Abstract
This study demonstrates volitional arm and hand motions restored to a person living with complete tetraplegia due to high cervical spinal cord injury. Selective intramuscular functional electrical stimulation (FES) of paralyzed muscles throughout the upper extremity powered multiple reaching and grasping movements. An intracortical brain computer interface (iBCI) recorded neural signals from the participant’s contralateral motor cortex, extracted movement intentions from these signals, and commanded FES patterns to generate these intended movements. As a result of the combined technological approach, the participant could volitionally reach, grasp, and drink from a cup, demonstrating the feasibility of this FES + iBCI system to restore cortically-controlled functional arm and hand movements in persons with extensive paralysis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spinal cord injury (SCI)
- Intracortical brain computer interface (iBCI)
- Functional electrical stimulation (FES)
- Tetraplegia
- Microelectrode arrays
1 Clinical Significance and Background
Spinal cord injury (SCI) resulting in paralysis affects over 250,000 people nationwide with over 12,000 new cases each year. Slightly more than half of all SCI cases occur at cervical levels (tetraplegia). Incomplete and complete tetraplegia have accounted respectively for 34 and 18% of all SCI cases since 2000, with less than 1% of all cases achieving full recovery [1]. Even with a caregiver, many of these individuals experience a lower quality of life due to loss of personal independence from the inability to perform standard activities-of-daily-living (ADL) on their own, such as reaching to grasp objects for drinking and self-feeding. People with low cervical SCI (C5-C7) resulting in chronic hand paralysis can regain assisted arm reaching and simple hand grasp function by using functional electrical stimulation (FES) neuroprostheses, using their residual voluntary movement to command the neuroprosthesis. FES, in the absence of descending cortical commands, applies spatially and temporally coordinated patterns of electrical stimulation to peripheral nerves and muscles to reanimate paralyzed limbs and allow for performance of simple but functional and meaningful arm and hand movements [2,3,4,5]. In particular, FES has been used to restore simple arm and hand function to persons with cervical SCI using position transducer and muscle (electromyogram, or EMG) command interfaces [2, 4, 6]. In contrast, people with high cervical SCI (C1-C4), resulting in chronic tetraplegia, have limited residual voluntary movement post-injury to use as a command source, and hence cannot adequately command even simple FES restored movements without implementing a more suitable command interface, such as a brain interface.
Recent investigations have shown the efficacy of using intracortical brain-computer interfaces (iBCIs) to decipher intended movement, from the electrical activity of intact cortical networks, to command various simple external devices. The possibility of naturally commanding neuroprosthetic devices, such as FES arm and hand systems, using an iBCI offers significant potential benefit to people with chronic and complete tetraplegia. Current rehabilitation command options for people with high cervical SCI have historically included mouthsticks, chin-controlled joysticks, sip-and-puff [7], and voice recognition systems. However, these systems are slow and limited in functionality, which makes them unsuitable for commanding neuroprostheses to perform multi-dimensional coordinated and dexterous actions. An iBCI commanded neuroprosthesis offers the possibility of harnessing intact cortical activities that remain even many years after injury. Patterns of cortical activation related to reaching and grasping have been validated in both non-human primate models [8, 9] and human participants. By recording these cortical patterns, extracted intended movement signals have been used to command higher dimensional prosthetic systems [10, 11]. Persons with paralysis are very knowledgeable of, and highly amenable to, receiving iBCIs for commanding neuroprosthetic upper extremity movements, provided that there are substantial performance gains over less invasive options [12, 13]. Hence iBCIs, when combined with FES, may offer a user an acceptable means of providing advanced functional arm and hand movement restoration. Persons with arm and hand paralysis have stated that they would much prefer to regain command over their own limbs (reanimated via FES) than other types of movement assistive devices, such as robotic limbs [12, 14]. There is a potential psychological benefit to seeing one’s own limb move [15], possibly contributing to this preference. Furthermore, robotic assistants have met with limited acceptance in the past [16, 17] because of the inconvenience of setup and limited portability [18].
Our team of investigators at Case Western Reserve University demonstrated that a person with chronic and complete tetraplegia could use an invasive BCI to command both reaching and grasp movements of his paralyzed arm, restored by FES [19]. The study, described in detail in the remainder of this chapter, used intramuscularly implanted FES electrodes in combination with dual intracortical microelectrodes and a cortically commanded motorized arm support to restore movements of the shoulder, elbow, wrist, and hand grasping. We show that, using this combined FES + iBCI technology, the study participant regained the ability to perform functional and meaningful reaching and grasping movements. This study is the first demonstration of an implantable BCI with an implanted stimulation system, and shows the potential for a totally implanted BCI and FES system that would restore cortically commanded whole arm and grasping movements to persons with paralysis.
2 System Description and Scientific Approach
The study participant (identified as T8) was enrolled into the BrainGate2 pilot clinical trial (ClinicalTrials.gov NCT00912041) and gave informed consent for medical and research procedures as approved by the Institutional Review Boards of University Hospitals Case Medical Center (Cleveland, OH) and Massachusetts General Hospital (Boston, MA). At the time of this study, T8 was a 53-year-old man with high cervical SCI (C4, AIS A) that occurred 8 years prior to enrollment. On his right side (contralateral to the intracortical implant), T8 retained some limited voluntary shoulder girdle motion, but no voluntary glenohumeral, elbow, or hand function. T8 underwent three separate surgical procedures. First, he received two 96-channel microelectrode arrays (Blackrock Microsystems, Salt Lake City, Utah) [20] that were implanted into the hand area on the precentral gyrus [21] of his motor cortex (Fig. 1b). During two subsequent procedures, occurring respectively four and nine months post implantation of the arrays, T8 received a total of thirty-six percutaneous muscle stimulating electrodes (Synapse Biomedical, Oberlin, OH) [4] (Fig. 1c) in his right (dominant) upper and lower arm. These included four percutaneous anodic current return electrodes. Implanted muscles include those for finger (flexor digitorum superficialis, extensor digitorum communis), thumb (flexor pollicis longus, adductor pollicis, extensor pollicis longus), wrist (extensor carpi radialis/ulnaris, flexor carpi radialis/ulnaris), elbow (biceps, triceps), and shoulder (anterior, posterior deltoids, pectoralis major) functions. By stimulating the thumb and finger muscles, we could restore a lateral hand grasp (where the thumb pad opposed the lateral surface of the proximal phalanx of the index finger), which can be used to complete a wide range of functional tasks [22, 23]. All implanted muscles were exercised 2–3 times per week, 2–4 h per session using cyclical electrical stimulation patterns to improve strength, range of motion, and fatigue resistance. Electrical stimulation resulted in restoration of 76 degrees of flexion/extension of the elbow, and a lateral grasp that could close with enough force to securely hold several objects, including a coffee mug.
Overview of the FES + iBCI system. a Illustration of how the system components are connected, b [left] structural MRI scan of T8’s brain. The implant locations of the two intracortical arrays are indicated with red squares. [top right] Photo of microelectrode arrays and wire bundles shortly after intracortical implantation. [bottom right] SEM of an example microelectrode recording array (photo courtesy of Blackrock Microsystems), c example FES Implanted lead and electrode
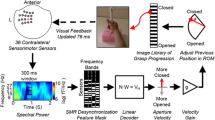
Figure 1a illustrates the percutaneous FES + iBCI system. The implanted microelectrode arrays recorded electrical activity from multiple neurons. A neural decoder then translated the recorded neural activity patterns into command signals for controlling the stimulation of biceps, triceps, forearm, and hand muscles to produce coordinated reaching and grasping movements. An external stimulator delivered charge-balanced, biphasic, constant-current stimulation through the percutaneous muscle stimulation electrodes, with fixed current amplitude (20 mA) and frequency (12.5 Hz), and variable pulse duration of 0–200 μs, to produce muscle contractions and subsequent limb movement. The duration of the current pulse (“pulse-width”) applied at a given electrode determined the strength of the muscle contraction, and different muscles were activated in a coordinate manner to produce functional arm and hand movements. Thus, we varied the pulse-widths and muscle combinations as functions of T8’s cortically-derived movement commands to enable graded control of functional motions. To support the paralyzed arm against gravity during movement, T8 was fitted with a Mobile Arm Support (MAS) (Focal MEDITECH, Tilburg, Netherlands). The MAS also provided a motorized vertical arm motion that was used to restore self-initiated, cortically-controlled shoulder elevation. Instrumented goniometers were fitted onto T8’s elbow, wrist, and/or hand to measure the range of motion of each actuated movement.
A neural decoder was calibrated at the beginning of each experimental session to translate patterns of cortical neural activity into command signals for the FES system. The decoder used two neural features from each electrode of the intracortical microelectrode arrays: (1) the threshold crossing (TX) “firing” rate, determined by counting the number of action potentials present in a 20 ms time window that crossed a preset noise threshold, and (2) the average spectral high frequency power (HFP, 250–3000 Hz) in a 20 ms time window. The decoder used a linear transformation function, similar to the Kalman filter used in recent iBCI applications [24], to map the 384 features (192 firing rates and 192 spectral high frequency power values) to three movement commands. Each command determined the stimulation level corresponding to a specific pattern of muscles (either a hand open/close pattern or an elbow flexion/extension pattern) or the actuation level of the mobile arm support. Figure 2a illustrates the decoding process.
Overview of how the FES + iBCI system translates cortical neural activity into FES stimulus parameters. a Neural activity is decoded into a single command signal for each joint (“stimulation pattern %”). A pattern mapper then converts this command signal into the appropriate pulse widths to apply to each individual FES electrode, enabling the participant to coordinate the action of multiple electrodes and muscles using only a single command. b Example neural activity during elbow flexion/extension commands (electrode 67) and hand opening/closing commands (electrode 81). c The stimulation pattern used for converting a decoded elbow movement command into the stimulation pulse widths required to produce the commanded movement. d Example threshold crossing rates, decoded command, and pulse widths over time as T8 made elbow flexion and extension movements
To initialize the neural decoder, we used neural data recorded while T8’s arm was automatically driven by the FES system to make elbow, hand or shoulder movements, and he was verbally instructed to simultaneously attempt to control the observed arm motions. We then refined the decoder [25] by using neural data recorded while T8 actually controlled his FES-powered arm movements, using the initial decoder to volitionally make those same movements. After calibration and refinement, the neural decoder was held constant for the remainder of the research session while T8 made several single joint movements and completed a functional coffee drinking task.
3 Results
Neural activity recorded from many of the intracortical electrodes was strongly related to T8’s intended movement commands when he attempted to use the FES + iBCI system. Figure 2b illustrates how the threshold crossing rates observed on two example electrodes changed as a function of the movement that T8 was attempting in response to verbal instruction. On one electrode, substantially more threshold crossings were observed during attempted elbow flexion as opposed to extension, and on another electrode, substantially more threshold crossings were observed during attempted hand opening as opposed to closing. Of the 192 electrodes, we identified a neural feature (either threshold crossing or spectral high frequency power) that coded for hand opening and closing on 36 electrodes, for elbow flexion and extension on 45 electrodes, and for mobile arm support elevation on 37 electrodes. We considered a neural feature to “code” for a certain movement if that feature’s mean value was significantly different (t-test, p < 10−4) between the two opposing commands, such as hand opening versus closing.
T8 completed a series of self-initiated, iBCI-commanded FES arm reaching movements involving 2D (elbow, grasp) and 3D (elbow, shoulder, grasp) joint movements. Sitting upright in his wheelchair, T8 first qualitatively demonstrated the ability to command movement of each degree-of-freedom independent. The first successful demonstration of T8 cortically commanding movements of his reanimated arm and hand occurred 7 days post-implant of the FES electrodes. After demonstrating robust control of single degree-of-freedom arm and hand movements, T8 then demonstrated his ability to perform an activity-of-daily living (ADL) task in which he acquired a cup of coffee and took a drink (Fig. 3A). The task required T8 to use the FES + iBCI system to (1) extend his elbow, (2) open his hand, (3) grasp the cup securely, (4) flex his elbow to transport it close to his mouth, 5) take a drink, 6) extend his elbow to return the cup, and 7) release his grasp. T8 required between 20 to 40 s to complete the drinking task and was successful in 11 of the 12 attempts made during the illustrated session (Fig. 3b). His success demonstrates for the first time that a person with extensive paralysis, and nine years post injury, can perform cortically-controlled volitional functional movements involving both arm reaching and hand grasping with an FES + iBCI system. When asked to describe how he commanded the FES arm movements, T8 replied, “It’s probably a good thing that I’m making it move without having to really concentrate hard at it.…I just think ‘out’ and… it just goes.” T8 was completely unable to perform the same task with the FES system turned off (Fig. 3c); his minor residual motion of his shoulder girdle could only cause a small, uncontrolled jerk of his elbow and could not move his hand at all, despite T8’s attempts to command the required arm movements.
T8 using the FES + iBCI system to take a drink of coffee. a T8 reaching out to grasp the cup of coffee (left) and bringing it to his mouth to take a drink (right). b The length of time it took T8 to complete each phase of the drinking task. Data is shown for 12 trials completed within a single experimental session; only one trial was failed when T8 dropped the cup. c Example time series of T8’s elbow and hand motion when the FES + iBCI system was turned on (left) and when the FES system was turned off (right). When the system was on, the decoded neural commands (blue) and the elbow and hand joint angles (orange) changed appropriately as T8 moved through the phases of the task, enabling him to take a drink of coffee. When the system was off, T8 could only make small, uncontrolled elbow jerks caused by his residual shoulder motion and could not move his hand at all
4 Discussion and Future Direction
We have demonstrated, for the first time, that cortically-controlled volitional arm and hand function can be restored to a person with chronic tetraplegia by simultaneously (1) reanimating multiple, functionally meaningful motions of the limb through intramuscular FES of paralyzed muscles and (2) enabling control of these FES-restored motions by extracting multiple movement intention commands from intracortical recordings in real time. The simultaneous implementation of iBCI and FES technologies represents a neurotechnology-based bridging of the participant’s spinal cord injury and demonstrates a significantly more intuitive command interface than those currently available to persons with extensive (whole arm) paralysis. With the FES + iBCI system, T8 was once again able to just “think” about moving his arm and hand, and the movement intentions decoded from the recorded neural activity were sufficient to create the desired arm and hand movements via the FES system.
By restoring iBCI-commanded and FES-driven motion of both the arm and hand in a human participant, the present work significantly extends previous iBCI research performed in intact [26,27,28] and temporarily paralyzed non-human-primates [8, 9], as well as research done in individuals with paralysis controlling cursors or robotic limbs [10, 11, 29, 30]. Our present FES + iBCI system restores both reaching and grasping to an individual with complete motor paralysis of the hand and arm, while following a viable path to clinical relevance through the use of implantable stimulation technology.
The performance of the FES + iBCI system used here was somewhat limited by SCI-related conditions such as muscle atrophy, denervation, and joint contractures, and by the difficulty of precisely targeting desired muscles when inserting FES electrodes percutaneously rather than in an open surgical procedure. However, these limitations are addressable by currently available, implantable FES technologies (e.g., precisely located intramuscular electrodes and peripheral nerve cuff electrodes) and associated techniques (model-based optimization of muscle stimulation patterns, muscle tendon transfers to replace the functions of denervated muscles, and more extensive exercise programs). Advances in intracortical electrodes to enhance long-term recording stability (through increased mechanical and biological viability [31, 32]), and to enable a fully-implanted brain recording interface [33] may also increase the clinical viability of an FES + iBCI system. Nevertheless, (1) T8 had a high rate of success in performing a reaching and grasping task using his current percutaneous FES-activated arm and hand, and (2) remaining performance limitations can be largely addressed using existing (but permanent) technologies. The movements afforded to T8 by the current system allowed him to take a drink of coffee, with his own arm and hand, solely of his own volition (reaching out, grasping, reaching back to the face). These actions are representative of movements needed to perform a wide range of reaching tasks [34], suggesting that more functional activities may be achieved with the current system. The present study used percutaneous, removable technologies both for the brain recordings and the FES system in order to first evaluate the feasibility of the approach. Future systems inspired by this project are being designed to provide full-time, reliable, effective, intuitive control of the arm and hand, and may thus enable restoration of a much wider range of functional activities.
References
NSCISC, Spinal cord injury, facts and figures at a glance. J. Spinal Cord. Med. 36, 1–2 (2013)
K.L. Kilgore, H.A. Hoyen, A.M. Bryden, R.L. Hart, M.W. Keith, P.H. Peckham, An implanted upper-extremity neuroprosthesis using myoelectric control. J. Hand. Surg. [Am.] 33, 539–550 (2008)
P.H. Peckham, M.W. Keith, K.L. Kilgore et al., Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch. Phys. Med. Rehabil. 82, 1380–1388 (2001)
W.D. Memberg, K.H. Polasek, R.L. Hart et al., Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch. Phys. Med. Rehabil. 95, 1201–1211 (2014)
P.H. Peckham, J.S. Knutson, Functional electrical stimulation for neuromuscular applications. Ann. Rev. Biomed. Eng. 7, 327–360 (2005)
Kirsch RF (2008) Restoration of hand and arm by functional neuromuscular stimulation. In: Neural Interfaces Conference. Cleveland, OH, 2008
P.A. Lathem, T.L. Gregorio, S.L. Garber, High level quadriplegia: an occupational therapy challenge. Am. J. Occup. Ther. 39, 705–714 (1985)
C. Ethier, E.R. Oby, M.J. Bauman, L.E. Miller, Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485, 368–371 (2012)
C.T. Moritz, S.I. Perlmutter, E.E. Fetz, Direct control of paralysed muscles by cortical neurons. Nature 456, 639–642 (2008)
L.R. Hochberg, M.D. Serruya, G.M. Friehs et al., Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006)
L.R. Hochberg, D. Bacher, B. Jarosiewicz et al., Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012)
C.H. Blabe, V. Gilja, C.A. Chestek, K.V. Shenoy, K.D. Anderson, J.M. Henderson, Assessment of brain-machine interfaces from the perspective of people with paralysis. J. Neural Eng. 12, 043002 (2015)
J. Lahr, C. Schwartz, B. Heimbach, A. Aertsen, J. Rickert, T. Ball, Invasive brain–machine interfaces: a survey of paralyzed patients’ attitudes, knowledge and methods of information retrieval. J. Neural Eng. 12, 043001 (2015)
J.L. Collinger, M.L. Boninger, T. Bruns, K. Curley, W. Wang, D.J. Weber, Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J. Rehabil. Res. Dev. 50, 145–160 (2013)
R.H. Nathan, A. Ohry, Upper limb functions regained in quadriplegia: a hybrid computerized neuromuscular stimulation system. Arch. Phys. Med. Rehabil. 71, 415–421 (1990)
J. Hammel, K. Hall, D. Lees et al., Clinical evaluation of a desktop robotic assistant. J. Rehabil. Res. Dev. 26, 1–16 (1989)
J.M. Hammel, H.F. Van der Loos, I. Perkash, Evaluation of a vocational robot with a quadriplegic employee. Arch. Phys. Med. Rehabil. 73, 683–693 (1992)
R. Mahoney, Robotic products for rehabilitation: status and strategy. In: International Conference on Rehabilitation Robotics. Bath, UK, 1997: 1–6
A.B. Ajiboye, F.R. Willett, D.R. Young et al., Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 6736, 1–10 (2017)
E.M. Maynard, C.T. Nordhausen, R.A. Normann, The Utah intracortical electrode array: a recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 102, 228–239 (1997)
T.A. Yousry, U.D. Schmid, H. Alkadhi et al., Localization of the motor hand area to a knob on the precentral gyrus. A New Landmark. Brain 120, 141–157 (1997)
K.L. Kilgore, P.H. Peckham, G.B. Thrope, M.W. Keith, K.A. Gallaher-Stone, Synthesis of hand grasp using functional neuromuscular stimulation. IEEE Trans. Biomed. Eng. 36, 761–770 (1989)
University of California LAD of E. Studies to Determine the Functional Requirements for Hand and Arm Prosthesis: The Final Report Covering Work During the Year 1946–1947, Under Subcontract No. 17 of Prime Contract VAm-21223 with the National Academy of Sciences. 1947
D. Bacher, B. Jarosiewicz, N.Y. Masse, et al., Neural point-and-click communication by a person with incomplete locked-in syndrome. Neurorehabil Neural Repair 2014; published online Nov. https://doi.org/10.1177/1545968314554624
B. Jarosiewicz, N.Y. Masse, D. Bacher et al., Advantages of closed-loop calibration in intracortical brain—computer interfaces for people with tetraplegia. J. Neural Eng. 10, 046012 (2013)
D.M. Taylor, S.I. Helms-Tillery, A.B. Schwartz, Direct cortical control of 3D neuroprosthetic devices. Science (80–) 296, 1829–1832 (2002)
M.D. Serruya, N.G. Hatsopoulos, L. Paninski, M.R. Fellows, J.P. Donoghue, Instant neural control of a movement signal. Nature 416, 141–142 (2002)
M. Velliste, S. Perel, M.C. Spalding, A.S. Whitford, A.B. Schwartz, Cortical control of a prosthetic arm for self-feeding. Nature 453, 1098–1100 (2008)
J.L. Collinger, B. Wodlinger, J.E. Downey et al., High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564 (2013)
T.N. Aflalo, S. Kellis, C. Klaes et al., Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Sci Mag 348, 906–910 (2015)
M. Jorfi, J.L. Skousen, C. Weder, J.R. Capadona, Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 12, 011001 (2015)
J.C. Barrese, N. Rao, K. Paroo et al., Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 10, 066014 (2013)
D. Borton, M. Yin, J. Aceros, A. Nurmikko, An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10, 026010 (2013)
A.S. Cornwell, J.Y. Liao, A.M. Bryden, R.F. Kirsch, Standard task set for evaluating rehabilitation interventions for individuals with arm paralysis. J. Rehabil. Res. Dev. 49, 395 (2012)
Acknowledgements
We thank the study participant for his pioneering efforts participating in the present study. We thank the Louis Stokes Cleveland VA Medical Center Cares Tower Residence Center, for space and logistical support. We additionally thank the members of the BrainGate2 Consortium for their feedback and support of the research efforts.
Support for this work was provided by the National Institutes of Health under grants NIH 1R01HD077220, NIH N01HD53403, NIH R01DC009899, and VA B6453R. The reported contents do not necessarily represent the views of the funding or parent institutions, or of the US Government. The funding sources had no role in the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bolu Ajiboye, A., Hochberg, L.R., Kirsch, R.F. (2020). Restoring Functional Reach-to-Grasp in a Person with Chronic Tetraplegia Using Implanted Functional Electrical Stimulation and Intracortical Brain-Computer Interfaces. In: Guger, C., Allison, B.Z., Miller, K. (eds) Brain–Computer Interface Research. SpringerBriefs in Electrical and Computer Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-49583-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-49583-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49582-4
Online ISBN: 978-3-030-49583-1
eBook Packages: Computer ScienceComputer Science (R0)