Abstract
Benzodiazepines are one of the most commonly used sedatives in the pediatric intensive care units for intubation, status epilepticus, and muscle relaxation and now less rarely used for tolerance of mechanical ventilation due to concerns about delirium. This chapter discusses the basic mechanism of action, uses, dosing, and drug interactions of commonly used benzodiazepines in the PICU.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Overview of Benzodiazepines and Mechanism of Action
Benzodiazepines are the most commonly used sedatives in the pediatric intensive care units [1]. In the pediatric and adult intensive care units, benzodiazepines are used for variety of indications such as anxiolysis, sedation, anterograde amnestic effects, seizures, and to treat alcohol withdrawal syndrome.
Benzodiazepines have specific action at gamma-aminobutyric acid (GABA) receptors . The mechanism of action of this sedative agonist is to improve GABAergic transmission. To better understand the mechanism of action of benzodiazepine, it helps to understand gamma-aminobutyric acid (GABA) neurotransmitter.
GABA is the most widely distributed inhibitory neurotransmitters in the central nervous system (CNS) and limits the excitability of neuronal activity in all areas of the brain. Increasing GABAergic activity results in sedation, amnesia, and ataxia, whereas a decrease in the GABAergic signal results in arousal, anxiety, restlessness, and insomnia [2]. The GABA receptors are widely distributed and utilized throughout the central nervous system and have two subtypes, GABAA and GABAB. GABA functions as the major inhibitory neurotransmitter and controls the excitability of neurons by binding to the GABAA receptor. The GABAA receptor is the major molecular target for the action of many drugs in the brain including the benzodiazepines. The binding promotes an increased influx of chloride ions hyperpolarizing the cell membrane and preventing the generation of an action potential. This effect leads to a minor communication between neurons and, therefore, has a calming effect on many of the functions of the brain [3]. Figure 5.1 shows the GABAA receptor, which is further broken down into several subtypes two α1 subunits, two β2 subunits, and one γ2 subunit. The receptor also had a single binding site for the benzodiazepines.
Gamma-aminobutyric acid (GABA) receptor . (Source: Bertram G, Katzung, Anthony J. Trevor: Basic & clinical pharmacology, 14th ed. www.academia.edu. Copyright © McGraw-Hill Education. All rights reserved)
Benzodiazepines are allosteric modulators that require GABA to be bound to its receptor. When benzodiazepines bind to the benzodiazepine receptor on a GABA receptor, they do not stimulate it directly. Instead, it increases the frequency with which the chlorine channel opens when GABA binds its receptor resulting in an increase amount of chloride ions in the postsynaptic neuron that immediately hyperpolarizes this neuron and decreases the excitability. Benzodiazepine’s advantage compared to other drugs, i.e., barbiturates, is it acts in the same receptor and decreases the activity of neurons [2,3,4,5,6,7,8]. Benzodiazepines are the only drugs that give GABA more affinity for its receptor and act as an allosteric modulator. They do not provide a higher activation of GABA itself. This results in less toxicity with the benzodiazepines. Sedation, anterograde amnesia, and anticonvulsant activity are promoted through α1 receptors, whereas anxiolytic and muscle relaxation are promoted by the α2 GABAA receptor (Table 5.1). In addition to their action on the central nervous system, benzodiazepines have a dose-dependent ventilatory depressant effect, and they also cause a modest reduction in arterial blood pressure and an increase in pulse rate as a result of a decrease of systemic peripheral resistance [1]. It is through this mechanism that sedation, hypnosis, muscle relaxation, anxiolytic, anterograde amnesia, and anticonvulsant effects occur. The most commonly used benzodiazepines for sedation in the pediatric intensive care are midazolam, lorazepam, and diazepam [4].
Pharmacokinetic and Pharmacodynamic Considerations
All benzodiazepines enhance the binding of gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter, to the GABA-A subtype of GABA receptors, resulting in GABAergic neurotransmission. All the agents in the benzodiazepine drug class have similar clinical effects. However, they differ in their pharmacokinetic profiles, such as rate of absorption, elimination half-life, and onset and duration of action. Details of benzodiazepine pharmacodynamics and pharmacokinetics are provided in Tables 5.2 and 5.3. [18].
Benzodiazepines undergo Phase I and Phase II metabolic pathways: hepatic oxidation and reduction by cytochrome P450 and glucuronide conjugation. Alprazolam, midazolam, and diazepam undergo hydroxylation, while clonazepam undergoes nitroreduction [5].
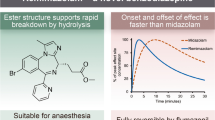
Lorazepam displays low hepatic metabolism and does not have active metabolites. Diazepam is rapidly absorbed and has the most rapid onset of action but also the greatest abuse/dependence potential. Diazepam is considered a long-acting benzodiazepine and associated with accumulation, which may result in sedation, cognitive impairment, and psychomotor retardation. Lorazepam and midazolam are considered short-acting benzodiazepine agents. The search for a water-soluble benzodiazepine with clinical properties similar to diazepam but without its potential for venous irritation led to the development of midazolam [6].
Midazolam is the most commonly used benzodiazepine in pediatric anesthesia. It is administered orally, nasally, and rectally as well as intravenously and intramuscularly. When administered midazolam causes anterograde amnesia, sedation, and anxiolysis. Midazolam is a water-soluble benzodiazepine that has various clinical advantages over diazepam. It is not painful when administered intravenously or intramuscularly. Midazolam is FDA approved as premedication in children and is the only benzodiazepine approved by the FDA for use in neonates. Its clearance in adults (1.8–6.4 hours) is reduced compared with children (1.4–4.0 hours). Midazolam clearance is reduced even more in neonates and preterm infants compared to toddlers and older children (6–12 hours). The elimination half-life is less in preterm infants less than 32 weeks’ gestational age [7, 8]. Any factor that impairs hepatic blood flow (e.g., cardiac surgery with bypass compared with cardiac surgery without bypass) may decrease elimination including hypovolemic states and patients receiving vasopressors. Midazolam has the best pharmacokinetic profile for neonates compared to other benzodiazepines because the active metabolite exhibits minimal clinical activity and has a half-life similar to the parent compound. Midazolam has been administered as a continuous infusion both in the operating room and in the intensive care unit. The depth of sedation correlates with plasma concentrations of midazolam. Prolonged use does lead to tolerance, dependency, and benzodiazepine withdrawal [9]. Long-term infusions (i.e., ≥5 days) should be tapered over days while carefully monitoring for signs of withdrawal [10]. Benzyl alcohol toxicity is a theoretical concern associated with midazolam that can cause metabolic acidosis and gasping syndrome in neonates and infants. Toxicity should not occur when midazolam is given according to the recommended dosing guidelines. Cytochrome P450 3A4 metabolizes midazolam into an active metabolite. Therefore, midazolam will interact with those drugs/foods that are CYP 3A4 inhibitors like grapefruit juice, erythromycin, calcium channel blockers, and protease inhibitors. The interaction causes prolonged duration of action of midazolam [11].
Lorazepam was approved by the FDA in September 1977 and is a benzodiazepine with sedative and antianxiety effects. It is administered orally, intramuscularly, or intravenously. Lorazepam, like diazepam, is virtually insoluble in water. Peak plasma levels of lorazepam are seen in 60–90 minutes. Lorazepam has a slower onset of action and longer duration of action compared to the other benzodiazepines. When administered IV, lorazepam produces little or no clinical effect for about 5 minutes, with its maximum effect occurring approximately 20 minutes after administration. The duration of action of lorazepam following IM administration is approximately 6–8 hours. The major side effect is excessive sleepiness and a prolonged amnesic period. Because lorazepam is dissolved in propylene glycol, it can accumulate to produce metabolic acidosis and renal dysfunction [11]. The amnesic properties of lorazepam are impressive and include both anterograde and a degree of retrograde amnesia. Lack of recall is maximal approximately 15–20 minutes after IV administration and may include events occurring throughout the treatment day [6, 12].
Diazepam has been used extensively as a premedication, adjunct to anesthesia, for sedation, amnesia, and control of seizures. It can be administered orally, intravenously, and rectally to children. Diazepam is rapidly absorbed after oral administration, with peak plasma concentrations at 30–90 minutes. In children, the absorption rate is more rapid compared to adults. IM administration is painful and results in irregular absorption and should be avoided. Rectal diazepam is used for prehospital treatment of pediatric status epilepticus [5]. IV diazepam is associated with extravasation and tissue necrosis. Administering IV lidocaine before the diazepam and administering diazepam slowly through a rapidly flowing IV catheter minimizes this pain and risk. Diazepam is highly plasma bound, with a serum half-life ranging from 20 to 80 hours. Its half-life is reduced in younger adults and children to ~18 hours. Hepatic disease may decrease the elimination of diazepam. Diazepam undergoes oxidative metabolism by demethylation (CYP 2C19) to its active metabolite, desmethyldiazepam. The active metabolite has potency similar to the parent compound and a half-life greater than the parent compound. Therefore, caution is required when using diazepam in neonates [5].
The preservative benzyl alcohol is present in many formulations of diazepam. This preservative should not be used in neonates because it is difficult to metabolize, can cause metabolic acidosis, and is associated with kernicterus. The amount of benzyl alcohol contained in a standard dose of diazepam would likely be insufficient to cause harm to a neonate [6].
Adverse Reactions, Drug-Drug Interactions, and Monitoring Parameters
Adverse reactions to benzodiazepines are usually dose dependent with more severe adverse effects occurring when doses are increased. Dose-dependent adverse reactions to benzodiazepines include CNS effects and respiratory depression. Benzodiazepine exposure has also been associated with the development and longer duration of delirium and lower likelihood of ICU discharge in critically ill infants and young children [13, 14, 17].
The most frequent adverse reactions reported when using benzodiazepines include sedation, dizziness, weakness, fatigue, muscle weakness, ataxia, and iatrogenic withdrawal symptoms on abrupt discontinuation or weaning [15, 17].
Other reactions to benzodiazepines include:
-
Central Nervous System: confusion, depression, dysarthria, headache, slurred speech, tremor, vertigo
-
Gastrointestinal System: constipation, nausea, gastrointestinal disturbances, change in appetite, jaundice, increase in bilirubin, increase in liver transaminases, increase in alkaline phosphatase
-
Cardiovascular System: hypotension
-
Psychiatric and Paradoxical Reactions: stimulation, restlessness, anxiety, excitation, agitation, hostility, aggression, rage, irritability, rage, hallucinations, delusions, increased muscle spasticity, nightmare, sleep disturbances or insomnia
-
Urogenital System: incontinence, urinary retention, changes in libido
-
Dermatological Symptoms: allergic skin reactions, alopecia
Certain medications can influence the pharmacokinetics of benzodiazepines. Medications that are known to be inhibitors may result in increased and prolonged sedation due to a decrease in plasma clearance of benzodiazepines. Medications that are known to be inducers may result in a reduced sedation due to an increase in plasma clearance of benzodiazepines. Table 5.5 shows common drug interactions with benzodiazepines. Additionally, grapefruit juice may increase serum concentrations of midazolam. It is recommended to avoid concurrent use of grapefruit juice with oral midazolam.
Contraindications to the use of benzodiazepine include:
-
1.
Benzodiazepines are contraindicated in patients with a known hypersensitivity to the drug or to any components of the formulation.
-
2.
Paradoxical reactions such as anxiety, agitation, hostility, aggression, excitation, sleep disturbances, insomnia, and hallucinations have been reported during benzodiazepine use. Benzodiazepine should be discontinued if the patient experiences this reaction.
-
3.
Midazolam is not used for intrathecal or epidural administration due to presence of the preservative benzyl alcohol in the dosage form.
-
4.
Diazepam is contraindicated in patients with severe respiratory insufficiency, sleep apnea syndrome, severe hepatic insufficiency, and myasthenia gravis.
-
5.
Lorazepam should be used with caution with compromised respiratory function such as sleep apnea.
Benzodiazepine and Opioids
Adjuvant use of benzodiazepines and opioids increases the risk of respiratory depression due to reactions at different receptor sites in the central nervous system that control respiration [16]. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly increase opioid-related respiratory depression exists. Dosage and duration of concurrent use of benzodiazepines and opioids should be limited, and patients should be monitored closely for increased respiratory depression and sedation.
Administration of benzodiazepines with other central nervous system depressants such as alcohol, barbiturates, antipsychotics, sedative, hypnotics, anxiolytics, antidepressants, narcotic analgesics, sedative antihistamines, and anticonvulsants produces CNS-depressant effects.
Breastfeeding/Pregnancy Considerations
Pregnancy Considerations
Teratogenic effects have been reported with some benzodiazepines. The incidence of premature birth and low birth weights may be increased due to maternal use of benzodiazepines. Hypoglycemia and respiratory problems in the neonate may occur following exposure late in pregnancy. Neonatal withdrawal symptoms may occur within days to weeks after birth in babies exposed to some benzodiazepine in utero.
Breastfeeding Considerations
Breastfeeding is not contraindicated in women using benzodiazepines. Sedative effects of benzodiazepine exposure through breast milk appear to present minimal risk of CNS depression in infants. Infant sedation is also more likely in mothers taking a greater number of concomitant CNS depressants [2].
In Summary
Benzodiazepines are the most commonly used sedatives in the pediatric intensive care units. Recent studies have highlighted the strong association between the use of benzodiazepines and ICU-acquired delirium. Additionally, abrupt cessation of benzodiazepines will result in iatrogenic withdrawal syndrome.
References
Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community*. Crit Care Med. 2014;42(7):1592–600.
Nutt D. GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med. 2006;2(2):S7–11.
Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–23.
Vet NJ, Kleiber N, Ista E, de Hoog M, de Wildt SN. Sedation in critically ill children with respiratory failure. Front Pediatr. 2016;4:89.
Cote CJ, Lerman J, Ward RM, Lugo RA, Goudscouzian N. Pharmacokinetics and pharmacology of drugs used in children. In: A practice of anesthesia for infants and children. 4th ed. Philadelphia: Saunders/Elsevier; 2009. p. 89–146.
Malamed S. Pharmacology. In: Sedation: a guide to patient management. 6th ed. St. Louis: Elsevier, Inc; 2018. p. 319–58.
Gorski JC, Hall SD, Jones DR, VandenBranden M, Wrighton SA. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;47(9):1643–53.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67.
Blumer JL. Clinical pharmacology of midazolam in infants and children. Clin Pharmacokinet. 1998;35(1):37–47.
Best KM, Boullata JI, Curley MA. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model. Pediatr Crit Care Med. 2015;16(2):175–83.
Duceppe MA, Perreault MM, Frenette AJ, et al. Frequency, risk factors and symptomatology of iatrogenic withdrawal from opioids and benzodiazepines in critically ill neonates, children and adults: a systematic review of clinical studies. J Clin Pharm Ther. 2019;44(2):148–56.
Pentikainen PJ, Valisalmi L, Himberg JJ, Crevoisier C. Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J Clin Pharmacol. 1989;29(3):272–7.
Chicella M, Jansen P, Parthiban A, et al. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med. 2002;30(12):2752–6.
Kraus JW, Desmond PV, Marshall JP, Johnson RF, Schenker S, Wilkinson GR. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411–9.
Traube C, Silver G, Reeder RW, et al. Delirium in critically ill children: an international point prevalence study. Crit Care Med. 2017;45(4):584–90.
Traube C, Greenwald BM. “The times they are a-changin”: universal delirium screening in pediatric critical care. Pediatr Crit Care Med. 2017;18(6):594–5.
Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28(6):2122–32.
Horn E, Nesbit SA. Pharmacology and pharmacokinetics of sedatives and analgesics. Gastrointest Endosc Clin N Am. 2004;14(2):247–68.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Moore, W., Adu, O., Haire-Kendall, S. (2021). Sedative Agents (Benzodiazepines). In: Kamat, P.P., Berkenbosch, J.W. (eds) Sedation and Analgesia for the Pediatric Intensivist. Springer, Cham. https://doi.org/10.1007/978-3-030-52555-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-52555-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52554-5
Online ISBN: 978-3-030-52555-2
eBook Packages: MedicineMedicine (R0)