elegans (C. elegans) one-cell embryo, that revealed several master regulators of polarization, encoded by six Partitioning-defective genes (Par1 to Par6) (Kemphues et al. 1988; Etemad-Moghadam et al. 1995; Watts et al. 2000). Since the initial discovery of the Par genes, it has been recognized that many of the key regulators have been conserved across protostomes and deuterostomes. Accordingly, studies of the mechanisms underlying polarization have extended into mammalian epithelial tissue culture experiments, mouse genetic experiments, and Drosophila genetics. The power of Drosophila genetics, in particular, has contributed to a rapid expansion of our knowledge of the molecular underpinnings of cellular polarization.
Because of numerous studies, three key protein complexes or “modules” have emerged as central to the establishment and maintenance of apical-basolateral polarity in diverse species: the PAR, CRUMBS, and SCRIBBLE complexes (Pieczynski and Margolis 2011). The PAR complex, which is essential for the establishment of the apical pole, is composed of Par3, Par6, and the protein kinase, aPKC (atypical protein kinase C). Par3 and Par6 are linker proteins holding the complex together and mediating interactions with lipids and other proteins. Particularly important are the interactions of the Par complex with Cdc42 (cell division control protein 42), a small, signal transducing GTPase (Nunes de Almeida et al. 2019), and some authors regard it as an integral component of the complex (Pichaud 2018). Much of the polarizing activity of the PAR complex is mediated through the actions of Cdc42 and aPKC on a large array of effector proteins, particularly proteins affecting remodeling of the actomyosin cytoskeleton. The CRUMBS complex, which works with the PAR complex to maintain the apical pole, is comprised of the proteins Crumbs, PALS1 (protein associated with lin-7, also called in various species, stardust, sdt, or MPP-5), and PATJ (Pals1-associated tight junction protein) (Ivanova et al. 2015). While Crumbs is a transmembrane protein, PALS1 and PATJ are cytoplasmic linker proteins (Sen et al. 2012). The last of these three complexes, the SCRIBBLE complex, is essential for maintenance of the basolateral pole and is comprised of the Dlg (discs large), Scribble, and Lgl (lethal giant larvae—the human equivalents are Llgl proteins) proteins, all three of which are large cytoplasmic scaffold proteins. As will be presented in this chapter, evidence is mounting for the importance of a fourth complex, a complex comprised of Cdc42, Par6 and aPKC (Nunes de Almeida et al. 2019).
The nomenclature of polarity proteins varies for human, mouse, and C. elegans and D. melanogaster. For ease of interpretation, in this chapter, wherever we are discussing a protein in a species-specific situation, we will use the species-specific name followed by the generic term. For example, for the Drosophila orthologue of Par3, called Baz or Bazooka, we will refer to as Baz/Par3 throughout the text.
3.2 Major Molecular Determinants of Polarity
Apical-basolateral polarization is a product of the interactions of two systems. One system, comprised of the PAR, CRUMBS and SCRIBBLE complexes, controls the behavior and localization of cytoskeletal elements, particularly the actomyosin circumferential belt associated with adherens junctions. The second system, a system of transport vesicles, targets the insertion of membrane proteins and phospholipids in a polarized fashion, to membrane domains either apical or basolateral relative to the adherens junctions. The interactions of these two systems are complex, although in at least some examples of polarized cells, genetic studies point to the PAR/CRUMBS/SCRIBBLE system, which directly regulates the cytoskeletal structure, as being the predominant system for the establishment of polarity (Nelson 2009). Further complexity is added by uncertainty as to the degree to which apical-basolateral polarization is cell autonomous. It is likely, therefore, that the actual initiating event for apical-basolateral polarization may vary significantly with cell type, development stage, physical location, environmental factors, species, and in the presence of a disease state, pathophysiology (St Johnston and Ahringer 2010a). Indeed, genetic studies have established that the relative importance of particular polarizing factors does vary between tissue types and developmental stages (Franz and Riechmann 2010). However, what is clear is that many of the decision points in apical-basolateral polarity are mutually re-enforcing, co-operating to cement the location of the apical-basolateral boundary. Due to the complexity of the apical-basolateral system, we will first consider the individual properties of the major players before assembling their interactions to describe the polarization process.
3.2.1 The aPKC Protein Kinases
The protein kinase C family is comprised of fifteen members in humans. The family includes well-known components of G-protein-coupled protein receptor (GPCR) and receptor tyrosine kinase (RTK) signal transduction pathways (Newton 2018). In these pathways, the protein kinase C activity is activated by a combination of diacylglycerol (DAG) and calcium ions, both of which are the products of the activation of enzymes of the phospholipase C family. The DAG is a direct product of the action of phospholipase C on the membrane phospholipid, phosphatidylinositol 3,4-bisphosphate (PIP2), while the calcium ions are released from intracellular stores (chiefly in the endoplasmic reticulum), in response to the presence of inositol-3-phosphate, another product of the phospholipase C reaction. However, the prominent PKC family members in epithelial polarization are aPKCs, or atypical PKCs (Hong 2018), which are distinctive in that they require neither DAG, nor calcium for activation. Two of the human PKCs, PKCiota and PKCzeta, fall into this category. The aPKCs are activated through protein-protein interactions. In terms of the apical-basolateral system, Par3, Par6, and Cdc42 are key regulators of aPKC activity. For the most part, the literature reflects a stimulatory role for Cdc42, an inhibitory role for Par3, and Par6 acting as an inhibitor or activator depending upon circumstances (Graybill et al. 2012; Rodriguez et al. 2017). Variability in the effect of these aPKC interactors reflects the fact that the details of apical-basolateral polarization vary depending on tissue type, developmental stage, organism and environmental cues.
A large proportion of apical-basolateral determinant proteins are aPKC substrates, as would be expected of a critical kinase in a complex, multi-faceted process. Among the relevant known substrates of aPKCs are Lgl/Llgl, Par1, Par2, Par3, Par6, and Crumbs (Hong 2018). Lgl/Llgl (and a number of other target proteins that are inhibited by aPKC-mediated phosphorylation) depends upon a highly basic region for functioning (see Fig. 3.2). The basic region promotes the electrostatic interaction of Lgl/Llgl with the negatively-charged inner leaflet of the membranes. A critical aPKC-mediated phosphorylation site on Lgl/Llgl is in the basic domain, well placed to interfere with the electrostatic attractions (Bailey and Prehoda 2015; Hong 2018).
For other proteins inhibited by aPKC, such as the basolateral-specific kinase Par1, the inhibition depends upon the phosphorylation of Par1 creating a binding site for the 14-3-3 protein Par5. Binding by 14-3-3 prevents the migration of active Par1 into the apical domain (Suzuki et al. 2004; Motegi et al. 2011; Jiang et al. 2015; Wu et al. 2016). Regulation of protein activity by phosphorylation-dependent sequestration by 14-3-3 proteins is a well-established mechanism of regulation for a wide range of proteins.
Phosphorylation of Baz/Par3 by aPKC is important for epithelial cell maturation as it inhibits the association of Baz/Par3 with apical complexes, facilitating the movement of Baz/Par3 to the adherens junction. However, at least in Drosophila follicular cells, there are questions as to the actual importance of aPKC-mediated phosphorylation of Baz/Par3. Cells expressing a kinase-dead mutant form of aPKC display normal distribution of Baz/Par3 (Kim et al. 2009).
3.2.2 The Small Signal-Transducing GTPases
Many stages of apical/basolateral polarization are under the control of signal transducing GTPases (often called small, monomeric G-proteins) of the RAS (rat sarcoma virus oncogene) superfamily (Colicelli 2004). Like other signal-transducing GTPases, members of the RAS superfamily are only active when bound to GTP, not GDP. Like other GTPases, these small GTPases function through protein-protein interactions that activate effector proteins.
Activation happens through guanine-nucleotide exchange factors (GEFs), proteins which catalyze the exchange of guanine nucleotides on the small GTPases. As cytoplasmic GTP levels exceed GDP levels, free exchange favors the formation of the active form of the protein. The GTPases are negatively-self-regulating as they possess a GTPase activity that restores the protein to the inactive state. The GTPase activity of these proteins, therefore, is not involved in the activation of effector molecules, but is instead purely regulatory. The endogenous GTPase activity is typically weak in the absence of stimulatory cofactor proteins called GTPase activating proteins (GAPs) (Toma-Fukai and Shimizu 2019). Hence, through balancing the local activities of GEFs and GAPs, the cell can fine tune the level and localization of activity of RAS superfamily of GTPases (Goryachev and Leda 2019).
While the classic small GTPases are the products of the RAS proto-oncogenes, the RAS superfamily members centrally involved in epithelial cell polarization belong to the Rho (RAS homologue) and Rab (RAS genes from the rat brain) families. Rho family members, particularly Rac1 (RAS-related C3 botulinum toxin substrate 1), RhoA (RAS homologue 1), and Cdc42, are key controllers of the cytoskeleton, particularly the actomyosin skeleton (Quiros and Nusrat 2014). Rab family members on the other hand are central to the differential delivery of proteins and lipids to the apical or basolateral membranes, through their targeting of transport vesicles to membranes (Li and Marlin 2015). Members of both families function through attachment to the inner leaflet of the plasma membrane. However, these are large families of signaling proteins and their regulatory mechanisms vary significantly both within and between the families. In both families, members are negatively regulated in the GDP-bound form by GDI proteins (GDP disassociation inhibitors), that localize the GTPases away from the plasma membrane. The GTP-bound, membrane-bound forms are free of inhibitors and able to interact with a wide range of effectors.
Rho family members are well-known regulators of actin cytoskeleton dynamics. In total there are now twenty known Rho family members expressed in mammals (Aspenström 2019). RhoA is known for its role in the formation of focal adhesions and stress fibers, Cdc42 for its role in the formation of filipodia, and Rac1 for its role in the formation of lamellipodia (Ridley 1995). Clearly, these functions are not directly relevant to the role of these proteins in producing the polarization of stable epithelial sheets, where their roles pertain more to mediating phenomena such as positioning the adherens junction, and thereby the apical-basolateral boundary. Nonetheless, some of the biochemical activities used by these proteins to reshape the migrating cell are no doubt relevant to their roles in epithelial cell polarization. Rho family members are often activated in response to extracellular signals. For example, RhoA is regulated by phosphorylation of one of its GEFs, in response to activation of Eph (erythropoietin-producing hepatocellular) RTKs.
The Rho family members act in cell polarity chiefly through dictating the arrangement of the cell’s actomyosin skeleton, contributing to the localization of the cellular junctions. Through the re-arrangement of the cytoskeleton, the Rho family members also impact the delivery of vesicle cargo to target membranes (Croisé et al. 2014). Their effect on the cytoskeleton is mediated through the localization or activation of a wide variety of effector proteins. The number of Rho family effector molecules is indeed large, and clearly an area of on-going study, with some effectors much better understood than others (Watson et al. 2017). Among the better understood families of effectors are the actin cytoskeleton re-modelling proteins of the WASP (Wiskott-Aldrich Syndrome Protein) and formin (limb deformity homology proteins) families, and protein kinases of the PAK (p21 activated kinases), MRCK (myotonic dystrophy kinase related Cdc42-binding kinases), PKC (protein kinase C), and ROCK (Rho-associated protein kinase) families. The WASP proteins are particularly important effectors for Cdc42, which activates WASP proteins through inducing dramatic changes in their conformation (Rohatgi et al. 2000). The conformational changes lead to the localization and activation of the ARP 2/3 (actin-related proteins 2 and 3) complex, an actin nucleator. Because the ARP2/3 complex associates with the lateral surface of existing microfilaments, its actions encourage the arrangement of actin into branched, dendritic structures (Goode and Eck 2007).
A particularly important effect of Rho family members on epithelial polarization is the RhoA-dependent formation of the peri-junctional actomyosin ring associated with the tight and adherens junctions (and thereby, the formation of the apical-basolateral border). The p114RhoGEF is localized to regions closely associated with tight junctions, causing the localized activation of RhoA. In columnar epithelial Caco-2 and HCE epithelial cells, depletion of p114RhoGEF resulted in an altered distribution of ZO-1 (zona occludins protein-1—ZO proteins are cytoplasmic proteins closely associated with the tight junctions), reduced peri-junctional actin microfilaments, and increased formation of stress fibers (Terry et al. 2011). Depletion of p114RhoGEF also resulted in a redistribution of activated RhoA from junctional complexes to more basal locations in the cell and also in the redistribution of myosin II away from points of cell contact (Terry et al. 2011). Consistent with a Rho-mediated activation of Myosin II, artificial overexpression of p114RhoGEF led to a contracted peri-junctional ring (Terry et al. 2011).
A similar story has emerged in Drosophila, where two apically activated Rho-GEFs, Wireless/p114RhoGEF and RhoGEF2, control the formation of actomyosin structures. While Wireless/p114RhoGEF is needed for proper formation of the actomyosin belt associated with adherens junctions, RhoGEF2 is needed for the actomyosin cytoskeleton that is more medially located in the apical domain (Garcia De Las Bayonas et al. 2019; Levayer et al. 2011), which is associated with the formation of structures such as microvilli and cilia.
The Rab family members are specific for particular transport vesicles, and are typically involved in directing those vesicles to fuse with very particular target membranes (Lamber et al. 2019; Bento et al. 2013). There are more than 60 Rab family members, and their association with the cytoplasmic side of vesicles provide specificity to the vesicles, particularly in terms of site of origin. Rab and Rab-effector proteins guide vesicles to their targets. Of particular importance in apical-basolateral polarization are Rab5, Rab7, Rab8 and Rab11. Rab5 and Rab7 are markers of early and late endosomes, respectively. Rab8 functions in the targeting of secretory vesicles from the trans Golgi network to the plasma membrane. Rab11 is a marker of recycling endosomes. Like most RAS superfamily members, Rab activation depends upon associated GEFs. However, some Rab-GEFs play additional roles in the physical targeting of Rabs to the correct organelle or membrane domain (Lamber et al. 2019).
Rab actions require the activities of effector proteins. Many Rab effector proteins are tethering proteins involved in direct physical interactions between Rab-marked vesicles and molecular markers on the target membranes. For example, effector EEA1 (early endosome antigen-1), which is recruited to vesicles by activated Rab5, identifies target membranes by binding to a phospholipid, phosphatidylinositol 3,4,5-triphosphate (PIP3). In an extended conformation, EEA1 can capture a membrane at a distance. Rab5 can then induce conformational changes in EEA1 that cause it to collapse, drawing the two membranes closer together (Murray et al. 2016).
After Rabs and Rab effector proteins initiate the first interactions between a vesicle and a target membrane, SNARE (SNAP receptor) proteins mediate vesicle fusion (Ungermann and Kümmel 2019). SNARES on a vesicle intertwine with SNARES on the target membrane, and catalyze the membrane fusions. During docking, a Rab-GAP protein causes the hydrolysis of GTP, leading to the inactivation of the Rab and its disassociation from the vesicular membrane. While SNARES drive the membrane fusion reaction, Rab effector proteins provide much of the specificity that ensures the correct targeting of vesicles.
The importance of Rab GTPases to the polarization process is highlighted by the actions of Rab8 and Rab11 in positioning components of the PAR complex at the apical surface (Bryant et al. 2010; Colombié et al. 2017). This process can be studied in MDCK (Madin-Darbin Canine Kidney) epithelial cells plated in 3D culture, which undergo a reversal of polarity as aggregates of cells form lumens. Because polarity-reversal requires a transcellular transport of membrane components, the involvement of Rab11 is not surprising—Rab11 has been observed functioning in multiple examples of similar processes. Reduced expression of Rab11 and Rab8 in MDCK cells in 3D culture caused disorganized cell aggregates with multiple lumens (Bryant et al. 2010). Complementation experiments indicated that Rab8 probably acts downstream of Rab11 in the MDCK-3D system, most probably through a GEF-mediated recruitment of active Rab8 to sub-apical Rab11-positive vesicles. The sub-apical vesicles are positioned for fusion with the apical membrane and cargo deposition. In combination, Rab8 and Rab11 target Par3 to the apical membrane, and control the apical activation of Cdc42. The process of reverse polarization of the epithelial cells involves Par3 moving to the new apical membrane, and subsequently to junction sites as the lumen expands (Bryant et al. 2010). The self-re-enforcing nature of polarity determination is emphasized by the dependence of the actions of Rab11 upon Par3 function, indicating a circular inter-regulation between Par3 and the Rab protein (Bryant et al. 2010). A similar process of basal to apical transport of Baz/Par3 has also been observed as an early component of epithelial morphogenesis in Drosophila (McKinley and Harris 2012).
3.2.3 Par3 and Par6: Scaffold Proteins of the PAR Complex
Many of the components of the three master regulator complexes, PAR, CRUMBS, and SCRIBBLE, are scaffold proteins, proteins with many protein-protein interaction domains, that facilitate the juxtaposition of signaling components (see Fig. 3.2). Without doubt, the capability of these proteins to form functional conglomerations is central to the polarizing capabilities of the three complexes. The scaffold proteins of the complexes include Par3, Par6, Crumbs, PALS1, PATJ, Dlg, Scribble and Llgl. Crumbs is distinguished from the others in being a transmembrane protein.
The Par3 protein includes three PDZ (Psd95-Dlg/ZO1) domains, a phosphatidyl-inositol phosphate binding domain, an oligomerization domain (designated CR1), and an aPKC binding domain (Yu et al. 2014). PDZ domains are typically protein-binding domains that specialize in binding the carboxy-terminal tail of other proteins, frequently the carboxy-terminal tail of transmembrane proteins. However, certain PDZ domains can also bind lipids (Wu et al. 2007). The first PDZ domain of Par3 is required for the binding of Par3 to Par6. While the second PDZ domain of Par3 binds a broad array of phosphoinositol phospholipids, including PIP2 and PIP3, the third PDZ domain binds the PIP3 phosphatase, PTEN (phosphatase and tensin homologue) phosphatase (Wu et al. 2007).
Par6 has one PDZ domain, one CRIB domain (Cdc42 and Rac-Interactive Binding motif domain), and one PB1 (Phox and Bem1) domain. The PDZ domain can bind to the C-terminal tail of the transmembrane Crumbs protein, a component of the complex interactions between the two apical complexes. Importantly, binding of activated (i.e., GTP-bound) Cdc42 to the CRIB domain of Par6 stabilizes the interaction of Par6 and Crumbs protein (Whitney et al. 2016). This provides the potential for a regulated physical interaction between the two major apical complexes. Par6 also binds aPKC through the Par6 PB1 domain binding the aPKC PB1 domain (Graybill et al. 2012).
3.2.4 Crumbs and the Scaffold Proteins of the CRUMBS Complex
The CRUMBS complex is composed of the transmembrane protein Crumbs, and two associated cytoplasmic scaffold proteins, PALS1/Sdt/Stardust and PATJ. Crumbs itself is important for the localization of the complex, and other proteins, to the regions of the adherens and tight junctions. PALS1/Sdt/Stardust binds Par6 through conserved amino-terminal regions of PALS1 called ECR1 and ECR2 (evolutionarily conserved regions 1 and 2) (see Wang et al. 2004; Gao and Macara 2004; Koch et al. 2016). PALS1 also has two L27 domains, the first of which is essential for the interaction with PATJ, binding to the L27 domain of PATJ. PALS1 has a single PDZ domain which can bind either the Crumbs protein or the Baz/Par3 protein (Sen et al. 2015). Additionally, PALS1 has GUK (guanylate kinase like) and SH3 (src-homology-3) domains that stabilize the interaction with the Crumbs protein (Li et al. 2014).
As mentioned previously, PATJ has a L27 domain that mediates binding to PALS1. Additionally, depending upon the species, PATJ has ten to thirteen PDZ domains, creating an enormous capacity for complex assembly. Included in its binding partners are the tight-junction associated scaffold protein ZO-3 and nectins (nectin cell adhesion molecules) (Adachi et al. 2009).
3.2.5 The Scaffold Proteins of the SCRIBBLE Complex
The major components of the Scribble complex, Scribble protein, Dlg, and Llgl/Lgl, are large scaffold proteins present throughout the animal kingdom. All three were originally discovered through genetic screens in Drosophila - Drosophila embryos with mutations in the genes encoding any of the three proteins display aberrant, excessive, cellular proliferation (Mechler et al. 1985; Woods and Bryant 1989; Bilder and Perrimon 2000).
Scribble protein includes a leucine-rich repeat domain and four PDZ domains (Caria et al. 2019). While most of Scribble’s protein interactions are mediated by its PDZ domains, the leucine-rich repeats mediate the interaction with Lgl/Llgl (Stephens et al. 2018). Dlg includes one L27 domain, three PDZ domains, an SH3 domain, and a GUK domain. Lgl/Llgl interacts with other proteins through a WD40 repeat region, containing up to fourteen repeats, that potentially forms two seven-bladed WD40 propellers (Cao et al. 2015; Jossin et al. 2017; Stephens et al. 2018). The combined direct interactions of the three Scribble complex proteins comprises of an enormous number of binding partners, with several of significant import to apical/basolateral axis formation. Amongst those with significant roles in the establishment or maintenance of polarity are Rab5, beta-catenin, PTEN, ZO-2 (zona-occludens protein-2, an adaptor protein associated with tight junctions), E-cadherin, Par6, Myosin II, and the t-SNARE, syntaxin-4 (Stephens et al. 2018).
3.2.6 The Phosphatidyl-Phosphoinositols
Localization of the three master modules is partially dictated by the distribution of phosphatidyl phosphoinositols, particularly PIP2 and PIP3 (Martin-Belmonte et al. 2007; Ruch et al. 2017). PIP2 is enriched in the apical membrane, while PIP3 is enriched in the basolateral membrane. The two phospholipids are readily interchangeable, with PI3 kinase creating PIP3 from PIP2, and PTEN phosphatase restoring PIP3 back to PIP2. The polarized distribution of these lipids reflects the localized distributions of PI3 kinase and PTEN phosphatase activities. These localizations of PI3 kinase to the basolateral compartment and PTEN phosphatase to the apical compartment are directed by small GTPases of the Rho family. As phosphatidyl phosphoinositols play roles in the regulation of vesicular transport, this provides a second mechanism, in addition to cytoskeletal remodeling, by which Rho family GTPases may regulate transport (Croisé et al. 2014).
The importance of PIP2 and PIP3 in polarization also provides a mechanism for external signals to impact polarization. PI3 kinase activity is highly regulated by receptor tyrosine kinases (including receptors for many growth factors and insulin), by GPCRs, and by integrins (Guo and Giancotti 2004; Fritsch et al. 2013). The PIP2 at the apical domain can stabilize the location of the PAR module through a bridging molecule, annexin-2, linking the PIP2 to the Cdc-42 component of the PAR module.
3.3 Co-ordination of the Molecular Interactions That Prescribe the Apical-Basolateral Axis
As mentioned previously, the key processes in the establishment and maintenance of apical-basolateral polarity is a competition between two apical protein complexes, the PAR and CRUMBS complexes, and a basolateral complex, SCRIBBLE. While the apical complexes confer appropriate biochemical properties to the apical membrane and specify the location of the tight and adherens junctions, the basolateral complex prevents the invasion of apical proteins and structures into the basolateral domain. The apical-basolateral polarization therefore is the product of a finely-tuned balance between the three complexes.
This chapter is focused upon the segregation of the epithelial cell into apical and basolateral divisions, because of the relevance of this division to channel and transporter biology. However, in reality more sub-divisions of the epithelial cell membrane unquestionably exist, particularly in the region of the junctional complexes (St Johnston and Ahringer 2010a).
3.3.1 Initialization of Axis Formation
It is natural to ask whether one or other of the key modules has primacy, initiating the polarization process and dictating the cellular localization and behavior of the others. As will be outlined in this section, current evidence suggests the processes vary significantly according to species, developmental stage and context.
Central to the question of apical-basolateral axis morphogenesis is the positioning of the boundary between the two domains, corresponding to the positioning of the adherens junctions, and the associated tight or septate junctions. In Drosophila, part of the maturation process for a developing epithelium is the dislocation of Baz/Par3 from apical domains to associate with the intercellular junctions, specifically the adherens junctions (Harris and Peifer 2005; Walther et al. 2016). This dislocation process for Baz/Par3, which is dependent upon aPKC-mediated phosphorylation, is observed in several examples of epithelial morphogenesis in Drosophila, including the development of the follicular epithelium and of the photoreceptors (Walther and Pichaud 2010; Krahn et al. 2010; Morais-de-Sá et al. 2010). This localization of Baz/Par3, which mediates formation and stability of the adherens junctions, positions Baz/Par3 basal to both Crumbs and Par6, and presumably only allows interactions at a border region where their localizations intersect (Bryant et al. 2010; Walther et al. 2016).
In mammalian cells, the localization of Par3 to junctions during epithelial morphogenesis is critical for tight junction formation. At tight junctions, Par3 functions by activating PI3kinase, by spatially restricting PTEN, and by controlling the activity of Tiam1 (a Rac1 GEF protein), and Bcr1 (a Rac1 GTPase activating protein). These proteins function in the polarization of a range of cell types (Narayanan et al. 2013). The Par3 regulation of Rac1, through Tiam1 and Bcr1, is essential for tight junction assembly (Chen and Macara 2005).
However, studies of the developing Drosophila photoreceptor have provided strong genetic evidence for an additional, sufficient pathway for adherens junctions positioning and maintenance. In addition to the pathway dependent upon Baz/Par3 (presumably acting after disassociation from the PAR complex), an alternative pathway dependent upon a Cdc42-dependent kinase (Mbt, mushroom bodies tiny, the Drosophila equivalent of Pak4) is also functional in specifying the adherens junctions localization (Walther et al. 2016). The Mbt/Pak4 functions to stabilize E-cadherin at the junctions through phosphorylation of beta-catenin (Selamat et al. 2015). Both Baz/Par3 and Mbt/Pak4 dependent localizations are dependent upon an apical localization of activated Cdc42.
A recently proposed model of polarization, based on experiments in the Drosophila photoreceptor, suggests a certain level of primacy for Cdc42, at least in this particular tissue. The proposed model begins with an apical localization of Cdc42 that is independent of Par6, Baz, aPKC, or Crumbs (Nunes de Almeida et al. 2019). The apically located Cdc42 is active and acts to recruit PAR complex (that is, Baz/Par3-Par6-aPKC). An exchange occurs in which Par6-aPKC is transferred from the Par complex to Cdc42 to form an alternative apical complex (Cdc42-Par6-aPKC). Subsequently, in tissue morphogenesis, Cdc42 facilitates the transfer of Par6-aPKC to the transmembrane molecule Crumbs, which in turn stabilizes Crumbs at the membrane. This model emphasizes the importance of Cdc42, both as an initiator and as a mediator of many subsequent steps (Nunes de Almeida et al. 2019). This model also invokes Par6-aPKC being shuttled sequentially between three complexes—the classic PAR module, a complex including Cdc42 and a complex involving the Crumbs protein (possibly the CRUMBS complex). The proposition of a Cdc42-Par6-aPKC complex is also supported by the observation of an equivalent complex in C. elegans zygotes, where the distribution of Par and PKC proteins reflect an anterior-posterior polarization (Rodriguez et al. 2017; Wang et al. 2017). The traditional view of discrete PAR and CRUMBS complexes may need to be revisited as the interactions of Crumbs protein, Par3, Par6, aPKC, and Cdc42 increasingly appear fluid and dynamic (Walther et al. 2016; Rodriguez et al. 2017; Pichaud et al. 2019).
How might Cdc42 be localized to the apical membrane, independent of PAR or CRUMBS complexes? One possibility is suggested by a different polarized system, the migrating astrocyte, in which Cdc42 is specifically delivered to the leading edge. Delivery of Cdc42 is mediated by endosomes, and is dependent upon Rab5 (Osmani et al. 2010; Baschieri et al. 2014). Alternatively, apical localization of appropriate GEFs could mediate an apical distribution of activated Cdc42 (Bryant et al. 2010; Zihni et al. 2014). Similarly, localization of Cdc42 GAP activity has been observed to localize activated Cdc42 through localized inactivation (Wells et al. 2006; Anderson et al. 2008; Elbediwy et al. 2012; Beatty et al. 2013; Klompstra et al. 2015). Finally, annexin-2 is thought to cross-link Cdc42 to PIP2, allowing the polarized distribution of phosphatidyl phosphoinositols to contribute to Cdc42 distribution (Pinal et al. 2006; Nunes de Almeida et al. 2019).
While the Drosophila photoreceptor system points to the early importance of Cdc42, two other systems emphasize the early importance of Par3 (or its Drosophila equivalent, Baz). MDCK cells initiating epithelialization reveal the importance of both Par3 and of intercellular interactions. Amongst the earliest events in these cells is the homophilic, intercellular interactions of nectin proteins. Subsequently, Par3 localizes to the nectin junctions through interactions of the first PDZ domain of Par3 with the carboxy-terminal tails of the nectins (Takai et al. 2008). The genesis of the primary epithelium of Drosophila also reflects an early importance for Baz/Par3. However, in this system polarization seems to be much more cell autonomous. The Baz/Par3 protein is delivered to the nascent apical surface by microtubules that are oriented by their attachment to the centrosome, which is situated above the nucleus (Harris and Peifer 2004; McGill et al. 2009). Baz/Par3 subsequently recruits cadherins to form adherens junctions.
The tissue-specific nature of epithelial polarization is also highlighted by the distribution of phosphatidyl phosphoinositols in Drosophila epithelial retinal cells. While in many epithelia, the localization of PIP2 to the apical membrane is critical for normal polarization, in these retinal cells, the apical membranes are enriched for PIP3 (Pinal et al. 2006).
In summary, therefore, current evidence suggests that the initiating events do indeed vary with environment, tissue-type, and developmental stage (Kim et al. 2009; Chen et al. 2018). Variation occurs as to the degree of cell autonomy, as to the requirement for either cell-cell or cell-matrix contacts, and as to the sequential ordering and hierarchy of polarizing factors. However, a consistent feature of most of the studied programs is the restricted localization of components of the PAR complex or its close associate Cdc42 early in polarity formation.
While PAR complex proteins appear to be common features of most, possibly even all examples of polarizing cells, the CRUMBS complex (Crumbs protein with PALS1 and PATJ), is more specifically important to epithelial polarization (St Johnston and Ahringer 2010a). In Drosophila, Crumbs protein is essential for the formation of the apical domain. Over-expression of Crumbs protein in Drosophila epithelial cells leads to an expansion of the apical domain (Wodarz et al. 1995), and its overexpression in MDCK cells disrupts the normal polarization process (Lemmers et al. 2004; Roh et al. 2003). Crumbs protein binds spectrin, connecting the CRUMBS complex to the actomyosin cytoskeleton (Izaddoost et al. 2002). PALS1 on the other hands binds to the tight junction-associated proteins ZO-3 and claudin. Combined with the localization of the CRUMBS complex to the lateral, not central, regions of the apical membrane, these protein associations suggest the complex may be responsible for positioning and stabilizing the tight and adherens junctions (Shin et al. 2005). Supporting this hypothesis, ectopic expression of Crumbs protein in mammalian cells is sufficient to cause the formation of tight junction-like structures in cells that do not normally have them (Fogg et al. 2005).
An important function of Crumbs protein is the exclusion of Baz/Par3 from binding to Par6, which results from competition for binding sites on Par6 with Crumbs protein (Morais-de-Sá et al. 2010). This allows for aPKC to phosphorylate Crumbs protein, which activates it, and also allows for the movement of Baz/Par3 to a location that is slightly more basal (as previously mentioned, a feature of matured, fully polarized epithelia) (St Johnston and Ahringer 2010a). The “less-apically” localized Par3 collaborates with Crumbs protein to stabilize the tight and adherens junctions, through Par3 mediated-localization of active Rac1.
3.3.2 SCRIBBLE Complex Function
SCRIBBLE complex components such as Lgl/Llgl, function in restricting apical factors such as the PAR complex to the apical domain, and antagonize PAR activity (Bilder and Perrimon 2000; Hutterer et al. 2004; Yamanaka et al. 2006). Lgl/Llgl and the basolateral-specific kinase, Par1, both contribute to the exclusion of Par3 and Par6 from the basolateral domain of epithelia and from the posterior domain of Drosophila oocytes (in many ways a molecular parallel to the basolateral domain of epithelia) (Bayraktar et al. 2006; Tian and Deng 2008; Doerflinger et al. 2003, 2006, 2010). Par1 phosphorylates Par3, a phosphorylation that excludes PAR complex from Par1 domains. Reciprocally, Lgl/Llgl is excluded from the apical or anterior domains by a mechanism that is probably dependent upon aPKC phosphorylation (Benton and St Johnston 2003; Plant et al. 2003; Suzuki et al. 2004; Hutterer et al. 2004; Tian and Deng 2008).
As would be expected, the SCRIBBLE complex components not only prevent basolateral spread of PAR and CRUMBS complex components, but are also responsible for localization of basolateral membrane components. In mammals, as is typical for many proteins, several equivalents to Scribble protein are present. In humans, for example, three Scribble proteins are present, human Scribble (Scrib), Erbin and Lano. Knockout studies in DLD1 cells (a human colorectal cancer cell line) indicate that the three proteins complement each other in apical-basolateral polarization (Choi et al. 2019). Basolateral to the adherens junctions in these cells are a second group of adherens junctions, the spot-like adherens junctions (SLAJ). Single knockouts of Scribble family members do not affect the formation of SLAJs, but combined Scrib/Erbin/Lano knockouts result in mis-localization of SLAJs. Consistent with Drosophila data, the triple knockout Scribble family members also affect the cellular distribution of PAR and CRUMBS components, allowing Par6, aPKC, and Pals1 to move basolaterally.
Genetic studies have revealed that SCRIBBLE-independent pathways in Drosophila embryos can drive formation of functioning basolateral domains, including a pathway dependent upon a Crumbs-binding protein, Yurt (Tanentzapf and Tepass 2003; Laprise et al. 2009; Perez-Vale and Peifer 2018). This pathway appears more important in the later stages of development.
3.3.3 The Microtubule Cytoskeleton Directs Cargo to Apical and Basolateral Membranes
Within columnar epithelial cells, the bulk of the microtubules run in an apical-basal orientation, with the positive end oriented towards the basal membrane (Müsch 2004). Most remaining microtubules form two web-like horizontal structures, one adjacent to the apical membrane, the other adjacent to the basal membrane. An important step therefore in the establishment of a differentiated epithelial cell is the redistribution of microtubules away from centrosomes, with most moving towards apically located non-centrosomal MTOCs (microtubule organizing centers).
The orientation and polarity of the apical-basolateral microtubules dictate the polarized delivery of cargo to the apical or basolateral membrane domains, which is essential for their biochemical and functional differentiation. Not surprisingly, therefore, the correct orientation and structure of the microtubules is controlled by the polarity complexes, in both non-epithelial and epithelial systems (Doerflinger et al. 2003, 2010). In Drosophila oogenesis, all normal microtubule polarization is lost in mid-development eggs with mutated forms of Par1 that cannot be phosphorylated by aPKC. Similarly, normal microtubule polarization is also lost in eggs with Baz/Par3 mutations that cannot be phosphorylated by Par1. Epistasis experiments indicated that Par1 acts downstream of Baz/Par3 to direct microtubule organization and polarity (Doerflinger et al. 2010). A critical component of establishing the polarized distribution of microtubules in Drosophila oocytes is the Par1-mediated polarized distribution of a spektraplakin, Short stop (Shot). Shot localizes cortically due to an F-actin-binding activity, and is excluded from regions where Par1 is active. Together with the microtubule binding protein Patronin, Shot forms non-centrosomal MTOCs, anchoring the minus ends of microtubules to the anterior cortex in Drosophila oocytes, and the apical cortex of epithelial cells (Nashchekin et al. 2016). A mammalian homologue of Patronin, CAMSAP3, is required for the apical-basal orientation of microtubules in epithelial cells, suggesting this model of microtubule polarization may be evolutionarily conserved (Toya et al. 2016). The kinase aPKC is also important for the formation of non-centrosomal MTOCs, as in early embryonic epithelia in Drosophila, aPKC is necessary to facilitate a movement of microtubules away from centrosomes to more cortical locations (Harris and Peifer 2007).
3.4 The Horizontal Axis of Polarization
Discussions around epithelial polarization have classically focused on the apical-basolateral axis. However, for many epithelial tissues, cellular polarization within the horizontal plane is also central to function. In most epithelia, the most obvious cytological indicator of horizontal plane polarization is the positioning of cilia or hairs on the apical membrane surface (Devenport 2014). This horizontal polarization, referred to as planar cell polarity (PCP), is central to the function of many epithelial tissues as it regulates the positioning, orientation, rotation, and co-ordination of the cilia, amongst other structures. As PCP is not as functionally important for ion channel and transporter function as apical-basolateral patterning, only a brief overview of the establishment and control of PCP will be provided here (the current state of PCP establishment and maintenance has been provided elsewhere—see Butler and Wallingford 2017; Blair and McNeill 2018; Humphries and Mlodzik 2018). The chief concern here will instead be on the interactions of PCP with the apical-basolateral system.
3.4.1 Establishment of Planar Cell Polarity in Epithelial Sheets
The core components regulating the establishment and maintenance of PCP are transmembrane molecules that are asymmetrically distributed in the horizontal plane, at the apical end of the cell (Devenport 2014). Marking one side of the apical membrane is the transmembrane protein Frizzled (Fz). Marking the opposite end of the apical membrane is the transmembrane protein Van Gough (Vang) (see Fig. 3.3). Both Fz and Vang interact with a third transmembrane protein, Flamingo (Fmi). While Fz/Fmi complexes are concentrated on one surface by interactions with the intracellular proteins Dishevelled (Dsh) and Diego (Dgo), Vang/Fmi complexes are concentrated on the opposite surface by interactions with the intracellular protein prickle (Pk). Although these interactions have largely been discerned through Drosophila genetics, particularly studies of the developing Drosophila wing and epidermis, clear support exists for homologous proteins establishing and maintaining horizontal polarization in many vertebrate systems (Theisen et al. 1994; Usui et al. 1999; Henderson et al. 2018).
Schematic of special relationships of key planar cell polarity (PCP) proteins to each other in a planar polarized epithelium. Schematic view looking down on the apical face of a planar polarized epithelium. Note the separation in the plane of the membrane protein Vang and associated cytoplasmic proteins, from the membrane protein Frizzled and associated proteins. Cilia are displayed in orange. Pk, Prickle; Dsh, Dishevelled; Dgo, Diego
For many purposes, functionality of a field of planar polarized cells requires that all the cells of the field be polarized in the same orientation. Therefore, intercellular interactions between epithelial cells are essential for the co-ordination of polarity across an epithelial field. These intercellular interactions occur at the intercellular junctions, and involve Fmi, Fz, and Vang. Fmi forms intercellular homophilic interactions that facilitate the transmission of the polarity information from one cell of a field to the immediately adjacent cells (Devenport and Fuchs 2008; Vladar et al. 2012; Devenport 2014). The nature of the signaling Fmi, and therefore the cellular asymmetry, is dependent upon the other proteins with which it is complexed.
External factors impinge upon, and where needed, alter the PCP of particular epithelia. These external factors include signaling from the Wnt family of secreted signaling molecules and anisotropic strain across the epithelial sheet (Butler and Wallingford 2017). A second PCP system, based on different molecules from the core PCP system, is now recognized as dominant in certain processes. This second system, the Fat, Dachsous, and Four-Jointed PCP (Ft-Ds-Fj) system, interacts with the original core system through currently unresolved mechanisms (Matakatsu and Blair 2004; Casal et al. 2006; Butler and Wallingford 2017).
3.4.2 Points of Intersection Between the Two Plains of Polarization
The PCP polarization is almost certainly downstream of the apical-basolateral (A-BL) polarization. This is borne out by the numerous examples of genetic lesions causing lost or inappropriate PCP patterning of bristles, hair or cilia, with the structures still forming at the apical end of the epithelial cell (Gubb and García-Bellido 1982). At the molecular level, there are also clear examples of components of the A-BL system affecting the PCP system (Courbard et al. 2009; Djiane et al. 2005). For example, a key mechanism for the regulation of PCP by Wnt5A requires the Par6 protein. Signaling induced by Wnt5A induces the formation of a complex including Par6 that directs the degradation of PK1 (a mouse homologue of Pk) (Narimatsu et al. 2009, Liu et al. 2014).
Curiously, genetic ablation of Par3 in two mammal systems resulted in disruption of PCP, but no overt disruption of A-BL axis formation (Hikita et al. 2018; Malt et al. 2019). One of these two systems is murine endothelial vasculature. Endothelial cells normally align organelles such as the Golgi with the direction of flow, but failed to do so in a Par3 conditional mutant in some regions of the vasculature. Similarly, conditional knockout of Par3 disrupted the planar cell polarity of murine inner ear hair cells. Mutagenesis of Scrib1, a murine homologue of the gene encoding the Scribble protein also interferes with planar cell polarity in the cochlea (Montcouquiol and Kelley 2003). This phenomenon of A-BL determinants assuming PCP functions in mammals is clearly an area for further investigation.
While the impact of A-BL components upon PCP is clear, the reverse is less clear. Nonetheless, the processes controlling the genesis of the two axes clearly converge upon Rho family GTPases as key components of both of their outputs (Strutt et al. 1997; Zihni et al. 2017). For both processes, rearrangements of the same cytoskeletal structures manifest their respective polarizations. Hence, each program must impact the other at a functionally meaningful level.
3.5 Outputs of Polarization
Polarization impacts every aspect of epithelial structure. This section will expand on the importance of polarization to four examples of epithelial cell function—tissue morphogenesis, mitotic spindle orientation, basement membrane formation, and solute transepithelial transport.
3.5.1 Tissue Morphogenesis
Polarization drives many of morphological specializations of epithelial tissues. For instance, polarization drives the formation of a medial, apical actomyosin belt which is necessary for the creation of microvilli in intestinal cells (Schneeberger et al. 2018). However, a more sophisticated level of tissue morphogenesis can occur through coordinated shape changes mediated by the polarization systems acting across an epithelial sheet. Indeed, co-ordination of the polarization systems across cells plays a major role in critical morphogenenic processes, including lumen formation and convergent extension (Navis and Nelson 2016; Huebner and Wallingford 2019). Convergent extension involves the extension of a field of epithelial cells in one plane, while it narrows in a second. While gross cell migration can be involved in convergent extension, the major component of convergent extension seems to be an intercalation of the epithelial cells. The intercalation results from small, polarized movements of cells relative to each other (Huebner and Wallingford 2019). The intercalation allows for tissue elongation, without new cell proliferation. Most famously, convergent extension controls change in the neuroepithelium of the vertebrate central nervous system (CNS), including changes that elongate the CNS and changes that facilitate neural tube closure (Juriloff and Harris 2012).
The necessary coordinated changes in cell shape to achieve convergent extension are largely under the control of components of the PCP pathway. Consistent with the importance of the pathway for convergent extension, one in five human newborns with a very severe neural tube defect (craniorachischisis) have deleterious mutations in components of the PCP pathway (Juriloff and Harris 2012). Similarly, neural tube defects are common features of mice with knock-outs of genes encoding components of the pathway, including mutations in Scrib1, the murine orthologue of Scribble. Once again, this would appear to reflect a vertebrate-specific role for Scribble protein in PCP, rather than its classical role in apical-basolateral axis formation (Juriloff and Harris 2018).
3.5.2 Mitotic Spindle Orientation
A second point of functional interaction between the two planes of polarization is cell division (Ségalen and Bellaïche 2009). Cells in simple epithelia mostly divide in the plane of the epithelial sheet (mitotic spindle aligns in the plane of the epithelial sheet, cytokinesis occurs in the plane of the apical-basolateral axis). However, cells in certain complex epithelia, such as the vertebrate embryonic epidermis, regularly divide in either a planar axis or a perpendicular axis. When the cells divide in a perpendicular axis, the mitotic spindle aligns with the apical-basolateral axis, and cytokinesis is in the same plane as the cell sheet. Planar divisions are typically symmetrical, and can be used for example for the expansion of an epithelial layer’s stem cell population. Perpendicular divisions are typically asymmetrical, and often are used to generate cells committed to differentiation, such as the keratinocytes of the stratum spinosum. Cells committed to mitotic division within a complex epithelial layer, therefore, must make a choice between planar and perpendicular division.
Proper tissue formation clearly depends upon an appropriate relative number of planar and perpendicular divisions. These decisions act through changes in the location of spindle anchoring protein complexes, particularly mInsc/Insc-LGN/Pins-NuMA/Mud complex (Bergstralh et al. 2013; Williams et al. 2014; Saadaoui et al. 2014).
Apical localization of mInsc/Insc-LGN/Pins-NuMA/Mud favors perpendicular division. Apical polarity factors, such as Par3/Baz, recruit mInsc/Insc to the apical pole, which in turn recruits LGN/Pins and NuMA/Mud, forming the complex that anchors spindle astral microtubules to the cell cortex (Williams et al. 2014; Saadaoui et al. 2014, 2017). The actions of polarization factors may integrate with information from cellular architecture or tissue mechanical tension to dictate the orientation of cellular division (Dias Gomes et al. 2019; Box et al. 2019; Li et al. 2019; Finegan and Bergstralh 2019).
Lateral localization of LGN/Pins-NuMA/Mud favors planar cell division. In most tissues, this basal localization is regulated through the localization of the dynein-interacting protein LGN/Pins, which can be achieved by an inhibitory phosphorylation by the apical kinase, aPKC, (Hao et al. 2010) or through interactions with the basolateral determinant Dlg (Bergstralh et al. 2013).
Drosophila genetic evidence also points to a role for the planar cell polarity regulating spindle orientation (Bellaïche et al. 2001a, b, 2004; Ségalen et al. 2010). Similarly, mice defective in Vangl2 (a mouse homologue of the PCP gene encoding Vang) display increased numbers of perpendicular divisions and as a result a hyper-thickened epidermis (Box et al. 2019). However, PCP pathway disruptions may interfere with the spindle orientation indirectly, through effects on tissue architecture (Box et al. 2019).
3.5.3 Formation of the Basement Lamina
Amongst the most important products delivered to the basolateral membrane are the components of the basement membrane, otherwise known as the basement lamina. Epithelial cell sheets attached basally to the basement membrane, and depend heavily on it for strength (Halfter et al. 2015). The predominant proteins of the basal lamina (the component of the basement membrane produced by the epithelial cells) are the Col IV, Laminin, Nidogen, and heparan sulfate proteoglycans. Advances have indicated that the basal membrane is more complicated than once thought, with a polarity of its own. While the epithelial-oriented face is rich in components that make contact with epithelial-presented proteins such as integrins, the stromal-oriented face is rich in components that interact with stroma (Halfter et al. 2015). It is interesting to question whether the integrin-basal lamina interactions represent a positively re-enforcing loop for basolateral membrane identity (O’Brien et al. 2001; Schneider et al. 2006). Integrin signaling has the potential to stimulate PI3kinase, leading to a localized enrichment of PIP3, a critical identifier of the basolateral membrane domain.
A fascinating aspect of basal lamina synthesis can be observed in Drosophila egg chamber morphogenesis—namely that endoplasmic reticulum and the Golgi apparatus are also polarized, in the sense that specific subdomains of each are responsible for the production and sorting of basal lamina proteins (Lerner et al. 2013). This localized translation depends upon the presence of a specialized discontinuous Golgi apparatus (Kondylis and Rabouille 2009). A similar specialized translation zone may act in the polarized distribution of at least some other proteins, as has been described for Wingless protein destined for apical secretion (Wilkie and Davis 2001; Herpers and Rabouille 2004; Bonifacino 2014). The basal lamina components are transported to the basal membrane and exported through a specialized Rab10-dependent vesicular transport designed to handle exceptionally large cargo proteins (Lerner et al. 2013; Devergne et al. 2014).
In many tissues, the presence of the basement membrane re-enforces the existing apical-basolateral axis decision, and may be important for its reinstatement following cell division. In these tissues the basal localization of laminin is probably decisive in determining polarization, as is the basal localization of the integrins that interact with it (Yu et al. 2005).
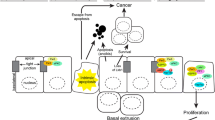
The movement of a cell towards cancer depends upon breaking interactions with the basal extracellular matrix (ECM), as well as disrupting other basal structures such as the desmosome. Hence, basal changes are important components of the epithelial-mesenchymal transition (EMT). Full or partial EMT contribute to most examples of metastasis (Heikenwalder and Lorentzen 2019). The importance of basal interactions in preventing the EMT process may contribute to the tumor suppressive activities of the three components of the basal SCRIBBLE complex. Each of Scribble, Lgl/Llgl, and Dlg were first identified through their inhibition of inappropriate cell proliferation in Drosophila embryos. The expression of human homologues of each of these proteins has been observed to be decreased in certain cancers (Lin et al. 2015). More recently, it has become recognized that Scribble and Dlg proteins are both important targets for HPV E6-mediated degradation, a phenomenon typically associate with proteins that are important tumor suppressors (Nakagawa and Huibregtse 2000, Matsumoto et al., 2006). Hence, the SCRIBBLE complex and its derived structures would appear to be particularly important in the suppression of cancer.
3.5.4 Transepithelial Solute Transport
As stated at the outset of this chapter, solute transport across an epithelial sheet must have the capacity to be vectorial to be effective in most of its functions. Vectorial transport depends upon apical-basolateral axis formation, and a resulting differential localization of pumps, transporters and channels to the apical and basolateral domains. This chapter will not review the specific pathways currently known for the localization of these proteins, which better belongs in chapters devoted to each. In broad overview these proteins are directed to the appropriate membrane subdomains through the interactions of Rab GTPases, myosins (including myosin II and myosin Vc) and other motor proteins, actin microfilaments and microtubules. The combination of these factors indicates several points at which the apical-basolateral complex may affect the transport of carriers, pumps, and channels to specific membrane subdomains. For example, the apical-basolateral axis predetermines the polarity of microtubules, the distribution of microfilaments, and the activity of Rho family effectors of myosin activity such as MLCK. Interested readers are directed to Chap. 5 (this volume) by Heike Fölsch for information about sorting of proteins to the apical or basolateral membranes of epithelial cells.
3.6 Conclusions
It is now increasingly clear that polarization is an aspect of most, possibly all, Eukaryotic cells, regardless of cell type or circumstance (Heikenwalder and Lorentzen 2019). Therefore, the early experiments in C. elegans and D. melanogaster should be viewed as visionary, and have appropriately inspired vibrant research communities. Enormous progress has resulted, but some areas clearly remain to be addressed. In particular, future comparisons of system specialities, such as comparing differences in the details of polarization between different epithelial tissues will deepen further the understanding of the polarization process.
Cells undergoing epithelial meseschymal transitions (EMT) have an apical-basolateral polarization, which morphs into an anterior-posterior patterning following the transition (Heikenwalder and Lorentzen 2019). Thus, the cellular manipulation of polarity is central to the process of becoming migratory, and therefore also central to the process of becoming metastatic. As such, proteins directing apical-basolateral patterning would seem to present potential targets for chemotherapeutic drug development. However, investigations into this field have been relatively limited. In part this reflects questions whether polarity proteins can be used to specifically target cancer cells, avoiding excessive adverse effects. In part, it may also reflect that metastasis in general has gained relatively little attention as a property of cancer to target, compared to other properties such as sustained proliferation (Steeg 2016). However, the potential large number of new targets should encourage pursuit of polarity factors as the basis of new treatments for cancer and other ailments, and some development has occurred for a small number of polarity factors including aPKC (Lin et al. 2018). Interest in polarity proteins as targets should also be spurred by the recent recognition of the role of polarity in genome stability (Dias Gomes et al. 2019).
References
Adachi M, Hamazaki Y, Kobayashi Y, Itoh M, Tsukita S, Furuse M, Tsukita S (2009) Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol Cell Biol 29:2372–2389. https://doi.org/10.1128/MCB.01505-08
Anderson DC, Gill JS, Cinalli RM, Nance J (2008) Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320:1771–1774. https://doi.org/10.1126/science.1156063
Aspenström P (2019) The Intrinsic GDP/GTP exchange activities of Cdc42 and Rac1 are critical determinants for their specific effects on mobilization of the actin filament system. Cells 8:759. https://doi.org/10.3390/cells8070759
Bailey MJ, Prehoda KE (2015) Establishment of Par-polarized cortical domains via phosphoregulated membrane motifs. Dev Cell 35:199–210. https://doi.org/10.1016/j.devcel.2015.09.016
Baschieri F, Confalonieri S, Bertalot G, Di Fiore PP, Dietmaier W, Leist M, Crespo P, Macara IG, Farhan H (2014) Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun 5:4839. https://doi.org/10.1038/ncomms5839
Bayraktar J, Zygmunt D, Carthew RW (2006) Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J Cell Sci 119:711–721. https://doi.org/10.1242/jcs.02789
Beatty A, Morton DG, Kemphues K (2013) PAR-2, LGL-1 and the CDC-42 GAP CHIN-1 act in distinct pathways to maintain polarity in the C. elegans embryo. Development 140:2005–2014. https://doi.org/10.1242/dev.088310
Bellaïche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F (2001a) Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol 3:50–57. https://doi.org/10.1038/35050558
Bellaïche Y, Radovic A, Woods DF, Hough CD, Parmentier ML, O’Kane CJ, Bryant PJ, Schweisguth F (2001b) The partner of inscuteable/discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell 106:355–366. https://doi.org/10.1016/s0092-8674(01)00444-5
Bellaïche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F (2004) The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development 131:469–478. https://doi.org/10.1242/dev.00928
Bento CF, Puri C, Moreau K, Rubinsztein DC (2013) The role of membrane-trafficking small GTPases in the regulation of autophagy. J Cell Sci 126:1059–1069. https://doi.org/10.1242/jcs.123075
Benton R, St Johnston D (2003) Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691–704. https://doi.org/10.1016/s0092-8674(03)00938-3
Bergstralh DT, Haack T, St Johnston D (2013) Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R Soc Lond B Biol Sci 368. https://doi.org/10.1098/rstb.2013.0291
Bilder D, Perrimon N (2000) Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403:676–680. https://doi.org/10.1038/35001108
Blair S, McNeill H (2018) Big roles for Fat cadherins. Curr Opin Cell Biol 51:73–80. https://doi.org/10.1016/j.ceb.2017.11.006
Bonifacino JS (2014) Adaptor proteins involved in polarized sorting. J Cell Biol 204:7–17. https://doi.org/10.1083/jcb.201310021
Box K, Joyce BW, Devenport D (2019) Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. Elife. https://doi.org/10.7554/eLife.47102
Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE (2010) A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12:1035–1045. https://doi.org/10.1038/ncb2106
Butler MT, Wallingford JB (2017) Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 18:375–388. https://doi.org/10.1038/nrm.2017.11
Cao F, Miao Y, Xu K, Liu P (2015) Lethal (2) giant larvae: an indispensable regulator of cell polarity and cancer development. Int J Biol Sci 11:380–389. https://doi.org/10.7150/ijbs.11243
Caria S, Stewart BZ, Jin R, Smith BJ, Humbert PO, Kvansakul M (2019) Structural analysis of phosphorylation-associated interactions of human MCC with Scribble PDZ domains. FEBS J. https://doi.org/10.1111/febs.15002. epub ahead of print
Casal J, Lawrence PA, Struhl G (2006) Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133:4561–4572
Chen X, Macara IG (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7:262–269. https://doi.org/10.1038/ncb1226
Chen J, Sayadian AC, Lowe N, Lovegrove HE, St Johnston D (2018) An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol 16:e3000041. https://doi.org/10.1371/journal.pbio.3000041
Choi J, Troyanovsky RB, Indra I, Mitchell BJ, Troyanovsky SM (2019) Scribble, Erbin, and Lano redundantly regulate epithelial polarity and apical adhesion complex. J Cell Biol 8:2277–2293. https://doi.org/10.1083/jcb.201804201
Colicelli J (2004) Human RAS superfamily proteins and related GTPases. Sci STKE RE13. https://doi.org/10.1126/stke.2502004re13
Colombié N, Choesmel-Cadamuro V, Series J, Emery G, Wang X, Ramel D (2017) Non-autonomous role of Cdc42 in cell-cell communication during collective migration. Dev Biol 423:12–18. https://doi.org/10.1016/j.ydbio.2017.01.018
Courbard JR, Djiane A, Wu J, Mlodzik M (2009) The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev Biol 333:67–77. https://doi.org/10.1016/j.ydbio.2009.06.024
Croisé P, Estay-Ahumada C, Gasman S, Ory S (2014) Rho GTPases, phosphoinositides, and actin: a tripartite framework for efficient vesicular trafficking. Small GTPases 5:e29469. https://doi.org/10.4161/sgtp.29469
Devenport D (2014) The cell biology of planar cell polarity. J Cell Biol 207:171–179. https://doi.org/10.1083/jcb.201408039
Devenport D, Fuchs E (2008) Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol 10:1257–1268. https://doi.org/10.1038/ncb1784
Devergne O, Tsung K, Barcelo G, Schüpbach T (2014) Polarized deposition of basement membrane proteins depends on Phosphatidylinositol synthase and the levels of Phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A 111:7689–7694. https://doi.org/10.1073/pnas.1407351111
Dias Gomes M, Letzian S, Saynisch M, Iden S (2019) Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity. Nat Commun 10:3362. https://doi.org/10.1038/s41467-019-11325-3
Djiane A, Yogev S, Mlodzik M (2005) The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121:621–631. https://doi.org/10.1016/j.cell.2005.03.014
Doerflinger H, Benton R, Shulman JM, St Johnston D (2003) The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development 130:3965–3975. https://doi.org/10.1242/dev.00616
Doerflinger H, Benton R, Torres IL, Zwart MF, St Johnston D (2006) Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr Biol 16:1090–1095. https://doi.org/10.1016/j.cub.2006.04.001
Doerflinger H, Vogt N, Torres IL, Mirouse V, Koch I, Nüsslein-Volhard C, St Johnston D (2010) Bazooka is required for polarisation of the Drosophila anterior-posterior axis. Development 137:1765–1773. https://doi.org/10.1242/dev.045807
Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS (2012) Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol 198:677–693. https://doi.org/10.1083/jcb.201202094
Etemad-Moghadam B, Guo S, Kemphues KJ (1995) Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83:743–752. https://doi.org/10.1016/0092-8674(95)90187
Finegan TM, Bergstralh DT (2019) Division orientation: disentangling shape and mechanical forces. Cell Cycle 18:1187–1198. https://doi.org/10.1080/15384101.2019.1617006
Fogg VC, Liu CJ, Margolis B (2005) Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci 118:2859–2869. https://doi.org/10.1242/jcs.02412
Franz A, Riechmann V (2010) Stepwise polarisation of the Drosophila follicular epithelium. Dev Biol 338:136–147. https://doi.org/10.1016/j.ydbio.2009.11.027
Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J (2013) RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 153:1050–1063. https://doi.org/10.1016/j.cell.2013.04.031
Gao L, Macara IG (2004) Isoforms of the polarity protein par6 have distinct functions. J Biol Chem 279:41557–41562. https://doi.org/10.1074/jbc.M403723200
Garcia De Las Bayonas A, Philippe JM, Lellouch AC, Lecuit T (2019) Distinct RhoGEFs activate apical and junctional contractility under control of G Proteins during epithelial morphogenesis. Curr Biol 29:3370–3385. https://doi.org/10.1016/j.cub.2019.08.017
Goode BL, Eck MJ (2007) Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem 76:593–627. https://doi.org/10.1146/annurev.biochem.75.103004.142647
Goryachev AB, Leda M (2019) Autoactivation of small GTPases by the GEF-effector positive feedback modules. F1000Res pii: F1000 Faculty Rev-1676. https://doi.org/10.12688/f1000research.20003.1
Graybill C, Wee B, Atwood SX, Prehoda KE (2012) Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J Biol Chem 287:21003–20111. https://doi.org/10.1074/jbc.M112.360495
Gubb D, García-Bellido A (1982) A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol 68:37–57
Guo W, Giancotti FG (2004) Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5:816–826. https://doi.org/10.1038/nrm1490
Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, Plodinec M (2015) New concepts in basement membrane biology. FEBS J 282:4466–4479. https://doi.org/10.1111/febs.13495
Hao Y, Du Q, Chen X, Zheng Z, Balsbaugh JL, Maitra S, Shabanowitz J, Hunt DF, Macara IG (2010) Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr Biol 20:1809–1818. https://doi.org/10.1016/j.cub.2010.09.032
Harris TJ, Peifer M (2004) Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol 167:135–147. https://doi.org/10.1083/jcb.200406024
Harris TJ, Peifer M (2005) The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol 170:813–823
Harris TJ, Peifer M (2007) aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell 12:727–738. https://doi.org/10.1016/j.devcel.2007.02.011
Heikenwalder M, Lorentzen A (2019) The role of polarisation of circulating tumour cells in cancer metastasis. Cell Mol Life Sci 76:3765–3781. https://doi.org/10.1007/s00018-019-03169-3
Henderson DJ, Long DA, Dean CH (2018) Planar cell polarity in organ formation. Curr Opin Cell Biol 55:96–103. https://doi.org/10.1016/j.ceb.2018.06.011
Herpers B, Rabouille C (2004) mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol Biol Cell 15:5306–5317. https://doi.org/10.1091/mbc.e04-05-0398
Hikita T, Mirzapourshafiyi F, Barbacena P, Riddell M, Pasha A, Li M, Kawamura T, Brandes RP, Hirose T, Ohno S, Gerhardt H, Matsuda M, Franco CA, Nakayama M (2018) PAR-3 controls endothelial planar polarity and vascular inflammation under laminar flow. EMBO Rep 19:e45253. https://doi.org/10.15252/embr.201745253
Hong Y (2018) aPKC: the kinase that phosphorylates cell polarity. F1000Res 7 F1000 Faculty Rev-903. https://doi.org/10.12688/f1000research.14427.1
Huebner RJ, Wallingford JB (2019) Coming to consensus: a unifying model emerges for convergent extension. Dev Cell Vol 48:126. https://doi.org/10.1016/j.devcel.2018.12.006
Humphries AC, Mlodzik M (2018) From instruction to output: Wnt/PCP signaling in development and cancer. Curr Opin Cell Biol 51:110–116. https://doi.org/10.1016/j.ceb.2017.12.005
Hutterer A, Betschinger J, Petronczki M, Knoblich JA (2004) Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell 6:845–854. https://doi.org/10.1016/j.devcel.2004.05.003
Ivanova ME, Fletcher GC, O’Reilly N, Purkiss AG, Thompson BJ, McDonald NQ (2015) Structures of the human Pals1 PDZ domain with and without ligand suggest gated access of Crb to the PDZ peptide-binding groove. Acta Crystallogr D Biol Crystallogr 71:555–564. https://doi.org/10.1107/S139900471402776X
Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW (2002) Drosophila crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416:178–183. https://doi.org/10.1091/mbc.e03-04-0235
Jiang T, McKinley RF, McGill MA, Angers S, Harris TJ (2015) A Par-1-Par-3-centrosome cell polarity pathway and its tuning for isotropic cell adhesion. Curr Biol 25:2701–2708. https://doi.org/10.1016/j.cub.2015.08.063
Jossin Y, Lee M, Klezovitch O, Kon E, Cossard A, Lien WH, Fernandez TE, Cooper JA, Vasioukhin V (2017) Llgl1 connects cell polarity with cell-cell adhesion in embryonic neural stem cells. Dev Cell 41:481–495. https://doi.org/10.1016/j.devcel.2017.05.002
Juriloff DM, Harris MJ (2012) A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol 94:824–840. https://doi.org/10.1002/bdra.23079
Juriloff DM, Harris MJ (2018) Insights into the etiology of mammalian neural tube closure Defects from developmental, genetic and evolutionary studies. J Dev Biol 6:pii: E22. https://doi.org/10.3390/jdb6030022
Kemphues KJ, Priess JR, Morton DG, Cheng N (1988) Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52:311–320. https://doi.org/10.1016/s0092-8674(88)80024-2
Kim S, Gailite I, Moussian B, Luschnig S, Goette M, Fricke K, Honemann-Capito M, Grubmüller H, Wodarz A (2009) Kinase-activity-independent functions of atypical protein kinase C in Drosophila. J Cell Sci 122:3759–1771. https://doi.org/10.1242/jcs.052514
Klompstra D, Anderson DC, Yeh JY, Zilberman Y, Nance J (2015) An instructive role for C. elegans E-cadherin in translating cell contact cues into cortical polarity. Nat Cell Biol 17:726–735. https://doi.org/10.1038/ncb3168
Koch L, Feicht S, Sun R, Sen A, Krahn MP (2016) Domain-specific functions of Stardust in Drosophila embryonic development. R Soc Open Sci 3:160776. https://doi.org/10.1098/rsos.160776
Kondylis V, Rabouille C (2009) The Golgi apparatus: lessons from Drosophila. FEBS Lett 583:3827–3838. https://doi.org/10.1016/j.febslet.2009.09.048
Krahn MP, Bückers J, Kastrup L, Wodarz A (2010) Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J Cell Biol 190:751–760. https://doi.org/10.1083/jcb.201006029
Lamber EP, Siedenburg AC, Barr FA (2019) Rab regulation by GEFs and GAPs during membrane traffic. Curr Opin Cell Biol 59:34–39. https://doi.org/10.1016/j.ceb.2019.03.004
Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U (2009) Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 459:1141–1145. https://doi.org/10.1038/nature08067
Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Médina E, Arsanto JP, Le Bivic A (2004) CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15:1324–1333. https://doi.org/10.1091/mbc.e03-04-0235
Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S (2013) A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell 24:159–168. https://doi.org/10.1016/j.devcel.2012.12.005
Levayer R, Pelissier-Monier A, Lecuit T (2011) Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol 13:529–540. https://doi.org/10.1038/ncb2224
Li G, Marlin MC (2015) Rab family of GTPases. Methods Mol Biol 1298:1–15. https://doi.org/10.1007/978-1-4939-2569-8_1
Li Y, Wei Z, Yan Y, Wan Q, Du Q, Zhang M (2014) Structure of Crumbs tail in complex with the PALS1 PDZ-SH3-GK tandem reveals a highly specific assembly mechanism for the apical Crumbs complex. Proc Natl Acad Sci U S A 111:17444–17449. https://doi.org/10.1073/pnas.1416515111
Li J, Cheng L, Jiang H (2019) Cell shape and intercellular adhesion regulate mitotic spindle orientation. Mol Biol Cell 30:2458–2468. https://doi.org/10.1091/mbc.E19-04-0227
Lin WH, Asmann YW, Anastasiadis PZ (2015) Expression of polarity genes in human cancer. Cancer Inform 14(Suppl 3):15–28. https://doi.org/10.4137/CIN.S18964
Lin CM, Titchenell PM, Keil JM, Garcia-Ocaña A, Bolinger MT, Abcouwer SF, Antonetti DA (2018) Inhibition of atypical protein kinase C reduces inflammation-induced retinal vascular permeability. Am J Pathol 188:2392–2405. https://doi.org/10.1016/j.ajpath.2018.06.020
Liu C, Lin C, Gao C, May-Simera H, Swaroop A, Li T (2014) Null and hypomorph Prickle1 alleles in mice phenocopy human Robinow syndrome and disrupt signaling downstream of Wnt5a. Biol Open 3:861–870. https://doi.org/10.1242/bio.20148375
Malt AL, Dailey Z, Holbrook-Rasmussen J, Zheng Y, Hogan A, Du Q, Lu X (2019) Par3 is essential for the establishment of planar cell polarity of inner ear hair cells. Proc Natl Acad Sci U S A 116:4999–5008. https://doi.org/10.1073/pnas.1816333116
Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128:383–397. https://doi.org/10.1016/j.cell.2006.11.051
Matakatsu H, Blair SS (2004) Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development 131:3785–3794. https://doi.org/10.1242/dev.01254
Matsumoto Y, Nakagawa S, Yano T, Takizawa S, Nagasaka K, Nakagawa K, Minaguchi T, Wada O, Ooishi H, Matsumoto K, Yasugi T, Kanda T, Huibregtse JM, Taketani Y (2006) Involvement of a cellular ubiquitin-protein ligase E6AP in the ubiquitin-mediated degradation of extensive substrates of high-risk human papillomavirus E6. J Med Virol 78:501–507. https://doi.org/10.1002/jmv.20568
McGill MA, McKinley RF, Harris TJ (2009) Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol 185:787–796. https://doi.org/10.1083/jcb.200812146
McKinley RF, Harris TJ (2012) Displacement of basolateral Bazooka/PAR-3 by regulated transport and dispersion during epithelial polarization in Drosophila. Mol Biol Cell 23:4465–4471. https://doi.org/10.1091/mbc.E12-09-0655
Mechler BM, McGinnis W, Gehring WJ (1985) Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J 4:1551–1557
Montcouquiol M, Kelley MW (2003) Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci 23:9469–9478. https://doi.org/10.1523/JNEUROSCI.23-28-09469.2003
Morais-de-Sá E, Mirouse V, St Johnston D (2010) aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 143:509–523. https://doi.org/10.1016/j.cell.2010.02.040
Morrissey MA, Sherwood DR (2015) An active role for basement membrane assembly and modification in tissue sculpting. J Cell Sci 128:1661–1668. https://doi.org/10.1242/jcs.168021
Motegi F, Zonies S, Hao Y, Cuenca AA, Griffin E, Seydoux G (2011) Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat Cell Biol 13:1361–1367. https://doi.org/10.1038/ncb2354
Murray DH, Jahnel M, Lauer J, Avellaneda MJ, Brouilly N, Cezanne A, Morales-Navarrete H, Perini ED, Ferguson C, Lupas AN, Kalaidzidis Y, Parton RG, Grill SW, Zerial M (2016) An endosomal tether undergoes an entropic collapse to bring vesicles together. Nature 537:107–111. https://doi.org/10.1038/nature19326
Müsch A (2004) Microtubule organization and function in epithelial cells. Traffic 5:1–9
Nakagawa S, Huibregtse JM (2000) Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol 20:8244–8253. https://doi.org/10.1128/mcb.20.21.8244-8253.2000
Narayanan AS, Reyes SB, Um K, McCarty JH, Tolias KF (2013) The Rac-GAP Bcr is a novel regulator of the Par complex that controls cell polarity. Mol Biol Cell 24:3857–3868. https://doi.org/10.1091/mbc.E13-06-0333
Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL (2009) Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137:295–307. https://doi.org/10.1016/j.cell.2009.02.025
Nashchekin D, Fernandes AR, St Johnston D (2016) Patronin/Shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev Cell 38:61–72. https://doi.org/10.1016/j.devcel.2016.06.010
Navis A, Nelson CM (2016) Pulling together: tissue-generated forces that drive lumen morphogenesis. Semin Cell Dev Biol 55:139–147. https://doi.org/10.1016/j.semcdb.2016.01.002
Nelson WJ (2009) Remodeling epithelial cell organization: transitions between front–rear and apical–basal polarity. Cold Spring Harb Perspect Biol 1:a000513. https://doi.org/10.1101/cshperspect.a000513
Newton AC (2018) Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol 53:208–230. https://doi.org/10.1080/10409238.2018.1442408
Nunes de Almeida F, Walther RF, Pressé MT, Vlassaks E, Pichaud F (2019) Cdc42 defines apical identity and regulates epithelial morphogenesis by promoting apical recruitment of Par6-aPKC and Crumbs. Development 146:dev175497 https://doi.org/10.1242/dev.175497
O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE (2001) Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3:831–838. https://doi.org/10.1038/ncb0901-831
Osmani N, Peglion F, Chavrier P, Etienne-Manneville S (2010) Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 191:1261–1269. https://doi.org/10.1083/jcb.201003091
Perez-Vale KZ, Peifer M (2018) Modulating apical-basal polarity by building and deconstructing a Yurt. J Cell Biol 217:3772–3773. https://doi.org/10.1083/jcb.201810059
Pichaud F (2018) PAR-complex and crumbs function during photoreceptor morphogenesis and retinal degeneration. Front Cell Neurosci 12:90. https://doi.org/10.3389/fncel.2018.00090
Pichaud F, Walther RF, Nunes de Almeida F (2019) Regulation of Cdc42 and its effectors in epithelial morphogenesis. J Cell Sci 132 pii: jcs217869. https://doi.org/10.1242/jcs.217869
Pieczynski J, Margolis B (2011) Protein complexes that control renal epithelial polarity. Am J Physiol Renal Physiol 300:F589–F601. https://doi.org/10.1152/ajprenal.00615.2010
Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F (2006) Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol 16:140–149
Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T (2003) A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 5:301–308. https://doi.org/10.1038/ncb948
Quiros M, Nusrat A (2014) RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin Cell Dev Biol 36:194–203. https://doi.org/10.1016/j.semcdb.2014.09.003
Ridley AJ (1995) Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev 5:24–30. https://doi.org/10.1016/s0959-437x(95)90049-7
Rodriguez J, Peglion F, Martin J, Hubatsch L, Reich J, Hirani N, Gubieda AG, Roffey J, Fernandes AR, St Johnston D, Ahringer J, Goehring NW (2017) aPKC cycles between functionally distinct PAR protein assemblies to drive cell polarity. Dev Cell 42:400–415. https://doi.org/10.1016/j.devcel.2017.07.007
Roh MH, Fan S, Liu C, Margolis B (2003) The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci 116:2895–2906. https://doi.org/10.1242/jcs.00500
Rohatgi R, Ho HY, Kirschner MW (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 150:1299–1310. https://doi.org/10.1083/jcb.150.6.1299
Ruch TR, Bryant DM, Mostov KE, Engel JN (2017) Par3 integrates Tiam1 and phosphatidylinositol 3-kinase signaling to change apical membrane identity. Mol Biol Cell 28:252–260. https://doi.org/10.1091/mbc.E16-07-0541
Saadaoui M, Machicoane M, di Pietro F, Etoc F, Echard A, Morin X (2014) Dlg1 controls planar spindle orientation in the neuroepithelium through direct interaction with LGN. J Cell Biol 206:707–717. https://doi.org/10.1083/jcb.201405060
Saadaoui M, Konno D, Loulier K, Goiame R, Jadhav V, Mapelli M, Matsuzaki F, Morin X (2017) Loss of the canonical spindle orientation function in the Pins/LGN homolog AGS3. EMBO Rep 18:1509–1520. https://doi.org/10.15252/embr.201643048
Schneeberger K, Roth S, Nieuwenhuis EES, Middendorp S (2018) Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease. Dis Model Mech 11 pii: dmm031088. https://doi.org/10.1242/dmm.031088
Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S (2006) Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development 133:3805–3815. https://doi.org/10.1242/dev.02549
Ségalen M, Bellaïche Y (2009) Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 20:972–977. https://doi.org/10.1016/j.semcdb.2009.03.018
Ségalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, Doe CQ, Bellaïche Y (2010) The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell 19:740–752. https://doi.org/10.1016/j.devcel.2010.10.004
Selamat W, Tay PL, Baskaran Y, Manser E (2015) The Cdc42 effector kinase PAK4 localizes to cell-cell junctions and contributes to establishing cell polarity. PLoS One 10:e0129634. https://doi.org/10.1371/journal.pone.0129634
Sen A, Nagy-Zsvér-Vadas Z, Krahn MP (2012) Drosophila PATJ supports adherens junction stability by modulating Myosin light chain activity. J Cell Biol 199:685–698. https://doi.org/10.1083/jcb.201206064
Sen A, Sun R, Krahn MP (2015) Localization and function of Pals1-associated tight junction protein in Drosophila is regulated by two distinct apical complexes. J Biol Chem 290:13224–13233. https://doi.org/10.1074/jbc.M114.629014
Shin K, Straight S, Margolis B (2005) PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol 168:705–711
Steeg PS (2016) Targeting metastasis. Nat Rev Cancer 16:201–218. https://doi.org/10.1038/nrc.2016.5
Stephens R, Lim K, Portela M, Kvansakul M, Humbert PO, Richardson HE (2018) The scribble cell polarity module in the regulation of cell signaling in tissue development and tumorigenesis. J Mol Biol 430:3585–3612. https://doi.org/10.1016/j.jmb.2018.01.011
St Johnston D, Ahringer J (2010a) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141:757–774. https://doi.org/10.1016/j.cell.2010.05.011
St Johnston D, Ahringer J (2010b) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141:757–774. https://doi.org/10.1016/j.cell.2010.05.011
Strutt DI, Weber U, Mlodzik M (1997) The role of RhoA in tissue polarity and Frizzled signalling. Nature 387:292–295. https://doi.org/10.1038/387292a0
Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S (2004) aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol 14:1425–1435. https://doi.org/10.1016/j.cub.2004.08.021
Takai Y, Ikeda W, Ogita H, Rikitake Y (2008) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342. https://doi.org/10.1146/annurev.cellbio.24.110707.175339
Tanentzapf G, Tepass U (2003) Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol 5:46–52. https://doi.org/10.1038/ncb896
Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K (2011) Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 13:159–166. https://doi.org/10.1038/ncb2156
Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL (1994) Dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 120:347–360
Tian AG, Deng WM (2008) Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development 135:463–471. https://doi.org/10.1242/dev.016253
Toma-Fukai S, Shimizu T (2019) Structural insights into the regulation mechanism of small GTPases by GEFs. Molecules 24:3308. https://doi.org/10.3390/molecules24183308
Toya M, Kobayashi S, Kawasaki M, Shioi G, Kaneko M, Ishiuchi T, Misaki K, Meng W, Takeichi M (2016) CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc Natl Acad Sci U S A 113:332–337. https://doi.org/10.1073/pnas.1520638113
Ungermann C, Kümmel D (2019) Structure of membrane tethers and their role in fusion. Traffic 20:479–490. https://doi.org/10.1111/tra.12655
Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98:585–595. https://doi.org/10.1016/s0092-8674(00)80046-x
Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD (2012) Microtubules enable the planar cell polarity of airway cilia. Curr Biol 22:2203–2212. https://doi.org/10.1016/j.cub.2012.09.046
Walther RF, Pichaud F (2010) Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol 20:1065–1074. https://doi.org/10.1016/j.cub.2010.04.049
Walther RF, Nunes de Almeida F, Vlassaks E, Burden JJ, Pichaud F (2016) Pak4 is required during epithelial polarity remodeling through regulating AJ stability and Bazooka Retention at the ZA. Cell Rep 15:45–53. https://doi.org/10.1016/j.celrep.2016.03.014
Wang Q, Hurd TW, Margolis B (2004) Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J Biol Chem 279:30715–30721. https://doi.org/10.1074/jbc.M401930200
Wang SC, Low TYF, Nishimura Y, Gole L, Yu W, Motegi F (2017) Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization. Nat Cell Biol 19:988–995. https://doi.org/10.1038/ncb3577
Watson JR, Owen D, Mott HR (2017) Cdc42 in actin dynamics: an ordered pathway governed by complex equilibria and directional effector handover. Small GTPases 8:237–244. https://doi.org/10.1080/21541248.2016.1215657
Watts JL, Morton DG, Bestman J, Kemphues KJ (2000) The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127:1467–1475
Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T (2006) A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125:535–548. https://doi.org/10.1016/j.cell.2006.02.045
Whitney DS, Peterson FC, Kittell AW, Egner JM, Prehoda KE, Volkman BF (2016) Binding of crumbs to the Par-6 CRIB-PDZ module is regulated by Cdc42. Biochemistry 55:1455–1461. https://doi.org/10.1021/acs.biochem.5b01342
Wilkie GS, Davis I (2001) Drosophila wingless and pair-rule transcripts localize apically by dynein-mediated transport of RNA particles. Cell 105:209–219. https://doi.org/10.1016/s0092-8674(01)00312-9
Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, Fuchs E (2014) Par3-mInsc and Gαi3 cooperate to promote oriented epidermal cell divisions through LGN. Nat Cell Biol 16:758–769. https://doi.org/10.1038/ncb3001
Wodarz A, Hinz U, Engelbert M, Knust E (1995) Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 14:67–76. https://doi.org/10.1016/0092-8674(95)90053-5
Woods DF, Bryant PJ (1989) Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev Biol 134:222–235. https://doi.org/10.1016/0012-1606(89)90092-4
Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M (2007) PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28:886–898. https://doi.org/10.1016/j.molcel.2007.10.028
Wu JC, Espiritu EB, Rose LS (2016) The 14-3-3 protein PAR-5 regulates the asymmetric localization of the LET-99 spindle positioning protein. Dev Biol 412:288–297. https://doi.org/10.1016/j.ydbio.2016.02.020
Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S (2006) Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119:2107–2118. https://doi.org/10.1242/jcs.02938
Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM (2005) Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 16:433–445. https://doi.org/10.1091/mbc.e04-05-0435
Yu CG, Tonikian R, Felsensteiner C, Jhingree JR, Desveaux D, Sidhu SS, Harris TJ (2014) Peptide binding properties of the three PDZ domains of Bazooka (Drosophila Par-3). PLoS One 9:e86412. https://doi.org/10.1371/journal.pone.0086412
Zihni C, Munro PM, Elbediwy A, Keep NH, Terry SJ, Harris J, Balda MS, Matter K (2014) Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell Biol 204:111–127. https://doi.org/10.1083/jcb.201304064
Zihni C, Vlassaks E, Terry S, Carlton J, Leung TKC, Olson M, Pichaud F, Balda MS, Matter K (2017) An apical MRCK-driven morphogenetic pathway controls epithelial polarity. Nat Cell Biol 19:1049–1060. https://doi.org/10.1038/ncb3592
Acknowledgements
I would like to thank Dr. Dee Silverthorn and Dr. Kirk Hamilton for their helpful suggestions and editing of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The American Physiological Society
About this chapter
Cite this chapter
Bergemann, A.D. (2020). Establishment and Maintenance of Epithelial Polarization. In: Hamilton, K.L., Devor, D.C. (eds) Basic Epithelial Ion Transport Principles and Function. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-52780-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-52780-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52779-2
Online ISBN: 978-3-030-52780-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)