Abstract
The major target organ of progesterone is the reproductive system. Progesterone, in association with estrogen, is involved in the development and sexual maturation of the reproductive organs and orchestrates the menstrual cycle. Progesterone takes part in all the processes from the preparation of the uterine decidua, myometrium and cervix during the menstrual cycle through blastocyst implantation and is the key hormone in pregnancy maintenance, sustaining of myometrial quiescence, cervical competence and modulation of the maternal immune system during pregnancy. Accumulating evidence suggests that, in humans, progesterone withdrawal during parturition is probably functional and involves a shift in the balance between progesterone and cortisol, as well as changes in the genomic and non-genomic effects of progesterone at the cellular level. This chapter describes the specific effects of progesterone on the uterus and the cervix during the normal menstrual cycle, in the maintenance of normal pregnancy, and during parturition. Progesterone also has numerous systemic effects and influences other organs outside the female reproductive tract.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Functional progesterone withdrawal
- Menstrual cycle
- Parturition
- Pregnancy
- Progesterone
- Progesterone receptor
- Reproductive tract
1 Introduction
The corpus luteum was first discovered in 1672 by Reinier de Graaf and named in 1689 by Marcelo Malpighi. Malpighi proposed that the corpus luteum produces the ovarian follicles and that the yellow substance, like egg yolk, serves to nourish the ovum. In 1903, Fraenkel demonstrated that excission of the corpora lutea of rabbits, before implantation, prevented implantation. Moreover, lutectomy in early pregnancy (<14 days) resulted in pregnancy loss. In 1929, Corner and Allen reported that injecting extracts of the corpus luteum into castrated adult female rabbits induces a characteristic alteration of the endometrium identical to progestational proliferation, previously shown to be due to the presence of corpora lutea in the ovaries. Allen and Corner subsequently demonstrated that in ovariectomized rabbits (at the 18th hour of pregnancy), the presence of progestational proliferation induced by corpus luteum extracts may sustain normal implantation as well as embryo survival and growth; whereas in the absence of progestational proliferation, the embryos never survived beyond the fourth day. Therefore, the extracts of corpus luteum were essential for both implantation and early pregnancy maintenance.
In 1930, Allen proposed the name “progestin” to refer to the hormone responsible for these biological effects. In 1934 four different groups reported purification and characterization of “progestin”. Each group suggested a different name to refer to the main corpus luteum hormone, and the name “progesterone” came by consensus after a meeting of the League of Nation’s Health Organization in 1935.
The major target organ of progesterone is the reproductive tract; however, progesterone has a systemic effect and influences other organs including, but not limited to, the mammary glands, the nervous system and brain, the heart, the bones, and the endocrine and immune systems (Fig. 1.1) [1, 2]. In the reproductive system, progesterone, in association with estrogen, is involved in the development and sexual maturation of the reproductive organs and orchestrates the menstrual cycle [3,4,5]. This chapter describes the specific effects of progesterone on the uterus (myometrium and endometrium) and the cervix during the normal menstrual cycle and pregnancy. Detailed discussion on the effect of progesterone on organs outside the female reproductive tract is described in other chapters of this book.
Tissue and cell types expressing progesterone receptors. (Modified from Graham JD, Clarke CL [185])
2 The Mechanisms of the Cellular Action of Progesterone
Progesterone can evoke genomic or non-genomic responses upon its interaction with target cells. The term “genomic actions” refers to the cellular (nuclear) response involved in the activation of the genetic machinery, resulting in modulation of DNA expression. The genomic actions of progesterone (the Classical pathway) are largely, but not only, mediated by the progesterone receptor (PR) [6]. The term “non-genomic actions” (the Non-Classical pathways) indicates the cellular responses to progesterone that involve alternative pathways, such as the activation of signal-transduction cascades, the generation of intracellular second messengers, and the modulation of protein kinases and ion fluxes (Fig. 1.2) [7, 8].
Central Paradigm for genomic and non-genomic progesterone actions on myometrial cells. Reproduced with permission from Thieme Publishers: Mesiano S. Myometrial progesterone responsiveness [186]
2.1 Genomic Actions of Progesterone and the Cytosolic Progesterone Receptor
The classical cytosolic PR, [6] a member of the steroid/nuclear receptor superfamily of ligand-activated nuclear transcription factors is a mediator of the genomic actions of progesterone. In resting conditions, this receptor is localized in the cytosol, within a large complex of proteins, including heat shock proteins and FK506-binding proteins, contributing to maintaining it in a transcriptionally inactive state (Fig. 1.3) [9].
Progesterone activation of the cytosolic progesterone receptor. Reproduced with permission from Elsevier: Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids, 2003. 68:761–770 [187]
The cytosolic receptor can be activated by ligand-dependent [9] and ligand-independent mechanisms [10]. In the ligand-dependent pathway, progesterone gains access into the cell through passive diffusion or facilitated transport, and binds to the receptor, which changes its conformation (including dimerization and shedding of the heat shock proteins) [11]. This process allows the dissociation of the PR from the chaperone complex, its translocation into the nucleus, and finally its interaction with the DNA, where binding in a homodimeric form to cis-acting DNA progesterone response elements (PREs) modulates the transcription of target genes [9]. The ligand-independent activation of the PR, instead, is the result of cross-talk between membrane receptors and intra-cellular kinases, including a cAMP-dependent kinase, the cyclin A/cyclin-dependent kinase-2 (Cdk2), the mitogen-activating protein kinase (MAPK), the stress-activated p38 MAPK, and the protein kinases A and C [12].
The progesterone receptor gene (PGR) encoding the human nuclear PR is located on chromosome 11q22.1 and has eight exons. Alternative splicing allows for the synthesis of different isoforms of the receptor [13]. The two major isoforms of the PR are progesterone receptor-A (PR-A) and B (PR-B). These isoforms, although characterized by a different length, do not differ in their amino acid sequence: PR-B is 933 amino acids in length, while PR-A lacks 165 amino acids at the N-terminal [14]. In vitro, PR-B is a stronger trans-activator than PR-A, whereas PR-A acts as a trans-repressor of PR-B and of other steroid receptors [15, 16]. Structurally, both isoforms consist of an amino-terminal region, a centrally located DNA binding domain, and a carboxy-terminal hinge region containing nuclear localization signals as well as the ligand-binding domain. Three transcription activation function (AF) domains have been identified within the PR amino acid chain. AF-1 is located upstream of the DNA-binding domain, while AF-2 is located in the ligand-binding domain [17]. AF-3 is unique to the PR-B isoform and is located within the N-terminal region [15, 18]. In addition, an inhibitory function region, located between the AF-1 and AF-3 domains, has been proposed to be responsible for the auto-inhibition and trans-repression of the PR [19]. Interestingly, most of the evolutionary changes in the human PR took place in this region [20]. (For more information on the structure of the Human Progesterone Receptor gene, see http://www.ncbi.nlm.nih.gov/gene/5241).
A third isoform of the PR, the PR-C, was also described [21, 22]. The PR-C is a 60kD N-terminally truncated isoform, lacking the DNA-binding domain, but containing the hormone-binding region with the sequence for dimerization and nuclear localization [21, 23]. The cytoplasmatic PR-C has been suggested to inhibit PR-B activity by sequestrating the locally available progesterone [23]. The nuclear PR-C can form heterodimers with PR-B, therefore interfering with its binding to the response elements in the DNA [23]. In contrast, PR-C can enhance the progestin-induced transcriptional activity of the PR-A and PR-B isoforms, either by sequestrating the co-repressors and/or by increasing the capacity of the heterodimers of PR-A or PR-B with PR-C to recruit co-activators [21]. In this manner, PR-C could be involved in the modulation of the transcriptional activity of PR-A and PR-B, contributing to the pleiotropic effects of progestins [21]. Additional isoforms of the PR, such as PR-S [24] and PR-M [25] have also been identified and partially characterized. It has been proposed that the tissue responses to progesterone may be affected by changes in the expression ratio of the different isoforms [22].
Importantly, the validity of the immunoassay used in the identification of some of the PR isoforms, such as PR-C and PR-M, has been questioned, [26, 27] as the nuclear PR antibodies used may cross-react with cytoskeletal proteins (α-actinin, desmin and vimentin). Hence, these antibodies are not specific for these PR isoforms [27].
2.2 The Role of Co-Regulators in Progesterone Signaling
The activity of the nuclear PR is regulated not only by the hormone itself but also by co-regulators (co-activators and co-repressors) as well as by chromatin modifiers [28]. Co-regulators can enhance or inhibit gene transcription by creating a functional link between the ligand-activated receptors, the DNA and the transcription factors [29]. The existence of “intermediary factors” in the PR nuclear signaling was described more than four decades ago by the group of B.W. O’Malley [30]. Since then, the interest in co-regulators has increased, given their possible involvement in the “transcriptional interference” in the tissue-specific responses, evoked by nuclear receptor ligands and selective receptor modulators (i.e., Tamoxifen and Raloxifene), and their roles in the pathogenesis/progression of neoplastic disease [31]. Thus, the possible involvement of progesterone co-regulators in the modulation of myometrial progesterone action should be taken into account [32, 33].
Progesterone co-activators include members of the “Steroid Receptor Co-activator” (SRC/p160) family, [34] such as SRC-1, SRC-2, and SRC-3, which share a strong sequence homology [34, 35]. The involvement of these progesterone co-activators in normal growth, puberty, and female reproductive function, as well as in mammary gland development, is supported by studies of genetically modified animals. SRC-1 [36, 37] is an important co-activator in the uterus, whereas SRC-3 is in the mammary gland, [37, 38] and SRC-2 is in both organs [32, 33]. Of note, SRC-1 and SRC-2 knockout mice manifest a deficient uterine response to progesterone stimulation. However, SRC-1 knockout mice preserved their fertility, [36] whereas SRC-2 knockout mice had an early block of embryo implantation [32]. Progesterone receptor co-activators share an NRbox (also called the LXXLL motif) necessary for binding to the “co-activator binding groove” in the receptor [35, 39].
Co-repressors of the progesterone receptors inhibit transcription factor recruitment and down-regulate the receptor-dependent gene expression. This is accomplished preferentially by recruiting histone deacetylases, [40] which enhance tight nucleosome-DNA interactions and increase chromatin compaction [35]. However, the molecular basis of the interactions between steroid receptors and co-repressors is not well-defined [35].
2.3 Non-genomic Actions of Progesterone
The identification of steroid receptors on cells lacking a functional nucleus, (i.e., spermatozoa, erythrocytes and platelets) suggests non-genomic steroid actions. This is a fast-track rapid response system, in contrast to the long response time (i.e., hours/days) of the “genomic” pathways [7, 8]. The first evidence in support of the existence of non-genomic progesterone actions came from the study of progesterone responses in germ cells (oocytes and spermatocytes). Some of the non-genomic actions exerted by progesterone on these germ cells include changes in intracellular calcium concentrations, [41, 42] promotion of Na+ [43] and Cl−[44] fluxes, inhibition of adenylate cyclase activity with a consequent decrease in intracellular cAMP levels, [45] and the involvement in G proteins-phospholipase C- inositol trisphosphate, and diacylglycerol signaling [46, 47]. Fig. 1.3.
“Membrane-initiated steroid signaling” defines the non-genomic activities of progesterone that are secondary to the activation of membrane-bound progesterone receptors (mPRs) [7, 8]. Evidence supporting the idea that at least some of the non-genomic actions of progesterone are mediated by mPRs includes: (1) progesterone application outside the cell is more effective in decreasing intracellular cAMP concentrations than upon its cytoplasmic microinjection; [48] (2) progesterone activity is sustained after conjugation with synthetic polymers [49, 50] or its covalent binding to large molecules, such as albumin, [46, 51] which prevent progesterone access into the cytosol; (3) progesterone effects are reduced in the presence of antibodies directed toward progesterone membrane-binding proteins; [42] and (4) the non-genomic activities of progesterone, such as Ca2+ influx, are not affected by inhibitors of genomic progesterone responses, including RU38486 and RU486 [41, 52] Fig. 1.4.
The effect of progesterone and progesterone induced blocking factor (PIBF) on maternal immune system during pregnancy. Reproduced with permission from Elsevier: Walch KT, Huber JC, Progesterone for recurrent miscarriage: truth and deceptions. Best Pract Res Clin Obstet Gynaecol, 2008. 22:375–89 [59]
Progesterone high-affinity binding proteins and receptors have been identified on the cellular membranes of a variety of cells such as spermatozoa, [41, 42, 52] porcine liver microsomes, [53, 78] and porcine vascular muscle cells [54] as well as in the brains of female mice knocked out for the classical PR [55]. Some researchers have previously argued that the existence of a progesterone-binding site does not necessarily indicate that the receptor is functionally active in terms of cellular signaling, and that a characterization of non-classical receptors is still required [7].
Non-genomic progesterone receptors display different affinities, binding capacities, and dose response/competition curves for progesterone and other molecules sharing progestin structure. For example, the recombinant human mPRγ, produced in an E. coli expression system, has a high-affinity, saturable, single binding site for progesterone and several of its hydroxylated derivatives; however, recombinant human mPRγ does not bind and has no affinity for synthetic progestins and anti-progestins [56]. Similarly, there is evidence indicating the presence of at least two distinct membrane-surface progesterone receptors in capacitated human spermatozoa: a high-affinity site specific for progesterone and a low-affinity site that binds with equal affinity to 11β-hydroxyprogesterone and 17α-hydroxyprogesterone [41].
3 The Physiologic Effects of Progesterone
3.1 The Effect of Progesterone on the Immune System
The immune system in the female reproductive tract faces two opposing challenges: the consistent exposure to infectious pathogens and, in contrast, the need to be tolerant to both the allogenic spermatozoa and the semi-allogenic fetus. To overcome these challenges, the female sex steroids (i.e., estrogen and progesterone) control the function of the innate and adaptive immune systems in the reproductive tract according to the changes occurring through the menstrual cycle and during pregnancy [57, 58]. Indeed, in the rat uterus, major histocompatibility complex (MHC) class II positive cells, macrophages, granulocytes, and dendritic cells were more abundant in the endometrial stroma and around the uterine glandular epithelium in the estrus stages of the menstrual cycle relative to the diestrus stages in which progesterone is the dominant hormone [59]. Moreover, ovariectomy in mice results in a decrease in the number of uterine macrophages that can be restored by hormonal treatment [2].
The uterine/decidual natural killer (uNK) cells, which are different from the peripheral NK cell population, [1] are also affected by progesterone. uNK cells have a role in promoting blastocyst implantation and maintenance of pregnancy [60, 61]. During the mid-late luteal phase, the numbers of this unique population of NK cells is elevated [62, 63] as a result of the increased decidual concentrations of interleukin (IL)-15, and IL-15 mRNA [64]. Their number further increases during the early stages of pregnancy and decreases from mid-gestation to term [62]. The immunologic recognition of pregnancy also leads to a higher expression of PR on the membrane of uterine NK cells [65] and decreased cytotoxic activity in comparison to the non-pregnant state [66].
During normal pregnancy, progesterone diverts the T-cell response toward Th-2 rather than Th-1, leading to higher secretion of IL-6 and IL-10, as well as supporting B-cell antibody production [67,68,69]. During pregnancy, there is also a change in the antibody population and a shift toward asymmetric “blocking” antibodies (i.e., those glycosylated by mannose-rich oligosaccharide on only one of the Fab regions; although asymmetric antibodies can combine with antigens, they poorly activate phagocytosis, complement fixation, and cytotoxicity). The prevalence of asymmetric antibodies increases from 9% in the non-pregnant state to 29% in pregnant women [70]. Yet, they can compete with symmetric and competent antibodies having the same specificity and thus block the actions of symmetric antibodies. Asymetric antibodies may be a mechanism to reduce the antibodies’ mediated response against the invading trophoblast during pregnancy and to control the equilibrium of maternal anti-fetal immune responses [71].
It has been reported that the effects of progesterone on the T-cell response, B-cell activity, generation of asymmetric antibodies, and NK cytotoxicity are mediated by a progesterone-induced blocking factor (PIBF) (Fig. 1.4), a 34 kDa immunoregulatory protein synthesized by PR-positive lymphocytes and CD56+ decidual cells [72]. The actions of PIBF include: (1) enhancement of the production of asymmetric antibodies; [73] (2) diverting the T-helper response toward Th-2 activity, resulting in increased concentrations of IL-3, IL-4, and IL-10 as well as decreased IL-12 production; [74] and (3) the latter, combined with the inhibition of perforin secretion by PIBF in a dose-dependent manner, reduces the cytotoxic activity of NK cells [74, 75]. In summary, progesterone affects all arms of the immune system and propagates maternal tolerance to the semi-allogenic fetus.
3.2 The Role of Progesterone in Non-pregnant Women
3.2.1 Progesterone and the Menstrual Cycle
Progesterone participates in the control of ovulation, the preparation and stabilization of the endometrium before implantation, the regulation of the implantation process, and the maintenance of pregnancy [76]. During the follicular phase of the menstrual cycle, estrogen predominates and has a major role in the proliferation of the endometrium while progesterone concentration is relatively low. Progesterone predominates during the secretory phase (maximal concentrations occur in the mid-luteal phase), inhibits the endometrial proliferation induced by estrogen, and changes the endometrial morphology to the secretory type [77]. However, the glandular and vascular elements continue to grow, resulting in progressive tortuosity [77]. Progesterone stimulates glycogen vacuole formation within glandular cells, resulting in the active secretion of glycoproteins and peptides by the glands into the endometrial cavity as well as in edema of the endometrial stromal tissue [78]. In the mid-luteal phase, progesterone is responsible for the transformation of stromal cells into decidual cells, which is critical for the establishment of pregnancy. In the absence of conception, the degeneration of the corpus luteum exerts a physiological progesterone withdrawal resulting in menstruation [76].
Previous exposure to estrogen is essential to stimulate synthesis of PR in endometrial cells. Progesterone can then exert its anti-estrogenic effect on the endometrium [79] through several proposed potential mechanisms, such as the down regulation of estrogen receptor expression, [80] the conversion of estradiol to a less active form (estrone sulphate) via the stimulation of 17-hydroxysteroid dehydrogenase and sulfotransferase, and the suppression of estrogen-mediated synthesis/secretion of specific proteins (e.g., transcription of the proto-oncogene c-fos mRNA) [81].
In addition, progesterone increases the expression of tissue factor (TF) and plasminogen activator inhibitor-1 during decidualization [82]. It has been suggested that an increase in decidual TF concentration is needed to secure rapid hemostasis during blastocyst implantation and placentation as well as the control of postpartum hemorrhage [82]. The association between the decidual expression of TF and progesterone was established by the differences in TF expression in confluent stromal cell cultures derived from proliferative phase endometrium. Stromal cell cultures treated with mifepristone did not increase their TF expression; moreover, administration of mifepristone to cell cultures previously exposed to estradiol+MPA (medroxyprogesterone acetate) or estradiol+progesterone decreased their TF content and TF mRNA expression [83]. Therefore, a low progesterone concentration could contribute to less-effective decidual hemostasis, which may lead to increased decidual bleeding, and a subsequent spontaneous miscarriage or preterm delivery.
3.2.2 Progesterone and the Myometrium in the Non-pregnant Uterus
Uterine contractile activity throughout the menstrual cycle is partially regulated by estrogen and progesterone [84, 85]. This process has been proposed to be mediated by cyclic changes in estrogen and progesterone receptor expression in the endometrium and sub-endometrium [86]. The decrease in the progesterone concentration in the transition from the luteal phase of one menstrual cycle to the follicular phase of the subsequent cycle is followed by increased uterine contractility, which aids in clearing menstrual contents [85]. The rise in estrogen concentration during the late follicular phase further increases uterine contractility, preparing the uterus to facilitate sperm motility toward the Fallopian tube [85]. During the luteal phase, following an increase in progesterone concentration, the uterus is relatively quiescent [84, 85].
Of note, studies in non-pregnant women demonstrated that plasma progesterone concentrations do not reflect the actual progesterone concentrations in the myometrium. Akerlud et al. [87] measured the estrogen and progesterone concentrations in the non-pregnant uterus of women with normal menstrual cycles, demonstrating that there is no correlation between plasma and tissue progesterone concentrations in the same individual, although the progesterone concentrations in the plasma and myometrial tissue change during the menstrual cycle. The authors reported that in peri- and postmenopausal women, the myometrial concentration of progesterone remains comparable to those in menstruating women, despite a substantial decline in the plasma concentration of progesterone, suggesting that the myometrial uptake of ovarian hormones may be saturated even if plasma concentrations are relatively low. Moreover, the myometrial/plasma ratio of progesterone decreases significantly during the luteal phase [87]. This decrease may be due to down-regulation of the myometrial progesterone receptors following accumulation of progesterone in the myometrium [88].
3.2.3 The Effect of Progesterone on the Uterine Cervix during the Menstrual Cycle
The uterine cervix is a primary end organ that is responsive to pubertal hormonal action, cyclical changes in sex hormones during the menstrual cycle, pregnancy, labor, and menopause [89,90,91]. The expression of PR changes significantly in the glandular epithelium of the cervix, reaching its peak in the early secretory phase and declining sharply afterward [92]. Progesterone has a dramatic effect on the constituents of the cervical mucus and, hence, on the function of cervical secretions [93, 94]. The cervical mucus becomes thick and sticky under the influence of progesterone. Indeed, one of the suggested mechanisms by which progestins exert their contraceptive effect is through changes in the chemical properties of mucus [95, 96].
The available data suggest that progesterone has an effect on the cervix in the non-pregnant state; however, how and to what extent this effect is important in physiologic and pathologic conditions have yet to be determined.
3.3 Progesterone and Pregnancy
3.3.1 The Role of Progesterone in the Maintenance of Normal Pregnancy and Parturition
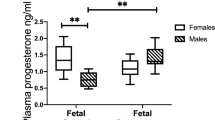
Estrogen and progesterone play a central role in pregnancy [97]. The corpus luteum is the main source of progesterone until the seventh week of gestation; then the placenta takes over as the main source of progesterone between 7 and 9 weeks of gestation, a transition termed the “luteal-placental shift” [98, 99] (Fig. 1.5). Indeed, ovariectomy before 8 weeks of gestation results in abortion, but the procedure has no effect on the pregnancy if performed after 9 weeks of gestation. Maternal plasma progesterone concentrations rise during pregnancy from 40 ng/mL in the first trimester to 160 ng/mL in the third trimester [99]. At term, the placenta produces approximately 250 mg of progesterone per day, of which 90% is secreted to the maternal circulation and only 10% into the fetal circulation. However, the fetal plasma progesterone concentration is seven-fold higher than the maternal concentration, probably due to the differences in their volume of distribution [100]. However, neither parturition at term [101] nor preterm [102] are associated with significant changes in amniotic fluid progesterone concentrations.
The luteal-placental shift in progesterone production during pregnancy. Reproduced with permission from Elsevier: Yen SSC. Endocrine-metabolic adaptation in pregnancy, in Reproductive endocrinology, Yen SCC Jaffe RB, Editors. 1991, W.B. Saunders: Philadelphia. p. 936–971 [188]
During pregnancy, progesterone is thought to maintain myometrial quiescence and inhibit cervical ripening, while estrogens have been implicated in increasing myometrial contractility and excitability as well as in the induction of cervical ripening prior to the onset of labor [103, 104]. However, before spontaneous parturition, the changes in sex-steroid serum concentrations differ between different species. In many species, a fall in maternal serum progesterone concentration occurs prior to the onset of parturition.
Luteolysis is a crucial component in the mechanism of parturition in the rat, mouse and rabbit [105, 106]. An increase in local progesterone metabolism in both the uterus [107] and cervix [108] was associated with the onset of labor in mice. In sheep and goats, an increase in fetal plasma cortisol induces the placental production of P450 C17 enzymes (17α-hydroxylase and C17–20 lyases), which catalyze the conversion of progesterone to androstenedione, which is transformed into estrogen by aromatases [109]. However, in primates (including humans) and guinea pigs, there is no apparent change in the circulating maternal progesterone concentration before parturition. The human placenta, lacks P450 C17 enzymes and, therefore, cannot synthesize estrogen and androstenedione from C21-progestins; thus, progesterone is the final product of the human placenta.
A serum “progesterone withdrawal” has not been demonstrated in humans or guinea pigs; yet, progesterone is considered important in pregnancy maintenance because inhibition of its action could result in parturition in both species. Administration of anti-progestins [i.e., mifepristone or onapristone] to pregnant women, [110] primates [111] or guinea pigs [103] can induce abortion and/or labor.[97, 127] Alternative mechanisms for the suspension of progesterone action without a serum progesterone withdrawal have been proposed, including: (1) binding of progesterone to a high-affinity protein that reduces the functional active form; [112] (2) an increase in cortisol concentration during late pregnancy, which may compete with progesterone binding to the glucocorticoid receptors, resulting in a functional progesterone withdrawal; [113] and (3) the conversion of progesterone to an inactive form within the target cell before interacting with its receptor. Indeed, the human amnion and chorion can convert progesterone to the inactive 20α-dihydroxyprogesterone, and this metabolite increases with gestational age and around the time of parturition [114, 115]. However, none of these hypotheses have been proven; [116] therefore, the focus of investigation has shifted to the abundance and modulation of estrogen-progesterone receptor expression and to progesterone’s binding capability to its nuclear response element.
3.3.2 The Nuclear Progesterone Receptor in the Myometrium during Pregnancy and Parturition
Conflicting results have been reported regarding the role of the PR in the myometrium during human pregnancy and parturition [116, 117]. The conflicting results may be due to the existence of multiple receptor isoforms, whose myometrial expression is spatially and temporally regulated throughout gestation [118]. Thus, it is likely that the results of the studies may be affected by the sampling site and the specificity of the assay. Of note, initial studies on PR expression in the human myometrium did not distinguish between the different isoforms and were performed on biopsies isolated from the lower uterine segment. In contrast, more recent studies tested the expression of the different receptor isoforms and focused preferentially on the fundal myometrium. The latter is more likely to reflect the molecular changes that mediate uterine contractility than the lower uterine segment, which reacts in favor of dilatation [22]. Finally, the non-genomic progesterone actions have broadened the research on the mechanism of labor toward identification of membrane progesterone receptors that may participate in the suspension of progesterone action.
The key mechanisms explaining the functional progesterone withdrawal include either a reduction of the total number of progesterone receptors within the target tissue or a relative increase of inhibitory PR isoforms. Rezapour et al. [119] investigated the expression of progesterone receptors in the myometrium of women at term not in labor and in the active phase of spontaneous labor, and found significant changes in the distribution of receptors after the onset of labor. The active normal labor group had a higher receptor concentration in the upper uterine segment as well as a higher upper-to-lower uterine segment receptor ratio than the not-in-labor group. Of interest, myometrial PR concentrations were lower in oxytocin-resistant labor than in normal labor. Although progesterone is involved in labor-associated changes in the myometrium through receptor-mediated processes, Rezapour et al. [119] suggested that progesterone is not an inhibitor of myometrial contractility and thus, not consistent with the progesterone withdrawal theory. However, there are in vitro reports indicating that progesterone stimulates myometrial tonus and frequency of contractions [120] and that it has an anti-tachyphylactic effect on oxytocin-induced myometrial contractions [121].
Pieber et al. [116] analyzed the labor-associated changes in the expression of PR-A and PR-B in myometrial samples obtained during term cesarean deliveries from women not in labor and in labor. While PR-A expression was detected only in the presence of effective labor, PR-B was equally expressed in labor and not-in-labor samples. Transient transfection of myometrial cells with PR-A and PR-B confirmed that the over-expression of PR-A has a dominant repressive effect on the transcription of progesterone sensitive genes within human term myometrial cells. The authors interpreted that the expression of PR-B occurs throughout gestation and is required for pregnancy maintenance, whereas a higher expression of PR-A in the presence of effective labor at term may contribute to “functional progesterone withdrawal” [116]. The increase in the expression of the inhibitory PR-A, [116, 122] and in the PR-A/PR-B ratio in the human myometrium, was interpreted as the possible underlying mechanism of the “functional progesterone withdrawal”.
The change in the PR-A/PR-B ratio occurs at the mRNA level [123]. The abundance of mRNAs encoding for PR-A and PR-B and estrogen receptors (ERα and β) were compared in the lower uterine segment in women at term in labor and not in labor. The mRNA levels of ERα and of the homeobox gene HOXA10 were used as markers of progesterone responsiveness. In the laboring myometrium, the mean relative abundance of mRNAs encoding for PR-A, PR-B, and ERα was significantly increased compared to non-laboring tissue, whereas ERβ was low and did not differ between the groups. There was a significant two- to three-fold increase in the PR-A/PR-B ratio in laboring compared to non-laboring specimens. Of interest, in non-laboring myometria, the PR-A mRNA levels and the PR-A/PR-B mRNA ratio positively correlated with mRNA of ERα and HOXA10 in the laboring myometrium. These positive correlations were interpreted as an indicator that progesterone responsiveness is inversely related to the PR-A/PR-B gene expression ratio and decreases at the onset of labor. Moreover, ERα could be an early gene, whereas HOXA10 may possibly be a late gene responding respectively to changes in the PR-A/PR-B expression ratio. The positive correlation detected in non-laboring myometria between ERα mRNA levels and those of contraction-associated genes, such as cyclooxygenase-2 (COX-2), and the oxytocin receptor, suggests that the process of human parturition is initiated within myometrial cells well before the onset of active labor [123].
3.3.3 The Membrane Progesterone Receptor during Pregnancy and Parturition
The first report on the existence of high-affinity membrane-associated progesterone binding sites within the uterine tissue dates back to the 1984 study of Haukkamma et al. [124]. It was noted that uterine membrane-associated receptors differ from their soluble cytosolic counterparts, previously identified in the human uterus, in terms of the specificity of their ligands.
Labor and sex-steroids differentially modulate the mPRs. Karteris et al. [125] reported the expression of two different functional mPRs (mPRα and mPRβ) in the myometrial cells of pregnant humans that are directly coupled to G-inhibitory proteins. This expression results in the inhibition of adenyl cyclase, a subsequent decline in cAMP concentrations and increased phosphorylation of the myosin light chain, which facilitates myometrial contractions. The authors proposed that, during labor, progesterone acts preferentially on its membrane receptors, a modus operandi that promotes the shift from quiescence to a contractile state. This change results from the altered PR-B/PR-A ratio, the changes in sex-steroids, and the existence of complex cross-talk between the nuclear and membrane progesterone receptors [125].
Fernandes et al. [126] combined bioinformatic analyses with the expression profile of mPRs to define their role in cycling human endometrium and gestational tissues. Sequence analysis suggested that these receptors belong to the “progestin and adiponectin receptors” family. The onset of parturition was associated with a marked reduction in myometrial mPRα and mPRβ transcripts. Of interest, the levels of mPRα expression were high in the placenta, and inversely correlated with that of the nuclear PR, indicating that mPRα may have an important functional role, particularly in reproductive tissues expressing low levels of nuclear PR [126].
3.3.4 Progesterone Oxytocin Responsiveness and Ca2+ Fluxes
Progesterone reduces the myometrial responsiveness to oxytocin through genomic [127] and non-genomic [128, 129] pathways. However, the exact mechanisms by which progesterone blunt uterine responsiveness to oxytocin is unclear. Three potential mechanisms have been proposed: (1) progesterone represses oxytocin receptor synthesis through its genomic action; [127] (2) direct interaction between progesterone and its metabolites with the oxytocin receptor; [130] or (3) the continuous presence of intracellular high progesterone concentrations may alter the responsiveness of the oxytocin receptor through non-genomic effects [131].
The oxytocin receptor needs a cholesterol-rich microenvironment to become stable in its high-affinity state [132]. The intracellular binding of progesterone to the multi-drug-resistant P-glycoprotein interferes with cholesterol transport to and from the plasma membrane, and higher intracellular concentrations of progesterone inhibit cholesterol esterification, [133] which reduces cholesterol concentrations in the plasma membrane [134]. Additionally, progesterone also increases the activity of 3-hydroxy-3-methylglutaryl (HMG-CoA) reductase, increasing the synthesis and membrane concentrations of the cholesterol precursors that are less active in their support of the high-affinity oxytocin receptor [135]. The depletion of active membranous cholesterol forms leads to a low-affinity mode of the oxytocin receptor, which may reduce its intracellular activity [136]. A decrease in the intracellular progesterone concentrations restores the cholesterol transport, leading to an increase in the active cholesterol concentration in the plasma membrane that supports the activity of the high-affinity oxytocin receptor, thus regaining its uterotonic effect [137].
Some of the activities of progesterone on the myometrium may be mediated by its effects on the activity and metabolism of cAMP [138] and the inhibition of trans-membrane Ca2+ entry [139]. Treatment of human myometrial smooth muscle cells with MPA resulted in a significant reduction in the oxytocin-mediated increase in intracellular Ca2+ concentration [139].
3.3.5 The Interplay Between NFκB and Progesterone in Pregnancy Maintenance and in the Onset of Labor
Nuclear factor kappa B (NFκB) is a transcription factor family classically associated with inflammation. Data indicate that myometrial NFκB activity changes with labor and its activation is regulated in a spatio-temporal fashion. It has been proposed that NFκB is an upstream regulator of multiple labor-associated processes, including the formation of contraction-associated proteins, inflammatory mediators (e.g., cytokines), uterotonic phospholipid metabolites (e.g., prostaglandins), and the induction of extracellular matrix remodeling [140, 141]. The stimuli and mechanisms responsible for NFκB activation in spontaneous labor have not yet been elucidated. Increasing local concentrations of surfactant protein A, [142] accumulation of advance glycation end-products, [143] the amnion cells’ mechanical stretch, [144, 184] and the paracrine or autocrine pro-inflammatory effects of the corticotrophin-releasing hormone [145] have been proposed as potential candidates.
NFκB activation favors the myometrial expression of inhibitory isoforms of the PR. Evidence in support of this role includes: (1) a spatial correlation is suggested by the enhanced expression of PR-B and PR-C along with NFκB activation during labor, and these changes are selective to the fundal human myometrium; [22] (2) a temporal correlation has been proposed given the correlation of PR isoform expression and local NFκB activation in the pregnant mouse uterus and in the human fundal myometrium; [22, 142] (3) intra-amniotic injection of surfactant protein A to pregnant mice promoted uterine NFκB activation and preterm labor as well as a rapid increase in uterine levels of PR-B and PR-C; [22, 142] (4) intra-amniotic injection of the NFκB inhibitor (SN50) caused a decrease in the uterine levels of PR-B and PR-C; [22, 142] and (5) in vitro models demonstrated that the activation of the NFκB pathway in response to IL-1β treatment is associated with an increased expression of all three PR isoforms (PR-A, PR-B and PR-C) in myometrial cells [22].
The PR-mediated activation of target genes that modulate uterine contractility is antagonized by NFκB. Kalkhoven et al. [146] reported the existence of a mutual trans-repression between the PR and the RelA(p65) subunit of NFκB in different cell lines. This repression was independent from the PR isoforms and the cell type. The authors suggested that the most likely mechanism involved is a direct interaction between the two proteins that would result in an inactive heterodimeric complex on the DNA, which prevents co-factors and members of the basal transcriptional machinery from initiating transcription [146]. Other possible explanations for the mutual repression of RelA(p65) and PR include binding of these transcription factors to their respective cognate DNA elements, or competition for the same co-activators or transcription intermediary factors (transcriptional interference or squelching) [146]. A similar mutual negative interaction between NFκB and PR activity was reported in human amnion cells [147]. Stimulation of these cells with IL-1β resulted in NFκB activation, followed by a repression of progesterone-dependent transcription, even in the presence of excess PR [147]. This may be the case during spontaneous labor in humans: indeed, the constitutive activity of NFκB reported in human amnion cells in the presence of labor may contribute to the loss of myometrial quiescence, both by repressing the PR activity and increasing the expression of COX-2. The authors proposed that the increase in NFκB activity, near to, or at the time of labor, may represent a “watershed point at which labor becomes inevitable” [147].
The anti-inflammatory activity of progesterone may contribute to the prolongation of pregnancy by direct or indirect attenuation of the NFκB-mediated inflammatory cascade. Several observations support this view: (1) over-expression of the PR in amnion cells was associated with significant repression of NFκB reporter expression; [147] (2) the IL-1β induced up-regulation of COX-2 mRNA in immortalized human fundal myometrial cells was suppressed by exogenous administration of progesterone and associated with a rapid induction of the NFκB transactivation inhibitor, kBα; [148] (3) progesterone down-regulates cytokine production by human leukemia cell lines, mediated, at least in part, by suppression of NFκB activity; [149] and (4) physiological concentrations of progesterone suppress both the spontaneous and the IL-1(α and β)-mediated production of IL-8 by the uterine cervical fibroblasts in pregnant rabbits [150].
In contrast, Vidaeff et al. [151] demonstrated that pre-treatment of HeLa cells with progesterone before exposure to IL-1β resulted in a significant decrease in NFκB protein subunit p65 in the cytoplasm. However, pre-treatment of HeLa cells did not reduce the amount of nuclear p65 or affect the nuclear translocation of p65. The authors suggested that any possible role played by progesterone in preterm labor prevention is not exerted through anti-inflammatory mechanisms of NFκB down-regulation [151].
3.3.6 Changes in Myometrial Progesterone Co-Regulators during Pregnancy
The possibility that changes in the activity of co-regulators can contribute to the functional progesterone withdrawal is currently an object of investigation. Condon et al. [152] proposed that a decline in the levels of PR co-activators in the pregnant uterus at term may antagonize PR function and contribute to the initiation of labor. Analysis of the mRNA and protein expression of PR co-activators in the fundal myometrium of 12 women in labor and 12 women not in labor revealed that the laboring myometrium was associated with a lower mRNA and protein expression of SRC-2, SRC-3, and CBP (the cAMP-response element-binding protein) than non-laboring myometrial samples, while SRC-1 expression was relatively unchanged before and after the onset of labor.
Term gestation was associated with a decrease in the levels of histone H3 acetylation in the human and mouse uteri. Treatment of pregnant mice with trichostatin, a histone deacetylase inhibitor, delayed the onset of labor by 24 to 48 hours. Altogether, these results suggested that reduced uterine expression of progesterone co-activators at term would lead to a reduction in histone acetylation, thus resulting in an impaired PR responsiveness and a functional progesterone withdrawal [152].
In 2005, a novel progesterone co-repressor, polypyrimidine tract-binding protein-associated splicing factor (PSF), was identified in the rat myometrium: [153] its mRNA expression increased as term approached and was up-regulated prior the onset of labor. PSF interferes with PR binding to its DNA response element and enhances PR degradation. Within the human myometrium, PSF expression was significantly up-regulated as pregnancy progressed, particularly within the upper uterine region, and levels remained elevated in labor. Co-immunoprecipitations and DNA-binding assays showed that PSF directly interacts with the nuclear PR and its glucocorticoid receptor and specific co-regulatory proteins within the human myometrium [154]. These findings are suggestive of a role for myometrial PSF as a nuclear co-regulator and a potential contributor to functional progesterone withdrawal [153,154,155].
3.3.7 Progesterone Receptor in Fetal Membranes, Decidua, Placenta
There is evidence that all three isoforms, PR-A, PR-B and PR-C, are expressed in the decidua and fetal membranes, yet there is still controversy concerning the predominant isoform [156,157,158,159]. A Western Blot analysis conducted by Goldman et al. [156] revealed that the major isoform in the human decidua is PR-B, whereas in the human amnion, it is PR-C. In contrast, in a review on this subject, Taylor et al. [159] reported that immunohistochemical, Western blotting and real-time reverse transcription polymerase chain reaction techniques provide evidence that the major PR isoform in the human decidua is PR-A, whereas in the human term fetal membranes and syncytiotrophoblast, it is PR-C [159].
The quantitative and qualitative expression of the PR isoforms in the decidua and fetal membranes may be subject to significant changes during labor [156, 157]. Our group reported that, in fetal membranes obtained from women in labor, there is a PR-A predominance and a higher PR-A/PR-B ratio than in women not in labor, in which PR-B is the predominant isoform [157]. Similarly, Mesiano et al. [123] reported that mRNA encoding for PR-A and the PR-A/PR-B expression ratio increased significantly in the human myometrium at term in association with labor. Although the role of progesterone receptors in the fetal membranes has not yet been elucidated, it has been proposed that a shift in progesterone isoform expression may be part of a “feto-maternal signaling pathway in the initiation of labor” [158].
3.3.8 The Effect of Progesterone on the Cervix During Pregnancy
Progesterone exerts biological effects in the uterine cervix, and a withdrawal (in rats, rabbits and sheep) or decline in progesterone action (guinea pigs and primates) [109, 139] has been proposed as a key control mechanism for cervical ripening [103, 150, 160, 161]. Evidence in support of this view includes the following: (1) administrat ion of anti-progestins to women in the mid-trimester and at term induces cervical ripening [162] but not labor, [103] which may not begin at all or may be delayed by days or weeks after cervical ripening has been accomplished; and (2) the administration of a PR antagonist to pregnant guinea pigs, [163, 164] and old-world monkeys [165]. Cervical responsiveness to anti-progestins increases with advancing gestational age, and the effect of anti-progestins in the cervix is not always accompanied by changes in myometrial activity. Indeed, Stys et al. [166] demonstrated a dissociation between the effects of progesterone in the myometrium and those in the cervix. Contrary to the acute nature of uterine contractions, the process of cervical ripening is gradual in normal pregnancies and may start weeks before labor and delivery [167].
Cervical ripening is a multifactorial process affected by a myriad of factors [104, 168] and characterized by slow changes in the composition of the extra-cellular matrix. The precise mechanisms by which a blockade of progesterone action may induce cervical changes are poorly understood. Both in vitro and in vivo studies have supported the key role of progesterone in this process. A decline in progesterone action may induce cervical changes through pro-inflammatory mediators, including IL-8, [169] nitric oxide, [161] prostaglandins [169] and matrix-degrading enzymes [170]. Cervical remodeling and ripening may be influenced by NFκB, which can oppose progesterone action, [22, 107, 146,147,148, 151] thus providing a link between inflammation, a decline in progesterone action and cervical ripening. The effects of progesterone on cellular and extra-cellular components of the cervix are discussed below.
3.3.8.1 Collagen Remodeling
The mechanical properties of the cervix are largely determined by collagen and proteoglycans that comprise the extra-cellular matrix of the connective tissue. Shortly before labor, the cervix’s collagen fibers become less densely packed and the collagen concentration decreases [168]. These changes are mediated, in part, by increased collagenase activity [171]. In pregnant women, there is a decrease of 30% to 50% in the collagen concentration of the uterine cervix in comparison to the non-pregnant state [172].
Winn et al. [173] have investigated the individual and combined effects of relaxin, estrogen and progesterone on growth, softening and histological characteristics of the cervix of ovariectomized non-pregnant gilts. When administered alone, progesterone had no effect on cervical growth and only a modest effect on cervical softening; when progesterone was administered with relaxin, there was an increased extensibility of the cervix in comparison to progesterone alone or to its combination with estrogen. In addition, the combination of progesterone and relaxin maximally decreased the collagen/amorphous ground substance [173]. The administration of mifepristone to pregnant rats at mid-gestation was associated with marked cervical changes, including decreased tensile strength, reduced collagen organization, and increased matrix metalloproteinase-2 (MMP)-2 mRNA expression [174]. Additionally, the collagen fibrils in the cervix had a shorter mean length and smaller mean diameter after mifepristone treatment. Collectively, this evidence suggests that progesterone suppresses cervical collagenolysis, one of the major processes of cervical ripening before labor [174].
3.3.8.2 Changes in Glycosaminoglycans
Glycosaminoglycans (GAGs) are an important component of connective tissues and the extracellular matrix. Softening of the cervix is associated with changes in GAG, [175] specifically, an increase in total GAGs, hyaleronic acid and water content, while sulphated GAGs decrease [176, 177]. Carbonne et al. [167] determined the effects of progesterone on PGE2-induced changes in GAG synthesis in human cervical cell cultures. Progesterone did not prevent changes in GAG production (usually considered to reflect cervical ripening); moreover, a high concentration of this hormone even favored these changes. The authors hypothesized that this paradoxical finding may account for the early changes in the consistency of the cervix and for the alteration in GAG content that can be observed as early as the first trimester [177]. Another explanation for this counterintuitive finding is that progesterone has a different effect on the cervix and on the body of the uterus. Increasing concentrations of progesterone during pregnancy may play a key role in the gradual ripening of the cervix and promote the myometrial quiescence and down-regulation of gap junctions in the uterus.
3.3.8.3 Suppression of Metalloproteinases
The degradation of collagen in the cervix is mediated primarily by MMPs, and their effects can be repressed by their endogenous inhibitors (TIMPs) [178]. In the cervical fibroblast of rabbits, progesterone decreases the levels of proMMP-1, proMMP-3, and the steady-state levels of the respective mRNAs in the culture media, and increases the concentrations of TIMPs more effectively than that of estradiol-17 beta [179]. Similarly, Imada et al. [178] reported that physiological concentrations of progesterone suppressed IL-1-mediated production of proMMP-9 and its mRNA in a dose-dependent manner. The authors concluded that, in the rabbit uterine cervix, progesterone is a physiological suppressor of the proMMP-9 production at the transcriptional level [178]. However, the nature of the effect of progesterone may vary according to its concentration.
The effect of anti-progestins on cervical MMP expression has also been studied. Onapristone augmented the expression of MMP-3 mRNA in rabbits [170]. In contrast, mifepristone increases the expression of MMP-2 mRNA, but not of MMP-9 or MMP-3, [174] suggesting that the anti-progestins differ not only in their specificity to progesterone but they may also differ in the mechanism by which their effect is exerted.
3.3.8.4 Modulation of the Inflammatory Response in the Uterine Cervix
Macrophages, neutrophils and eosinophils are thought to play a central role in the remodeling of the cervical connective tissue by production of cytokines and proteolytic enzymes in response to inflammatory stimuli, and they have a regulatory role in cervical ripening [180, 181]. Ramos et al. [182] investigated the mechanism through which eosinophilic invasion is modulated during the second half of pregnancy in rats. Exposure to 17β-estradiol together with progesterone resulted in very poor eosinophilic infiltration, but the progesterone inhibition of eosinophilic infiltration was reversed by co-administration of mifepristone. The authors suggested that the progesterone effect is mediated through the progestin receptor [182].
Human cervical cells release IL-8, [183] a neutrophilic chemotactic and activating agent, [184] which is thought to initiate cervical ripening by promoting neutrophils chemotaxis to the cervix and their activation within the cervical stroma [183, 184]. Denison et al. [169] demonstrated that the release of IL-8 by cervical explants was significantly stimulated by PGE2 and inhibited by progesterone. The release of the secretory leukocyte protease inhibitor (an inhibitor of neutrophil function) by cervical explants was significantly stimulated by progesterone and inhibited by PGE2 [169].
4 Conclusions
Progesterone is the key hormone in pregnancy maintenance; it is involved in all processes during pregnancy, from the preparation of the uterine decidua, myometrium and cervix during the menstrual cycle through blastocyst implantation, sustaining myometrial quiescence, cervical competence and modulation of the maternal immune system. There is accumulating evidence that progesterone withdrawal during parturition in humans is probably functional and involves a shift in the balance between progesterone and cortisol as well as in the changes in the genomic and non-genomic effects of progesterone at the cellular level.
References
Szekeres-Bartho J, Schindler AE. Progestogens and immunology. Best Pract Res Clin Obstet Gynaecol. 2019;60:17–23.
Jure I, De Nicola AF, Labombarda F. Progesterone effects on the oligodendrocyte linage: all roads lead to the progesterone receptor. Neural Regen Res. 2019;14:2029–34.
DeMayo FJ, et al. Mechanisms of action of estrogen and progesterone. Ann N Y Acad Sci. 2002;955:48–59.
Catt KJ IV. Reproductive endocrinology. Lancet. 1970;1(7656):1097–104.
An BS, et al. Differential role of progesterone receptor isoforms in the transcriptional regulation of human gonadotropin-releasing hormone I (GnRH I) receptor, GnRH I, and GnRH II. J Clin Endocrinol Metab. 2005;90:1106–13.
Williams SP, Sigler PB. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393(6683):392–6.
Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev MolCell Biol. 2003;4:46–56.
Garg D, et al. Progesterone-mediated non-classical signaling. Trends Endocrinol Metab. 2017;28:656–68.
Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86.
Power RF, Conneely OM, O’Malley BW. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992;13:318–23.
DeMarzo AM, et al. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc. Natl. Acad.Sci.U.S.A. 1991;88(1):72–6.
Brosens JJ, et al. Steroid receptor action. Best Pract Res Clin Obstet Gynaecol. 2004;18:265–83.
Kastner P, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms a and B. EMBO J. 1990;9:1603–14.
Patel B, et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–73.
Meyer ME, et al. A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem. 1992;267:10882–7.
Vegeto E, et al. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55.
Hirata S, et al. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab. 2003;14:124–9.
Sartorius CA, et al. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol. 1994;8:1347–60.
Huse B, et al. Definition of a negative modulation domain in the human progesterone receptor. Mol Endocrinol. 1998;12:1334–42.
Wildman DE, et al. Evolutionary history of the progesterone receptor in primates. J Soc Gynecol Invest. 2006;13:238A.
Wei LL, et al. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–87.
Condon JC, et al. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75.
Wei LL, Norris BN, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62:287–97.
Hirata S, et al. The novel isoform of the estrogen receptor-alpha cDNA (ERalpha isoform S cDNA) in the human testis. J Steroid Biochem Mol Biol. 2002;80:299–305.
Saner KJ, et al. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–63.
Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–87.
Madsen G, et al. Progesterone receptor or cytoskeletal protein? Reprod Sci. 2007;14:217–22.
Kumar R, et al. The clinical relevance of steroid hormone receptor corepressors. Clin Cancer Res. 2005;11:2822–31.
Lee K, et al. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol. 2006;102:41–50.
Spelsberg TC, Steggles AW, O’Malley BW. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971;246:4188–97.
Gao X, Loggie BW, Nawaz Z. The roles of sex steroid receptor coregulators in cancer. Mol Cancer. 2002;1:7.
Mukherjee A, et al. Steroid receptor coactivator 2 is essential for progesterone-dependent uterine function and mammary morphogenesis: insights from the mouse--implications for the human. J Steroid Biochem Mol Biol. 2006;102:22–31.
Fernandez-Valdivia R, et al. Progesterone-action in the murine uterus and mammary gland requires steroid receptor coactivator 2: relevance to the human. Front Biosci. 2007;12:3640–7.
McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–74.
Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71.
Xu J, et al. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279(5358):1922–5.
Han SJ, et al. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55.
Xu J, et al. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad.Sci.U.S.A. 2000;97:6379–84.
Heery DM, et al. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387(6634):733–6.
Aoyagi S, Archer TK. Dynamic histone acetylation/deacetylation with progesterone receptor-mediated transcription. Mol Endocrinol. 2007;21:843–56.
Luconi M, et al. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab. 1998;83:877–85.
Falkenstein E, et al. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+−fluxes in sperm. Endocrinology. 1999;140:5999–6002.
Patrat C, Serres C, Jouannet P. Induction of a sodium ion influx by progesterone in human spermatozoa. Biol Reprod. 2000;62:1380–6.
Turner KO, Meizel S. Progesterone-mediated efflux of cytosolic chloride during the human sperm acrosome reaction. Biochem Biophys Res Commun. 1995;213:774–80.
Finidori-Lepicard J, et al. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981;292(5820):255–7.
Grosse B, et al. Membrane signalling and progesterone in female and male osteoblasts. I. Involvement Of intracellular Ca(2+), inositol trisphosphate, and diacylglycerol, but not cAMP. J Cell Biochem. 2000;79:334–45.
Le Mellay V, Lieberherr M. Membrane signaling and progesterone in female and male osteoblasts. II. Direct involvement of G alpha q/11 coupled to PLC-beta 1 and PLC-beta 3. J. Cell Biochem. 2000;79:173–81.
Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–8.
Ishikawa K, et al. Primary action of steroid hormone at the surface of amphibian oocyte in the induction of germinal vesicle breakdown. Mol Cell Endocrinol. 1977;9:91–100.
Baulieu EE, et al. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978;275(5681):593–8.
Meizel S, Turner KO. Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol. 1991;77:R1–5.
Blackmore PF, Lattanzio FA. Cell surface localization of a novel non-genomic progesterone receptor on the head of human sperm. Biochem Biophys Res Commun. 1991;181:331–6.
Meyer C, et al. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–31.
Falkenstein E, et al. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229:86–9.
Krebs CJ, et al. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc. Natl. Acad.Sci.U.S.A. 2000;97:12816–21.
Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad.Sci.U.S.A. 2003;100:2237–42.
White HD, et al. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37:30–8.
Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–8.
Walch KT, Huber JC. Progesterone for recurrent miscarriage: truth and deceptions. Best Pract Res Clin Obstet Gynaecol. 2008;22:375–89.
Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–74.
Croy BA, et al. Decidual natural killer cells: key regulators of placental development (a review). J Reprod Immunol. 2002;57:151–68.
Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22.
Roche SL, et al. Progesterone attenuates microglial-driven retinal degeneration and stimulates protective Fractalkine-CX3CR1 signaling. PLoS One. 2016;11:e0165197.
Verma S, et al. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–68.
Roussev RG, Higgins NG, McIntyre JA. Phenotypic characterization of normal human placental mononuclear cells. J ReprodImmunol. 1993;25:15–29.
Chao KH, et al. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J ReprodImmunol. 1995;34:274–80.
Piccinni MP, Maggi E, Romagnani S. Role of hormone-controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans. 2000;28:212–5.
Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95.
Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103.
Eblen AC, et al. Alterations in humoral immune responses associated with recurrent pregnancy loss. Fertil Steril. 2000;73:305–13.
Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005;97:389–96.
Szekeres-Bartho J, et al. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am J Reprod Immunol Microbiol. 1985;9:15–8.
Kelemen K, et al. A progesterone-induced protein increases the synthesis of asymmetric antibodies. Cell Immunol. 1996;167:129–34.
Faust Z, et al. Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am J Reprod Immunol. 1999;42:71–5.
Laskarin G, et al. Progesterone induced blocking factor (PIBF) mediates progesterone induced suppression of decidual lymphocyte cytotoxicity. Am J Reprod Immunol. 2002;48:201–9.
Jabbour HN, et al. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46.
Gambino LS, et al. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod. 2002;17:1199–206.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–3.
Lerner LJ. Hormone antagonists: inhibitors of specific activities of estrogen and androgen. Recent Prog Horm Res. 1964;20:435–90.
Hsueh AJ, Peck EJ, Clark JH. Progesterone antagonism of the oestrogen receptor and oestrogen-induced uterine growth. Nature. 1975;254(5498):337–9.
Kirkland JL, Murthy L, Stancel GM. Progesterone inhibits the estrogen-induced expression of c-fos messenger ribonucleic acid in the uterus. Endocrinology. 1992;130:3223–30.
Lockwood CJ, et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann N Y Acad Sci. 1994;734:57–79.
Lockwood CJ, Krikun G, Papp C, Aigner S, Nemerson Y, Schatz F. Biological mechanisms underlying RU 486 clinical effects: inhibition of endometrial stromal cell tissue factor content. J Clin Endocrinol Metab. 1994;79:786–90.
Cibils LA. Contractility of the nonpregnant human uterus. Obstet Gynecol. 1967;30:441–61.
de Ziegler D, Bulletti C, Fanchin R, Epiney M, Brioschi PA. Contractility of the nonpregnant uterus: the follicular phase. Ann. N.Y. Acad. Sci. 2001;943:172–84.
Noe M, et al. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendometrial unit. Hum Reprod. 1999;14:190–7.
Akerlund M, Batra S, Helm G. Comparison of plasma and myometrial tissue concentrations of estradiol-17 beta and progesterone in nonpregnant women. Contraception. 1981;23:447–55.
Batra S, Sjoberg NO, Thorbert G. Sex steroids in plasma and reproductive tissues of the female Guinea pig. Biol Reprod. 1980;22:430–7.
Cano A, et al. Expression of estrogen receptors, progesterone receptors, and an estrogen receptor-associated protein in the human cervix during the menstrual cycle and menopause. Fertil Steril. 1990;54:1058–64.
Gorodeski GI. Effects of menopause and estrogen on cervical epithelial permeability. J.Clin.Endocrinol.Metab. 2000;85:2584–95.
Odeblad E. Physical properties of cervical mucus. Adv Exp Med Biol. 1977;89:217–25.
Snijders MP, et al. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil. 1992;94:363–71.
Odeblad E. The physics of the cervical mucus. Acta Obstet Gynecol Scand Suppl. 1959;38(Supp 1):44–58.
Odeblad E. Undulations of macromolecules in cervical mucus. Int J Fertil. 1962;7:313–9.
Croxatto HB. Mechanisms that explain the contraceptive action of progestin implants for women. Contraception. 2002;65:21–7.
Erkkola R, Landgren BM. Role of progestins in contraception. Acta Obstet Gynecol Scand. 2005;84:207–16.
Mesiano S. Roles of estrogen and progesterone in human parturition. Front Horm Res. 2001;27:86–104.
Tulchinsky D, Hobel CJ. Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17 alpha-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. Am J Obstet Gynecol. 1973;117:884–93.
Johansson ED. Plasma levels of progesterone in pregnancy measured by a rapid competitive protein binding technique. Acta Endocrinol. 1969;61:607–17.
Tulchinsky D, Okada D. Hormones in human pregnancy. IV. Plasma progesterone. Am J Obstet Gynecol. 1975;121:293–9.
Ohana E, et al. Maternal plasma and amniotic fluid cortisol and progesterone concentrations between women with and without term labor. A comparison. J Reprod Med. 1996;41:80–6.
Mazor M, et al. Maternal plasma and amniotic fluid 17 beta-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Arch Gynecol Obstet. 1996;258:89–96.
Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–61.
Stjernholm Y, et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol. 1996;174:1065–71.
Karim SM, Hillier K. Prostaglandins in the control of animal and human reproduction. Br Med Bull. 1979;35:173–80.
Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–96.
Mendelson CR, Condon JC. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol. 2005;93:113–9.
Mahendroo MS, et al. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–92.
Bernal AL. Overview of current research in parturition. Exp Physiol. 2001;86:213–22.
Bygdeman M, et al. The use of progesterone antagonists in combination with prostaglandin for termination of pregnancy. Hum Reprod. 1994;9(Suppl 1):121–5.
Puri CP, et al. Effects of progesterone antagonist ZK 98.299 on early pregnancy and foetal outcome in bonnet monkeys. Contraception. 1990;41:197–205.
Westphal U, Stroupe SD, Cheng SL. Progesterone binding to serum proteins. Ann N Y Acad Sci. 1977;286:10–28.
Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. NatMed. 1996;2:556–60.
Milewich L, et al. Initiation of human parturition. VIII. Metabolism of progesterone by fetal membranes of early and late human gestation. Obstet Gynecol. 1977;50:45–8.
Mitchell BF, Wong S. Changes in 17 beta,20 alpha-hydroxysteroid dehydrogenase activity supporting an increase in the estrogen/progesterone ratio of human fetal membranes at parturition. Am J Obstet Gynecol. 1993;168:1377–85.
Pieber D, et al. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–9.
Tan H, et al. Progesterone receptor-a and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–30.
Challis JRG, et al. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–50.
Rezapour M, et al. Sex steroid receptors and human parturition. Obstet Gynecol. 1997;89:918–24.
Fu X, et al. Unexpected stimulatory effect of progesterone on human myometrial contractile activity in vitro. Obstet Gynecol. 1993;82:23–8.
Fu X, et al. Antitachyphylactic effects of progesterone and oxytocin on term human myometrial contractile activity in vitro. Obstet Gynecol. 1993;82(4 Pt 1):532–8.
Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform a inhibits isoform B-mediated transactivation in human amnion. Eur J Pharmacol. 2001;427:7–11.
Mesiano S, et al. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor a expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–30.
Haukkamaa M. High affinity progesterone binding sites of human uterine microsomal membranes. J Steroid Biochem. 1984;20:569–73.
Karteris E, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–34.
Fernandes MS, et al. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J Endocrinol. 2005;187:89–101.
Nissenson R, Fluoret G, Hechter O. Opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc Natl Acad Sci U S A. 1978;75:2044–8.
Soloff MS, et al. Regulation of oxytocin receptor concentration in rat uterine explants by estrogen and progesterone. Can J Biochem Cell Biol. 1983;61:625–30.
Larcher A, et al. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology. 1995;136:5350–6.
Grazzini E, et al. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392(6675):509–12.
Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83.
Gimpl G, et al. Oxytocin receptors and cholesterol: interaction and regulation. Exp Physiol. 2000;85:41S–9S.
Debry P, et al. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J Biol Chem. 1997;272:1026–31.
Smart EJ, et al. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–35.
Metherall JE, Waugh K, Li H. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J Biol Chem. 1996;271:2627–33.
Gimpl G, Fahrenholz F. Human oxytocin receptors in cholesterol-rich vs cholesterol-poor microdomains of the plasma membrane. Eur J Biochem. 2000;267:2483–97.
Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–93.
Kofinas AD, et al. Progesterone and estradiol concentrations in nonpregnant and pregnant human myometrium Effect of progesterone and estradiol on cyclic adenosine monophosphate-phosphodiesterase activity. J Reprod Med. 1990;35:1045–50.
Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Phys. 1999;276(Pt 1):C379–85.
Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–81.
Lappas M, Rice GE. The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta. 2007;28:543–56.
Condon JC, et al. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–83.
Lappas M, Permezel M, Rice GE. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Endocrinol. 2007;193:269–77.
Mohan AR, et al. The effect of mechanical stretch on cyclooxygenase type 2 expression and activator protein-1 and nuclear factor-kappaB activity in human amnion cells. Endocrinology. 2007;148:1850–7.
Karalis K, et al. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254(5030):421–3.
Kalkhoven E, et al. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–24.
Allport VC, et al. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–6.
Hardy DB, et al. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–33.
Srivastava MD, Anderson DJ. Progesterone receptor expression by human leukocyte cell lines: molecular mechanisms of cytokine suppression. Clin Exp Obstet Gynecol. 2007;34:14–24.
Ito A, et al. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301(Pt 1):183–6.
Vidaeff AC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappaB signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23–8.
Condon JC, et al. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A. 2003;100:9518–23.
Dong X, et al. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem. 2005;280:13329–40.
Tyson-Capper AJ, Shiells EA, Robson SC. Interplay between polypyrimidine tract binding protein-associated splicing factor and human myometrial progesterone receptors. J Mol Endocrinol. 2009;43:29–41.
Xie N, et al. Expression and function of myometrial PSF suggest a role in progesterone withdrawal and the initiation of labor. Mol Endocrinol. 2012;26:1370–9.
Goldman S, et al. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: possible mechanism for functional progesterone withdrawal. Mol HumReprod. 2005;11:269–77.
Oh SY, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am JObstetGynecol. 2005;193(Pt 2):1156–60.
Mills AA, et al. Characterization of progesterone receptor isoform expression in fetal membranes. Am J Obstet Gynecol. 2006;195:998–1003.
Taylor AH, et al. The progesterone receptor in human term amniochorion and placenta is isoform C. Endocrinology. 2006;147:687–93.
Facchinetti F, et al. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453–4.
Marx SG, et al. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–39.
Giacalone PL, et al. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–4.
Chwalisz K, et al. Cervical ripening in Guinea-pigs after a local application of nitric oxide. Hum Reprod. 1997;12:2093–101.
Hegele-Hartung C, et al. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4:369–77.
Wolf JP, et al. Progesterone antagonist (RU 486) for cervical dilation, labor induction, and delivery in monkeys: effectiveness in combination with oxytocin. Am J Obstet Gynecol. 1989;160:45–7.
Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978;130:414–8.
Carbonne B, et al. Effects of progesterone on prostaglandin E(2)-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Mol Hum Reprod. 2000;6:661–4.
Glassman W, Byam-Smith M, Garfield RE. Changes in rat cervical collagen during gestation and after antiprogesterone treatment as measured in vivo with light-induced autofluorescence. Am J Obstet Gynecol. 1995;173:1550–6.
Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol. 1999;180(Pt 1):614–20.
Imada K, et al. An antiprogesterone, onapristone, enhances the gene expression of promatrix metalloproteinase 3/prostromelysin-1 in the uterine cervix of pregnant rabbit. Biol Pharm Bull. 2002;25:1223–7.
Osmers R, et al. Collagenase activity in the cervix of non-pregnant and pregnant women. Arch Gynecol Obstet. 1990;248:75–80.
Danforth DN, Buckingham JC, Roddick JW Jr. Connective tissue changes incident to cervical effacement. AmJObstetGynecol. 1960;80:939–45.
Winn RJ, Baker MD, Sherwood OD. Individual and combined effects of relaxin, estrogen, and progesterone in ovariectomized gilts. I. Effects on the growth, softening, and histological properties of the cervix. Endocrinology. 1994;135:1241–9.
Clark K, et al. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. Am J Obstet Gynecol. 2006;194:1391–8.
Cabrol D, et al. Prostaglandin E2-induced changes in the distribution of glycosaminoglycans in the isolated rat uterine cervix. Eur J Obstet Gynecol Reprod Biol. 1987;26:359–65.
Danforth DN, et al. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120:641–51.
Osmers R, et al. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol. 1993;81:88–92.
Imada K, et al. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod. 1997;56:575–80.
Sato T, et al. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem J. 1991;275(Pt 3):645–50.
Junqueira LC, et al. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–81.
Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukotrienes and Essential Fatty Acids. 2004;70:207–22.
Ramos JG, et al. Estrogen and progesterone modulation of eosinophilic infiltration of the rat uterine cervix. Steroids. 2000;65:409–14.
Barclay CG, et al. Interleukin-8 production by the human cervix. Am J Obstet Gynecol. 1993;169:625–32.
Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9.
Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19.
Mesiano S. Myometrial progesterone responsiveness. SeminReprodMed. 2007;25:5–13.
Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–70.
Yen SSC. In: SSC Y, Jaffe RB, editors. Endocrine-metabolic adaptation in pregnancy, in Reproductive endocrinology: W.B. Saunders, Philadelphia; 1991. p. 936–71.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vaisbuch, E., Erez, O., Romero, R. (2021). Physiology of Progesterone. In: Carp, H.J. (eds) Progestogens in Obstetrics and Gynecology. Springer, Cham. https://doi.org/10.1007/978-3-030-52508-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-52508-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52507-1
Online ISBN: 978-3-030-52508-8
eBook Packages: MedicineMedicine (R0)