Abstract
The inhibition performance of Rosmarinus officinalis (RO) extract on the corrosion of carbon steel was examined using electrochemical and analytical techniques. Investigations were carried out in HCL 1 M solution at different temperatures. Results showed that the extract acted as a mixed-type inhibitor, and inhibition efficiency increased with increasing inhibitor concentration. The adsorption of the extract molecules onto the carbon steel surface followed the Langmuir adsorption model. To confirm these results, scanning electron microscopy (SEM), was applied.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Several protection methods have been applied to protect steel against corrosion. The use of inhibitors is the better-known one, especially in an acid medium. Inhibitors may reduce the rate of one or both of the partial reactions of the corrosion process, i.e. the anodic metal dissolution and the cathodic oxygen reduction [1]. However, unwanted side effects on the environment were noticed due to several inhibitors with high inhibition efficiency, namely chromate, phosphate and arsenic compounds [2]. Thus, the use of plant extracts as corrosion green inhibitors with a similar inhibition effect based on organic compounds has become increasingly necessary [3]. Many authors have reported the successful use of these substances to inhibit metal corrosion in acidic media [4].
2 Materials and Methods
Leaves of Rosmarinus officinalis were firstly cleaned with distilled water and dried at 313 K. The dried leaves were then crushed into a fine powder. A quantity of the dried powder was extracted with a mixture of water and methanol via stirring for 24 h at room temperature. The extract was filtered, dried and conserved at 277 K until use.
The inhibition efficiency of the plant extract was evaluated, in HCl 1 M at 298 K by weight loss measurements, potentiodynamic polarization curves, linear polarization resistance (RPL) and electrochemical impedance spectroscopy (EIS). Electrochemical measurements were carried out using a potentiostat-galvanostat SP 300, EC LAB software connected to a three-electrode cell with Ag/AgCl as a reference electrode and a Pt-mesh as an auxiliary electrode. The carbon steel XC38 used as a working electrode (0.28 cm2 active area).The chemical composition of the metal used was determined as (wt%): C 0.38, Mn 0.66, Si 0.27, Ni 0.02, Cr 0.21, Mo 0.02 and the balance Fe. The polarization curves were recorded at a constant scan rate of 1 mV/s. The potential range was ± 0.20 V versus(Ag/Ag Cl). EIS measurements were carried out at the open circuit potential with frequency ranged between 50 kHz and 10 MHz. The applied AC voltage was ± 10 mV.
3 Results
3.1 Weight Loss Methods
The effect of the inhibitor concentration on the steel corrosion in HCl 1 M was studied by weight loss measurements at 298°K. Results are shown in Fig. 1.
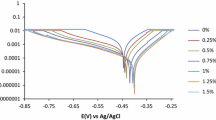
3.2 Electrochemical Measurements
The values of corrosion current density (icor) and the inhibition efficiency were estimated using the Tafel extrapolation method (Table 1).
3.3 Thermodynamic Investigations
The influence of temperature on the corrosion behavior of the steel working electrode under different experimental conditions was investigated at temperatures 303, 313 and 323 K the Thermodynamic parameters are regrouped in Table 2.
3.4 SEM Images
The SEM micrographs of XC48 steel samples before and after contact with 1 M HCl for 24 h, with and without 400 ppm of the RO extract are given in Fig. 2. Before the immersion, the XC48 steel sample is relatively smooth (Fig. 2a), however, the metallic surface of the working electrode (Fig. 2b) is seriously damaged after the exposure to the corrosive solution. In Fig. 2c, it can be observed that the surface of the steel immersed in acid solution with 400 ppm of RO extract is more homogeneous indicating that the adsorbed extract molecules protect the metal surface.
4 Discussion
The corrosion rate, calculated from weight loss investigations, decreased (Fig. 1), while the inhibition efficiency (IE %) increased in HCl solution with the addition of the Rosmarinus officinalis extract. This indicates the formation of a protective layer on the active surface of the working electrode [5]. The current density (icor) values decreased with the increase in Rosmarinus officinalis extract concentration (Table 1). Significant changes on the Tafel slopes (βa and βc) were also observed upon adding the extract, which indicates a mixed type inhibitor.
According to electrochemical impedance diagrams, the charge transfer resistance increases and the capacity of the double layer decreases with the increase of inhibitor concentration. The thermodynamic investigations showed that the adsorption of this inhibitor is spontaneous and obeys to the Langmuir model. The activation energies and the negative free energy of adsorption obtained indicate that the Rosmarinus officinalis extract is physically adsorbed on the surface of the steel and that the adsorption is strong and spontaneous [6].
5 Conclusions
Weight loss measurements and electrochemical investigations showed that Rosmarinus officinalis leaf extract was an effective inhibitor against XC38 steel corrosion in HCl medium. The extract acted as a mixed inhibitor, adsorbing on the steel surface according to the Langmuir isotherm model. Surface analysis technique supported electrochemical results and confirmed the adsorption of the active components of the RO leaf extract on the surface of the XC38 Steel.
References
El Kacimi, Y., Azaroual, M.A., Touir, R., Galai, M., Alaoui, K., Sfaira, M., Ebn Touhami, M., Kaya, S.: Corrosion inhibition studies for mild steel in 5.0 M HCl by substituted phenyltetrazole. Euro-Mediterranean J. Environ. Integr. 2, 1–11 (2017)
Ferkous, H., Zerroug, M., Radjai, M., Chaouch, M.A., Jebali, Z., Majdoub, H.: Electochemical and surface morphological studies of a carbon steel corrosion by natural product in acidic solution, Euro-Mediterranean J. Environ. Integr. 1291–1292, 2017
Quraishi, M. A., Singh, A., Singh, V.K., Yadav, D.K., Singh, A.K.: Materials chemistry and physics, 122(7), 114–122 (2010)
Ehsani, A., Mahjani, M.G., Hosseini, M., Safari, R., Moshrefi, R., Mohammad Shiri, H.: Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 490, 444–451 (2017)
Derfouf, H., Harek, Y., Larabi, L., Basirun, W., Ladan, M.: Corrosion inhibition activity of carbon steel in 1.0 M hydrochloric acid medium using Hammada scoparia extract:gravimetric and electrochemicalStudy. J. Adhes. Sci. Technol. 33(8), 808–883 (2019)
Larouj, M., Ourrak, K., El M’Rabet, K., Zarrok, H., Serrar, H., Boudalia, M., Boukhriss, S., Warad, I., Oudda, H., Toui, R.: Thermodynamic study of corrosion inhibition of carbon steel in acidic solution by new pyrimidothiazine derivative. J. Mater. Environ. Sci., 8(11), 3921–3931 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Belakhdar, A., Ferkous, H., Djellali, S., Lahbib, H., Amor, Y.B. (2021). Thermodynamic and Electrochemical Studies of Corrosion Inhibition of Carbon Steel by Rosmarinus Officinalis Extract in Acid Medium. In: Ksibi, M., et al. Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (2nd Edition). EMCEI 2019. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-51210-1_236
Download citation
DOI: https://doi.org/10.1007/978-3-030-51210-1_236
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51209-5
Online ISBN: 978-3-030-51210-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)