Abstract
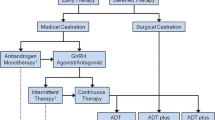
Androgen deprivation therapy (ADT) has been the standard of care for treating advanced prostate cancer for many decades. Prostate cancer cells are generally androgen-dependent, and most patients with advanced disease will respond to ADT in some form or another. Androgen deprivation therapy is utilized in the suppression of testosterone production. ADT may be used as a sole or primary treatment. It is utilized in men with prostate cancer who are of advanced age, who have significant comorbidities, and who decline curative therapy (Tanagho et al., Smith’s general urology. New York: McGraw-Hill Companies, Inc., 2008). There are four methods to block androgen: (1) removal of the sources of androgens, (2) use of LHRH agonists/GnRH antagonists, (3) use of antiandrogens, and (4) inhibition of androgen synthesis.
ADT has significant side effects such as hot flashes, decreased libido, osteoporosis, decreased sexual function and drive, and metabolic and cardiac effects. Most are mild to moderate and good quality of life can be achieved with its use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Androgen deprivation therapy
- Prostate cancer
- Advanced prostate cancer
- ADT side effects
- GnRH antagonist
- LHRH agonist
- Hormonal blockade
Introduction
Androgen deprivation therapy (ADT) has been the standard of care for treating advanced prostate cancer for many decades. Prostate cancer proliferation is, in part, attributed to male androgens. Androgen deprivation therapy is utilized in the suppression of testosterone production. Castrate levels of testosterone are defined as less than 50 ng/dl testosterone. The American Urologic Association is constantly revising its standards. Currently, 25 ng/ml of testosterone is being considered for a new castrate level. The first-line treatment methods of achieving castration target 90–95% of testosterone production in the testes. Many forms of hormone manipulation are used to achieve castrate levels of testosterone. These methods include surgical castration, luteinizing hormone-releasing hormone agonists, and gonadotropin-releasing hormone antagonists. Earlier use of synthetic estrogens, such as diethylstilbestrol (DES), was abandoned due to increased risk of mortality from cardiac causes [1].
There is a small amount of testosterone produced by the adrenal glands. The first-line agents do not suppress this production. This small amount of testosterone production is blocked by using steroidal and nonsteroidal antiandrogens. Complete testosterone blockade requires a multimodal approach.
As discussed in Chap. 5, it is the standard of care to offer adjuvant ADT to radiation as a primary treatment to patients with unfavorable intermediate-risk and high-risk, or very high-risk group [40]. Studies show that patients with higher risk disease undergoing radiation treatment have been shown to prolong survival with neoadjuvant and adjuvant ADT [2, 3]. Short-term ADT (4–6 months) is recommended in patients with intermediate disease. Long-term ADT (18–36 months) should be offered for high-risk and very high-risk patients [40]. One study compared the use of short-term ADT ( 4 months) versus long-term ADT (24 months) in men with locally advanced cancers (cT2c-T4N0-1M0 and Gleason scores 8–10). Men receiving long-term ADT to had an improved overall survival and disease specific. Short-term ADT consists of 2 months of neoadjuvant ADT and 2 months of adjuvant ADT [2]. Another study compared men with localized higher risk prostate cancer (PSA equal to or greater than 10 ng/mL and/or Gleason grade 7 or higher, cT3) receiving radiation monotherapy or combined radiation with 6 months of ADT. The study revealed that the overall and disease-specific survival rates were better in the men who received ADT [3].
ADT may be used as a sole or primary treatment. It is utilized in men with prostate cancer who are of advanced age, have significant comorbidities, and for those who decline curative therapy. One large study compared cancer-specific survival and overall survival in men with localized prostate cancer who received primary ADT versus men on surveillance. The study concluded that primary ADT did not provide a survival benefit in the majority of men compared to those on observation [4].
ADT has significant side effects such as hot flashes, decreased libido, osteoporosis, decline in sexual function and drive, and metabolic and cardiac effects. Most are mild to moderate and a good quality of life can be achieved with its use.
In this chapter, we review the general mechanism of action of ADT, side effect management, patient counseling, health lifestyle, and second-line hormonal manipulations.
Mechanism of Action
Regulation of Androgen Production
In order to understand the mechanism of action of ADT, it is important to review the testosterone production pathways. Testosterone is regulated by two mechanisms in the body. The hypothalamic–pituitary–gonadal (HPG) axis, see Fig. 7.1, is responsible for the production and regulation of testosterone by the testicles. The hypothalamic–pituitary–adrenal (HPA) axis, see Fig. 7.2, is responsible for a very small amount of testosterone released from the adrenal glands. Gonadotropin-releasing hormone (GnRH) and corticotrophin-releasing factor (CRF) are neurohormones produced in the hypothalamus. GnRH simulates in pulses the secretion of two gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland. CRF stimulates the release of adrenocorticotropic hormone (ACTH) also from the anterior pituitary gland. LH is responsible for 95% of circulating testosterone production through receptors on the surface of Leydig cells in the testes. ACTH acts on the adrenal glands to produce androstenedione and dehydroepiandrosterone. These intermediate metabolites become androgens that are more active in prostate tissue [5].
Testosterone levels are maintained within a narrow range by a negative feedback mechanism. When testosterone is decreased, GnRH and LH secretion are increased. When testosterone is elevated, GnRH and LH secretion are decreased. Continuous stimulation of the pituitary gland leads to an increase in the secretion of GnRH resulting in a decrease in secretion of LH and consequently a decrease in testosterone production.
Similarly, cortisol regulation is maintained by stimulation and inhibition of CRF and ACTH on the hypothalamus and pituitary gland [6, 7].
Agents of Treatment
Prostate cancer cells are generally androgen-dependent, and most patients with advanced disease will respond to ADT in some form or another [8]. There are four methods to block androgen: (1) removal of sources of androgens, (2) use of LHRH agonists/GnRH antagonists, (3) use of antiandrogens, and (4) inhibition of androgen synthesis.
Removal of Sources of Androgens
Bilateral orchiectomy historically was considered the gold standard for the treatment of advanced prostate cancer. Removal of the testes results in the inhibition of testosterone and dihydrotestosterone (DHT) and an increase in luteinizing hormone and follicle-stimulating hormone (FSH). This method results in a 95% reduction in testosterone hormone levels. One study revealed that greater than 90% of testosterone levels were reduced within 24 hours of castration [9]. Although more cost-effective, the major disadvantage of orchiectomy is its irreversibility. Treatment by orchiectomy should only be considered in men with advanced prostate cancer who require indefinite ADT.
Use of LHRH Agonists and GnRH Antagonists
An alternative to orchiectomy is pharmacologic androgen suppression using LHRH receptor agonists and GnRH antagonists. LHRH receptor agonists function to suppress androgens produced at the level of the testes, not the adrenal glands. Initially, agonist action causes a rise in LH, FSH, testosterone, and DHT. This elevation in hormone levels produces what is known as “testosterone surge” and may contribute to the exacerbation of clinical symptoms in men with bone metastases. LH increases up to ten-fold during the flare and may last as long as 10–20 days [10]. Testosterone flare is blocked by prior administration with an antiandrogen, which will be discussed later in this chapter.
The goal of androgen suppression is attaining a testosterone level less than 50 ng/dl within 3 weeks of administration. Chronic stimulation of the pituitary gland results in desensitizing and suppressing the LHRH receptors, which ultimately causes a decrease in hormone levels, see Fig. 7.3. FSH is only partially suppressed as compared to LH. FSH levels begin to rise after a few weeks to baseline concentration, which is known as the “FSH escape.” FSH receptors located on the surface of prostate cancer cells and blood vessels within tumors may promote progression of prostate cancer [11].
Examples of LHRH agonists, routes of administration, doses, and interval of administration are listed in Table 7.1. The most common agonist used is leuprolide acetate. It can be administered subcutaneously or intramuscularly.
GNRH receptor antagonists are the most recently introduced class of hormonal treatments. GnRH receptor antagonists rapidly and competitively bind to GnRH receptors in the pituitary, blocking the release of gonadotropins, causing a decrease in LH, FSH, and testosterone. See Fig. 7.4. Testosterone suppression occurs rapidly with the use of GnRH receptor antagonists and does not produce the testosterone flare associated with the receptor agonists; therefore, antiandrogens are not coadministered with the antagonists. GnRH antagonists are preferred for an initial treatment therapy for men with bone metastases. A recent study comparing the ability of GNRH antagonists and agonists to suppress FSH revealed that GNRH antagonists were superior at suppressing and maintaining lower FSH levels than the agonists, which could account for better prostate cancer control [12].
Firmagon® (degarelix) is the only GnRH receptor antagonist administered by subcutaneous injection monthly. The initial loading dose is 240 mg, given as two 120 mg injections administered simultaneously. The monthly maintenance dose is 80 mg, which is currently the only dose available.
The primary advantage of GnRH receptor antagonists is their ability to lower testosterone rapidly. In one clinical trial, the efficacy of degarelix compared to leuprolide in lowering testosterone to castrate levels was measured. During a period of 28 days, 620 patients were randomized to degarelix or leuprolide. Degarelix lowered testosterone to castrate levels in 52% of men after 1 day and 96% after 3 days. Leuprolide lowered testosterone to castrate levels in 18% of men after 14 days and 100% after 28 days [13].
Antiandrogens: Nonsteroidal and Steroidal Antiandrogens
There are two categories of antiandrogens: steroidal and nonsteroidal antiandrogens. Nonsteroidal antiandrogens, also called androgen receptor antagonists, bind to and inhibit the androgen receptor, thereby inhibiting activation of the receptor and limiting the androgens’ biological effects. Nonsteroidal antiandrogens do not inhibit the hypothalamic-pituitary axis from producing testosterone. Testosterone levels will not lower with the use of nonsteroidal antiandrogens. They are not recommended as a monotherapy for advanced prostate cancer.
First-generation nonsteroidal antiandrogens are often used as an adjuvant therapy with LHRH agonists to provide maximum androgen blockade. They are prescribed 2–3 weeks prior to LHRH receptor agonists to prevent the potential clinical effects of the testosterone flare in men with metastatic prostate cancer. Examples of nonsteroidal antiandrogens include bicalutamide, flutamide, and nilutamide. These are listed in Table 7.2 with their dosing information [14]. Enzalutamide and apalutamide, potent antiandrogens, will be discussed in another chapter.
Flutamide was the first nonsteroidal antiandrogen produced for the treatment of prostate cancer. Flutamide was prescribed in 250 mg doses three times daily due to its short half-life of 6 hours. Eliminated through renal excretion, flutamide is not recommended for patients with renal impairment. Flutamide is rarely used since the introduction of longer acting antiandrogens.
Another nonsteroidal antiandrogen, nilutamide, has a longer half-life of 56 hours. It is prescribed for once-daily dosing, which allows for better compliance. The initial dose is 300 mg for the first month, followed by 150 mg daily maintenance dose. Nilutamide should be taken with food. It is eliminated through hepatic clearance [15].
Bicalutamide is the most recently developed nonsteroidal antiandrogen. Bicalutamide is prescribed as a 50-mg dose for once a day. It has a half-life of 6 days. It may be taken with or without food. Metabolism occurs via the liver; however, patients with mild to moderate hepatic impairment are not excluded from taking bicalutamide [15]. Periodic liver function tests should be performed in patients with moderate hepatic impairment, who remain on chronic bicalutamide therapy. Bicalutamide is well tolerated with very few side effects. It is a more potent nonsteroidal antiandrogen than its predecessors, with a much greater binding affinity for the androgen receptor [16].
Steroidal antiandrogens inhibit the binding of dihydrotestosterone to prostate cancer cells. Unlike nonsteroidal antiandrogens, steroidal antiandrogens, such as cyproterone acetate (CPA), are involved in the negative feedback mechanism exerted on the hypothalamic–pituitary axis, which leads to decrease in LH and subsequently lowers testosterone. CPA is less effective at controlling prostate cancer than LHRH agonists and GnRH antagonists, and CPA has a higher side-effect profile including significant cardiovascular complications in up to 10% of men [17]. CPA is not available in the United States.
Antiandrogen Withdrawal
When a person has used combined androgen blockade using LHRH agonists and antiandrogens, a withdrawal effect can occur when the antiandrogen is removed from the combination. This withdrawal effect results in a decrease in the PSA. In this setting, a possible mutation in the androgen receptor allows the antiandrogen activity to shift from an antagonist to an agonistic exertion on prostate cancer cells [18]. This withdrawal phenomenon has been shown in flutamide, nilutamide, and bicalutamide antiandrogens. PSA decrease after flutamide withdrawal has been seen within 4 weeks, whereas nilutamide and bicalutamide withdrawal yielded a PSA decrease within 6 weeks [19]. Two studies revealed that antiandrogen withdrawal effect on PSA occurred in 15–30% of patients with a more than a 50% decrease in PSA level over an average of 3.5–5 months [20, 21].
Side Effects Management
Androgen deprivation therapy (ADT) has side effects for most men. The common side effects vary in intensity, frequency, and duration but most side effects reported in clinical trials were mild to moderate [13, 24]. Common side effects in order of their frequency include hot flashes, injection site pain, fatigue, weight gain, muscle loss, weakness, decrease of libido, sexual dysfunction, depression, osteoporosis, heart disease, and uncontrolled blood sugar [25]. The side effects in this discussion are not an exhaustive list. There are other side effects such as cognitive loss, gynecomastia, or anemia to name a few. They are not discussed in this chapter, as their rate of occurrence is very low and generally not a significant deterrent to treatment.
The most common side effects in the use of ADT are hot flashes. These are a vasomotor symptom of decreased testosterone. They are described as a short burst of heat generally originating in the head with or without perspiration [26]. They range in intensity, duration, and frequency, but the vast majority fall in the mild to moderate category. The rate of reported hot flashes varies depending on the drug given to start with and the study reviewed. Reports of the rate of hot flashes range from 20% to 88% of men depending on the drug and the study being reviewed [24, 26]. It is safe to assume that more than 30% of patients using ADT will experience hot flashes.
Patients report more hot flashes within the first 3 months with degarelix than leuprolide. This is due to the antagonistic effect of degarelix and the speed with which it reduces testosterone compared to slower acting leuprolide. After 3 months, it is unlikely for a patient to start experiencing hot flashes.
The main risk factor for developing hot flashes appears to be with men who have a higher BMI or slower heart rate. The exact reason for these predictors is speculative. Having a higher BMI is associated with lower testosterone levels at baseline and may hasten the suppression. For heart rate, it is thought that hypothyroid conditions may play a role in contributing to hot flashes [26].
There are several ways to manage hot flashes. The method of management should be commensurate with the severity, i.e., the more severe the hot flashes, the more invasive the treatment.
Studies in Europe have shown that cyproterone acetate, an antiandrogen/progestin, has a 95% success rate in resolving hot flashes. Cyproterone acetate is not used as a first line regardless of its efficacy in reducing hot flashes because it can interfere with the ADT therapy [25, 27].
Medroxyprogestrone acetate is 83% effective in resolving hot flashes and is considered the first-line treatment for hot flashes [25, 27]. Since depomedroxyprogesterone acetate (Depo-provera®) is readily available in 150 mg IM doses in the United States, it is most often dosed at 300IM q6mos as needed for hot flashes. Be aware that depomedroxyprogesterone has the potential for serious side effects. Thromboembolism, breast cancer, depression, seizures, bone density loss, and hepatic impairment are just a few [39].
Venlafaxine ER 75 mg daily is about 47% effective at reducing and/or eliminating hot flashes [27]. Venlafaxine has serious side effects of its own such as suicidal ideation and worsening depression, serotonin syndrome, seizures, and arrhythmias to name a few. Initially, close follow-up after starting this medication is recommended. This medicine must not be stopped abruptly, so refills need to be maintained on time and the patient needs to be compliant and reliable [28].
Alternative or complementary treatments such as soy, black cohosh, or Mexican yam have some anecdotal effect. However, this may be a placebo effect. In a very small study of 33 men undergoing ADT, soy protein did not show improvement in vasomotor symptoms. Acupuncture was tested and found to be effective with 95% improvement, but none of the acupuncture studies was randomized or placebo-controlled [25].
Injection site pain occurs more often with degarelix (35%) as opposed to leuprolide (1%). When experienced it is mostly mild to moderate and is usually in the first injection only. The first injection of degarelix is two180 mg doses vs. one 80 mg dose for maintenance. This medication forms a disc or nodule under the skin and is more viscous than a traditional vaccine. The best management is patient education. Tylenol is sufficient to help with residual pain. Over-the-counter hydrocortisone cream can also help with redness.
Fatigue is a noticeable side effect of ADT that can affect the quality of life. Fatigue, as a symptom of hypogonadism, is so recognizable that it is used as a marketable tool for men’s health clinics. Fatigue is reported about 3–11% depending on the study referencing [13, 24]. The only evidence-based management for ADT-induced fatigue is exercise. In a systematic review of all peer-reviewed articles published between 1980 and 2013 focusing on exercise and the treatment-related adverse effects of prostate cancer, none of the outcomes reported worsening fatigue with exercise. All studies reviewed showed improvement or equivocal results. The studies that showed improvement varied from as little as 30 min 3 days a week to 60 min 3–5 days a week. The one commonality the positive studies had was some form of resistance training to improve muscle mass/definition [29, 31].
After only 9 months of ADT, about 80% of men will have a decrease in bone mineral density (BMD) [32]. A decrease in BMD leads to osteopenia, osteoporosis, and increased risk for fractures. The risk for fractures increases with longer duration of use. The National Comprehensive Cancer Network (NCCN) guidelines recommend men have a serum 25-hydroxy vitamin D and a DEXA bone scan for baseline information [33]. The National Osteoporosis Foundation recommends men older than 50 years of age, have a daily intake of 1200 mg calcium, and 800–1000 IU vitamin D [25]. It is reasonable to recommend patients start a calcium and vitamin D supplement such as Caltrate® or Citracal®. The upper limit of safe vitamin D3 supplement in a 50-year-old or older with normal vitamin D levels is 4000 IU daily. If vitamin D levels drop below normal (<20 ng/ml), the patient should be replenished [33]. In high-risk patients, such as ADT-treated patients, vitamin D is often replaced when levels drop below 30 ng/ml. vitamin D3 is sold in stores in 2000 IU and 4000 IU doses. The common practice is to recommend 2000 IU of vitamin D3 in addition to the calcium and vitamin D supplement. If a severe deficiency occurs <10 ng/ml, 50,000 IU of vitamin D2 once a week should be prescribed. Repeat 25-hydroxy levels 3 months after treatment, is recommended to ensure they have returned to normal. If vitamin D levels fail to increase with over-the-counter D3 supplementation, 50,000 IU of vitamin D2 weekly should be prescribed. Patients who have 25-hydroxy serum levels <10 ng/ml at the baseline evaluation are at risk for osteomalacia, referral to their primary care for further evaluation is recommended [33].
There is no evidence-based guideline set by the AUA or NCCN on how often vitamin D should be monitored. The NCCN suggests yearly screening at a minimum. Conservative management practices choose to monitor every 6 months.
The NCCN guidelines recommend denosumab (60 mg subcutaneously every 6 months), for men who have a 10-year risk of fracture ≥3%, based on the Fracture Risk Assessment Tool (FRAX) algorithm, or if the baseline DEXA scan shows osteopenia. The FRAX algorithm can be accessed at https://www.sheffield.ac.uk/FRAX/. When using the FRAX algorithm, select yes for “secondary osteoporosis” due to ADT [25, 34].
A cascade of side effects occurs with the increase in fat mass, loss of lean muscle mass, and increase in waist circumference. Men are at risk of having metabolic syndrome, which is a significant risk factor for diabetes and heart disease [35]. Studies have shown that as little as 12 weeks of ADT can result in a decrease in insulin sensitivity and an increase in plasma insulin. In multiple studies, greater than 1 year of ADT, 28–44% of the men had fasting glucose in the diabetic range [25]. No definitive prevention or guidelines have been set to address the risk of diabetes. Some preliminary studies have shown positive outcomes with metformin usage [25, 30]. Prescribing this is not the standard of care to date, and managing diabetes or prediabetes is not in the purview of urology.
Regular monitoring of blood glucose level every 6 months is a sound clinical decision. Also working in concert with a patient’s primary care provider will help ensure patient health while on ADT.
The FDA has required the drug manufacturers of leuprolide to list an association with increased risk in cardiac harm. The studies regarding this have mixed results. There are multiple studies to show an association between ADT use and increased incidents of coronary artery disease (CAD), myocardial infarction (MI), and congestive heart failure (CHF). The cause for this is unknown. Lack of testosterone alone may not be the culprit. Men who have undergone orchiectomies do not show an increased risk for CAD. This would suggest the problem lies with the pharmacology of the medication or a secondary problem arising from the metabolic effects of weight gain, decreased lean muscle mass, increase in central obesity, and increase in serum lipids. All of which have known cardiovascular risk factors. The use of ADT does not increase the overall morbidity due to CAD unless the patient had CAD, CHF, or a history of MI prior to start of ADT [25, 30, 36]. Careful patient selection and monitoring are key to the prevention of CAD complications.
The use of ADT can significantly affect the quality of life with decreased libido and erectile dysfunction. This can lead to loss of feeling of masculinity and depression [37].
Decrease in testosterone results in a loss of libido. Over time, it can increase venous leakage, decrease arterial flow, and impair nitric oxide leading to sexual dysfunction. There is also significant atrophy of the penis and testes [25]. This can lead to feelings of loss of masculinity, particularly in men who are younger and define masculinity with sexual function. Men who have erectile dysfunction prior to treatment due to age, medication, or comorbid conditions are less bothered by this. Loss of sexual function can be particularly stressful in men who are single or not in long-term relationships. Navigating a new relationship can be tricky when one partner cannot perform sexually and may lead men to avoid social situations or dating. Men in relationships, also worry about their partner leaving them if they cannot satisfy them [37].
This loss of masculinity and self-identity can lead to depression. Men should be screened for depression and thoughts of suicide. If patients are willing to admit to thoughts of depression, sadness, or loss of self-worth, referral to their primary care providers for antidepressants is recommended. Three studies have shown that moderate to high-intensity exercise can improve sexual function in men. This translates to ≥3000 kcal/week or 450 kcal/day [30]. The average man would need to walk briskly for an hour a day to achieve this. That is a lot of exercise, and most Americans do not have the self-discipline or physical health to accomplish this.
Healthy Lifestyle
It is important that patients adopt a healthy lifestyle while receiving ADT. Exercise, both aerobic and strength training, is important in mitigating side effects such as fatigue, erectile dysfunction, and hot flashes. Weight-bearing exercises are important for bone density. Consistent aerobic exercise is a known benefit for cardiovascular health. Diet, as a solution for the side effects of ADT, is under continual study. Challenges arise when studying diet, mainly in compliance and reporting. There are to date no known foods that will reverse hot flashes or improve ED [29]. We know that patients who have a waist circumference of greater than 40 inches are at higher risk for metabolic syndrome and CAD. A heart-healthy diet low in refined carbohydrates and sugars can help reduce waist circumference. We have discussed that patients with a BMI greater than 30 and regular alcohol consumption are more prone to hot flashes. Adopting a healthy lifestyle with exercise and diet are the keys to reducing the risks of side effects of ADT, but there is no proof that these will eliminate them all together [25, 30]. At the end of the day, eating healthy and exercising regularly will improve a patient’s outlook and mood. That may be the best way to ensure good quality of life despite the side effects.
Patient Counseling
Many large urology groups have dedicated Advance Prostate Cancer Clinics (APCC) where an MD or an APC spends 30–40 minutes educating patients when they start an ADT medication. It is important to manage patient expectations when starting this medication. Patients should be aware of all of the side effect potentials with this treatment.
By educating the patients about the risks that ADT presents, they can keep open dialogue with all their providers.
Knowing the cardiac and metabolic effects can help patients understand the role this treatment will play in managing their existing cardiac or diabetic conditions. Feelings of depression or loss of quality of life can be discussed without shame when patients know the medicine is causing their problems. Patients need to know there are potential solutions before the symptoms arise to prevent frustration and noncompliance with treatment.
Discussing sexual function and depression are sensitive intimate discussions. Taking time to review this treatment helps the provider and patient build a relationship that fosters open communication. This will lead to more “shared decision-making” (SDM). SDM leads to higher patient satisfaction scores, and patients report better quality of life if they feel they have chosen their treatments. When patients feel that they have control of their healthcare, they will be more likely to be compliant with treatment regimens [38].
Second-Line Hormone Manipulation
Ketoconazole
Historically, second-line therapies such as ketoconazole have been used off-label in men with castrate-resistant prostate cancer before and after chemotherapy. One example of a second-line hormonal therapy is ketoconazole, which is a nonselective steroid 17α-hydroxylase/17, 20 lyase (CYP17A1) inhibitor that blocks the synthesis of adrenal testosterone. It is prescribed at 200 mg or 400 mg three times daily along with prednisone due to adrenal suppression. Testosterone suppression to castrate levels is immediate. One study noted that castrate levels of testosterone occurred within 4 hours [22]. Currently, ketoconazole is utilized as palliative therapy for patients with advanced prostate cancer with symptomatic spinal cord compression and in a setting with limited access to GnRH receptor antagonists [23]. More recent second-line therapies (abiraterone, enzalutamide, and apalutamide) have superseded the need for ketoconazole beyond the urgent need for castration in the symptomatic spinal cord compression patient and will be discussed in another chapter.
Clinical Pearls
-
Start ADT treatment with a GNRH antagonist or a nonsteroidal antiandrogen if the patient is metastatic to avoid “surge.”
-
Choose ADT recipients carefully. Men with uncontrolled diabetes or heart disease may not be good candidates.
-
Monitor bone health with DEXA scans and serum vitamin D.
-
Side effects can be improved with exercise and heart-healthy diet.
References
Nelson JB. Hormone therapy for prostate cancer. In: Campbell-Walsh urology, vol. 3. 10th ed. Amsterdam: Elsevier Inc; 2012. p. 2935.
Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM, Shipley WU, Radiation Therapy Oncology Group. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21(21):3972–8.
D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–7.
Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, Yao SL. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300(2):173–81. https://doi.org/10.1001/jama.300.2.173.
Denmeade SR, Isaacs JT. Overview of regulation of systemic androgen levels. In: Holland-Frei cancer medicine. 6th ed. Hamilton: BC Decker Inc; 2003.
Up To Date. Physiology of gonadotropin-releasing hormone. http://www.uptodate.com. Accessed 29 Dec 2018.
Up To Date. Adrenal steroid biosynthesis. http://uptodate.com. Accessed 29 Dec 2018.
Tanagho EA, McAninch JW. Smith’s general urology. 17th ed. New York: McGraw-Hill Companies, Inc; 2008. p. 367.
Maatman TJ, Gupta MK, Montie JE. Effectiveness of castration versus intravenous estrogen therapy in producing rapid endocrine control of metastatic cancer of the prostate. J Urol. 1985;133(4):620–1.
Weckermann D, Harzmann R. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. Eur Urol. 2004;46(3):279–83.
Lepor H, Shore ND. LHRH agonists for the treatment of prostate cancer: 2012. Rev Urol. 2012;14(1–2):1–12.
Crawford ED, Tombal B, Boccardo F, Miller K, Shore N, Moul JW, Damber JE, Collette L, Persson BE. FSH suppression and tumour control in patients with prostate cancer during androgen deprivation with a GnRH agonist or antagonist. Scand J Urol. 2019;9:1–9. https://doi.org/10.1080/21681805.1522372.
Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schroder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–8. https://doi.org/10.1111/j.1464-410X.2008.08183.x.
Up To Date. ADT plus first-generation antiandrogens. Http://www.uptodate.com. Accessed 16 Jan 2019.
Mahler C, Verhelst J, Denis L. Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer. Clin Pharmacokinet. 1998;34(5):405–17.
Mukherjee A, Kirkovsky L, Yao XT, Yates RC, Miller DD, Dalton JT. Enantioselective binding of Casodex to the androgen receptor. Xenobiotica. 1996;26(2):117–22.
de Voogt HJ, Smith PH, Pavone-Macaluso M, DePauw M, Suciu S. Cardiovascular side effects of diethylstilbestrol, cyproterone acetate, medroxyprogesterone acetate and estramustine phosphate used for the treatment of advanced prostate cancer: results from European Organization for Research on Treatment of Cancer trials 30761 and 30762. J Urol. 1986;135(2):303–7.
Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–8.
Nieh PT. Withdrawal phenomenon with the antiandrogen casodex. J Urol. 1995;153(3 Pt 2):1070–2.
Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149(3):607–9.
Small EJ, Srinivas S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer. 1995;76(8):1428–34.
Trachtenberg J, Halpern N, Pont A. Ketoconazole: a novel and rapid treatment for advanced prostate cancer. J Urol. 1983;130(1):152–3.
Patel V, Liaw B, Oh W. The role of ketoconazole in current prostate cancer care. Natl Rev Urol. 2018;15(10):643–51. https://doi.org/10.1038/s41585-018-0077-y.
Spitz A, Young JM, Larsen L, Mattia-Goldberg C, Donnelly J, Chwalisz K. Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2012;15(1):93–9. https://doi.org/10.1038/pcan.2011.50. Epub 25 Oct 2011. PubMed PMID: 22025196; PubMed Central PMCID: PMC3278745.
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36. Epub 2 Aug 2014. Review. PubMed PMID: 25097095. https://doi.org/10.1016/j.eururo.2014.07.010.
Iversen P, Karup C, van der Meulen E, Tankó LB, Huhtaniemi I. Hot flushes in prostatic cancer patients during androgen-deprivation therapy with monthly dose of degarelix or leuprolide. Prostate Cancer Prostatic Dis. 2011;14(2):184–90. Epub 29 Mar 2011. PubMed PMID: 21445092. https://doi.org/10.1038/pcan.2011.11.
Irani J, Salomon L, Oba R, Bouchard P, Mottet N. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial. Lancet Oncol. 2010;11(2):147–54. Epub 4 Dec 2009. PubMed PMID: 19963436. https://doi.org/10.1016/S1470-2045(09)70338-9.
Up To Date. Venlafaxine drug information Lexicomp. 1978-2018. http://www.uptodate.com. Accessed 12 Dec 2018.
Moyad MA, Newton RU, Tunn UW, Gruca D. Integrating diet and exercise into care of prostate cancer patients on androgen deprivation therapy. Res Rep Urol. 2016;8:133–43. eCollection 2016. Review. PubMed PMID: 27574584; PubMed Central PMCID: PMC4993404. https://doi.org/10.2147/RRU.S107852.
Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32(4):335–46. Epub 16 Dec 2013. Review. PubMed PMID: 24344218. https://doi.org/10.1200/JCO.2013.49.5523.
Yu EY, Kuo KF, Gulati R, Chen S, Gambol TE, Hall SP, Jiang PY, Pitzel P, Higano CS. Long-term dynamics of bone mineral density during intermittent androgen deprivation for men with nonmetastatic, hormone-sensitive prostate cancer. J Clin Oncol. 2012;30(15):1864–70. Epub 9 Apr 2012. PubMed PMID: 22493411; PubMed Central PMCID: PMC3383183. https://doi.org/10.1200/JCO.2011.38.3745.
Up To Date. Vitamin D deficiency. http://www.uptodate.com. Accessed 12 Dec 2018.
Principals of androgen deprivation therapy. NCCN guidelines version 3.2016, Prostate cancer. 2016. Http://NCCN.org. Accessed 12 Dec 2018.
Ziehr DR, Chen MH, Zhang D, Braccioforte MH, Moran BJ, Mahal BA, Hyatt AS, Basaria SS, Beard CJ, Beckman JA, Choueiri TK, D'Amico AV, Hoffman KE, Hu JC, Martin NE, Sweeney CJ, Trinh QD, Nguyen PL. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015;116(3):358–65. Epub 29 Oct 2014. PubMed PMID: 25124891. https://doi.org/10.1111/bju.12905.
Nguyen PL, Jarolim P, Basaria S, Zuflacht JP, Milian J, Kadivar S, Graham PL, Hyatt A, Kantoff PW, Beckman JA. Androgen deprivation therapy reversibly increases endothelium-dependent vasodilation in men with prostate cancer. J Am Heart Assoc. 2015;4(4):e001914. PubMed PMID: 25896892; PubMed Central PMCID: PMC4579953. https://doi.org/10.1161/JAHA.115.001914.
Jones JM, Kohli M, Loprinzi CL. Androgen deprivation therapy-associated vasomotor symptoms. Asian J Androl. 2012;14(2):193–7. Epub 30 Jan 2012. Review. PubMed PMID: 22286861; PubMed Central PMCID: PMC3338189. https://doi.org/10.1161/JAHA.115.001914.
Chambers SK, Chung E, Wittert G, Hyde MK. Erectile dysfunction, masculinity, and psychosocial outcomes: a review of the experiences of men after prostate cancer treatment. Transl Androl Urol. 2017;6(1):60–8. Review. PubMed PMID: 28217451; PubMed Central PMCID: PMC5313306. https://doi.org/10.21037/tau.2016.08.12.
Martinez LS, Schwartz JS, Freres D, Fraze T, Hornik RC. Patient-clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Educ Couns. 2009;77:384.
Pfizer.com, Depo Provera injection. http://labeling.pfizer.com/ShowLabeling.aspx?id=522. Accessed 2 Nov 2019.
NCCN Guidelines version 4.2018, prostate cancer, risk stratification and staging work up, p 11–17. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 2 Dec 2019.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Reyes, C., Groshel, C., Given, R. (2021). Androgen Deprivation Therapy. In: Trabulsi, E.J., Lallas, C.D., Lizardi-Calvaresi, A.E. (eds) Chemotherapy and Immunotherapy in Urologic Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-52021-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-52021-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52020-5
Online ISBN: 978-3-030-52021-2
eBook Packages: MedicineMedicine (R0)