Abstract
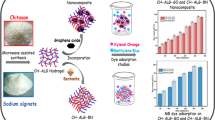
Wastewater treatment has become immensely challenging. Adsorption process has an obvious drawback since it does not lead to dyes degradation in the end. A novel Polypyrrole/Chitosan/exfoliated Grapheneoxide/Montmorillonite (PPy@CS/MMT/GO) nanohybrid was synthesized using in situ polymerization of pyrrole on a high surface area CS/MMT/GO nanocomposite to give multi-functional adsorbent with high ability for removal of neutral, anionic, and cationic dyes. The PPy@CS/MMT/GO surface morphology was characterized by various instrumental techniques such as SEM, TEM, and XRD. The removal efficiency for Biebrich scarlet (99%), Eosin Y (97%), neutral red (95%), Safranin O (66%), and Titan yellow (85%) was estimated. The adsorption trend was rapid in the case of Biebrich scarlet, Eosin Y, and neutral red, consequently. The R2 values for second-order model indicated the high reliability for the developed regression models in explaining experimental data of adsorption of Saf-O and Tit-Y onto PPy@CS/MMT/GO. Langmiur and Freundlich adsorption models were used to describe the equilibrium isotherm for Tit-Y and Saf-O. Thermodynamic studies demonstrated that the adsorption of Saf-O and Tit-Y onto PPy@CS/MMT/GO was feasible endothermic and spontaneous. The reutilization of the nanohybrid which adsorbed the dyes was examined.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
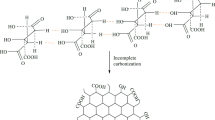
Water contamination by dyes has become one of the main environmental issues. The wastewater discharged from industries may enter the environment without adequate treatment process causes severe toxicity to living organisms due to their non-biodegradability and stability. Several technologies have been developed for the removal of dyes such as tricking filters activated sludge, carbon adsorption and photo-degradation process. Among these techniques, adsorption is the most widely used method because it is economical, simple, can be handled fairly, achieves fast flow rates, does not result in harmful substances such as free radicals or ozone, produced during photo-degradation techniques [1]. However, adsorption still has an obvious drawback since this process does not lead to the degradation of the dyes at the end. Therefore, removing these dyes before discharging into the environment is a great demand for safety and reutilization of the dyes after adsorption is considered as a great demand [2,3,4]. Chitosan has attracted significant interest in water treatment due to its good biocompatibility, biodegradability, and presence of multiple functional groups in the main chain [5]. However, the gel formation below pH 5.5 limits the use of CS as an adsorbent in removing the dyes since the dying process is usually adjusted in a 3 to 4 pH range. The incorporation of conducting polymers into CS produces a new material that brings together some advantages of both polymers and limits the disadvantages of CS due to its solubility in an acidic medium [6]. Two-dimensional single-layered graphene oxide and clay especially montmorillonite have attracted tremendous interest due to their low cost, unique structure, mechanical properties, ease of intercalation, and/or exfoliation. The intrinsic van der Waals interaction between layers of graphene results in agglomeration. However, the dispersion of these fillers on a molecular level by polymer intercalation through chemical bonding or strong intermolecular forces unexpected new properties that might be achieved. It was reported that [7] montmorillonite-pillared graphene oxide provided higher surface area (972 m2g−1) in comparison with GO (611 m2g−1) and MMT (243 m2g−1) and has a high adsorption capacity to cationic and anionic dyes.
To our best knowledge, no reports on the effect of combining graphene oxide with MMT to improve the adsorption properties of PPy/CS have been published followed by the utilization of nanohybrid-loaded dye for the synthesis of Polymethylmethacrylate nanocomposites. Here, the polymerization of pyrrole in the presence of CS/MMT/GO was achieved resulting in a multi-functional nanohybrid. The adsorption performance of PPy@CS/MMT/GO nanohybrid to remove different kinds of dyes including biebrich scarlet, Eosin Y, neutral red, Safranine-O, and Titan yellow as models from aqueous solution was studied. Besides, adsorption isotherms, kinetics and thermodynamics were studied.
2 Experimental
2.1 Materials
Titan yellow (Tit-Y), Safranine-O (Saf-O), Biebrichscarlet, Eosin Y, Neutral red, Methylviolet (Sigma-Aldrich, USA); Sodium montmorillonite (Gonzales, TX, USA) under trade name of mineral colloid BP, Graphite flakes, methylmethacrylate (Across, USA), pyrrole (Mallinckrodt, USA), Chitosan (Across, USA) 100,000–300,000 were purchased.
2.2 Preparation of PPy@CS/MMT/GO
GO was prepared from graphite flakes by the modified Hummer’s method [8, 9]. PPy@CS/MMT/GO was prepared by a simple solution mixing-evaporation method. Accordingly, 0.8 g MMT was swelled in 40 ml of water and sonicated for 15 min. Afterward, the MMT suspension was added into chitosan solution (2 g CS in a 100 ml of aqueous acetic acid solution), stirred at 27 °C for 24 h. Subsequently, 1.5 g GO suspended in 150 ml H2O was added into the suspension and cooled to 2 °C. Then, 0.4 ml pyrrole was added to the mixture. The oxidative polymerization of pyrrole was started after adding 2.2 g of FeCl3. Stirring was continued for 24 h then the mix was centrifuged and washed with sodium hydroxide NaOH (0.001 M). Finally, the precipitate was washed three times with H2O, ethanol and oven dried at 70 °C.
2.3 Adsorption of Dyes onto PPy@CS/MMT/GO Nanohybrid
The adsorption performance of PPy@CS/MMT/GO for five different dyes using batch experiments was performed by stirring 0.007 g of adsorbents (200 rpm) with 10 ml of dyes, the concentration was determined spectrophotometrically at λmax (Table 1) and the removal efficiency and equilibrium adsorption capacity were calculated from the following Eqs. (1, 2)
Removal efficiency
Equilibrium adsorption capacity
where Co and Ce (ppm) are the initial and equilibrium concentrations of dyes, V is the volume of dye solution, w is the adsorbent mass.
Then, the resulting nanohybrid-loaded dyes were incorporated into polymethylmethacrylate.
3 Results
3.1 Characterization of the Nanohybrid
The SEM image of PPy@CS/MMT/GO (Fig. 1a) exhibited a layered morphology (MMT) with sheets (GO) but without any agglomeration. In addition, a sphere-like morphology was observed confirming the polymerization of pyrrole into a spherical shape with a range size from 80 to 110 nm. The TEM image of PPy@CS/MMT/GO (Fig. 1B) shows the GO sheets and layered MMT were dispersed homogeneously in the matrix. Moreover, the presence of PPy within the hybrid can be confirmed by the appearance of dark spherical shape surrounded by a shell of CS with a diameter that ranges from 5 to13 nm.
XRD pattern of PPy@CS/MMT/GO (Fig. 2) shows that the characteristic peak (001) of MMT (2θ = 9.1°) disappeared due to intercalation with CS and PPy, the characteristic peaks of CS (2θ = 10°) disappeared, peak at 2θ = 19.7° interfere with 110 plane of MMT (2θ = 19.8°) after intercalation process. The peak characteristic to GO at (2θ = 11.8°) also disappeared.
3.2 Adsorption Process
The removal efficiencies are shown in Table 1. The results demonstrate that Biebrich scarlet, Eosin y, and Neutral red are adsorbed rapidly, while Saf-O and Tit-Y dyes adsorbed slowly. Consequently, the efficiency of GO, MMT, CS, and PPy@CS/MMT/GO as adsorbents were carefully investigated for the removal of Tit-Y dye and Saf-O dye from aqueous solutions. Clearly, for the same dye, the removal efficiency of PPy@CS/MMT/GO is higher than the individual components. The removal efficiency of Tit-Y and Saf-O was found to be 85.5% and 66.9% in comparison with 25.4%, 60.6% for GO, 33.4%, 61.9% for MMT and 42.6, 22.9% for CS, respectively. The equilibrium adsorption capacity of PPy@CS/MMT/GO toward Tit-Y (62.7 mg/g) and Saf-O dye (17.8 mg/g) are higher than other adsorbents (Qe of Tit-Y and Saf-O = 26.4, 15.9 mg/g for MMT; 33.8, 5.8 mg/g for CS; 20.2, 15.5 mg/g for GO, respectively). Adsorption of Saf-O and Tit-Y onto the hybrid can be applied for their kinetics and other effects. The dye removal % decreases with increasing the initial dye concentration due to the occupation of all active sites on the nanohybrid which lack of available sites required for high initial concentration of both dyes. The removal efficiency% of Tit-Y decreases with increasing the pH value. On the other hand, Saf-O removal% improved by increasing the pH of the medium. The R2 values (Table 1) for second-order model indicated the high reliability for the developed regression models in explaining experimental data of adsorption Saf-O and Tit-Y onto PPy@CS/MMT/GO. Langmuir isotherm models fit better with experimental data of adsorption of Tit-Y onto PPy@CS/MMT/GO. However, Freundlich isotherm fits better the adsorption of Saf-O onto PPy@CS/MMT/GO (Table 1). From Van,t Hoff plot ΔH° was calculated to be 64150.47,108457.33 kJ/mol and ΔG° to be −4591.13, −3312.35 kJ/mol, ΔS° to be 227.06, 368.28 kJ/mol for Tit-Y and Saf-O, respectively.
4 Discussion
In the present work, the incorporation of GO into chitosan intercalated montmorillonite nanocomposite was used followed by in situ polymerization of pyrrole using FeCl3 as oxidant to obtain PPy@CS/MMT/GO nanohybrid. The combination of GO and MMT into PPy@CS revealed the excellent adsorption of neutral, cationic, and anionic dyes in comparison with GO, MMT, and CS. Interestingly, the Qmax for different adsorbents toward Saf-O dye was estimated [10], e.g.,. kaolonite clay (16.23 mg/g). The cationic Saf-O dye was adsorbed with high efficiency toward GO and MMT due to the presence of negative charged site on their surfaces. In contrast, CS showed high efficient adsorbents toward anionic dye. On the other hand, the PPy@CS/MMT/GO could be easily used as efficient adsorbent for cationic and anionic dyes due to the synergistic effect of MMT/GO and PPy/CS polymer. Obviously, examining the morphology of the nanohybrid revealed the absence of any aggregation of MMT. In addition, exfoliation of GO and MMT improved the surface area and presence of functional groups on the surface of nanohybrid favored the adsorption process. In strong acidic medium. the positively charged site in emeraldine salt polypyrrole favored the interaction with anionic dye; however, at high pH the emeraldine base inhibited the interaction due to depletion of active sites in the polymer skeleton. The adsorption of Saf-O and Tit-Y onto PPy@CS/MMT/GO was feasible endothermic and spontaneous. The resulting nanohybrid-loaded dye was incorporated onto polymethylmethacrylate to impart the yellow color for the polymer. The utilization of nanohybrid-loaded dye for synthesis polymethylmethacrylate nanocomposites solves the problem of adsorption process that does not lead to the dyes degradation in the end [8].
5 Conclusion
A facile in situ polymerization of pyrrole in the presence of CS/MMT/GO to form PPy@CS/MMT/GO for the removal of neutral, anionic, and cationic dyes from aqueous solution was demonstrated. PPy@CS/MMT/GO is an excellent adsorbent for different kinds of dyes with high removal efficiency for (Biebrich scarlet, Eosin y, Neutral red, Safranin O, Titan yellow). PPy@CS/MMT/GO has high capacity of adsorption for Safranin O and Titan yellow dyes when compared to its components. The R2 values for second-order model indicated the high reliability for the developed regression models in explaining experimental data of adsorption Saf-O and Tit-Y onto PPy@CS/MMT/GO. The Langmuir isotherm models fit better with experimental data of adsorption of Tit-Y onto PPy@CS/MMT/GO; however, Freundlich isotherm fits better for adsorption of Saf-O onto PPy@CS/MMT/GO. The thermodynamic studies demonstrated that the adsorption of Saf-O and Tit-Y onto PPy@CS/MMT/GO was feasible endothermic and spontaneous. Hybrid loaded dyes were added to methylmethacrylate during polymerization to impart the yellow color for the polymer.
References
Kim, T.-Y., Park, S.-S., Cho, S.-Y.: Adsorption characteristics of Reactive Black 5 onto chitosan beads cross-linked with epichlorohydrin. J. Ind. Eng. Chem. 18(4), 1458–1464 (2012)
Touati, A., et al.: Photocatalytic degradation of sulfur black dye over Ce-TiO 2 under UV irradiation: removal efficiency and identification of degraded species. Euro-Mediterranean J. Environ. Integr. 4(1), 4 (2019)
Arfi, R.B., et al.: Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-Mediterranean J. Environ. Integr. 2(1), 20 (2017)
Dasgupta, J., et al.: Poly (sodium-4-styrenesulfonate) assisted ultrafiltration for methylene blue dye removal from simulated wastewater: Optimization using response surface methodology. J. Environ. Chem. Eng. 4(2), 2008–2022 (2016)
Prashanth, K.H., Tharanathan, R.: Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Sci. Technol. 18(3), 117–131 (2007)
Sajesh, K., et al.: Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 62, 465–471 (2013)
Liu, L., et al.: Simultaneous removal of cationic and anionic dyes from environmental water using montmorillonite-pillared graphene oxide. J. Chem. Eng. Data 60(5), 1270–1278 (2015)
Chen, J., et al.: An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64, 225–229 (2013)
Salahuddin, N. et al.: Synthesis and efficacy of PPy/CS/GO nanocomposites for adsorption of ponceau 4R dye. Polymer 146, p. 291 (2018)
Adebowale, K.O., Olu-Owolabi, B.I., Chigbundu, E.C.: Removal of safranin-O from aqueous solution by adsorption onto kaolinite clay. J. Encapsul. Adsorption Sci. 4(03), 89 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Salahuddin, N.A., EL-Daly, H.A., El Sharkawy, R.G., Nasr, B.T. (2021). Adsorption of Dyes from Aqueous Solutions onto Multi-functional PPy/CS Exfoliated Nanohybrid for Fashionable Layered Polymer Nanocomposites. In: Ksibi, M., et al. Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (2nd Edition). EMCEI 2019. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-51210-1_76
Download citation
DOI: https://doi.org/10.1007/978-3-030-51210-1_76
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51209-5
Online ISBN: 978-3-030-51210-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)