Abstract
A new XPS methodology to analyze the atomic and electronic structure of a wide-gap dielectric zircon has been developed. A high spectral resolution with the O1s-line width of 1.3 eV and volume (near surface) sensitivity have been achieved by using a spectrometer with a dual charge compensation system and monochromatic Al\(K_{\upalpha}\)-excitation as well as by the measurement (electron exit angle 90°, spot diameter 100 μm) and sample preparation (fresh cleavage) conditions. The possibility of analysing the structure of short-range order, in particular, diagnosing the formation of Si-O-Si and Si-OH fragments occurring in the bulk and/or surface layers of zircon under the influence of external (e.g., radiation, chemical) factors, is shown.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
X-ray photoelectron spectroscopy (XPS) is based on recording the energy spectrum of electrons emitted from the surface layers of solid samples under the impact of X-ray radiation. The method along with the quantitative analysis of the chemical composition of the layer, allows the measurements of Eb binding energies of core level and valence band electrons to be carried out. The latter depends on the structure of short-range order, effective charge and chemical bond characteristics (Zigban et al. 1971; Nefedov and Vovna 1987). The layer thickness analysed by XPS depends on the photoelectron energy and does not exceed several hundred atomic layers (~20 nm) (Zigban et al. 1971). Photoelectrons from various depths can be recorded in the spectra by choosing the measurement conditions (see, for example, reviews by (Hochella 1988; Fadley 2010)). It has been shown that, when using Al\(K_{\upalpha}\) X-ray radiation and high values of the photoelectron collection angle, over 90% of the measured intensity is provided by the layers lying deeper than the surface monolayer (Nesbitt and Bancroft 2014). When analysing appropriately prepared mineral surfaces (fresh cleavage), the information obtained can be correlated with their bulk properties (Zakaznova-Herzog et al. 2005, 2006).
Photoionization and emission of both Auger and secondary electrons lead to positive charging of the dielectric material surface layer, thus resulting in a decrease in the kinetic energy of photoelectrons and overestimation of the calculated binding energy. A serious problem is the uneven charging of imperfect surfaces resulting in the broadening and distortion of the XPS peak shape and, as a result, the impossibility of separating the contributions from atoms with small differences in chemical shift. In modern spectrometers, high-energy resolution is provided by the use of monochromatic X-ray radiation and efficient charge compensation systems (Nesbitt and Bancroft 2014), as well as by specific surface preparation.
In mineralogy, XPS has so far been primarily used to study the surface properties and reactivity of natural oxides, sulfides and silicates and, to a lesser extent, to study the composition, structure and chemical bonds in the mineral bulk (see, for example, reviews by Hochella (1988); Fadley (2010)). It has been shown that the XPS spectra of silicates and phosphates in the amorphous and crystalline state (with typical O1s oxygen line widths in the order of 2 eV or greater, using an ESCALAB-MKII spectrometer and the analysis of mechanically processed or natural surfaces) reflect qualitatively the changes in the short-range order in the mineral bulk (Gubanov et al. 1991; Zatsepin et al. 1997; Shchapova et al. 2010; Votyakov et al. 2012). This approach was developed in connection with the introduction of high-resolution spectrometers (Zakaznova-Herzog et al. 2005, 2006; Fadley 2010; Nesbitt et al. 2011). Similar values of O1s, Si2p, Mg2p and Ca2p line FWHM (~1.3 eV) were obtained for quartz, olivine, diopside and bronzite, and their vibrational broadening was suggested. Three structurally nonequivalent oxygen atoms were assigned in the diopside: bridging and non-bridging MgOSi and CaOSi/FeOSi (Zakaznova-Herzog et al. 2005, 2006). The leaching of olivine, bronzite, and diopside was studied by Zakaznova-Herzog et al. (2008). The high resolution of the O1s spectrum allowed an additional type of OH− oxygen to be diagnosed, arising from the exchange of H+ with near-surface cations. Different types of oxygen in silicate glasses were studied (Nesbitt et al. 2011; Sawyer et al. 2015); the concentration dependences of the energy of oxygen O1s levels of various types and Si2p levels were used to interpret the concentration changes in the Raman spectra of stretching vibrations of SiO4 tetrahedra; their relationship with the electron density on oxygen atoms was revealed (Nesbitt et al. 2017).
For zircon, the XPS method was previously used to study the Eb elemental chemical shifts relative to SiO2 and ZrO2 simple oxides, with the possibility of using O1s Eb values to assess the Si-O covalence in silicates being discussed (Guittet et al. 2001). The effect of argon ion irradiation (8 keV) on the chemical composition of the ZrSiO4 surface layers was studied, and a significant loss of SiO2 and a strong reduction of Zr after irradiation was observed (Iacona et al. 1999). The effect of weathering of the zircon surface with various degrees of metamictization on the XPS spectra was considered; no significant changes in the chemical composition were detected, and the relationship between the changes in the O1s spectra was attributed to kaolinite particles (Balan et al. 2001). The XPS was used to study the flotation properties of zircon after plasma cleaning (Marshall et al. 2014) and surface modification by non-thermal treatment with electromagnetic pulses due to a change in the ratio of different types of hydroxyl groups (Chanturia et al. 2017). In (Shchapova et al. 2010), changes in the zircon O1s, Si2p and the valence band spectra due to radiation damage of its structure were observed; the changes were attributed to the partial polymerization of silicon-oxygen tetrahedra in the damaged structure. It should be noted that there are XPS studies of radiation damage of other natural orthosilicates, in particular, olivine, for which radiation-induced reduction of metallic Fe has been reported (Dukes et al. 1999; Loeffler et al. 2009).
Thus, XPS is a promising method for the analysis of structural and chemical transformations of the surface and bulk (sub-surface) layers of minerals. Modern equipment allows the spectra to be obtained with high-spectral resolution and the atomic and electronic structures of the bulk minerals to be analyzed using appropriate measurements and sample preparation techniques. The application of XPS to study the structure and chemical bonding of dielectric silicate minerals requires a detailed consideration of methodological issues.
This work is aimed at solving methodological issues of surface preparation, selecting XPS spectral measurement modes with the use of a PHI XPS Versaprobe 5000 high-resolution spectrometer, as well as processing and analysing the results to study the oxygen sublattice of silicate minerals, in particular zircon; minimizing the influence of surface states and the differential surface charge on O and Si spectral shape; and testing the methodology using highly crystalline zircon as an example.
2 Materials and Methods
2.1 Samples and Sample Preparation
Highly crystalline Ratanakiri zircon aged 0.92 ± 0.07 Ma (Zeug et al. 2018) from Cambodian secondary deposits of alkaline basalt rocks having a low (<0.1 wt%) amount of trace elements except hafnium (~0.7 wt% HfO2) was studied. The test sample was previously annealed under reducing conditions at 900–1000 °C. According to our data from the electron microprobe and Raman analyses, the sample was homogeneous in the major and trace element composition and structure. In order to reduce the contribution of the modified surface layers, the measurements were performed on a fresh cleavage, after which the sample was immediately placed in the vacuum chamber of the spectrometer. The orientation of the sample surface was not taken into account.
2.2 Analytical Equipment and Measurement Modes
The studies were performed using a PHI XPS 5000 VersaProbe spectrometer (ULVAC-Physical Electronics, USA, 2011). The spectrometer was operated according to the classical X-ray optical scheme using a quartz monochromator with a working range of the binding energy analyser of 0–1500 eV. The device used an electrostatic type of focusing and magnetic shielding of the main camera. The mentioned features of the spectrometer provided high energy resolution (\(\Delta\)E ≤ 0.5 eV for Al\(K_{\upalpha}\) excitation), high spatial selectivity (minimum diameter of X-ray Al\(K_{\upalpha}\) probe d ≤ 10 \(\upmu\)m) and high elemental sensitivity with a significant signal-to-noise ratio when studying a wide range of objects. An important feature of the spectrometer was a system of two-channel neutralization of the electrostatic charge arising during the registration of XPS spectra of dielectric samples. The neutralization was carried out using both an electron source with a thermal cathode (~1 eV) and low-energy ions (≤200 eV). The spectrometer had an oil-free two-stage vacuum pumping system based on turbomolecular and magnetic discharge pumps, which effectively maintained a working vacuum not exceeding 10−7 Pa. In this case, sample contamination with fore-vacuum oil vapour in the preparation chamber, which usually occurs when the standard vacuum-pumping scheme is used for vapour-oil fore-pumps, was completely excluded.
The measurements were performed at an electron exit angle of 90°, corresponding to the maximum bulk sensitivity. The X-ray spot diameter was 100 \(\upmu\)m. The X-ray radiation power on the sample did not exceed 25 W. In the survey spectral mode, the energy window width was equal to 187.85 eV, in the high-resolution mode in the region of O1s, Si2p, Zr3d, C1s lines it was 23.5 eV. The accumulation time at one point in a single pass was 50 ms. The measurements were carried out in vacuum with a residual pressure of argon ions used for neutralizing of about 1 × 10−6 Pa. The typical signal-to-noise ratio was of the order of 10,000/3 for core level lines. The spectrometer energy scale was calibrated using the Au 4f7/2 and Cu 2p3/2 lines; the effect of the energy window width on the FWHM was calibrated using Ag 3d5/2line. The initial processing of the spectra was carried out using the ULVAC-PHI MultiPak 9.8 software. The charge of the sample was taken into account by the C1s level binding energy of 284.8 eV (Moulder et al. 1992; https://xpssimplified.com/elements/carbon.php).
3 Results and Discussion
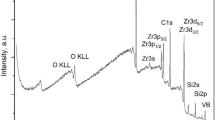
The survey spectrum of Ratanakiri zircon is shown in Fig. 1; electron binding energies of 525–535 eV (O1s), 425–445 (Zr 3 s), 325–355 (Zr 3p3/2, 3p1/2), 275–290 (C1s), 176–190 (Zr 3d5/2, 3d3/2), 145–160 (Si2s), 95–110 (unresolved Si 2p3/2, 2p1/2), <60 eV (Zr valence band 4s, 4p, 4d; Si3s, 3p; O3s, 3p) are typical for crystalline zircon (Iacona et al. 1999; Guittet et al. 2001; Shchapova et al. 2010). The chemical composition of the surface cleavage, determined by the ratio of the Zr3d, Si2p, O1s, and C1s lines using photoionization cross sections (Moulder et al. 1992), is close to stoichiometric with the exception of uncontrolled surface carbon: 14, 15.3, 61.6, 9 at. % for Zr, Si, O and C, respectively.
The O1s, Si2p, and Zr3d5/2 spectra of zircon are presented in Figs. 2, 3 and 4; the binding energies of O1s, Si2p, and Zr3d5/2 are shown in Table 1. The O1s spectrum is represented by a narrow, slightly asymmetric line with a tail in the high-energy region (Fig. 2a). The approximation of the entire line by one Voigt profile (R = 0.99487) gives the values of Eb(O1s) = 530.86 ± 0.01 eV and FWHM (O1s) = 1.32 ± 0.02 eV, but does not reproduce the line shape accurately. O1s binding energy is close to that obtained previously (531.3 and 530.95 eV) for the three-coordinated O (Si, Zr, Zr) atom in synthetic and highly crystalline zircon from kimberlites, respectively (Guittet et al. 2001; Shchapova et al. 2010), as well as to 531.0 eV in Mg olivine (Zakaznova-Herzog et al. 2005), in the structure of which, similar to zircon, isolated SiO4 tetrahedra are identified. Small discrepancies in zircon Eb(O1s) can be partially explained by small differences in the reference C1s binding energy (Greczynski and Hultman 2018). The obtained FWHM (O1s) of full contour ~1.3 eV in zircon is close to FWHM (O1s) = 1.24–1.27 eV in quartz, pyroxene and olivine (Kratos Axis Ultra spectrometer with magnetic retention charge compensation system, Al\(K_{\upalpha}\)—radiation, spectrometer spectral resolution 0.35 eV) (Nesbitt et al. 2004, Zakaznova-Herzog et al. 2005, 2006; Bancroft et al. 2009). In the cited papers, the nature of the O1s and Si2p spectral broadening has been discussed in detail and the following main factors have been named in conclusion: (i) phonon broadening (decrease in the lifetime of the excited state) leading to a temperature-dependent symmetric Gaussian diffusion of the contour, and (ii) vibronic splitting of the excited state (FSVB—final state vibrational broadening) first noted by (Siegbahn 1974), which leads to temperature-independent asymmetric broadening of the contour. It has also been noted that FWHM (O1s) ~1.3 eV is the minimum possible measured value for silicates at spectral resolution of ~0.35 eV at room temperature. Taking into account these considerations and the Versaprobe spectral resolution (not exceeding ~0.5 eV), the obtained zircon FWHM (O1s) = 1.32 ± 0.02 eV confirms that the selected measurement and sample preparation conditions truly provide the effective compensation of the surface differential charge, and the technique can be used further to study the effects of changes in line profile.
Zircon O1s spectrum (filled circles) and its approximation over full profile a and over its low-energy part (526.00–530.86 eV) by Voigt profile (black line) b. Empty circles—difference between experimental spectrum and approximation; red line—approximation of the difference spectrum by Voigt profile. Vertical dotted lines—binding energy Eb range (filled) of O1s for Si-OH coordination in olivine (532.2–532.7 eV) and Eb value of O1s for Si-O-Si coordination in quartz (530.8 eV) according to (Duval et al. 2002; Zakaznova-Herzog et al. 2008; Guittet et al. 2001)
Zircon Si2p spectrum (filled circles) and its approximation by one (black line) and two Voigt profiles corresponding to Si2p3/2 and Si2p1/2 contributions with fixed maxima at 101.6 and 102.2 eV (profiles and envelope curve—grey dotted lines) a and by three Voigt profiles corresponding to Si-O-Zr, Zr; Si-OH and Si-O-Si contributions (red, green and blue lines) with fixed values of the two latter maxima at 103.0 and 103.6 eV b. Black line—envelope curve
To obtain the information on the possible contribution of any other O atoms in different from O (Si, Zr, Zr) coordination to the high-energy O1s spectral tail, we have used the procedure for approximating the spectrum in its low-energy part (Fig. 3b) described in (Nesbitt and Bancroft 2014). An approximation by the Voigt profile (R = 0.99931) in the range of 526.00–530.86 eV gives maximum values of 530.80 ± 0.01 eV and FWHM = 1.24 ± 0.02 eV and clearly reveals the asymmetry of the O1s line. The additional high-energy part (~10% of the total integrated O1s intensity) is described by the Voigt profile with a maximum of 532.0 eV and FWHM 1.6 eV. A similar “additional” spectrum with a maximum at 532.2–532.7 eV and FWHM 1.2–1.6 eV has been observed in the initial and leached olivine, where it is assigned to Si—OH fragments (Zakaznova-Herzog et al. 2008). According to the type of the O1s spectrum in Ratanakiri zircon, it can be assumed that the additional high-energy part of the band is associated with both the FSVB effect of asymmetric line broadening (Bancroft et al. 2009) and the possible contribution of O atoms to Si-OH coordination (Eb = 532.2–532.7 eV (Duval et al. 2002; Zakaznova-Herzog et al. 2008). The latter can occur on the surface of the cleaved sample in the atmosphere. The contribution of O atoms to the coordination of Si–O–Si (Eb = 530.8 eV (Guittet et al. 2001)), if present, is at a very low amount. Thus, the application of the fresh cleavage technique makes it possible to identify the contributions of (i) sub-surface “bulk” and (ii) surface zircon layers altered by adsorption. Therefore, this technique is acceptable for studying the changes in the state of oxygen atoms induced by various factors (genetic features of the mineral).
The Si2p spectrum can be approximated by one Voigt profile with a maximum of Eb(Si2p) = 101.69 ± 0.01 eV and FWHM (Si2p) = 1.46 ± 0.01 eV, however, the line shape is not exactly reproduced (Fig. 3a). The peak parameters are close to those for olivine 101.7 and 1.29 eV (for comparison, 103.6 and 1.38 eV in quartz) (Zakaznova-Herzog et al. 2005). The spectrum is slightly asymmetric, but to a lesser extent, as compared to O1s. This may be due to the masking of asymmetry caused by the non-elementary nature of the band, because it represents an unresolved (splitting ~0.6 eV) Si2p3/2 and Si2p1/2 spin—orbit doublet (Bancroft et al. 2009). The deconvolution into two components, schematically simulating Si2p3/2 and Si2p1/2 contributions (Si2p3/2 and Si2p1/2 binding energies for olivine are taken from (Zakaznova-Herzog et al. 2005)) gives good agreement with the experiment, but does not contain useful information. Therefore, by analogy with Iacona et al. (1999); Balan et al. (2001); Chanturia et al. (2017), hereinafter, the Si2p3/2 and Si2p1/2 doublet is simulated by one profile. In this case, an additional intensity in the high-energy region can be related to the integral contribution of Si2p of Si atoms being in a certain new coordination. Figure 3b shows the approximation of the spectrum by three Voigt profiles: Si-O-Zr, Zr (parameters determined by fitting), Si-OH (fixed binding energy of 103.0 eV (Zakaznova-Herzog et al. 2008; Chanturia et al. 2017)) and Si-O-Si (fixed binding energy of 103.6 eV (Zakaznova-Herzog et al. 2005)). In this approximation, the Si–O–Zr, Zr linewidth is 1.40 eV, which is close to the data for olivine (see table). The contribution of Si-OH is ~5%, while the contribution of Si-O-Si is negligible. The result obtained is consistent with the analysis of the Ratanakiri zircon O1s spectrum. It confirms the absence of a significant differential surface charge and shows the presence of an insignificant amount of OH groups that can occur on the surface of the cleaved sample in the atmosphere.
The Zr3d spectrum is represented by two lines from the well-resolved components of the Zr3d5/2 and Zr3d3/2 spin-orbit splitting (Fig. 4a). The Voigt approximation gives Eb(Zr3d5/2) = 182.84 ± 0.01 eV (Fig. 4b), which is in good agreement with Guittet et al. (2001); Shchapova et al. (2010). FWHM (Zr3d5/2) = 1.16 ± 0.01 eV is significantly lower than in the cited works. There are no signs of OH groups. It has been noted by Balan et al. (2001) that chemical exposure and weathering have a weak effect on the shape of the Zr3d line in zircon; however, a strong effect of Ar ion irradiation on the line shape has been found by Iacona et al. (1999) due to a change in the charge state of some Zr atoms. The high resolution of the Zr3d spectrum achieved in this work allows the possibility of detecting radiation damage to be predicted not only in the silicon-oxygen, but also in the zirconium sublattice of zircon.
The valence band spectrum (Fig. 5) reproduces the known features of the zircon electronic structure (Rignanese et al. 2002; Shchapova et al. 2010; Du et al. 2012). Note that high kinetic energies of valence electrons (1446–1486 eV) provide the greatest depths of electron exit; in this case, the contribution of the surface layers is less than that for the core electrons. The achieved high spectral resolution allows subsequently tracing changes in chemical binding under the influence of external factors (zircon genetic characteristics). The contribution of bulk layers can be considered dominant.
Zircon valence band spectrum and the assignment of electronic state contributions according to Shchapova et al. (2010)
4 Conclusions
The XPS analysis methodology of the atomic and electronic structure of a wide-gap dielectric zircon has been developed. The minimization of the influence of surface states and the differential surface charge has been achieved by: (1) using a spectrometer with a dual charge compensation system and monochromatic Al\(K_{\upalpha}\)-excitation; (2) the selection of measurement conditions, in particular, a maximum increase in the exit angle of the measured electrons and a small spot diameter of 100 \(\upmu\)m; (3) a surface preparation technique—fresh cleavage in the atmosphere immediately before measurement. Various processing options of O1s, Si2p and Zr3d spectra have been tested using highly crystalline Ratanakiri zircon (Cambodia) from secondary deposits of alkaline basalt rocks, annealed under reducing conditions at 900–1000 °C. FWHM (O1s) = 1.3 eV measured using the developed methodology corresponds to the minimum possible for silicates when operating on high-resolution serial spectrometers. Such a result has been obtained for zircon for the first time. The possibility of analyzing the structure of short-range order, in particular, diagnosing the formation of Si-O-Si and Si-OH fragments occurring in the bulk and/or surface layers of zircon under the influence of external (e.g., radiation, chemical) factors, has been shown.
References
Balan E, Trocellier P, Jupille J, Fritsch E, Muller J-P, Calas G. Surface chemistry of weathered zircons. Chemical Geology. 2001;181(1–4):13–22.
Bancroft GM, Nesbitt HW, Ho R, Shaw DM, Tse JS, Biesinger MC. Toward a comprehensive understanding of solid-state core-level XPS linewidths: Experimental and theoretical studies on the Si 2p and O 1 s linewidths in silicates. Physical review B. 2009;80:075405.
Chanturia VA, Bunin IZh, Ryazantseva MV, Chanturia EL, Khabarova IA, Koporulina EV, Anashkina NE. Modification of Structural, Chemical and Process Properties of Rare Metal Minerals under Treatment by High-Voltage Nanosecond Pulses. Journal of Mining Science. 2017;53(4):718–733.
Du J, Davanathan R, René CL, Weber WJ. First-principles calculations of the electronic structure, phase transition and properties of ZrSiO4 polymorphs. Computational and Theoretical Chemistry. 2012;987:62–70.
Dukes CA, Baragiola RA, McFadden LA. Surface modification of olivine by H+ and He+ bombardment. Journal of Geophysical Research: Planets. 1999;104(E1):1865–1872.
Duval Y, Mielczarski JA, Pokrovsky OS, Mielczarski E, Ehrhardt JJ. Evidence of the existence of three types of species at the quartz-water solution interface at pH 0–10: surface group quantification and surface complexation modelling. J. Phys. Chem. 2002;B202:2937–2945.
Fadley CS. X-ray photoelectron spectroscopy: Progress and perspectives. Journal of Electron Spectroscopy and Related Phenomena. 2010;178–179:2–32.
Greczynski G, Hultman L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Applied Surface Science. 2018;451:99–103.
Gubanov VA, Zatsepin, AF, Kortov VS, Novikov DL, Friedman SP, Cherlov GB, Shchapova UV. Electronic states spectrum for lead silicate glasses with different short-range order structures. Journal of Non-Crystalline Solids. 1991;127(3):259–266.
Guittet MJ, Crocombette JP, Gautier-Soyer M. Bonding and XPS chemical shifts in ZrSiO4 versus SiO2 and ZrO2: Charge transfer and electrostatic effects. Physical Review B. 2001;63:125117-1–125117-7.
Hochella MF. Auger electron and X-ray photolectron spectroscopies. Reviews in Mineralogy. 1988;18:573–637.
Iacona F, Kelly R, Marletta G. X-ray photoelectron spectroscopy study of bombardment-induced compositional changes in ZrO2, SiO2, and ZrSiO4. Journal of Vacuum Science and Technology A: Vacuum, Surfaces, and Films. 1999;17(5):2771–2778.
Loeffler MJ, Dukes CA, Baragiola RA. Irradiation of olivine by 4 keV He+: Simulation of space weathering by the solar wind. Journal of Geophysical Research. 2009;114:E03003.
Marshall GM, Patarachao B, Moran K, Mercier PHJ. Zircon Mineral Solids Concentrated from Athabasca Oil Sands Froth Treatment Tailings: Surface Chemistry and Flotation Properties. Minerals Engineering. 2014;65:79–87.
Moulder JF, Stickle WF, Sobol PE, Bomben KD. Handbook of X-ray Photoelectron Spectroscopy. Perkin-Elmer Corp.: Eden, Prairie, MN, US. 1992.
Nefedov VI, Vovna VI. Elektronnaya struktura khimicheskikh soedinenii. M.: Nauka, 1987. 347 p. [Nefedov VI, Vovna VI. Electronic structure of chemical compounds. Moscow: Nauka, 1987. 347 p. (In Russian)].
Nesbitt HW, Bancroft GM. High resolution core- and valence-level XPS studies of the properties (structural, chemical and bonding) of silicate minerals and glasses. Reviews in Mineralogy and Geochemistry. 2014;78:271–329.
Nesbitt HW, Bancroft GM, Davidson R, McIntyre NS, Pratt AR. Minimum XPS core-level line widths of insulators, including silicate minerals. Am Mineral. 2004;89:878–882.
Nesbitt HW, Bancroft GM, Henderson GS, Ho R, Dalby KN, Huang Y, Yan Z. Bridging, non-bridging and free (O2–) oxygen in Na2O-SiO2 glasses: An X-ray Photoelectron Spectroscopic (XPS) and Nuclear Magnetic Resonance (NMR) study. Journal of Non-Crystalline Solids. 2011;357(1):170–180.
Nesbitt HW, Henderson GS, Bancroft GM, O’Shaughnessy C. Electron densities over Si and O atoms of tetrahedra and their impact on Raman stretching frequencies and Si-NBO force constants. Chemical Geology. 2017;461:65–74.
Rignanese G-M, Detraux F, Gonze X, Bongiorno A, Pasquarello A. Dielectric Constants of Zr Silicates: A First-Principles Study. Phys. Rev. Letters. 2002;89(11):117601(4).
Sawyer R, Nesbitt HW, Bancroft GM, Thibault Y, Secco RA. Spectroscopic studies of oxygen speciation in potassium silicate glasses and melts. Canadian Journal of Chemistry. 2015;93(1):60–73.
Siegbahn K. Electron spectroscopy-an outlook. Journal of Electron Spectroscopy and Related Phenomena. 1974;5(1):3–97.
Shchapova YuV, Votyakov SL, Kuznetsov MV, Ivanovsky AL. Influence of Radiation Defects on Electronic Structure of Zircon from X-Ray Photoelectronic Spectroscopy Data. Zh. Strukt. Khimii. 2010;4:687–692.
Votyakov SL, Schapova YuV, Hiller VV, Vinogradova NS, Glavatskikh SP, Porotnikov AV, Galakhova OL, Vovkotrub EG, Strekalovsky VN, Ivanovsky AL, Kuznetsov MV, Ryzhkov MV, Shalaeva EV. Spektroskopiya i fizika monatsita: sostoyanie i perspektivy ispol’zovaniya v reshenii geokhronologicheskikh problem Urala. Litosfera. 2012;4:158–172. [Votyakov SL, Schapova YuV, Hiller VV, Vinogradova NS, Glavatskikh SP, Porotnikov AV, Galakhova OL, Vovkotrub EG, Strekalovsky VN, Ivanovsky AL, Kuznetsov MV, Ryzhkov MV, Shalaeva EV. Spectroscopy and physics of monazite: state and prospects of use in solving the geochronological problems of the Urals. Litosfera. 2012;4:158–172. (In Russ.)].
Zakaznova-Herzog VP, Nesbitt HW, Bancroft GM, Tse JS, Gao X, Skinner W. High-resolution valence-band XPS spectra of the nonconductors quartz and olivine. Physical review B. 2005;72:205113.
Zakaznova-Herzog VP, Nesbitt HW, Bancroft GM, Tse JS. High resolution core and valence band XPS spectra of non-conductor pyroxenes. Surf Sci. 2006;600:3175–3186.
Zakaznova-Herzog VP, Nesbitt HW, Bancroft GM, Tse JS. Characterization of Leached Layers on Olivine and Pyroxenes Using High-Resolution XPS and Density Functional Calculation. Geochimica and Cosmochimica Acta. 2008;72:69–86.
Zatsepin AF, Kortov VS, Shchapova YuV. Electronic structure of phosphate glasses with a complex oxygen sublattice structure. Phys. Solid State. 1997;39(8):1212–1217.
Zeug M, Nasdala L, Wanthanachaisaeng B, Balmer WA, Corfu F, Wildner M. Blue Zircon from Ratanakiri, Cambodia. The journal of gemmology. 2018;36(2):112–132.
Zigban K, Nordling K, Falman A. i dr. Electronnaya spektroskopiya. M.: Mir, 1971. 495 p. [Siegbahn K, Nordling K, Fahlman A. et al. Electron spectroscopy. Moscow: Mir. 1971. 495 p. (In Russ.)].
Acknowledgements
This work was supported by the Russian Foundation for Basic Research, project No. 18-15-01153. The implementation of electron microprobe analysis was supported by the theme of state assignment of IGG UB RAS (number AAAA-A19-119071090011-6). The authors are grateful to Christoph Lenz for providing a zircon sample for research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Shchapova, Y.V., Zamyatin, D.A., Votyakov, S.L., Zhidkov, I.S., Kukharenko, A.I., Cholakh, S.O. (2020). Atomic and Electronic Structure of Zircon According to High-Resolution X-Ray Photoelectron Spectroscopy: Methodological Aspects. In: Votyakov, S., Kiseleva, D., Grokhovsky, V., Shchapova, Y. (eds) Minerals: Structure, Properties, Methods of Investigation. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-49468-1_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-49468-1_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49467-4
Online ISBN: 978-3-030-49468-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)