Abstract
Following the initial treatment of prostate cancer, men can be divided into one of three monitoring categories: no evidence of disease, disease status unknown (biochemical recurrence), and evidence of recurrence (measurable disease). Irrespective of initial treatment, monitoring is based on interval measurement of serum prostate-specific antigen (PSA). Multiple options for monitoring these men should be considered based on the patient’s pathology at diagnosis, stage, and primary treatment. Two national guidelines provide evidence-based guidance for the care of these patients. Concern for biochemical recurrence or recurrent disease is based on a rise, and ultimately, the doubling time of PSA. Additional diagnostic testing is then obtained to differentiate biochemical recurrence versus measurable disease. Chest imaging, computerized tomography (CT) or magnetic resonance imaging (MRI), and bone imaging are used to characterize a metastatic disease. If imaging studies fail to identify metastases, positron emission tomography (PET)-CT or PET-MRI can be considered. When imaging identifies probable metastatic disease, then histologic confirmation is obtained to guide additional oncologic treatment. The particular pathway and its timing are influenced by multiple factors such as risk level, PSA doubling time, age, and general health. Throughout this process, shared decision-making guides the individual pathway and timing of ongoing monitoring, additional diagnostic evaluation for metastatic disease, and treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prostate cancer
- Prostate cancer recurrence
- Salvage therapy
- Surveillance
- Biochemical recurrence
- Prostate-specific antigen monitoring
- Prostate-specific antigen doubling time
Introduction
In 2018, approximately 174,650 men will be diagnosed with prostate cancer and 31,620 will die because of prostate cancer [1]. Research has shown that men with prostate cancer are living longer following initial treatment. Comparison of cancer-free survival among men undergoing surgery versus radiotherapy is challenging because recurrence is defined differently based on treatment modality. For men undergoing radical prostatectomy as definitive therapy 28–35% will experience a rising prostate-specific antigen (PSA)/biochemical recurrence within 10 years [2, 3]. Similarly, among men managed with radiotherapy for initial treatment, 28–39% will develop a rising PSA/biochemical recurrence within 5 years [4]. Regardless of initial treatment or PSA/biochemical recurrence, 10-year cancer-specific survival rates are 92% for men treated with radical prostatectomy and 92% for radiotherapy with androgen deprivation therapy (ADT), versus for 88% for radiotherapy alone [5].
Monitoring men with prostate cancer may take multiple forms based on the patient’s pathology at diagnosis, stage, and primary treatment. Advanced practice providers (APPs) are increasingly playing a key role in the monitoring, management, and treatment of men with prostate cancer following primary intervention. In addition, APPs are providing care for men experiencing recurrence of prostate cancer, and they are using evidence-based guidelines for surveillance of these patients. This chapter will provide an overview of evidence and guideline-based approaches for monitoring and managing men who have completed initial staging and definitive treatment of prostate cancer, including those with recurrent prostate cancer. We will not discuss active surveillance prior to primary therapy or men who are not candidates for primary local therapy due to N1 or widely metastatic disease at initial diagnosis.
Monitoring Categories
Following the initial treatment of prostate cancer, men can be divided into one of three categories: no evidence of disease, disease status unknown (biochemical recurrence), and evidence of recurrence (measurable disease). Men categorized as having no evidence of disease have undergone surgery or radiation therapy for organ-confined disease, have a serum PSA that is deemed undetectable following surgery, or have a PSA within acceptable limits following radiotherapy over a defined period. Men with persistence or recurrence of cancer after radical prostatectomy have a detectable PSA increasing on two or more measurements [6]. In contrast, men with persistent or recurrence of cancer following radiotherapy have a PSA rise of 2 ng/ml above the nadir achieved after radiation. The Phoenix Consensus goes further and states that men with a rise in PSA above nadir, even if it has not risen 2 ng/ml, should be characterized as recurrent or persistent prostate cancer [4]. Men with a measurable disease have radiographic or biopsy-proven evidence of recurrence.
We searched the literature and identified two evidence-based, national guidelines for surveillance and management of men in these three categories. The National Comprehensive Cancer Network guidelines provide guidance for monitoring after initial management (definitive therapy) and for patients with recurrence [6]. In addition, 2017 guidelines from the American Urological Association (AUA), American Society for Radiation Oncology (ASTRO), and Society of Urologic Oncology (SUO) provide recommendations similar to the NCCN for monitoring men with recurrent disease in their combined guideline on clinically localized prostate cancer [7].
Monitoring for Biochemical Recurrence
Regardless of definitive treatment, monitoring for persistence or recurrence begins with the measurement of serum PSA. The timing of the first PSA after prostatectomy is based on the knowledge that its half-life is 2.5–3 days [8, 9]. Men with a higher pretreatment PSA may require a longer time period to achieve an undetectable level. The NCCN guidelines do not specify a time frame for measurement of the baseline PSA after initial treatment [6]. Nevertheless, a majority of clinicians obtain a serum PSA 4–6 weeks after surgery, although some delay as long as 12 weeks [8, 9].
Identifying persistence or recurrence of prostate cancer is more challenging following radiotherapy. Unlike men managed with surgery, the PSA will slowly fall to a nadir over a period of as long as 2–3 years, but it rarely falls to an undetectable level and ultimate treatment success is defined as a stable PSA ≥1.0 ng/ml [10]. Digital rectal examination (DRE) is also used to monitor for recurrence or persistence unless the PSA value is undetectable. In addition, 10–30% of men will experience a temporary elevation in PSA levels, without evidence of disease recurrence [11, 12]. This bounce in PSA may take up to 18 months to normalize or reach a new nadir. Because of this variability, NCCN guidelines suggest PSA measurement every 3–6 months. However, practice patterns vary widely based on expert opinion and patient’s risk factors.
Men defined as having no disease after radical prostatectomy, will undergo PSA measurement every 6–12 months for 5 years, then annually thereafter [6]. In men who are treated with radiotherapy and have an undetectable PSA, the DRE may be omitted. Some providers choose to monitor PSA every 3 months for 1 year following definitive therapy based on surgical pathology or other pretreatment risk factors. These include a pretreatment PSA >10 ng/ml, Gleason score ≥ 8, positive margins, perineural invasion, or positive lymph nodes on surgical pathology.
As noted earlier, differentiation of persistence versus recurrence is influenced by the initial treatment. For men undergoing radical prostatectomy (RP), biochemical recurrence is defined as an undetectable PSA after surgery that becomes detectable and subsequently rises on two or more measurements [8, 9]. In contrast, persistence is defined as failure of PSA to reach undetectable levels after radical prostatectomy. For men who underwent radiation therapy, persistent and recurrence are often used interchangeably when the PSA rises above nadir following radiation therapy versus failure to reach an acceptable nadir [3].
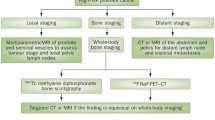
Men with rising PSA levels following initial surgery or radiation are the second largest group of men with prostate cancer [13]. Prostate-specific antigen doubling time (PSADT) is defined as the number of months needed for serum PSA to increase twofold [13]. It is used as first-line monitoring for recurrence because a change in PSA levels, rather than the absolute baseline value, may be the only manifestation indicating biochemical recurrence. Of the factors used to predict local versus systemic progression, PSADT is essential because it enables providers to differentiate a local disease that may still be curable from a systemic disease that may not. There are multiple nomograms and techniques for measuring PSADT. Nevertheless, all require measurement of more than two PSA values, preferably over a time period of 12 months or greater. A diagnosis of recurrence is not only based on PSADT, it also incorporates findings from chest imaging (chest X-ray or computerized tomography [CT]), abdominopelvic CT or magnetic resonance imaging (MRI), bone imaging (whole-body bone scan), and/or transrectal ultrasound (TRUS) for recurrence [6]. If imaging studies suggest recurrence, prostate bed biopsy may be considered. This principle is based on evidence that a doubling time <12 months is a key indicator for obtaining further diagnostic testing for metastatic disease [13,14,15].
Risk Stratification and Additional Testing
Approximately, 15% of men with prostate cancer are considered as high risk for disease progression and metastases [16]. Multiple criteria have been used to identify this subgroup of men. Many providers use the D’Amico et al. taxonomy which has been adopted by the American Urological Association (AUA) [17, 18]. These criteria are clinical T stage ≥cT2c, Gleason score 8–10, or a PSA >20 ng/ml at the time of diagnosis. The NCCN defines high risk as T3a, Gleason score ≥ 8, or PSA ≥ 20 ng/ml, and very high risk as T3b or T4 disease [19]. The Cancer of the Prostate Risk Assessment (CAPRA) score is similar but it incorporates the percentage of positive biopsy scores in its assessment of risk. The shortcoming of these taxonomies is their potential for inaccurate determination of T stage [16].
Patients with biochemical progression and concerning PSADT require further evaluation to detect metastases. This evaluation includes imaging and/or tissue biopsy. The goal of imaging is to detect and characterize metastatic disease in order to select treatment or guide a change in management. Imaging techniques are used to evaluate anatomic or functional parameters. The selection of a specific test is based on risk level, PSADT, age, and general health. While prostate cancer can metastasize anywhere in the body, the most common sites are the lymph nodes, bones, lungs, and liver. The following tests may be used to identify and localize distant metastases in the face of PSA persistence/recurrence: chest X-ray or CT, bone imaging (whole-body bone scan), abdominopelvic CT or MRI, TRUS, C-11 choline or F-18 fluciclovine PET-CT or PET-MRI, molecular assay, or prostate bed biopsy [6]. Understanding the patient’s risk level is useful because it helps the clinician to decide which test and in which order to proceed when evaluating disease progression or metastatic disease.
Chest imaging begins with a chest X-ray; it is cost-effective and provides a reasonable screening strategy before considering CT. Findings from the chest X-ray are considered definitive only when it is read as entirely normal. Any abnormality (bone lesions or fractures, concern for nodules, fluid collections, and opacities in the lungs) provides a basis for obtaining a chest CT. In the presence of abnormalities on a chest X-ray, the CT is obtained because it provides definitive cross-sectional imaging needed to identify lesions of concern for metastatic disease. These findings are essential for determining the need for potentially morbid biopsy for histologic confirmation of metastatic disease. Nevertheless, patients at high risk may proceed to chest CT as the initial study.
In addition to chest imaging, abdominopelvic CT or MRI should be performed because they provide a high level of detail for the detection of gross extracapsular disease, nodal disease, or visceral metastases. Typically, providers choose a CT, but an MRI is a viable alternative because of its ability to provide high soft-tissue contrast/characterization and multiparametric image acquisition.
The initial test used for the detection of bony metastases is the whole-body bone scan [6]. A radionuclide tracer (technetium-99-MDP) is injected to identify areas of increased uptake in the bones, implying increased osseous turnover and possible metastatic disease. If the bone scan is negative, but clinical suspicion of bone metastases persists, an F-18 sodium fluoride PET-CT is considered. Similarly, PET-CT or PET-MRI with C-11 choline of F-18 fluciclovine radionuclides may be performed when chest imaging and abdominopelvic CT-MRI do not identify suspected recurrent disease in the nodes, bones, or viscera.
Molecular assays may be completed such as the Decipher Prostate RP® used in treatment decision-making in men who have detectable levels of PSA after radical prostatectomy [20]. Its use is a 2B recommendation from the NCCN Guidelines for postoperative patient treatment decision-making [6]. The tissue obtained from RP is evaluated for active genes that express levels of 22 RNA biomarkers linked to an increased risk for metastatic prostate cancer at 5 years [20]. Decipher® proved robust in differentiating men with an increased risk of metastasis from those without metastasis 5 years after surgery, but its use did not improve outcomes when guiding postoperative treatment planning. Nevertheless, the use of a genomic assay enables providers to engage in shared decision-making of adjuvant versus salvage radiation therapy. Shared decision-making is clinically relevant, given the potential adverse side effects of additional therapy on sexual function, continence, and overall health-related quality of life.
Histologic confirmation is obtained via nodal, soft tissue, or bone biopsy based on findings from cross-sectional imaging suggesting a target for biopsy. Nodal biopsies are usually performed under ultrasonic or CT guidance by an interventional radiologist. Soft tissue biopsy can be performed by an interventional radiologist or pathologist, or a pathology biopsy team based on local resources and institutional practice. Bone biopsies are usually performed by a musculoskeletal radiologic team with expertise in this area.
Transrectal ultrasound or prostate MRI is occasionally used when local recurrence in the prostate bed is suspected. Transrectal ultrasound is a lower cost alternative to MRI; however, its diagnostic accuracy is based on the availability of a skilled ultrasonographer and urologist familiar with this procedure. One or more tissue samples, guided by TRUS, are typically obtained under local anesthesia.
Evidence of Recurrence
When evidence of persistent (measurable) disease is present in men who underwent radical prostatectomy as definitive therapy, monitoring or treatment is based on evidence of local recurrence versus distant metastases. Local recurrence is defined as evidence of disease in the prostate bed or surrounding tissue. Based on a process of shared decision-making, management options include observation and salvage external beam radiation therapy with or without androgen deprivation therapy (ADT) [6]. In postsurgical patients evaluated for persistent or recurrent PSA whose studies are positive for distant metastasis, choices include observation or ADT with or without external beam radiation therapy to the site of metastases (symptomatic and/or weight-bearing bones). Refer Chap. 10 for a more detailed discussion of salvage therapy.
Unlike patients managed with surgery, PSA levels are not expected to be undetectable following definitive radiation therapy [6]. In this case, biochemical recurrence is defined as PSA persistence/recurrence with or without a positive DRE. Recurrence is defined as an increase in PSA of 2 ng/ml or more above PSA nadir. Evaluation for recurrence should be considered when PSA is increasing, even if the rise above nadir is less than 2 ng/ml, especially for candidates who can be considered for salvage local therapy (otherwise young and healthy men). The initial evaluation is the same as for patients who underwent surgery and have persistent PSA or recurrence. Shared decision-making for treatment is based on candidacy for local therapy (original clinical stage T1/T2, N × N0, life expectancy >10 years, PSA < 10 ng/ml) versus continued observation.
Candidates for local therapy are further categorized based on a positive result of TRUS biopsy or negative findings of imaging studies for distant metastases [6]. Shared decision-making options for this group are observation, radical prostatectomy and pelvic lymph node dissection, cryosurgery, high intensity focused ultrasound, or brachytherapy. Based on the decision to pursue a specific intervention, they are again monitored for progression as described earlier. Alternatively, patients with a negative TRUS biopsy or absence of distant metastases on imaging studies will travel a different pathway. Management options for this group are observation, ADT, or participation in a clinical trial. Observation includes individualized PSA monitoring based on PSADT. Serial cross-sectional or bone imaging intervals are determined based on PSADT and symptoms.
For candidates deemed ineligible for local therapy, their pathway is based on results of cross-sectional CT or MRI and bone imaging, along with PSADT. Treatment options for this group are observation, ADT, or participation in a clinical trial [6]. Treatment varies based on several factors including patient choice and access to community-based resources such as a urologist who offers systemic therapy. Others may receive treatment from a medical oncologist. Refer Chap. 10.
Conclusion
Men with recurrent prostate cancer are living longer. Since progression occurs over a period of 10 years or more, long-term monitoring is essential. Monitoring men for biochemical or recurrent disease is based on interval measurement of PSA and PSADT. Further diagnostic evaluation is considered when PSADT raises concerns. Additional diagnostic testing allows the patient and the provider to understand when PSA monitoring alone is inadequate because of measurable disease. Monitoring is based on building a trusting relationship and reaching consensus on a plan of care within a framework of shared decision-making. Shared decision-making is based on the need to seek additional diagnostic information that determines the timing of evaluation and treatment ensuring mutually established goals for care.
Pearls for the Advanced Practice Provider
-
Men with recurrent prostate cancer are living longer and require long-term monitoring.
-
Men can be divided into three monitoring categories: no evidence of disease, disease status unknown (biochemical recurrence/persistence), and evidence of recurrence (measurable disease).
-
Irrespective of initial treatment, monitoring is based on interval measurement of serum prostate-specific antigen (PSA).
-
After RP, serum PSA should be undetectable (often reported as ≤0.01 ng/ml).
-
After radiation, serum PSA should reach a nadir of ≤1.0 ng/ml.
-
Concern for biochemical recurrence or recurrent disease is based on PSADT.
-
Diagnostic testing for metastatic disease includes CT, MRI, bone scan, and PET-CT or PET-MRI.
-
Shared decision-making and establishing a trusting, working relationship enhance the patients’ experience as they traverse this pathway.
References
American Facts and Figures 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2019/cancer-facts-and-figures-2019.pdf accessed July 13,2020.
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsk PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9.
Zaorsky NG, Raj GV, Trabulsi EJ, Lin J, Den RB. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Semin Oncol. 2013;40(3):322–36.
Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74.
Boorjian SA, Karnes RJ, Viterbo R, et al. Long–term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–91.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer, Version 4.2018-August 15, 2018. Accessed 11 Nov 2018. PROS C. PROS F, PROS 10Y, 12s, 11 hh.
Sanda MG, Chen RC, Crispoino T, Freedland S, Greene K, Klotz LH et al. Clinically Localized Prostate cancer: AUA/ASTRO/SUO Guideline. Journel of Urology. 2018;199(3):683–90. https://www.auajournals.org/doi/10.1016/j.juro.2017.11.095.
Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. J Urol. 1994;152(5 Pt 1):1358–68.
Lange PH, Ercole CJ, Lightner DJ, et al. The value of serum prostate specific antigen determinations before and after radical prostatectomy. J Urol. 1989;141(940):873–9.
Sandler HM, Dunn RL, Mclaughlin PW, et al. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48(3):629–33.
Critz FA, Williams WH, Benton JB, et al. Prostate specific antigen bounce after radioactive seed implantation followed by external beam radiation for prostate cancer. J Urol. 2000;163(4):1085–9.
Cavanagh W, Blasko JC, Grimm PD, Sylvester JE. Transient elevation of serum prostate-specific antigen following (125)I/(103) Pd brachytherapy for localized prostate cancer. Semin Urol Oncol. 2000;18(920):160–5.
Vickers AJ, NIH Public Access, et al. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br J Med Surg Urol. 2012;5(4):162–8. https://doi.org/10.1016/j.bjmsu.2011.08.006.
Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11(24):8669–71.
Klayton TL, Ruth K, Buyyounouski MK, Uzzo RG, Wong YN, Chen DY, et al. PSA doubling time predicts for the development of distant metastases for patient who fail 3DCRT or IMRT using the phoenix definition. Pract Radiat Oncol. 2011;1(4):235–42.
Chang AJ, Autio KA, Roach M III, Scher IS. “High-risk” prostate cancer: classification and therapy. Nat Rev Clin Oncol. 2014;11(8):308–23.
D’Amico AV, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1988;280:969/74.
Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31. https://doi.org/10.1016/j.juro.2007.03.003.
Cooperberg MR, Pasta DJ, Elkin EP, The University of California, San Francisco, et al. Cancer of the prostate risk assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–4.
Marrone M, Potosky AL, Penson D, Freedman AN. A 22 gene –expression assay, Decipher ® (GenomeDX Biosciences) to predict five-year risk of metastatic prostate cancer in men treated with radical prostatectomy. PLoS Curr. 2015;7. https://doi.org/10.1371/currents.eogt.761b81608129ed61b0b42c04f92ae.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sims, T.W., Gray, M. (2021). Monitoring and Managing Men Following Initial Treatment of Prostate Cancer. In: Trabulsi, E.J., Lallas, C.D., Lizardi-Calvaresi, A.E. (eds) Chemotherapy and Immunotherapy in Urologic Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-52021-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-52021-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52020-5
Online ISBN: 978-3-030-52021-2
eBook Packages: MedicineMedicine (R0)