Abstract
Ethno-phytopharmacology studies the traditional use of plants for the prevention and cure of several diseases. It provides multidisciplinary research on components of medicinal plants, their identification and description, properties, modes of action and interactions with the human organism. Search for new bioactive drugs is another aim of these experimental investigations. Since the World Health Organization (WHO) supports and encourages the introduction of traditional medicine resources into health systems around the world, the use of medicinal plants has shown a marked increase. For this reason, interest in applying scientific methods to validate or refute the traditional use of these plants with the rigors of evidence-based medicine to assess safety, efficacy, and quality has become increasingly important. These three concepts govern the twenty-first century therapy inherent to any conventional drug and allow medicinal plants to aid in the development and advancement of modern medicine, serving as a starting point for the design of new, better, and healthier drugs. In this chapter, parameters to validate medicinal plant attributes such as selection and harvest, extraction and processing methods, analytical techniques to isolate and identify bioactive metabolites, biological activity screening, and other aspects are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Plants have been used as medicine throughout human history by different cultures. Through trial and error learning, the human being learned to differentiate plants with beneficial effects from those that were toxic or inactive as well as which combinations or processing methods had to be used to obtain the desired results. Their countless uses have been documented and transmitted through generations, including organized traditional medical systems (Ayurveda, Unani, Kampo, and traditional Chinese medicine, among others). On the other hand, herbalism, folklore, and shamanism are widely practiced in Africa and South America. These are based on a “confidential information learning system” that is only passed to the next generation by a shaman, healer, or herbalist; therefore, scientific validation and information on the plants are less documented than in other systems (Fabricant and Farnsworth 2001).
Plant-based prepared medicines contain a variety of chemical compounds, many of which contribute or are responsible for their medicinal benefits. The compound to which the activity is attributed is named as the active compound, and its presence and concentration depend on a series of factors, among them, the plant’s species, harvesting time, the type of soil, the part of the plant utilized, the processing, etc. The diverse composition, occasionally unknown, of products based on medicinal plants, makes it difficult to ensure the quality of the different production batches without adequate controls. In many countries, these products are launched onto the market without these controls and without the necessary safety and efficacy studies. The fact that a herbal product that has demonstrated to be safe and efficient during the validation process, having a defined and constant composition, is one of the most important prior qualifications to produce a quality medicament (Kunle et al. 2012).
According to the World Health Organization (WHO), approximately 80% of the world population, especially inhabitants of developing countries, use medicinal plants or products derived from them as part of their health care due to their lack of access to laboratory-derived drugs (WHO, IUCN, WWF 1993; WHO 2013). The “WHO Traditional Medicine Strategy 2002–2005” program aimed to keep natural medical practices as possible therapeutic alternatives. The “WHO Traditional Medicine Strategy 2014–2023” program reappraises and sets out the course for traditional and complementary medicine usage in the next decade (WHO 2002, 2013).
In this chapter, we discuss the concept and origin of ethno-phytopharmacology, and the basic parameters to validate the attributes of medicinal plants are described. These parameters include, information recompilation, selection, collection and processing of the plant material, extraction methods, evaluation of the biological activity with special emphasis on antimicrobial activity, isolation processes for, and identification of, active compounds, and, finally, safety and efficacy studies.
Ethno-Phytopharmacology
The encouragement in the research of bioactive compounds based on plants started at the beginning of the twenty-first century when Friedrich Sertürner managed to isolate the analgesic and the agent of sleep from the opium poppy, which he called morphium (morphine) in reference to the Greek god of dreams, Morpheus (Atanasov et al. 2015). Since then, medicinal plants played a key role in the development and advancement of modern medicine, acting as a starting point for the development of new drugs.
In the last 100 years, a great number of bioactive compounds based on medicinal plants have become essential for modern medicine, examples being digoxin from Digitalis spp. , vincristine and vinblastine from Catharanthus roseus , morphine and codeine from Papaver somniferum, and atropine from Atropa belladona . Additionally, it is estimated that 60% of antitumor and antiinfectious drugs that are already on the market or into the trial phase are derived from nature (Newman and Cragg 2020a). Recognized examples of antiparasitic agents (malaria effectively) are quinine from Cinchona spp. and artemisinin isolated from Artemisia spp. In 2015, the Nobel Prize for Physiology or Medicine was awarded in part to Dr. Youyou Tu in the ethnomedicine field for her contribution to drug therapy based on artemisinin (isolated from Artemisia annua) , which reduced malaria impact and mortality rates, saving millions of lives globally (Andersson et al. 2016).
Between 1981 and 2019, 1394 new chemical entities belonging to the small molecules group were approved, 65% were derived or inspired by nature. Most of these compounds come from microbial sources or as a result of the interaction of microorganisms with plants (Newman and Cragg 2020a). However, the potential use of plants as a source for new drugs is still not fully explored. It is estimated that only around 6% of the approximately 250,000–500,000 plant species have been well studied in terms of biological activity and 15% have been studied phytochemically. It has been determined that 80% of medicinal plants’ isolated bioactive compounds have an ethnomedical use identical or related to the instructions for which the plant has been prescribed (Fabricant and Farnsworth 2001).
The word ethno-phytopharmacology comes from the following Greek terms:
-
Ethnos = race, culture
-
Phyton = plant
-
Pharmakon = drug, medicine
-
Logos = discourse, explanation
Ethno-phytopharmacology comprises a multidisciplinary approach including studies about the traditional use of plants for the diagnosis, prevention, and treatment of diseases and also research on the medicinal plants’ components, their identification and description, biological properties, mode of action, and interaction with the human body.
Such studies allow the scientific validation of medicinal plants’ attributes that once rested solely on their habitual and popular use, but also they provide new bioactive drugs with the rigors of medicine based on evidence, providing safety, efficacy, and quality to the patient. These three concepts rule the current therapeutic use in the twenty-first century of any conventional drug. Ethno-phytopharmacology allows medicinal plants to intervene in the development and advancement of modern medicine, acting as a starting point for the development of new drugs.
Validation of Medicinal Plants
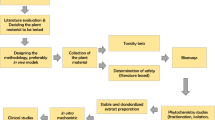
According to the Pan American Health Organization (PAHO) (Arias 1999), a medicinal plant is any wild or cultivated plant used for a medical purpose. A herbal medicine is a manufactured product that contains one or more therapeutically active components exclusively extracted from plants (aerial or non-aerial parts, juices, resins, oils, etc.) either raw or processed. However, these plant-based products must undergo a scientific validation process in terms of efficacy, safety, and quality. The belief that all plant-based products are a good treatment just because they are obtained from nature ought to be discarded. In many cases, these products are entirely ineffective and, occasionally, even toxic. At the same time, we should not ignore the possibility that many medicinal plants could be simple mediators acting as placebos in a cultural context and do not contain pharmacologically active molecules for the disease indicated by ethnomedical information (Gertsch 2009). Figure 17.1 shows the validation process stages for products based on medicinal plants; all of them are of great importance.
Selection of Plant Material
There are many approaches to select the vegetal species for the pharmacological study: the ethno-pharmacological approach, the random approach, the chemo-systematic approach, among others. Throughout millennia, human beings were improving their knowledge about medicinal plants by trial and error experience and transferring that knowledge. Therefore, the ethno-pharmacological approach is still the most efficient one for the discovery of new molecules. This approach is based on the selection of the plant species according to its traditional medical use (Atanasov et al. 2015). It is estimated that 74% of bioactive compounds derived from plants were discovered after checking the ethno-pharmacological approach (Ncube et al. 2008).
Information from the ethnic group about how they use the plant is extremely important. The part of the plant that they use (flowers, leaves, roots, etc.) and how they process it can give hints to the best method for the active compound extraction. The formulation and posology used will provide information about pharmacological activity, oral versus non-oral intake, and the doses to be tested. However, certain considerations have to be taken into account when the ethno-pharmacological approach is chosen to select the plant material; for example, each ethnic group has its own health and disease concepts, just as in different health systems. The signs and symptoms must be interpreted, translated, and related to medical concepts, allowing a study focused on a particular biological activity (Rates 2001).
Information from organized traditional medical systems (Ayurveda, Unani, Kampo, and Chinese traditional medicine), herbalism, folklore, and shamanism can be obtained from a number of sources, including books, articles about plants, review articles, footnotes made by the botanist on herbaria vouchers, fieldworks, and online database like NAPRALERT and USDA –Duke (Fabricant and Farnsworth 2001; Cos et al. 2006).
Information Search
Once the plant has been selected based on its ethnomedical knowledge, it is necessary to gather the greatest possible amount of scientific endorsement information. Aspects to consider in order to plan the collection of the plant to be utilized (Fabricant and Farnsworth 2001; WHO 2003) include:
-
Learning about its organography, morphology, and physiology. Allows determination of the variations that a plant can undergo during the different seasons and stages of life. The plant’s age and the moment of harvest could affect the chemical composition and influence the presence or absence of the active compound and, in consequence, its medicinal properties. Learning the plant’s morphology allows identification of the correct part that must be used
-
Knowing the botanical geography, meaning the place where the plant grows and its abundance, as well as the place where it will be harvested
-
Learning the legal aspects. If the plant is in danger of extinction or its cultivation is forbidden, obtaining the permits necessary to collect and study them
A global preliminary study about the plant is important because another aspect that determines chemical composition are its endophytic organisms, such as fungi and bacteria. Occasionally, compounds present in the collected plant material may be metabolites produced by endophytic organisms or induced products by the plant as a result of the interaction with the said organism (Giusiano et al. 2010; Atanasov et al. 2015). In fact, a good number of those thought to be “plant-derived” natural products have been shown to be metabolites produced as a result of interaction with endophytic and epiphytic microorganisms (Newman and Cragg 2020b).
Harvest
In most cases, the plants are directly collected from their natural habitat, so accurate identification is paramount. It is important that the botanist is capable of identifying the species and also prepare the material for the preservation of the herbarium so as to secure reference material (“voucher sample”). For unambiguous identification, it may be necessary to use a combination of methods, such as genetic and chemical analyses, in addition to the morphological and anatomic characterizations. On the other hand, the plants’ habitat and the legal permits, in particular that of protected species, must be respected when collecting from nature.
The WHO (2003) and the European Medicines Agency (EMA) (2006) developed guidelines on good agricultural and collection practices for medicinal plants in order to promote sustainable collection techniques and reduce environmental problems. In addition, the Convention on Biological Diversity (WHO 1992) and the Nagoya Protocol (Convention on Biological Diversity 2011) on access and benefit sharing must be respected.
The plant’s chemical composition depends not only on the identity of the species and the harvesting time, but also on the composition of the soil, the topography, geology, vegetation, altitude, weather and environmental conditions, daylight hours, among other variables; therefore, all this information must be gathered, and also obtain photographic records and specify the coordinates of the harvest site.
The harvest area must be far from pollution and agricultural crops since the presence of heavy metals and agrochemical compounds may affect the interpretation of the plant’s properties. Harvesting should be avoided near to drainage ditches, roadsides, mine tailings, garbage dumps, and industrial facilities that could produce toxic emissions. Similarly, harvesting medicinal plants in and around active pastures, including riverbanks downstream from pastures, should be avoided in order to avoid microbial contamination from animal waste (WHO 2003; EMA 2006; Atanasov et al. 2015).
The harvest plan must allude to the species, to the part of the plant (root, leave, fruit, etc.), and the amounts to be collected. Ecofriendly and nondestructive harvest systems, which will vary considerably from one species to another, must be applied. For instance, in the collection of trees and bushes roots, the main roots must not be cut off or unearthed and cutting off the central root must be avoided; only lateral roots ought to be located and collected (WHO 2003).
Based on the information compiled prior to collection, to secure the best quality and quantity of components with possible biological activity, the plant material must be collected during the right season or period, even the time of the day (as mentioned earlier under information). The components and/or concentrations may vary during the growth cycle (young or adult plant, or plant age) and even during the day. Additionally, it should be kept in mind that each part of the plant has its most appropriate harvest time when, in general, the active compound is in its highest concentration. For example, leaves are collected when photosynthesis is most active, meaning when they are green, mostly before or during the blooming phase, in dry weather (no rain), and in the morning when dew has evaporated (WHO 2003).
The harvested plant material should not come into direct contact with the soil and must be placed in baskets, mesh bags, or other clean and airy containers, without vegetal remnants from previous harvest activities. If the underground parts of the plant are the ones used (like the roots), the dirt residue must be removed (WHO 2003).
The use of plants under controlled conditions of cultivation ought to be considered over those harvested from nature. This allows the production of a homogeneous material, largely guaranteeing the chemical homogeneity and reducing the disadvantages associated with an uncontrolled environment (Rates 2001; WHO 2003). After harvest, transformation processes and compound degradation may occur making it a necessary requirement that the plant material goes to the processing stage as soon as possible.
Processing
The processes previous to extraction allow the removal of impurities and the avoidance of alterations on the plant material, so as to secure a high-quality crude material. Postharvesting alterations may be classified into external and internal alterations. External alterations are made by humidity, sunlight, heat, and the presence of strange material, such as dirt, insects, parasites, microorganisms, etc. Internal alterations may be caused by enzymatic reactions, autoxidation reactions, and reactions between components of the plant. These alterations can be reduced by following the harvest and processing stages step by step.
Plant material processing can be divided into five stages:
-
Classification
Every strange or contaminated material must be discarded and if it doesn’t comply with the organoleptic characteristics sought. A thorough material check has to be made, preventing it from being contaminated by insects, parasites, etc., either with fragments of other plants, or other parts of the itself plant that are not wanted, or decaying materials. An organoleptic evaluation must be made, including appearance, damage, size, color and odor (WHO 2003).
-
Washing and sanitizing
To get rid of debris that may be present on the surface and ensure the microbiological quality of the plant material, crude material must be subjected to a washing and sanitizing process after harvest. The washing must be done with plenty of potable water in order to remove soil debris, dust, spores, etc., that may affect the quality of the final product.
Sanitizing may be carried on through chemical or physical methods. The choice of the optimal method will depend on the type of material to be sanitized, on its volumes, and on its possible costs. Nevertheless, the chemical method by immersion in aqueous sodium hypochlorite solution (NaOCI) is still the most used. The solution concentration and the immersion duration time depend on the plant material. In general, the plant material is sanitized using an aqueous solution of NaOCI 0.5–2% over 5–10 minutes. Afterward, several rinses are performed with sterile purified water, followed by straining or centrifugation of the material to remove any remnants of NaOCI and water (Fuentes-Fiallo et al. 2000).
-
Drying
The aim of the drying or dehydrating process is to reduce the plant water content with the aim of preventing alteration of the active compound. It also allows its storage and conservation for a longer period. There exist a great number of drying techniques, and the choice will depend on the part of the plant, the material amount, and the advantages and disadvantages of each technique. Indigenous peoples generally use a natural outdoor drying process until it reaches a steady weight. They perform this process in a ventilated place and, depending on the part of the plant, this process is carried out in the sun or shade (Fuentes-Fiallo et al. 2000; WHO 2003).
Occasionally, herbal medicines are made out of recently harvested fresh plants as infusions, decoctions, or crushing the plant in a mortar or stone so as to apply it on skin or ingest it. In these cases, when traditional use warrants it, the drying process is omitted and can go straight to the extraction stage. To avoid alterations of unstable bioactive compounds that may be present, the time that goes by between the harvest and the extraction process must be the least possible. On the other hand, the fresh plant can undergo a stabilization process through freezing, lyophilization, use of alcohol vapors, etc. Stabilization causes the irreversible denaturation of the plant enzymes, allowing the maintenance of its relatively immutable chemical composition (Rates 2001).
-
Storage
Storage is not recommended when the active compound and stability of the plant material are unknown since the material can be exposed to alterations. But if storage is required, its conditions must be very well detailed. In general terms, the plant material must be stored labelled, protected from sunlight and contamination sources, in a cool and dry location, with humidity and temperature controls (WHO 2003).
-
Grinding and sifting
Occasionally, the plant material undergoes a grinding/milling and sifting process in order to improve the extraction of the compounds. When grinding/milling, smaller particles are obtained, increasing the contact surface and enhancing the extraction of the compounds; at the same time, sifting produces uniform sized particles. Generally, sieve sizes vary between 5 and 0.2 mm (Hilbay et al. 2016). However, if the particles are too fine, lumps/clumps can be formed and make the extraction difficult.
It is recommended to perform this process just before extraction. In contact with air, the plant material not ground/milled slowly loses its volatile compounds. Nevertheless, a higher reduction of these compounds is observed when it is ground/milled (Muñoz 1996).
Extraction
Several extraction techniques are available. Nevertheless, when the chemical nature of the involved components with biological activity is known (once more the information search is crucial), the extraction methods must lead to the acquisition of these components with the highest yield and purity possible. Traditional healers mainly use water to prepare the medicines, but it has been found that the majority of the active components that have been identified from plants are poorly soluble in water, while extracts from organic solvents have shown a greater biological activity (Ncube et al. 2008; Das et al. 2010). However, if the active compound is unknown, a generalized method cannot be described.
When the chemical composition is unknown, the extraction method can be based on the information about how the plant is used in popular medicine, or the investigator can perform several extractions with solvents of increasing polarity to make sure to extract most of the compounds. The extraction with solvents of increasing polarity is the most useful method since it allows one to separate the components of a complex mixture and see which is the fraction or fractions that show activity. On the other hand, plant essential oils, which are a complex mixture of volatile components, mainly terpenes and terpenoids are also worth recovering and to do this step, the most utilized technique is still steam distillation.
The extraction process is a very critical stage because the active components can be lost if the technique is not appropriate. Moreover, an inadequate extraction method can cause the decomposition of the natural product (Rates 2001; Ncube et al. 2008; Das et al. 2010; Nazzaro et al. 2017). Therefore, when the active compound is unknown, the plant material has to be separated into two parts: one part for obtaining the essential oils and the other for extraction with solvents of increasing polarity. It is important to determine the amount of plant material needed to obtain a sufficient amount of extract and/or essential oils in order to perform all subsequent tests. Successful extraction of active components will depend on the solvents used at this stage; thus, the solvent properties should include low toxicity, ease of evaporation, fast absorption, preservative action, and not cause the extract to complex or dissociate (Das et al. 2010).
The extracts (hexene, methanol, aqueous, etc.) can be obtained by maceration at room temperature for 12–24 hours with periodic stirring. The longer the solvent and plant material are in contact, the greater the extraction of the plant compounds until a chemical equilibrium is reached. Similarly, stirring the mixture and increasing the solvent-plant material ratio improves the extraction of the compounds. The ratio of solvent and dry plant material that is generally used is 10:1 (v/w). Depending on the plant, the part used, and the information gathered previously; the extraction time, the pH, the temperature, the size of the particle, and the relation between the amount of solvent and plant material can be modified (Das et al. 2010). Once the extract has been obtained, it must be filtered and then the solvent removed by concentration under vacuum and lyophilized until a dry residue is obtained. Extraction and evaporation should be performed at low temperatures so as not to alter thermolabile components. For aqueous extracts, only lyophilization is generally used (Brusotti et al. 2014). The recommended procedure for extracting compounds from medicinal plants is outlined in Fig. 17.2.
Evaluation of In Vitro Biological Activity: Emphasis on Antimicrobial Activity
Using the ethno-pharmacological approach as a guiding thread, the evaluation of the biological activity of plant-based products is necessary in order to validate the traditional use and to search for the most active extracts. Discovering active compounds from plants requires a multidisciplinary approach in which success depends on a well-chosen set of in vitro and in vivo tests.
The extracts obtained are assayed using biological tests selected based on the alleged bioactivity. For each biological property to be studied, the available in vitro assays should be used first since the in vitro bioassays are faster and require smaller amounts of samples (Cos et al. 2006; Brusotti et al. 2014). The choice should optimally combine simplicity with good sensitivity and reproducibility. Internationally accepted and standardized methods should be chosen.
Due to a great diversity of available assays for each biological property (antibacterial, antifungal, antiviral, antiparasitic, antineoplastic, antioxidant, etc.), this discussion will only focus on in vitro assays directed toward antibacterial and antifungal activities that meet these requirements. Other available in vitro antimicrobial activity methodologies are described in the review published by Balouiri et al. (2016).
Currently, there is no consensus on which is the most appropriate methodology to determine the antifungal and antibacterial activity of plant extracts, since they are complex mixtures of compounds. However, the Clinical & Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standardized reference methods to evaluate in vitro antibacterial and antifungal activities for pure compounds. Laboratory procedures and conditions set forth in these documents can be adapted and used for plant extracts to perform comparable and reproducible tests and results.
CLSI and EUCAST broth microdilution methods allow a quantitative evaluation of the in vitro inhibitory activities of a component against bacteria and fungi. The minimum inhibitory concentration (MIC) is obtained, defined as the lowest concentration of the compound or drug to inhibit the growth of microorganism, expressed in μg.mL−1 or mg.L−1. The main differences between the CLSI and the EUCAST methods lie in the inoculum size and the reading method, visual in the case of CLSI and spectrophotometric in EUCAST. To evaluate the biological activity of plant-based products, we recommend the CLSI document because the spectrophotometric reading could be affected by colored extracts or turbidity as a consequence of the chemical and hydrophobic nature of some components present in the extracts (Balouiri et al. 2016).
The broth microdilution method requires the preparation of two-fold serial dilutions of the “antimicrobial” to be tested (e.g., 64, 32, 16, 8, 4, 2, and 1 μg.mL−1) in a liquid culture medium dispensed in a 96-well microplate. Subsequently, the plates are inoculated with a standardized suspension of the microorganism to be tested. Once the incubation period is done, the plate is examined, and the MIC determined (Cantón et al. 2007; CLSI 2020). Following the steps set out in the CLSI documents allows the tests to be reproducible (inter- and intralaboratory), and the results to be comparable with other antimicrobial agents. Nevertheless, some modifications to the standardized protocol are necessary in order to test natural products. Those modifications must be the least possible alterations to the protocol. In the following section we give some considerations that have to be taken into account using these techniques:
-
For fastidious microorganisms with strict nutritional requirements, it is necessary to make some modifications, such as those proposed by Rojas et al. (2014) to study the susceptibility of Malassezia spp. Likewise, some authors have proposed modifications to improve the MIC reading based on the use of resazurin or tetrazolium salts (Balouiri et al. 2016); however, as it was previously said, the established protocol should be followed as strictly as possible.
-
To define the in vitro activity of an antimicrobial agent against a microorganism, reference methods include categories for clinical use, such as being susceptible, susceptible-dose dependent, resistant, or nonsusceptible. These categories allow one to infer whether a treatment at a defined dose of antimicrobial agent can stop the infection caused by a specific microorganism. Plant extracts and pure components isolated from plants are not discussed in the reference methods, therefore, can only be used to obtain MIC values and usually cannot make categorical interpretations (CLSI 2020).
-
In the reference documents, the MIC reading endpoint can be MIC-0, MIC-1, and MIC-2. These are defined as the lowest concentration of an antimicrobial agent capable of inhibiting 100%, ≥80% and ≥50% growth, respectively, as compared with the antimicrobial agent-free growth-control well. The MIC reading endpoint varies according to the drug and the microorganism to be tested; for example, for itraconazole, MIC-0 is used for filamentous fungi and MIC-2 for Candida spp. The selection of the MIC reading endpoint is based on the distribution of MIC, pharmacokinetic and pharmacodynamic parameters, animal models, treatment, and clinical evaluation (Espinel-Ingroff et al. 2012). Therefore, as there is no consensus for plant extracts, it is necessary to determine the three reading endpoints.
-
Dimethyl sulfoxide (DMSO) is recommended for use as a solvent for plant extracts and isolated compounds because of its advantages from other solvents. However, it is important to take into account that DMSO is toxic for many microorganisms; therefore, to avoid inaccurate results, the final concentration in each well should be ≤1% (CLSI 2020).
-
The test microorganisms will be determined after considering the traditional ethnomedical use of the plant. Sometimes it is necessary test the extract or the isolated compound activity against different genera and species of microorganisms (e.g., Gram-positive and Gram-negative bacteria, yeast, filamentous hyaline and dematiaceous fungi, etc.). To obtain conclusive statistical values, the test should be carried out against a considerable number of strains of the same species. An antimicrobial agent can show different MIC against different microorganisms even against isolates of the same species.
-
Reference strains and positive controls have to be included in order for the assay to be considered a reproducible and comparable method. The positive controls can be, for example, antimicrobial drugs in clinical use (penicillin, fluconazole, etc.). For a correct interpretation of the microplate, it is recommended to use the distribution showed in Fig. 17.3.
One key point in the evaluation of the in vitro activity of medicinal plant-based products is to establish the concentrations at which an extract is considered active for a particular biological activity and for a specific assay method. Often, different opinions are found on what is the significant concentration of the biological activity of an extract. Some authors use excessively high concentrations of plant extracts to identify a biological property. Paracelsus’ well-known quote reads “dosis sola facit venenum” (it is the dose which makes a thing poison). The problem is that high concentrations result in a markedly increased incidence of false positives in vitro tests. This lack of criteria causes loss of time and money. It should be understood that the concentration determines the meaning. Unfortunately, there are no standards; therefore, the biological activity criteria should be well supported.
The in vitro evaluation method for the biological activity must be as sensitive as possible to allow the detection of weakly active compounds or those in low proportions in the extract; at the same time false positives have to be recognized and eliminated (Gertsch 2009). Using the broth microdilution method, we can establish the criteria to determine the antibacterial and antifungal activity, having in mind the following points:
-
1.
Some authors consider that an extract is active with a value of MIC ≤1000 μg.mL−1 and ≤50 μg.mL−1 or ≤100 μg.mL−1 for a pure compound (Salvat et al. 2001; Holetz et al. 2002; Pessini et al. 2003; Svetaz et al. 2004; Malheiros et al. 2005; Sanches et al. 2005; Tanaka et al. 2006; Kuete 2010; Alvino Leite et al. 2015). Other authors establish more stringent values: MIC ≤100 μg.mL−1 for an extract and MIC ≤25 μM for a pure compound (Cos et al. 2006; Brusotti et al. 2014). Nevertheless, it is important to know that extracts are a complex mixture of compounds present in different proportions and that, in few occasions, the active compound is found in a proportion higher than 20%. On the other hand, according to CLSI, certain drugs categorized as susceptible against specific microorganisms present higher MIC values as it is the case of sulphonamides against Salmonella spp. and Staphylococcus spp. with a value of MIC ≤256 μg.mL−1 (CLSI 2020). Hence, by using too strict a criterion, the opportunity to detect potential active compounds could be missed.
-
2.
At MIC values ≥2000 μg.mL−1, the incidence of false positives increases significantly, that is, observation of growth inhibition as a result of using high and toxic concentrations. Moreover, technical difficulties may arise for the reading of the MIC due to turbidity or low solubility of the extract (Cos et al. 2006).
-
3.
CLSI documents test MIC values that are exponential values of the number 2 raised to a positive or negative integer, that is, 2x (CLSI 2018).
-
4.
Some authors make a classification of the active extracts (Holetz et al. 2002; Pessini et al. 2003; Tanaka et al. 2006; Kuete 2010; Alvino Leite et al. 2015).
-
5.
The application of a less rigorous endpoint, such as MIC-2, represents a better endpoint reading of the in vitro activity of some compounds (CLSI 2017a). On the other hand, CIM-2 and CIM-1 are associated with fungistatic/bacteriostatic drugs while CIM-0 is usually associated with drugs of biocidal action (CLSI 2017b, 2018). Therefore, use of CIM-2 would allow us to detect potential compounds with antimicrobial activity that would not be detected with CIM-0.
Based on the previous points, using the broth microdilution method, the antibacterial/antifungal activity of an extract can defined as follows.
The extract is considered:
-
Inactive: MIC-2 >1024 μg.mL−1
-
Low/weak activity: MIC-2 ≥512–1024 μg.mL−1
-
Moderate activity: MIC-2 ≥128–<512 μg.mL−1
-
Strong/good/significant activity: MIC-2 <128 μg.mL−1
However, the activity of the extract will be determined by the potency of the active compound and its proportion in the extract. Moreover, synergistic or antagonistic interactions among compounds present in the extract may occur.
A pure compound is considered:
-
Inactive: MIC-2 >256 μg.mL−1
-
Low/weak activity: MIC-2 ≥128–256 μg.mL−1
-
Moderate activity: MIC-2 ≥16–<128 μg.mL−1
-
Strong/good/significant activity: MIC-2 <16 μg.mL−1
For a better characterization of the activity, another parameter to evaluate is the minimum bactericidal concentration (MBC) or the minimum fungicidal concentration (MFC). MBC and MFC are defined as the concentration of antimicrobial agent needed to kill 99.9% of microorganisms compared to the initial inoculum. These parameters are determined from the microdilution plate, after the MIC reading, by subculturing the contents of the wells in which no growth was observed. After incubation, the number of colonies forming units per milliliter is calculated (UFC.mL−1) and the MBC or MFC is determined. In certain cases, MBC and MFC have proved to be better predictors of the clinical response. Currently, only the standardized reference method for determining the MBC has been developed (CLSI 1999); however, methods have been proposed to determine the MFC from modifications of the standardized method for bacteria (Espinel-Ingroff et al. 2002; Pfaller et al. 2004).
To estimate if an extract or a pure compound has bactericidal, bacteriostatic, fungicidal, or fungistatic action, the MBC/MIC or MFC/MIC ratios can be used (Hazen 1998; Pfaller et al. 2004; Meletiadis et al. 2007).
Ratio ≤4 is considered bactericide/fungicide
Ratio >4 is considered bacteriostatic/fungistatic
Nevertheless, the most recommended in vitro method to determine the type of action is the time-kill assay. The most appropriate methodology to perform this test is described in the CLSI M26-A document and provides information whether the antimicrobial effect is time dependent or concentration dependent. Modifications to this method have been proposed for use against fungi. This method also allows to determine if a combination of two compounds has a synergistic, antagonistic, or no interaction effect against a specific microorganism (Pfaller et al. 2004). Another method widely accepted to determine the interaction between two compounds is the checkerboard assay (Meletiadis et al. 2002; Odds 2003).
Before conducting the broth microdilution assay, in cases where one has several extracts or isolated compounds, it is advisable to perform a screening test to determine the most promising sample(s). This procedure “pre-test” will save time and money.
As a screening method, the agar dilution test described in CLSI M07 document (CLSI 2018) with modifications can be performed, which has shown to have a high correlation with the broth microdilution method. The “antimicrobial agent” to assay is incorporated into the nutrient agar medium in a concentration to obtain a qualitative evaluation (active or inactive), 1024 μg.mL−1 for extracts, and 256 μg.mL−1 for pure compounds. The inoculum is applied onto the agar surface using a replicator. After incubation, at least a 50% reduction in the growth of the tested microorganisms in comparison to the control must be observed. The main advantage of this test lies in the fact that it allows a qualitative evaluation of an extract against many microorganisms on the same plate. Modifications such as growth medium and incubation time may be necessary to perform a proper screening prior to performing the broth microdilution method.
Bioautography is another method that is used. It basically consists of a separation of the compounds present in the extract using thin-layer chromatography (TLC) and then detecting the fractions that produce microbial inhibition by overlaying on a microbial test plate. Even when bioautography separates and identifies the active fractions, it is necessary to determine the MIC using the broth microdilution method. Variations of this methodology are extensively described by Dewanjee et al. (2015).
Isolation and Identification of the Active Compound
Once the in vitro biological activity of extracts has been checked, the next step is to isolate and to identify the active compounds. In general, the integration of different separation methods is necessary. Otto Sticher (2008) reviewed aspects and practical applications of the main separation techniques.
Currently, bioassay-guided fractionation is one of the most modern techniques for identifying bioactive natural products. This approach involves the repetitive fractionation of the extracts and the evaluation of their biological activity until the isolation of the pure compounds with the selected biological activity. Brusotti et al. (2014) described different strategies of the bioassay-guided fractionation for the discovery of drugs from plant extracts.
The next step is to elucidate the structure of the active compound. Different techniques are applied, including HPLC- UV-DAD, HPLC-MS, GC-MS, HPLC-SPE-NMR, UHPLC-DAD-TOF-MS, among others. The selection of the techniques will depend on the chemical nature of the compounds and the equipment available. Combinations of sensitive analytic techniques (HPLC, GC) with spectroscopy methods (MS, NMR) allow faster identification of known components. HLPC and GC are widely used to create profiles and natural product fingerprints for quantitative analysis and the quality control. The HPLC connected to simple detectors can record chromatographic traces in order to create profiles or quantification (Brusotti et al. 2014).
In recent years, the application of modern analytical techniques in conjunction with metabolomics and network pharmacology would help to identify and discover new active compounds has been postulated. Natural product researchers begin to utilize collaborative computerized networks to identify relationships among metabolite data. This enabled to establish the world’s largest data warehouse for natural products. This repository, named Global Natural Products Social Molecular Networking (GNPS), is a web-based mass spectrometry ecosystem that aims to be an open-access knowledge base and share raw, processed, or identified tandem mass (MS/MS) spectrometry data. GNPS aids in identification and data from initial acquisition/analysis to post publication can be freely accessed at https://gnps.ucsd.edu (Newman 2020).
The fast identification of plant extract components is an important step in the medicinal plants’ validation process, allowing utilization of financial resources and effort only on the most promising compounds. However, attention should also be paid to known molecules that may have the biological property under study, but this activity was never reported.
The objective of isolation and identification of the active compound from the medicinal plant is based on being able to attribute a biological activity to one or more specific compounds present in the plant. When the active compound is isolated, we make sure to eliminate compounds that may have a toxic or antagonistic effect as well as to corroborate any existence of a synergistic effect between the components of a mixture. Furthermore, it allows an investigator to obtain standardized products based on medicinal plants. The standardization of plant-based products is the process that establishes a set of standards or inherent characteristics, constant parameters, definitive qualitative and quantitative values, which guarantee quality, efficacy, safety, and reproducibility. This involves the adjustment of the product’s preparation to give a defined compound amount with known biological activity, obtaining high quality products, and subjected to rigorous quality controls throughout the manufacturing process (Kunle et al. 2012).
Efficacy and Safety Assessment
To evaluate the safety profile and the active compound efficiency, a preclinical and a clinical phase must be designed. In general terms, the preclinical phase (set of in vitro tests and animal models) allows initial evaluation of the adverse effects. The aim is to reduce and anticipate the risk for humans. The clinical phase (clinical trials to human tests) seeks to prove the therapeutic efficacy. FDA and other drug-regulating organizations encourage researchers to contact them in every phase of the test. One of the most frequent causes of the interruption of the new drug development is the appearance of toxic effects while the lack of efficacy contributes in a smaller proportion.
The step toward animal testing depends on the bioactivity already demonstrated and the additional information on the animal with which the tests will be made. In the initial phases, in vitro tests take priority over studies in animal models. In vitro cytotoxicity evaluation assays against human cell lines represent a very important criterion in the validation of the safety of new drugs and should always be included in parallel with the in vitro biological activity evaluation tests. On the other hand, tests on lower animals should be prioritized over higher animals, for example: first tests are performed on Caenorhabditis elegans , Danio rerio (zebrafish), Drosophila melanogaster , or Galleria mellonella ; then on rodents (rats, mice, hamsters, guinea pigs, etc.) and, finally, on mammals such as rabbits, monkeys, etc. These decisions are generally based on scientific, economic, and ethical reasons (Atanasov et al. 2015). Nonmammal animal models have the advantage that they require less working time, cost, and space. Moreover, they provide a better statistical value because the usual number of experimental animals is 300 individuals for C. elegans versus 30–50 in mice, increasing the reliability of the results.
Rats and mice tests are crucial for the evaluation and validation of the biological activity of natural products and isolated compounds. These animal models have short reproduction cycles and human-like physiology. They provide a deeper pharmacological picture since they allow assessment of the efficacy and the pharmacokinetic, metabolic, and toxicological phenomena. In addition, the use of genetically modified rodents allows discovery of new pharmacological targets and to elucidate mechanisms of actions.
Nonrodent mammal species such as rabbits, pigs, and monkeys also are widely used in the preclinical phase. These animal models present limitations, not only a higher price, but also more pronounced ethical considerations. However, the FDA and EMA regulatory guidelines require safety tests on at least two mammalian species including a nonrodent before authorization of human trials (Atanasov et al. 2015). Due to considerations on animal welfare, high cost, and labor intensity, the number of tests in animal models is generally kept to a minimum.
During the verification of the compound efficacy in animal models, several parameters should be considered, such as the route of administration, the dose to be applied, the experimental reading, and the use of a positive control, if available. Besides, it should be borne in mind that reactions to treatment in animals cannot be extrapolated directly to humans in all cases since there are differences in the response between species. In addition, there are certain reactions that are difficult to determine in animals, such as headache and depression. Therefore, the step toward conducting human trials always constitutes a risk and in order to qualify for a clinical trial application there must be an adequate balance between benefit and risk.
Once the safety and efficacy of the compound in animals has been demonstrated, the next step is clinical trials. Clinical trials are divided into phase 0, I, II, and III trials. Postapproval surveillance trials are generally called phase IV trials. Characteristics of the different clinical trials phases are summarized in Table 17.1.
Concluding Remarks
The validation process of products based on traditional knowledge of medicinal plants is long, expensive, and faces many challenges. Most extracts and compounds obtained from medicinal plants are only evaluated in in vitro tests and do not follow the subsequent stages necessary to ensure their efficacy, safety, and quality. It is estimated that of 5000–10,000 compounds, only one reaches the market and the process takes approximately 15 years (Fig. 17.4). However, as the WHO Traditional Medicine Strategy 2014–2023 program emphasizes, this is the process necessary to validate or refute, with scientific language, the ethno-pharmacological properties of a medicinal plant and certify it. In addition, it processes favor the discovery of new chemical entities and the development of new drugs. Although natural products are not patentable, the use of a given natural product or a new synthetic compound using the information obtained from that natural product or the method of producing that substance can be patented (Tallmadge 2018).
Timeline of the discovery and development of new drugs from medicinal plants. (Adapted from Matthews et al. (2016))
References
Alvino Leite, M. C., de Brito Bezerra, A. P., de Sousa, J. P., & de Oliveira Lima, E. (2015). Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains. Medical Mycology, 53(3), 275–284.
Andersson, J., Forssberg, H., & Zierath, J. (2016). Avermectin and artemisinin – revolutionary therapies against parasitic diseases. Nobelprize.org. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/advanced-medicineprize2015.pdf
Arias, T. D. (1999). Glosario de medicamentos: desarrollo, evaluación y uso. Washington, DC: Organización Panamericana de la Salud (OPS). 333 p. ISBN 92 75 32305 4.
Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E.-M., Linder, T., Wawrosch, C., Uhrin, P., Temml, V., Wang, L., Schwaiger, S., Heiss, E. H., et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances, 33(8), 1582–1614.
Balouiri, M., Sadiki, M., & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6, 71–79.
Brusotti, G., Cesari, I., Dentamaro, A., Caccialanza, G., & Massolini, G. (2014). Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. Journal of Pharmaceutical and Biomedical Analysis, 87, 218–228.
Cantón, E., Msrtin, E., & Espinel-ingroff, A. (2007). Métodos estandarizados por el CLSI para el estudio de la sensibilidad a los antifúngicos (documentos M27-A3, M38-A y M44-A). Revista Iberoamericana de Micología, 15, 1. http://www.guia.reviberoammicol.com/Capitulo15.pdf.
Clinical and Laboratory Standards Institute (CLSI). (1999). Methods for determining bactericidal activity of antimicrobial agents (M26-A). Wayne, PA.
Clinical and Laboratory Standards Institute (CLSI). (2017a). Reference method for broth dilution antifungal susceptibility testing of yeasts (M27, 4th ed.). Wayne, PA.
Clinical and Laboratory Standards Institute (CLSI). (2017b). Reference method for broth dilution antifungal susceptibility testing of filamentous Fungi (M38, 3rd ed., p. 62). Wayne, PA.
Clinical and Laboratory Standards Institute (CLSI). (2018). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (M07, 11th ed.). Wayne, PA.
Clinical and Laboratory Standards Institute (CLSI). (2020). Performance standards for antimicrobial susceptibility testing (M100, 30th ed.). Wayne, PA.
Convention on Biological Diversity. (2011). Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising to the Convention on Biological Diversity. https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf
Cos, P., Vlietinck, A. J., Vanden, B. D., & Maes, L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. Journal of Ethnopharmacology, 106(3), 290–302.
Das, K., Tiwari, R. K. S., & Shrivastava, D. K. (2010). Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of Medicinal Plants Research, 4(2), 104–111.
Dewanjee, S., Gangopadhyay, M., Bhattacharya, N., Khanra, R., & Dua, T. K. (2015). Bioautography and its scope in the field of natural product chemistry. Journal of Pharmaceutical Analysis, 5(2), 75–84.
EMA. (2006). Committee on Herbal Medicinal Products: Guideline on good agricultural and collection practice for starting materials of herbal origin. London. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-agricultural-collection-practice-gacp-starting-materials-herbal-origin_en.pdf
Espinel-Ingroff, A., Fothergill, A., Peter, J., Rinaldi, M. G., & Walsh, T. J. (2002). Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for. Journal of Clinical Microbiology, 40(9), 3204–3208.
Espinel-Ingroff, A., Aller, A. I., Canton, E., Castañón-Olivares, L. R., Chowdhary, A., Cordoba, S., Cuenca-Estrella, M., Fothergill, A., Fuller, J., Govender, N., et al. (2012). Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrobial Agents and Chemotherapy, 56(11), 5898–5906.
Fabricant, D. S., & Farnsworth, N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environmental Health Perspectives, 109(1), 69–75.
Fuentes-Fiallo VR, Lemes Hernández CM, Rodriguez Ferradá CA, & Germosén-Robineau L. (2000). Manual de cultivo y conservacion de plantas medicinales. Tomo II: Cuba (p. 172). ISSN 1028-4796.
Gertsch, J. (2009). How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. Journal of Ethnopharmacology, 122(2), 177–183.
Giusiano, G., Rodolfi, M., Mangiaterra, M., Piontelli, E., & Picco, A. M. (2010). Hongos endófitos en 2 plantas medicinales del nordeste argentino. I: Análisis morfotaxonómico de sus comunidades foliares. Boletín Micológico, 25, 15–27.
Hazen, K. C. (1998). Fungicidal versus fungistatic activity of terbinafine and itraconazole: An in vitro comparison. Journal of the American Academy of Dermatology, 38, S37–S41.
Hilbay, R., Chamorro Armas, S., González, M., & Palacios, T. (2016). Reingeniería en los procesos de secado, molienda y tamizado de plantas aromáticas para mejorar la calidad de los derivados, caso: Empresa Jambi Kiwa. FIGEMPA Investig y Desarro, 1(6), 89–99.
Holetz, F. B., Pessini, G. L., Sanches, N. R., Cortez, A. G., Nakamura, C. V., & Dias Filho, B. P. (2002). Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias do Instituto Oswaldo Cruz, 97(7), 1027–1031.
Kuete, V. (2010). Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Medica, 76(14), 1479–1491.
Kunle, O. F., Egharevba, H. O., & Ahmadu, P. O. (2012). Standardization of herbal medicines – a review. International Journal of Biodiversity and Conservation, 4(3), 101–112.
Malheiros, A., Filho, V. C., Schmitt, C. B., Yunes, R. A., Escalante, A., Svetaz, L., Zacchino, S., & Monache, F. D. (2005). Antifungal activity of drimane sesquiterpenes from Drimys brasiliensis using bioassay-guided fractionation. Journal of Pharmaceutical Sciences, 8(2), 335–339.
Matthews, H., Hanison, J., & Nirmalan, N. (2016). “Omics” -informed drug and biomarker discovery: Opportunities, challenges and future perspectives. Proteomes, 4, 28. https://doi.org/10.3390/proteomes4030028.
Meletiadis, J., Meis, J. F. G. M., Mouton, J. W., & Verweij, P. E. (2002). Methodological issues related to antifungal drug interaction modelling for filamentous fungi. Reviews in Medical Microbiology, 13(3), 101–117.
Meletiadis, J., Antachopoulos, C., Stergiopoulou, T., Pournaras, S., Roilides, E., & Walsh, T. J. (2007). Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrobial Agents and Chemotherapy, 51(9), 3329–3337.
Muñoz, F. (1996). Plantas medicinales y aromáticas: estudio, cultivo y procesado (p. 343). Madrid: Mundi-Prensa Libros.
Nazzaro, F., Fratianni, F., Coppola, R., & De Feo, V. (2017). Essential oils and antifungal activity. Pharmaceuticals, 10(4), 1–20.
Ncube, N. S., Afolayan, A. J., & Okoh, A. I. (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African Journal of Biotechnology, 7(12), 1797–1806.
Newman, D. J. (2020). Modern traditional Chinese medicine: Identifying, defining and usage of TCM components. In Advances in pharmacology (Vol. 87, 1st ed., pp. 113–158). Elsevier Inc. https://doi.org/10.1016/bs.apha.2019.07.001
Newman, D. J., & Cragg, G. M. (2020a). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Products, 83(3), 770–803.
Newman, D. J., & Cragg, G. M. (2020b). Plant endophytes and epiphytes: Burgeoning sources of known and “unknown” cytotoxic and antibiotic agents? Planta Medica. Published online ahead of print, 2020 Feb 5, https://doi.org/10.1055/a-1095-1111
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. The Journal of Antimicrobial Chemotherapy, 52(1), 1. https://doi.org/10.1093/jac/dkg301.
Pessini, G. L., Dias Filho, B. P., Nakamura, C. V., & Garcia Cortez, D. A. (2003). Antibacterial activity of extracts and Neolignans from Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck. Memórias do Instituto Oswaldo Cruz, 98(8), 1115–1120.
Pfaller, M. A., Sheehan, D. J., & Rex, J. H. (2004). Determination of fungicidal activities against yeasts and molds: Lessons learned from bactericidal testing and the need for standardization. Clinical Microbiology Reviews, 17(2), 268–280.
Rates, S. M. K. (2001). Plants as source of drugs. Toxicon, 39, 603–613.
Rojas, F. D., Sosa, M. D. L. A., Fernández, M. S., Cattana, M. E., Córdoba, S. B., & Giusiano, G. (2014). Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Medical Mycology, 52, 641–646.
Salvat, A., Antonnacci, L., Fortunato, R. H., Suarez, E. Y., & Godoy, H. M. (2001). Screening of some plants from Northern Argentina for their antimicrobial activity. Letters in Applied Microbiology, 32(5), 293–297.
Sanches, N. R., Garcia Cortez, D. A., Schiavini, M. S., Nakamura, C. V., & Dias Filho, B. P. (2005). An evaluation of antibacterial activities of Psidium guajava (L.). Brazilian Archives of Biology and Technology, 48(3), 429–436.
Sticher, O. (2008). Natural product isolation. Natural Product Reports, 25, 517–554.
Svetaz, L., Tapia, A., López, S. N., Furlán, R. L. E., Petenatti, E., Pioli, R., Schmeda-Hirschmann, G., & Zacchino, S. A. (2004). Antifungal chalcones and new caffeic acids esters from Zuccagnia punctata acting against soybean infecting fungi. Journal of Agricultural and Food Chemistry, 52(11), 3297–3300.
Tallmadge, E. H. (2018). Patenting natural products after myriad. Harvard Journal of Law & Technology, 30(2), 569–600.
Tanaka, J. C. A., da Silva, C. C., de Oliveira, A. J. B., & Dias Filho, B. P. (2006). Antibacterial activity of indole alkaloids from Aspidosperma ramiflorum. Brazilian Journal of Medical and Biological Research, 39, 387–391.
Van Norman, G. A. (2016). Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC Basic to Translational Science, 1(3), 170–179.
WHO. (1992). Convention on biological diversity. https://www.cbd.int/doc/legal/cbd-en.pdf
WHO. (2002). WHO traditional medicine strategy 2002–2005. http://www.beovita.eu/pdf/WHO_EDM_TRM_2004.pdf
WHO. (2003). WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants. https://apps.who.int/medicinedocs/pdf/s4928e/s4928e.pdf
WHO. (2013). WHO traditional medicine strategy 2014–2023 (p. 76). Honk Kong. ISBN 9241506091, 9789241506090.
WHO, IUCN, WWF. (1993). Guidelines on the conservation of medicinal plants (pp 1–38). WHO, IUCN & WWF (Ed.). Gland. ISBN 2-8317-0136-8. https://apps.who.int/medicinedocs/documents/s7150e/s7150e.pdf
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mussin, J., Giusiano, G. (2020). Ethno–Phytopharmacology: Product Validation Process Based on Traditional Knowledge of Medicinal Plants. In: Chong, P., Newman, D., Steinmacher, D. (eds) Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery. Springer, Cham. https://doi.org/10.1007/978-3-030-51358-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-51358-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51357-3
Online ISBN: 978-3-030-51358-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)