Abstract
This chapter explores the clinical use of botulinum toxin therapy in ophthalmology and ophthalmic surgery. Ophthalmic conditions that can benefit from botulinum toxin therapy include blepharospasm, strabismus, nystagmus, lacrimal hypersecretion syndromes, eyelid retraction and spastic entropion among others. Since its first use in blepharospasm in 1983, toxin therapy has become the treatment of choice for blepharospasm with successful outcomes. This chapter also explores the techniques of injection, application and complications of toxin therapy in these conditions. Whilst generally considered safe, some of the complications of ophthalmic toxin therapy include ptosis, diplopia, dry eyes and epiphora.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Clinical use of botulinum toxin as medical therapy was first established in the treatment of strabismus by Alan Scott in 1980 [1]. Following successful clinical trials, botulinum toxin type A was approved by the U.S. Food and Drug Administration (FDA) for the treatment of strabismus, blepharospasm and hemifacial spasm in patients over 12 years of age in 1989. The early success of botulinum toxin therapy in treating strabismus bolstered research into additional therapeutic uses within the field of ophthalmology. Early botulinum toxin therapy within ophthalmology pioneered the expansion of its clinical application to the wide range of medical therapies we see today in multiple medical and surgical specialities.
The current application of toxin therapy within ophthalmology is extensive, as shown in the Table 13.1 below. Ophthalmic conditions that can benefit from botulinum toxin therapy include blepharospasm, strabismus, nystagmus, lacrimal hypersecretion syndromes, eyelid retraction and spastic entropion among others.
Periocular
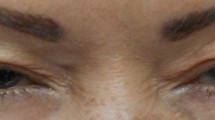
Blepharospasm (Image 13.1)
Benign essential blepharospasm is a distressing idiopathic dystonia involving the orbicularis oculi and upper facial muscles. It is characterised by involuntary muscle contraction resulting in the appearance of forceful, prolonged bilateral blinking. In severe cases, the increased force and frequency of blinking can render the patient functionally blind. This is a lifelong condition with significant impact on quality of life. Whilst the cause is not yet well understood, spasms can be triggered by activities that put strain on the eyes – reading, driving, stress and bright lights.
Botulinum toxin has been well established as in the treatment of blepharospasm, first shown to be effective in 1983. It has since become the treatment of choice, with very successful results [2,3,4,5,6,7,8,9,10,11,12,13,14].
Technique
Figure 13.1 illustrates the recommended injection sites for the treatment of blepharospasm. Care must be taken to direct the injecting needle away from the central region of the eyelid to avoid the levator palpebrae superioris, damage to which might result in ptosis.
Botox® (Botulinum Toxin Type A)
The initial recommended dose is 2.5 to 5 U injected into the medial and lateral pre-tarsal orbicularis oculi of the upper lid and the later pre-tarsal orbicularis oculi of the lower lid. The injections are given subcutaneously to ensure maximal effectiveness and safety. It is prudent to remain outside the orbital rim whilst injecting to avoid inadvertent weakening of the levator palpebrae superioris or the extraocular muscles.
The initial effect of the injections is seen within 72 hours and peaks at 1–2 weeks post injection. The effects last for approximately 13 weeks. The procedure can then be repeated indefinitely, with increasing doses if necessary. If the initial treatment does not last longer than 2 months, then a twofold increase in dosage is recommended. There have been some reports of decreasing effectiveness with prolonged use [15, 16], though this is rare and there is no evidence of any adverse effects with prolonged treatment. The cumulative dose of Botox® in a 30-day period should not exceed 200 U.
Dysport®
Initial recommended dose of 20 units should be injected medially 40 units laterally into the pre-tarsal region of both the upper and lower orbicularis oculi muscles of each eye. Symptomatic relief is expected between 2 and 4 days with maximal effect seen within 2 weeks. Injections should be repeated approximately every 8 weeks.
Myobloc® (Toxin Type B)
Type B toxin (Myobloc®) should be reserved for patients who develop resistance to type A toxin. The recommended dose of type B toxin is 1200–2500 U per eye, and the same injection technique and sites are used as in Botox®. Myobloc ® has a shorter duration of action of approximately 8–10 weeks, compared to 13 weeks in Botox®. Additionally, patients may report more pain due to the acidic nature of the preparations which has a tendency to diffuse into surrounding tissues (Table 13.2).
Complications
This is a safe and effective treatment of blepharospasm and is well tolerated. Some reports have described up to 30% [17] of patients experiencing side effects from botulinum toxin treatment of blepharospasm. Whilst this may seem like a large proportion, the side effects are often mild, transient and reversible, with symptoms subsiding with gradual recovery of muscle function. Table 13.3 lists some of the potential adverse effects following periocular botulinum toxin injection. The most common complication is ptosis, seen in around 13% of cases [18], and this risk significantly increases both with number of treatments [5] and dose [19]. Patients may not be able to distinguish the difference between eyelid closure secondary to ptosis or blepharospasm and thus may report ptosis as a failure of botulinum toxin treatment. It is therefore the clinician’s responsibility to determine the difference and identify the complication of ptosis where it occurs and avoid unnecessary early re-treatment and potential exacerbation of the ptosis.
Injecting botulinum toxin into the orbicularis muscle can result in a weakened blink as well as lagophthalmos. This can result in dry eyes, another commonly reported side effect of toxin therapy in blepharospasm. This can be overcome by using artificial tears routinely during toxin therapy.
Strabismus (Image 13.2)
The first therapeutic use of botulinum toxin in humans was for the treatment of strabismus. Strabismus is a common condition where there is an imbalance in extraocular muscle function, leading to misalignment of the eyes. The commonest strabismus causes are congenital whilst others may be acquired in adulthood, such as cranial nerve palsies. This deviation of ocular alignment may be intermittent or constant. Strabismus can also be subdivided into groups based on the direction of deviation: esotropia (inturning deviation), exotropia (out-turning deviation), hypertropia (upturning deviation), hypotropia (downturning deviation) or cyclotropia (rotatory deviation). Strabismus can be caused by pathologies affecting the extraocular muscles, the nerves that control these muscles or the central processing that directs eye movements at a cortical level. Toxin might be used as a diagnostic or a therapeutic tool in the management of strabismus.
Diagnostic
Botulinum toxin can be used preoperatively to detect whether fusion is present once the deviation is corrected. It may also help aid the prediction of surgical outcomes for incomitant deviations and to rule out the incidence of postoperative diplopia. It can also be used to investigate a possible postoperative slipped muscle and gauge the power of a paretic one.
Therapeutic
Strabismus treatment is targeted at aligning the visual axes. Conservative options include the use of prisms and orthoptic exercises to help establish binocular control of ocular alignment. Invasive options such as surgery are used to weaken or strengthen extraocular muscles and permanently change ocular alignment. Botulinum toxin therapy can be used to temporarily paralyse individual extraocular muscles in attempt to establish better ocular alignment. This effect may be permanent in children with neuroplasticity.
Botulinum toxin acts by weakening the muscle of interest that leads to a reduction, or in some cases complete correction, of the angle of deviation of strabismus. For example, to correct convergent squint caused by a sixth nerve palsy, the toxin would be injected into the medial rectus of the affected eye.
Once toxin is injected, paralysis sets in within 72 hours; the maximal effect of the toxin is usually seen around 14 days after injection and lasts for approximately 3 months. It is important to use electromyographic guidance to ensure the toxin is placed in the correct location and to use a small volume to prevent inadvertent involvement of other extraocular muscles or the levator muscle.
As the effect of the toxin sets in, it can cause an initial overcorrection of the deviation (a divergent deviation in the example of injection into the medial rectus in a sixth nerve palsy), but this effect is usually transient. It is important to monitor for unexpected vertical or horizontal deviations and ptosis which may occur if the solution extravasates during injection, affecting adjoining structures.
Aside from its use as primary treatment for strabismus, botulinum toxin can also be used as an adjunct to correct residual symptoms postoperatively in large-angle strabismus [20, 21]. It has also been used intraoperatively for large-angle strabismus, though this has not yet shown to improve outcomes over surgery alone [22].
Infantile Esotropia
Whilst botulinum toxin use in adults is a well-established alternative treatment option to surgical correction, it is not as well studied in children. It has been shown that early intervention with botulinum toxin therapy can re-establish motor and sensory fusion with good long-term results [23]. However, studies show varying results depending on the age of the patients and pattern of injection. The image below illustrates the injection of botulinum toxin into the medial rectus of a child under general anaesthetic (Image 13.3).
Sixth Nerve Palsy
Botulinum toxin has also been trialled in the treatment of acquired sixth nerve palsy. Injecting the medial rectus muscle has been shown to improve the rate of recovery. It also helps improve the deviation in the short term, lessening the need to use prisms or occlusion of the affected eye. However, the long-term outcomes are uncertain with many studies reporting equal efficacy between botulinum toxin therapy and conservative management of sixth nerve palsy.
Technique
To achieve paralysis of the muscle, botulinum toxin must be injected directly into the belly of an extraocular muscle. In order to correctly isolate the muscle in question, electromyography (EMG) is used (Image 13.4). One end of the EMG machine is connected to the base of the injecting needle and the other is connected to the patient’s forehead to complete the circuit. Topical anaesthesia is used to anaesthetise the conjunctiva. The needle is then passed through the conjunctiva posterior to the muscle insertion site on the sclera, staying superficial to the sclera to avoid penetration of the globe. The patient is first asked to look in the opposite direction to the action of the muscle being injected. When the needle is deemed to be in the muscle belly, the patient is then asked to look in the direction of action of the extraocular muscle being injected. This will result in an increased signal output from the EMG, confirming the correct location of the needle. The toxin is then slowly injected.
Different techniques for injecting botulinum toxin in strabismus:
-
1.
EMG-guided injection.
-
2.
Injection without EMG guidance.
-
3.
Injection under direct vision of the muscle during squint surgery.
-
4.
Injection through a conjunctival incision to visualise the muscle.
-
5.
Injection via a sub-Tenon’s lacrimal cannula alongside a muscle.
-
6.
Transconjunctival injection after grasping the muscle with forceps.
Indication | Botox® dose | Dysport |
|---|---|---|
Strabismus of less than 20 prism diopters | 1.25–2.5 U | 5–10 U |
Strabismus of 20–50 prism diopter | 2.5–5 U | 10–20 U |
VI nerve palsy | 1.25–2 U |
Myokymia
Eyelid myokymia is the involuntary contraction of the orbicularis muscle. It predominantly affects the lower eyelid and upper eyelid involvement is rare. It is a benign, non-progressive disorder that is often self-limiting and the underlying pathology is not fully understood. It can be triggered by stress, anxiety, fatigue, caffeine or alcohol. Botulinum toxin can be injected into the twitching orbicularis to temporarily paralyse and relax the muscle until spontaneous resolution is achieved. The recommended dose and technique is similar to that for the treatment of blepharospasm but only the upper or lower lid may need to be treated.
Chronic Dry Eyes
The aqueous component of the tear film is produced by the lacrimal gland and drained by the lacrimal pump. The lacrimal pump is located in the medial canthus and it is regulated by the orbicularis oculi. Thus, conditions causing an excess of blinking, such as blepharospasm or hemifacial spasm, can lead to a chronic dry eye state. Botulinum toxin injections can be used to reduce the blink rate and consequently improve ocular surface wetting [24]. Injecting the medial region of the orbicularis muscle of the lower lid has shown to reduce lacrimal pump action, increase tear output and improve dry eye symptoms [25].
Acquired Nystagmus
Acquired nystagmus is a rare condition caused by involuntary repetitive to-and-fro eye movements, leading to visual disturbances such as oscillopsia and blurred vision. Studies have shown success in treating nystagmus by injecting botulinum toxin directly into the rectus muscles [26,27,28]. However, the treatment should only be attempted in wheelchair-bound patients if all muscles are to be blocked as it may also inhibit the vestibular reflex or the ocular tilt reaction.
Cosmetic
Patients with blepharospasm treated with botulinum toxin were noted to have a stress-free appearance. This observation inspired research on the potential cosmetic applications of the toxin.
Botulinum toxin has revolutionised the cosmetic industry in recent years. The first cosmetically approved use for Botox® was for the treatment of glabellar furrows in 2002 and its cosmetic application has since expanded to treat lateral periocular rhytids (“crow’s feet”), perioral rhytids (“smoker’s lines”), mesolabial folds (“marionette lines”), transverse brow and forehead furrows and platysmal bands.
The safety profile for cosmetic Botox® is very promising, with no serious adverse events reported. Mild side effects include local pain and bruising, infection, brow and eyelid ptosis.
Complications
Botulinum toxin therapy is generally considered a safe and well-tolerated procedure.
Whilst side effects can occur, they are usually transient and rarely sight threatening.
Side effects secondary to the technique of administration are common to most procedures involving an injection. These include oedema, bruising, haemorrhage and mild pain. Bruising and haemorrhage can be minimised by stopping any anticoagulant medications 2 weeks prior to the injection. Additionally, use of ice packs prior to injection, careful placement of the needle and immediate gentle local pressure to the injection site can help prevent bruising.
The most common complication secondary to the chemodenervation associated with botulinum toxin therapy is ptosis. Diffusion of the neurotoxin into the orbital septum can result in impairment of the levator muscle. This can occur as early as 48 hours post injection and can last up to 12 weeks. Techniques to avoid the levator muscle such as avoiding the central portion of the upper lid are recommended to reduce the risk of ptosis.
Diplopia can occur, most commonly due inadvertent paralysis of the inferior oblique muscle, though this is rare. Additionally, unwanted horizontal deviation can occur, though uncommon.
Other common side effects of botulinum toxin therapy include dry eye and epiphora. Patients can develop lagophthalmos and impaired blinking due to orbicularis muscle weakening. This can lead to characteristic dry eye symptoms of burning, photophobia and redness. Conversely, toxin therapy can also result in epiphora by means of impaired lacrimal pump function secondary to reduced lower lid tone.
More severe reported side effects include incidences of acute angle-closure glaucoma [29, 30] and retinal detachment secondary to globe penetration associated with botulinum toxin injection [31]. However, these are very rare and often avoidable.
Bibliography
Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87(10):1044–9. https://doi.org/10.1016/s0161-6420(80)35127-0.
Taylor JD, Kraft SP, Kazdan MS, Flanders M, Cadera W, Orton RB. Treatment of blepharospasm and hemifacial spasm with botulinum A toxin: a Canadian multicentre study. Can J Ophthalmol. 1991;26(3):133–8. http://www.ncbi.nlm.nih.gov/pubmed/2054723. Accessed December 1, 2019
Scott AB, Stubbs HA, Kennedy RA. Botulinum a toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985;103(3):347–50. https://doi.org/10.1001/archopht.1985.01050030043017.
Tsoy EA, Buckley EG, Dutton JJ. Treatment of Blepharospasm with botulinum toxin. Am J Ophthalmol. 1985;99(2):176–9. https://doi.org/10.1016/0002-9394(85)90228-4.
Dutton JJ, Buckley EG. Long-term results and complications of botulinum A toxin in the treatment of blepharospasm. Ophthalmology. 1988;95(11):1529–34. https://doi.org/10.1016/s0161-6420(88)32977-5.
Calace P, Cortese G, Piscopo R, et al. Treatment of Blepharospasm with botulinum neurotoxin type A: long-term results. Eur J Ophthalmol. 2003;13(4):331–6. https://doi.org/10.1177/112067210301300401.
Osako M, Keltner JL. Botulinum A toxin (Oculinum) in ophthalmology. Surv Ophthalmol. 1991;36(1):28–46. https://doi.org/10.1016/0039-6257(91)90207-v.
Kraft SP, Lang AE. Botulinum toxin injections in the treatment of blepharospasm, hemifacial spasm, and eyelid fasciculations. Can J Neurol Sci. 1988;15(3):276–80. https://doi.org/10.1017/s0317167100027748.
Mauriello JA Jr. Blepharospasm, Meige syndrome, and hemifacial spasm: treatment with botulinum toxin. Neurology. 1985;35(0028-3878 (Print)):1499–500.
Jitpimolmard S, Tiamkao S, Laopaiboon M. Long term results of botulinum toxin type A (Dysport) in the treatment of hemifacial spasm: a report of 175 cases. J Neurol Neurosurg Psychiatry. 1998;64(6):751–7. https://doi.org/10.1136/jnnp.64.6.751.
Grandas F, Elston J, Quinn N, Marsden CD. Blepharospasm: a review of 264 patients. J Neurol Neurosurg Psychiatry. 1988;51(6):767–72. https://doi.org/10.1136/jnnp.51.6.767.
Cillino S, Raimondi G, Guépratte N, et al. Long-term efficacy of botulinum toxin A for treatment of blepharospasm, hemifacial spasm, and spastic entropion: a multicentre study using two drug-dose escalation indexes. Eye. 2010;24(4):600–7. https://doi.org/10.1038/eye.2009.192.
Elston JS. Long-term results of treatment of idiopathic blepharospasm with botulinum toxin injections. Br J Ophthalmol. 1987;71(9):664–8. https://doi.org/10.1136/bjo.71.9.664.
Grandas F, Elston J, Quinn JN, Marsden CD, Quinn N. Blepharospasm: a review of 264 patients. J Neurol Neurosurg Psychiatry. 1988;51:767–72. https://doi.org/10.1136/jnnp.51.6.767.
Engstrom PF, Arnoult JB, Mazow ML, et al. Effectiveness of botulinum toxin therapy for essential Blepharospasm. Ophthalmology. 1987;94(8):971–5. https://doi.org/10.1016/S0161-6420(87)33338-X.
Frueh BR, Musch DC. Treatment of facial spasm with botulinum toxin. An interim report. Ophthalmology. 1986;93(7):917–23. https://doi.org/10.1016/s0161-6420(86)33641-8.
Dutton JJ. Botulinum-A toxin in the treatment of craniocervical muscle spasms: short- and long-term, local and systemic effects. Surv Ophthalmol. 1996;41(1):51–65. https://doi.org/10.1016/s0039-6257(97)81995-9.
Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52(1):13–31. https://doi.org/10.1016/j.survophthal.2006.10.003.
Carruthers J, Stubbs HA. Botulinum toxin for benign essential blepharospasm, hemifacial spasm and age-related lower eyelid entropion. Can J Neurol Sci. 1987;14(1):42–5. https://doi.org/10.1017/s0317167100026159.
Owens PL, Strominger MB, Rubin PA, Veronneau-Troutman S. Large-angle exotropia corrected by intraoperative botulinum toxin a and monocular recession resection surgery. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 1998;2(3):144–6. https://doi.org/10.1016/s1091-8531(98)90004-0.
Khan AO. Two horizontal rectus eye muscle surgery combined with botulinum toxin for the treatment of very large angle esotropia. A pilot study. Binocul Vis Strabismus Q. 2005;20(1):15–20. http://www.ncbi.nlm.nih.gov/pubmed/15828866. Accessed December 4, 2019
Jain S, Anand SS, Jones A. Intraoperative botulinum toxin in large angle strabismus. J Am Assoc Pediatr Ophthalmol Strabismus. 2015;19(4):e12. https://doi.org/10.1016/j.jaapos.2015.07.007.
McNeer KW, Tucker MG, Guerry CH, Spencer RF. Incidence of stereopsis after treatment of infantile esotropia with botulinum toxin A. J Pediatr Ophthalmol Strabismus. 2003;40(5):288–92. http://www.ncbi.nlm.nih.gov/pubmed/14560837. Accessed December 4, 2019
de Oliveira FC, de Oliveira GC, Cariello AJ, Felberg S, Osaki MH. Botulinum toxin type A influence on the lacrimal function of patients with facial dystonia. Arq Bras Oftalmol. 2010;73(5):405–8. https://doi.org/10.1590/s0004-27492010000500003.
Sahlin S, Chen E, Kaugesaar T, Almqvist H, Kjellberg K, Lennerstrand G. Effect of eyelid botulinum toxin injection on lacrimal drainage. Am J Ophthalmol. 2000;129(4):481–6. https://doi.org/10.1016/s0002-9394(99)00408-0.
Carruthers J. The treatment of congenital nystagmus with Botox. J Pediatr Ophthalmol Strabismus. 1995;32(5):306–8. https://doi.org/10.3928/0191-3913-19950901-09.
Leigh RJ, Tomsak RL, Grant MP, et al. Effectiveness of botulinum toxin administered to abolish acquired nystagmus. Ann Neurol. 1992;32(5):633–42. https://doi.org/10.1002/ana.410320506.
Repka MX, Savino PJ, Reinecke RD. Treatment of acquired nystagmus with botulinum neurotoxin A. Arch Ophthalmol. 1994;112(10):1320–4. https://doi.org/10.1001/archopht.1994.01090220070025.
Corridan P, Nightingale S, Mashoudi N, Williams AC. Acute angle-closure glaucoma following botulinum toxin injection for blepharospasm. Br J Ophthalmol. 1990;74(5):309–10. https://doi.org/10.1136/bjo.74.5.309.
Zheng L, Azar D. Angle-closure glaucoma following periorbital botulinum toxin injection. Clin Exp Ophthalmol. 2014;42(7):690–3. https://doi.org/10.1111/ceo.12293.
Liu M, Lee HC, Hertle RW, Ho AC. Retinal detachment from inadvertent intraocular injection of botulinum toxin A. Am J Ophthalmol. 2004;137(1):201–2. https://doi.org/10.1016/s0002-9394(03)00837-7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jafari, K., Jain, S. (2020). Botulinum Toxin Treatment in Ophthalmology and Ophthalmic Surgery. In: Jabbari, B. (eds) Botulinum Toxin Treatment in Surgery, Dentistry, and Veterinary Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-50691-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-50691-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50690-2
Online ISBN: 978-3-030-50691-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)