Abstract
Bone marrow failure is a collective term used to describe a group of disorders which impair normal hematopoiesis, resulting in single or multiple blood cytopenias. Bone marrow failure varies in the degree and extent of its severity. There is a wide differential involved in evaluating and treating an adolescent female with bone marrow failure, and in this chapter, we are aiming to review the importance of establishing the inciting cause of marrow failure followed by focused discussion on the better studied and described genetic and acquired disorders associated with marrow failure. Details pertaining to the adolescent female are highlighted when relevant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bone marrow failure
- Acquired aplastic anemia
- Fanconi anemia
- Telomere biology disorders/dyskeratosis congenita
- Shwachman-Diamond syndrome
- Diamond-Blackfan anemia
Hematopoiesis and Bone Marrow Failure Mechanisms
Hematopoiesis is the process of blood cell formation. It is a complex and delicate process that starts from hematopoietic pluripotent stem cells, cells that are characterized by their unique ability for both self-renewal and differentiation. Committed progenitor cells are next in the hierarchy, display more limited ability for self-renewal, and undergo several steps of differentiation and maturation, ultimately leading to the production of an adequate number of mature peripheral blood cells. This process is under significant regulation by several factors such as chemokines, cytokines, growth factors, and the bone marrow microenvironment, all of which are essential in complete function of the bone marrow cells. A full review of hematopoiesis is beyond the scope of this chapter, and readers are directed to reviewing other didactic resources [1,2,3].
Bone marrow failure is a term used to describe the group of disorders whereby the process of hematopoiesis has been impaired enough to affect the sufficient production of mature peripheral blood leukocytes, erythrocytes, and/or platelets. Mechanistically, several models have been proposed to result in impaired hematopoiesis [4] as follows:
-
1.
An external injury is incurred, such as exposure of the bone marrow to irradiation or medications that halt the proliferation of stem cells. An example of this mechanism is the transient pancytopenia that occurs after a course of chemotherapy is administered to a patient with cancer.
-
2.
The hematopoietic stem cells themselves are abnormal and unable to proliferate properly and/or undergo premature apoptosis. Inherited bone marrow failure syndromes (IBMFS) are such disorders that display flawed survival ability.
-
3.
The immune system attacks the hematopoietic stem cells, resulting in exhaustion of the proliferative capacity of remaining cells.
-
4.
Abnormalities in bone marrow microenvironment, which in turn inhibit hematopoiesis.
It is noteworthy that these mechanisms are not all mutually exclusive, as a patient with genetically abnormal cells might accrue further injury at time of exposure to marrow suppressive medications, for example.
Clinical Evaluation of the Adolescent Female with Bone Marrow Failure
Patients with bone marrow failure usually present with symptoms related to the cytopenia(s), such as easy bruising, bleeding, fatigue, or evidence of infection, depending on the affected blood cell line. Occasionally, they might be asymptomatic of their cytopenia(s), yet abnormalities in blood counts are uncovered during medical evaluation of other conditions.
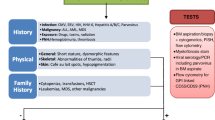
Upon presentation of an adolescent with single, bi-, or pancytopenia, a careful evaluation through comprehensive medical history, physical examination, and laboratory testing are required to first discern whether the etiology of the defect is due to impaired production of blood cells or due to increased destruction and then to discern whether the cause is inherited vs acquired (Table 26.1). Destructive causes (e.g., immune cytopenias) are not the focus of this chapter and therefore will not be discussed here. Once destructive causes are excluded, the decision to the exact timing of bone marrow evaluation through aspiration and biopsy with correlative cytogenetic testing is individualized based on the severity and extent of cytopenias. However, it is prudent to proceed with prompt examination in those with severe single cytopenia and in those with bi- or pancytopenia, as earlier diagnosis of bone marrow failure aids in proper administration of directed therapy and supportive care. Bone marrow examination allows assessment of cellularity and morphology of cells and excludes the presence of hematologic malignancy, myelodysplastic syndrome, or infiltrative disorders. Cytogenetics is important again to uncover presence of clones. Genetic testing through next-generation sequencing, deletion analysis, and whole exome sequencing is increasingly and more readily being used as an adjunct to all the above to confirm and better define various inherited conditions leading to, or associated with, cytopenias. Certain molecular tests are key to screen for some inherited marrow failure disorders, such as chromosome breakage testing for Fanconi anemia or measurement of telomere lengths to evaluate for a telomere biology disorder/dyskeratosis congenita.
The importance of distinguishing between inherited and acquired causes is very important for two main reasons:
-
1.
Treatment options are considerably different between inherited and acquired causes, as is described in depth in the following sections. Briefly, while immunosuppression might improve blood counts in a patient with idiopathic aplastic anemia, it is unlikely to result in any response in a patient with an inherited marrow failure syndrome. Additionally, while stem cell transplant might be curative of all causes of severe marrow failure, knowledge of an inherited cause would require adjustments to the stem cell transplant conditioning regimen. Transplant may require the use of reduced intensity regimens and, importantly, is vital in donor selection, for instance.
-
2.
When a genetic/inherited cause is diagnosed, counseling and genetic testing of siblings and parents are recommended, both for reasons of choosing an unaffected marrow donor and extending medical care, therapy, and surveillance to affected family members, as needed. For the adolescent female, knowledge of risk of transmittal to offspring is important for her to know, as she ages and considers having her own children.
In conclusion, the evaluation of an adolescent female with bone marrow failure ought to be detailed and comprehensive, as ramifications of the correct diagnosis are immense to the patient and extend even beyond her to her family. This group is unique in that medical providers might oversee the possibility of an inherited cause, yet absolutely should consider and evaluate for one.
Genetic Disorders Leading to Bone Marrow Failure
Fanconi Anemia (FA)
Fanconi anemia (FA) is a genetic disorder associated with variable congenital abnormalities, predisposition to bone marrow failure, as well as malignancy [5, 6]. The pathophysiology stems from chromosomal instability due to defects in proteins essential to DNA repair [7, 8]. Table 26.2 details modes of inheritance and genes implicated in FA, all of which comprise a pathway that repairs and removes DNA interstrand crosslinks, thereby allowing proper DNA replication and gene transcription [8]. The genes are grouped according to their function in the DNA repair pathway as upstream genes (FANCA, B, C, E, F, G, L, M, and T), ID2 complex (FANCD2 and I), and downstream genes (FANCD1, J, N, O, P, Q, R, S, U, V, and W) [9]. The upstream protein products combine to form the FA core complex upon DNA damage, which monoubiquitinates the ID2 complex, which in turn activates the downstream gene protein products, resulting in DNA repair [7, 8]. Chromosome breakage testing establishes the diagnosis of FA, where lymphoblastoid cell lines created from a proband display increased chromosomal breakage and radial ray forms when cells are exposed to diepoxybutane and mitomycin C [10]. Complementation analysis by somatic cell methods and next-generation sequencing looking for variants in genes involved in FA confirm the diagnosis and detail the genotype [11, 12].
FA is usually diagnosed in the first decade of life at the median age of 7 years, yet it has also been diagnosed in patients as young as newborn and in those older than 50 years [13]. FA is suspected in patients who present with single cytopenias, pancytopenia, or in those with red cell macrocytosis with or without anemia and with a high fetal hemoglobin [14,15,16]. Physical abnormalities are found in 75% of patients with FA, most commonly in the form of abnormal skin pigmentation, short stature, and upper extremity malformations [6, 17]. On occasion, malignancies such as myelodysplastic syndrome or squamous cell carcinoma of the head and neck in young adults prompt the diagnosis of FA [17].
From a hematologic standpoint, the severity of the marrow failure state guides consideration of the treatment route [14]. For example, periodic monitoring of blood counts and marrow cellularity might be sufficient in mild marrow failure, whereas more high-risk treatment as detailed below would be needed for patients in the moderate to severe marrow failure groups. The cytopenias described in the severity scale (Table 26.3; [18]) have to be persistent and otherwise unexplained. In addition to supportive care with blood transfusions when needed and iron chelation if a patient is needing chronic red cell transfusions, options for treatment of the FA-associated marrow failure include using:
-
1.
Androgens which may improve anemia and/or thrombocytopenia and, less so, neutropenia in 50–60% of treated patients [19, 20]. This improvement might be transient. Adverse effects observed and monitored for include virilization, accelerated growth spurt with premature closure of epiphyses, hypertension, and liver toxicity such as transaminitis, cholestatic jaundice, or hepatic adenoma development [20]. Of important note, use of androgens would not prevent development of MDS or AML [19, 20].
-
2.
Growth factors such as granulocyte colony-stimulating factor (GCSF) have been used in scenarios where patients have severe neutropenia along with a bacterial infection and usually only as a bridge to stem cell transplantation. Continued monitoring of the bone marrow for development of dysplasia or leukemia is warranted with prolonged use of GCSF [18].
-
3.
Hematopoietic stem cell transplantation (HSCT): Hematopoietic stem cell transplantation remains the curative option for FA-associated marrow failure but is a high-risk procedure given the inherent increased sensitivity that FA patients have to chemotherapy and irradiation. Conditioning regimens have been modified to reduce toxicities experienced by FA patients and improve survival, and the current recommendations are to use reduced intensity, fludarabine-based conditioning. Guidelines for HSCT timing and type are beyond the scope of this chapter, and readers are directed to review the consensus guidelines established by the Fanconi Anemia Research Foundation in 2014 (See https://www.fanconi.org/explore/clinical-care-guidelines) and other reviews [14, 18]. It is important to note that while HSCT cures the marrow failure state, it increases the risk of FA complications in other systems, and patients continue to warrant surveillance for organ dysfunctions as well as malignancy development [13, 21, 22].
The risk for malignancy development has come from analyses of data from several FA patient cohorts, which have compared the number of patients with certain cancers to the expected number in the database from the SEER (Surveillance, Epidemiology, and End Results) [23,24,25,26]. The information showed an overall risk of any cancer of 20- to 50-fold, solid tumors 20- to 40-fold, acute myelogenous leukemia 300- to 800-fold, head and neck squamous cell carcinoma (HNSCC) 200- to 800-fold, esophageal cancer 1300- to 6000-fold, vulvar cancer 500- to 4000-fold, and myelodysplastic syndrome more than 5000-fold [13, 23,24,25,26]. Patients with FA are at a very high risk of developing malignancies and develop them at an earlier age. Use of HSCT further increases the risk of HNSCC and gynecologic SCC, as well as non-melanoma skin cancers [5, 13].
For the adolescent female with FA-associated marrow failure, full discussion of the risks involved in each treatment modality is of paramount importance, e.g., some of the adverse effects of androgen use might not be desirable and the patient may opt to pursue HSCT instead. Alternatively, the patient might have undergone HSCT as a younger child and would need to receive education on the need for additional surveillance of the endocrine dysfunction or malignancy screening. Additionally, the hematologist often serves as the primary care provider for a patient with FA, and it is important that patients be referred to gynecologists and endocrinologists as they approach their teenage years. Issues such as delayed menarche, irregular menstruation, and excessive bleeding due to concurrent thrombocytopenia are a few examples of issue that might arise in FA adolescent female.
Telomere Biology Disorders/Dyskeratosis Congenita
Telomere biology disorders/dyskeratosis congenita (TBD/DC) are a heterogeneous group of disorders that affect multiple systems [27, 28]. The classical form includes a mucocutaneous triad of oral leukoplakia, lacy hyperpigmented skin changes, and dystrophic nails and includes a significant predisposition to marrow failure and cancer [27, 28]. Nonhematologic features are multiple and include strictures of lacrimal ducts, esophagus, or urethra; gastrointestinal enteropathies; abnormal teeth; early gray hair; and early hair loss [27, 28].
Over the last two decades, our understanding of the pathophysiology of TBD/DC has dramatically evolved due to improved understanding of telomeres and their role in human disease [27, 29]. Telomeres are specialized nucleoprotein structures at the end of eukaryotic chromosomes which protect chromosome ends, serve to solve the “end replication” problem, and are essential for maintenance of genetic stability [29]. In humans, telomeres consist of tandem repeats of TTAGGG nucleotides and a multi-protein complex called shelterin which protects telomere ends from DNA damage response [29, 30]. The normal length of telomeres is maintained through the enzyme telomerase reverse transcriptase (encoded by TERT), its RNA component (encoded by TERC), along with the function of several other proteins [27, 29, 31]. TBD/DC occurs when maintenance of normal telomere lengths is altered to where very short telomeres are observed, which in turn, results in cellular apoptosis or senescence [27, 32]. Genetic mutations in 14 genes affecting all components of the nucleoprotein complex have been observed in patients with TBD/DC, with significant variability in inheritance patterns as noted in Table 26.2 [27].
Bone marrow failure due to premature hematopoietic stem cell exhaustion is a prominent presentation of TBD/DC, where at least a single cytopenia rate of up to 80% by the age of 30 years has been observed [28, 33]. Patients may develop a single cytopenia (frequently thrombocytopenia [33]) before progressing to pancytopenia or later evolve into myelodysplastic syndrome [27].
Classic TBD/DC should be considered in patients with [27]:
-
1.
All three mucocutaneous changes described above, which are often subtle findings.
-
2.
Any one mucocutaneous change in combination with bone marrow failure and two other physical features consistent with TBD/DC.
-
3.
Bone marrow failure, myelodysplastic syndrome, and/or pulmonary fibrosis associated with previously described pathogenic germline mutation in a TBD-associated gene.
-
4.
Two or more features seen in TBD/DC associated with telomere length below the first percentile for age.
The testing of average telomere lengths involves flow cytometry with fluorescent in situ hybridization of telomeres in leukocyte subsets [34,35,36]. Using this method, measured telomere lengths of less than the first percentile in three of six of the lymphocyte subsets are both highly sensitive and specific for TBD/DC diagnosis [35, 36]. Use of this testing is widely being incorporated in assessing patients, not only with evidence of bone marrow failure and myelodysplastic syndrome but also in adults found with pulmonary fibrosis, unexplained liver disease, or early onset head and neck squamous cell carcinomas. Once a patient is identified to have very short telomeres, genetic testing further confirms the diagnosis and aids in genetic counseling being offered to affected families [27].
The management of the TBD/DC-associated marrow failure closely parallels that of patients with FA-associated marrow failure, as described above, with the following notable differences:
-
1.
Androgens may result in response rates in 70–80% of patients, but close attention is needed to ensure safety with close monitoring for liver toxicity [37].
-
2.
GCSF should not be used concurrently with androgens due to two reported cases of splenic peliosis and rupture [38].
-
3.
HSCT remains the only curative option. Similar to HSCT for FA, initial studies utilizing myeloablative regimens had extremely high mortality rates due to organ toxicity, particularly of the lungs, and endothelial injury [39]. The introduction of reduced intensity, fludarabine-based conditioning regimens has improved survival rates to about 65% [40], and more recent studies are studying whether elimination of DNA alkylating agents and radiation would reduce short- and long-term complications seen in TBD/DC patients post-transplant (Clinical trials.gov NCT01659606).
Patients with TBD/DC, similar to FA, have high risks of malignancies (fourfold increase) when compared to unaffected individuals. The types of cancers seen are head and neck (~70-fold), anogenital SCC (~50-fold), MDS (~500-fold), and AML (~70-fold) [5].
The adolescent female diagnosed with TBD/DC would need care to be delivered in a center that is familiar with all manifestations of TBD/DC. The same concepts described above for FA regarding discussion of treatment options of bone marrow failure apply. As hematologists often serve as providers of comprehensive care for TBD/DC, special care needs to be given to issues such as bone health and pulmonary and liver function. Care of a TBD/DC patient post-HSCT is also unique, and efforts have been made to delineate the surveillance and follow-up of this rare patient population [22].
Shwachman-Diamond Syndrome
Shwachman-Diamond Syndrome (SDS) is a disorder characterized by exocrine pancreatic insufficiency resulting in fat malabsorption, bone marrow dysfunction, and variable skeletal abnormalities [13, 41]. Patients have a high propensity to progress to myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) [41, 42].
Most patients with SDS are diagnosed in early childhood, although some may present in later childhood or even as adults, with the bone marrow failure component with or without mild pancreatic insufficiency [43, 44]. Diagnosing SDS has evolved over time and is reviewed in the draft consensus guidelines for diagnosis and treatment of SDS [44]. The clinical diagnosis briefly requires both of the following criteria [44]:
-
1.
Evidence of pancreatic exocrine dysfunction, typically by testing for pancreatic enzymes (amylase, lipase, trypsinogen) and evaluating for evidence of fat malabsorption. Exclusion of other known causes of pancreatic insufficiency (e.g., cystic fibrosis, Pearson syndrome) is warranted.
-
2.
Presence of hematological changes as described below, with exclusion of other known causes of bone marrow failure, such as FA.
Molecular diagnosis of SDS is established by presence of pathogenic biallelic mutations in SBDS, which is found in up to 90% of patients with SDS [41, 45]. In a small number of patients, newer genes implicated to result in disorders very similar to SDS are DNAJC21 [46], EFL1 [47], and SRP54 [48] (Table 26.2). SDS is a disorder of ribosomal function, where the protein product of SBDS gene cooperates with elongation factor-like GTPase 1 (EFL1) to catalyze release of the ribosome anti-association factor eIF6 and activate translation [49].
Failure to thrive due to pancreatic exocrine deficiency is often the presenting symptom in children with SDS, usually within the first year of life [44]. The fat malabsorption is treated with pancreatic enzymes taken with meals and snacks [41, 50]. Often improvement in pancreatic function is seen, and patients may not require enzyme therapy during adolescence and adulthood [41]. Patients have presented with bone marrow failure/MDS/AML without significant failure to thrive or pancreatic dysfunction.
From a hematological standpoint, nearly all patients with SDS have neutropenia of variable severity, which might be intermittent or persistent [44, 51]. In addition to the quantitative defect, neutrophils have been reported to have defects in migration or chemotaxis [52,53,54]. Other less commonly observed cytopenias include normo- or macrocytic anemia with high fetal hemoglobin, which again might be intermittent and of fluctuating severity and mild-moderate thrombocytopenia [44, 51]. A smaller number of patients have been reported with pancytopenia [55]. Bone marrow biopsies most often have hypocellularity with left shift in granulopoiesis, and fluctuating mild dysplasia is commonly observed [44, 51]. Persistent or progressive dysplasia is concerning for malignant transformation into MDS/AML. It is important to note that patients with SDS are prone to development of cytogenetic clones, most commonly del (20)(q11) and isochromosome 7q i(7)(q10) and that mere presence of such clones does not portend evolution of MDS/AML [44, 56,57,58].
Use of granulocyte colony-stimulating factor (GCSF) is considered for recurrent invasive bacterial and/or fungal infection due to severe neutropenia, with the goal to use the lowest dose possible to prevent infections rather than achieve normal hematological parameters [44]. Often doses may be given a few times a week and not needed daily [44]. Allogeneic HSCT remains a curative option but is being reserved for patients who have severe cytopenias or MDS/AML [41, 44], as its application is associated with increased transplant-related toxicities, particularly of the heart, lung, and liver [59]. Use of reduced intensity regimens in more recent years has improved outcome, but further larger studies are needed to confirm safety of wider application [60].
Diamond-Blackfan Anemia
Diamond-Blackfan anemia (DBA) is a clinically and genetically heterogeneous disorder, hematologically characterized by inherited red cell aplasia. Physical anomalies, including craniofacial defects, thumb deformities, and short stature, occur in about 50% of patients. Through studies conducted by the DBA Registry and the National Cancer Institute, DBA patients have been found to have a fivefold increased risk of cancer, where the most common cancers observed are MDS/AML, colon carcinoma, osteosarcoma, and genitourinary cancers [5, 61,62,63].
A consensus clinical diagnosis for classic DBA states that individuals with this disorder present within the first year of life and have normochromic, macrocytic anemia, reticulocytopenia, limited cytopenias of other lineages, and a visible paucity of erythroid precursor cells in the bone marrow [64]. Elevated erythrocyte adenosine deaminase (eADA) activity is observed in over 75–90% of cases [61]. About 10% of patients present later in childhood or adolescence, or even in adulthood, with macrocytic anemia and are found to have a hypocellular bone marrow with a paucity of red cell precursors.
DBA is a ribosomopathy, as 21 genes that either code for ribosomal subunits or indirectly alter ribosomal biogenesis have been reported to result in disease. About 25% of mutations affect RPS19 ([61]. The ribosomal gene mutations in DBA reported to date have all been heterozygous and include missense, nonsense, frameshift, and splice site mutations as well as large deletions [43, 61]. Genetic testing can currently provide a diagnosis in over 70% of cases [61, 65].
Regarding management of persistent and transfusion-dependent anemia, the majority of patients respond to glucocorticoids, with up to 80% achieving some improvement in erythropoiesis [66]. The exact mechanism by which glucocorticoids resolves the defect in erythropoiesis has been under investigation for quite some time. Evidence has shown that there is nonspecific anti-apoptotic effect on erythroid progenitors at the colony-forming unit-erythroid/proerythroblast progenitor interface [66]. In the mouse model Flygare et al. demonstrated increased red cell production through self-renewal of the burst-forming unit-erythroid [66,67,68]. Up to 20% of patients are able to enter into a state of remission by age 25 years, some of which might be spontaneous and not necessarily related to treatment with glucocorticoids [66]. Long-term administration of glucocorticoids, however, can be deleterious and has been reported to result in impaired bone health, hypertension, diabetes mellitus, and cataract formation, among other adverse effects in patients with DBA [69, 70]. Therefore, it is advisable to maintain patients on the lowest dose of glucocorticoids that allows for an adequate hemoglobin response [71].
Chronic red cell transfusions are needed in those patients whose erythroid defect is refractory to glucocorticoids, along with need for iron chelation to minimize the secondary effects of iron overload. Allogeneic SCT should be considered early in such patients, particularly if an unaffected matched sibling donor is available, as improved survival was noted when matched sibling donor SCT was implemented in patients younger than 9 years [71]. The role of unrelated donor SCT and exact timing of SCT consideration remains unclear and requires further larger studies, though use of treosulfan-based conditioning is showing promising results [60].
There are several considerations regarding DBA in the adolescent female. First, continued surveillance for adverse effects of chronic steroid use or iron overload is warranted, including involvement of subspecialists such as endocrinologists and gynecologists who are familiar with this disorder when possible, as some clinical observational studies suggest that females with DBA have an increased incidence of delayed puberty, irregular menstrual cycles, and decreased fertility [72,73,74]. Second, counseling is needed regarding the risk for malignancies as the onset of such malignancies may occur during adolescent or early adulthood years [62, 63] and the adolescent needs to report symptoms, such as extremity swelling, abdominal discomfort, and rectal bleeding, to allow for early diagnosis and treatment. Thirdly, recurrence of anemia after being in a remission or stable state on glucocorticoid therapy can occur and has been reported during puberty and pregnancy. During puberty, increases in the glucocorticoid doses may be necessary. However, if the dose increases over the maximum maintenance dose of 0.5 mg/kg/day, the patient may require transfusion therapy. Pregnancy in patients with DBA is considered high risk as it is associated with complications to the mother, fetus, or both [64, 75]. Transfusions are often needed in the patient with DBA to keep the hemoglobin >10 gm/dL while she is pregnant. Estrogen-containing oral contraceptives can disrupt the steady state of a non-transfused female with DBA. Progesterone-containing products have been better tolerated (personal observation). Lastly, genetic counseling about the autosomal dominant inheritance of this disorder and the 50% chance of an offspring being affected should be provided to the adolescent female as she enters young adulthood.
Acquired Causes of Bone Marrow Failure
Idiopathic Severe Aplastic Anemia (SAA)
In the adolescent female who presents with significant pancytopenia or bicytopenia, a major consideration is to elucidate whether the marrow failure state is genetic in nature or acquired. Inherited bone marrow failure disorders must be thoroughly considered and evaluated for, as described in the previous sections. Once an inherited cause has been eliminated, idiopathic severe aplastic anemia (SAA) might be the diagnosis, if the following diagnostic criteria are met, as set by Camitta [76]:
-
1.
Bone marrow hypocellularity, with less than 25% hematopoietic cells
-
2.
Two of the three peripheral blood cytopenias: absolute neutrophil count (ANC) less than 500/uL, platelet count less than 20,000/uL, and anemia with low absolute reticulocyte count of <60,000/uL
When ANC is less than 200/uL, very severe aplastic anemia is diagnosed.
Pathophysiology of SAA
Idiopathic SAA is believed to be the result of an immune attack, primarily by activated, oligoclonal cytotoxic T cells, which produce cytokines that cause apoptosis of the hematopoietic stem and progenitor cells through the Fas/Fas ligand pathway [77, 78]. The exact antigens that activate cytotoxic T cells remain unclear.
Supportive Care for SAA
The high risk of bacterial and fungal infections constitutes a major cause of mortality cause in SAA [79,80,81,82], and immediate evaluation of fever with judicious use of empiric antibiotics is warranted. Fungal infections, especially Aspergillus spp., should be evaluated in the patient with prolonged or recurrent unexplained fever [79,80,81,82]. The use of prophylactic antibiotics is variable across centers, except for use of prophylaxis for Pneumocystis jirovecii [83]. Another supportive care measure is the use of packed red cell and platelet transfusions to maintain adequate cardiopulmonary function and prevent bleeding, respectively. Blood products should be irradiated to prevent transfusion-associated graft vs host disease and to prevent allo-sensitization of patients [84].
Due to risk of significant bleeding with menstruation in severe thrombocytopenia, platelet transfusions might be needed more frequently during menstruation or menstrual suppression using oral contraception, or leuprolide may need to be instituted [85].
Iron overload may develop over time in patients who receive multiple red cell transfusions, and monitoring should occur so that iron chelation can be appropriately started. Serum ferritin is a crude marker of iron overload; however, when serum ferritin level is more than 1000 ug/L, liver and cardiac iron concentration should be checked with an MRI T2*or ferriscan. Chelation agents used vary between centers and are individually based, depending on anticipated toxicities with the available agents (e.g., deferiprone might be avoided due to risk of agranulocytosis; deferasirox might cause nephrotoxicity in those on cyclosporine) [86].
Definitive Therapies for SAA
SAA is a life-threatening disorder, and prompt institution of definitive therapy is paramount in the outcome of treated patients [87]. The two major pillars of definitive therapy for SAA are immunosuppressive therapy (IST) and hematopoietic stem cell transplantation (HSCT) [85]. The decision-making of which treatment to apply highly depends on the availability of a matching donor and is detailed in Fig. 26.1 and discussed below.
Schematic treatment algorithm for SAA in children. Grey boxes indicate insufficient information to recommend treatment and need for additional studies. Abbreviations CSA Cyclosporine A, hATG horse anti-thymocyte globulin, HLA human leukocyte antigen, HSCT hematopoietic stem cell transplantation, SAA idiopathic severe aplastic anemia, IST immunosuppressive therapy, MDS myelodysplastic syndrome, MSD matched sibling donor, MUD matched unrelated donor. *Use of eltrombopag is not recommended for patients with MDS
Immunosuppressive Therapy (IST)
The current standard of care for patients with SAA who lack a matched sibling donor is to receive immunosuppression with horse anti-thymocyte globulin (hATG) and cyclosporine A (CSA), which in combination have been effective in decreasing the activated cytotoxic T cells and dampening the ongoing marrow destruction [78, 85]. Due to the need for hematopoietic stem cells to recover hematopoiesis, response to treatment is generally assessed several months after initiation of IST and is measured by improvement of peripheral blood counts, and not by evaluation of the marrow cellularity [88]. Definitions of hematologic response differ between studies, but a refractory state is agreed on as continuing to meet criteria for SAA 6 months after initiation of IST. Complete response is achieved when blood counts return to normal or at least when the ANC increases to >1000/uL, hemoglobin is >10 gm/dL, and platelets are >100,000/uL without transfusions [85, 88]. Partial response is when peripheral cytopenias improve above the criteria for the SAA diagnosis but less than complete response. Several studies of the efficacy of IST with hATG and CSA have resulted in hematologic recovery in 60–70% of cases [89,90,91,92,93]. Specific to the adolescent group, Dufour et al. reported 3-year overall survival of 90% in patients who received IST alone [94].
Several postulations have been made regarding SAA that is refractory to IST, including misdiagnosis of a genetic etiology of the marrow failure or hypoplastic MDS, neither of which is responsive to immunosuppression. Alternatively, the immune mechanism causing marrow failure may not be susceptible to hATG/CSA. Lastly, the degree of hematopoietic destruction at time of IST treatment could be extensive to where recovery of remaining cells is minimal [95].
Aside from refractory SAA, the two other concerns with IST as the treatment option are the risks of relapse and clonal evolution [90, 96]. The rate of relapse of SAA at 5 years is 33%, which can be delayed, but not prevented, by a slow taper of CSA [97]. Clonal evolution of MDS or paroxysmal nocturnal hemoglobinuria (PNH) occurs in 15% of treated patients over the decade following IST [78, 88, 98]. It is important to note that intensification of immunosuppression by addition of other agents such as sirolimus [99] or mycophenolate mofetil [100] to the backbone of hATG and CSA has not decreased rates of relapse or clonal evolution, nor has it improved the rates of hematopoietic response.
Hematopoietic Stem Cell Transplantation (HSCT)
The utility of HSCT in treatment of SAA is derived from the need to replace a dysfunctional bone marrow of affected patients with hematopoietic stem cells derived from healthy donors, thereby restoring normal hematopoiesis. This treatment requires administration of conditioning regimens, which, in SAA patients, would mostly focus on immunosuppression of the host to allow engraftment of donor cells to occur. HSCT is associated with several short- and long-term risks that are beyond the scope of this chapter but briefly include graft failure, graft-vs-host disease (GvHD), infectious complications, and organ injury.
When an adolescent with SAA has an available HLA-matched sibling donor, hematopoietic stem cell transplantation (HSCT) should be the treatment of choice as it has been shown to be curative and the young age of transplanted patients is one of the factors associated with improved overall survival [101]. The European Group for Blood and Marrow Transplantation (EBMT) reviewed results of 940 matched sibling donor transplants of SAA, and overall survival was 84% in patients younger than 20 years, and a similar 3-year overall survival of 86% was found in a study of 537 adolescents, including 227 females [101]. Results from the Center for International Blood and Marrow Transplant Research (CIBMTR) showed a slightly higher overall survival of 90% in the 1013 patients younger than 18 years transplanted between 2006 and 2016 (D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2018. Available at https://www.cibmtr.org).
In patients who lack a matched sibling donor, IST remains the first treatment of choice. Matched unrelated donor HSCT (MUD HSCT) has been historically considered when IST fails to mount a hematologic response, when relapse post-IST occurs, or when hematologic clones evolve [95, 102]. Several reasons explain the delay in considering this treatment option, including the time lag to identifying an unrelated donor, as well as the inherent risks of GvHD, morbidity, and mortality after MUD HSCT observed in early reports [102]. However, there have been significant improvements in outcomes of MUD HSCT for SAA in recent years due to optimized HLA typing and the use of different conditioning regimens and GvHD prophylaxis. Bacigalupo et al. compared the outcomes of MUD HSCT to matched sibling donor HSCT and found that the outcome of the former was not statistically inferior, although there was greater risk of acute and chronic GvHD [101]. They additionally found that the use of peripheral stem cells is the strongest negative predictor of survival, followed by an interval of diagnosis to transplant of 180 days or more, older patients (>20 years), not using ATG in the conditioning regimen, and positive cytomegalovirus in either patient or donor [101]. A different study evaluating outcomes of the adolescent age group, specifically, also concluded that early transplant and use of bone marrow cells rather than peripheral stem cells were associated with improved outcome [94]. Furthermore, use of upfront MUD HSCT in about 30 children, including 8 adolescents, had excellent outcomes equaling survival of matched sibling donor HSCT, while at the same time showing decreased survival in those receiving MUD HSCT post-IST failure [103]. These results have prompted reconsideration of the treatment approach to include the possibility of MUD HSCT earlier in the course of SAA, if a donor is identifiable in a timely manner. Further larger studies are in progress to compare efficacy of upfront MUD HSCT to IST (ClinicalTrials.gov NCT02845596).
It is noteworthy that advances have also been made in alternative donor transplants for SAA, including successful use of haploidentical donor bone marrow cells followed by post-transplant cyclophosphamide for GvHD prophylaxis and graft rejection prevention [104]. Larger studies are being conducted to evaluate outcome of such alternative transplants, and it remains a second line treatment option at this point (ClinicalTrials.gov NCT02918292).
Considerations of Choice Between IST and HSCT
Matched sibling HSCT is preferred over IST as it restores hematopoiesis more rapidly, thereby reducing the risks of potentially life-threatening bacterial and fungal infections seen with prolonged neutropenia, and minimizes exposure to blood products with all the risks associated with transfusions over time, such as iron overload. It is also preferred as it would effectively eliminate the risks of later clonal evolution into MDS or PNH. However, these benefits need to be weighed against the potential for transplant-associated complications.
On the other hand, patients who receive and respond to IST have minimal to no long-term toxicity, and in most centers, institution of treatment does not require time delays. The downfalls include possible refractoriness to treatment with high potential of infections in the interim, as well as the need for long-term follow-up for onset of relapse and clonal evolution.
As is described above, there is a recent shift in paradigm of treatment choices to include earlier consideration of MUD HSCT when possible [103, 105], but more studies are needed to compare the treatment modalities more comprehensively. Due to the complexity of decision-making, it is highly recommended that patients with SAA are cared for by centers that are specialized in evaluation and care delivery to this unique population.
Alternative Treatments
The addition of hematopoietic-stimulating factors such as granulocyte-stimulating growth factor [106,107,108] and recombinant erythropoietin did not augment responses seen with IST [108]. However, the use of eltrombopag, a thrombopoietin mimetic, in 43 patients with refractory SAA, induced a durable hematologic response in 40% of patients in at least one blood lineage, while 8 patients developed clones [109]. A larger study of 88 adult patients who received eltrombopag at different time points of IST initiation showed an improved and quicker onset of response when eltrombopag was started at time of IST initiation [110]. Similar studies in children and adolescents are ongoing in both the upfront as well as the refractory and relapsed SAA settings (ClinicalTrials.gov NCT03025698).
References
Mills SE, editor. Histology for pathologists. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 2015.
Sieff CA, G.Q.D, Zon LI. Anatomy and physiology of hematopoiesis. In: Orkin SH, Fisher DE, Ginsburg D, Look AT, Lux SE, Nathan DG, editors. Nathan and Oski’s hematology and oncology of infancy and childhood. Philadelphia, PA: Saunders, Elsevier; 2015. p. 3–51. e21.
Akiko Shimamura DAW. Acquired aplastic anemia and pure red cell aplasia. In: Orkin SH, Fisher DE, Ginsburg D, Look AT, Lux SE, Nathan DG, editors. Nathan and Oski’s hematology and oncology of infancy and childhood. Philadelphia, PA: Elsevier; 2015. p. 161–81.
Alter BP, et al. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018;103(1):30–9.
Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24(3):101–22.
Wang AT, Smogorzewska A. SnapShot: Fanconi anemia and associated proteins. Cell. 2015;160(1–2):354–354 e1.
Rodriguez A, D’Andrea A. Fanconi anemia pathway. Curr Biol. 2017;27(18):R986–8.
Fiesco-Roa MO, et al. Genotype-phenotype associations in Fanconi anemia: a literature review. Blood Rev. 2019;37:100589.
Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysisz. Curr Protoc Hum Genet. 2015;85:8 7 1–8 7 17.
Ameziane N, et al. Genetic subtyping of Fanconi anemia by comprehensive mutation screening. Hum Mutat. 2008;29(1):159–66.
Ameziane N, et al. Diagnosis of fanconi anemia: mutation analysis by next-generation sequencing. Anemia. 2012;2012:132856.
Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Hematology Am Soc Hematol Educ Program. 2017;2017(1):88–95.
Dufour C. How I manage patients with Fanconi anaemia. Br J Haematol. 2017;178(1):32–47.
Butturini A, et al. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood. 1994;84(5):1650–5.
Kutler DI, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101(4):1249–56.
Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668(1–2):4–10.
Mehta PA, Tolar J. Fanconi Anemia. 2002 Feb 14 [Updated 2018 Mar 8]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1401/.
Scheckenbach K, et al. Treatment of the bone marrow failure in Fanconi anemia patients with danazol. Blood Cells Mol Dis. 2012;48(2):128–31.
Paustian L, et al. Androgen therapy in Fanconi anemia: a retrospective analysis of 30 years in Germany. Pediatr Hematol Oncol. 2016;33(1):5–12.
Dietz AC, et al. Current knowledge and priorities for future research in late effects after hematopoietic cell transplantation for inherited bone marrow failure syndromes: consensus statement from the second pediatric blood and marrow transplant consortium international conference on late effects after pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(5):726–35.
Dietz AC, et al. Late effects screening guidelines after hematopoietic cell transplantation for inherited bone marrow failure syndromes: consensus statement from the second pediatric blood and marrow transplant consortium international conference on late effects after pediatric HCT. Biol Blood Marrow Transplant. 2017;23(9):1422–8.
Alter BP, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179–88.
Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101(3):822–6.
Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511–7.
Tamary H, et al. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli Inherited Bone Marrow Failure Registry. Haematologica. 2010;95(8):1300–7.
Niewisch MR, Savage SA. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2019;12(12):1037–52.
Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110(4):768–79.
Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13(8):696–706.
de Lange T. Shelterin-mediated telomere protection. Annu Rev Genet. 2018;52:223–47.
Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015;29(11):1095–105.
Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60.
Dokal I. Dyskeratosis congenita. Hematology Am Soc Hematol Educ Program. 2011;2011:480–6.
Gutierrez-Rodrigues F, et al. Direct comparison of flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One. 2014;9(11):e113747.
Alter BP, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353–9.
Alter BP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439–47.
Khincha PP, et al. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol. 2014;165(3):349–57.
Giri N, et al. Splenic peliosis and rupture in patients with dyskeratosis congenita on androgens and granulocyte colony-stimulating factor. Br J Haematol. 2007;138(6):815–7.
de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11(6):584–94.
Gadalla SM, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol Blood Marrow Transplant. 2013;19(8):1238–43.
Nelson AS, Myers KC. Diagnosis, treatment, and molecular pathology of Shwachman-Diamond syndrome. Hematol Oncol Clin North Am. 2018;32(4):687–700.
Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Blood. 2017;130(21):2257–64.
Wilson DB, et al. Inherited bone marrow failure syndromes in adolescents and young adults. Ann Med. 2014;46(6):353–63.
Dror Y, et al. Draft consensus guidelines for diagnosis and treatment of Shwachman-Diamond syndrome. Ann N Y Acad Sci. 2011;1242:40–55.
Boocock GR, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33(1):97–101.
Dhanraj S, et al. Biallelic mutations in DNAJC21 cause Shwachman-Diamond syndrome. Blood. 2017;129(11):1557–62.
Stepensky P, et al. Mutations in EFL1, an SBDS partner, are associated with infantile pancytopenia, exocrine pancreatic insufficiency and skeletal anomalies in a Shwachman-Diamond like syndrome. J Med Genet. 2017;54(8):558–66.
Carapito R, et al. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J Clin Invest. 2017;127(11):4090–103.
Tan S, et al. EFL1 mutations impair eIF6 release to cause Shwachman-Diamond syndrome. Blood. 2019;134(3):277–90.
Mack DR, et al. Shwachman syndrome: exocrine pancreatic dysfunction and variable phenotypic expression. Gastroenterology. 1996;111(6):1593–602.
Hashmi SK, et al. Comparative analysis of Shwachman-Diamond syndrome to other inherited bone marrow failure syndromes and genotype-phenotype correlation. Clin Genet. 2011;79(5):448–58.
Rothbaum RJ, Williams DA, Daugherty CC. Unusual surface distribution of concanavalin A reflects a cytoskeletal defect in neutrophils in Shwachman’s syndrome. Lancet. 1982;2(8302):800–1.
Orelio C, et al. Altered intracellular localization and mobility of SBDS protein upon mutation in Shwachman-Diamond syndrome. PLoS One. 2011;6(6):e20727.
Stepanovic V, et al. The chemotaxis defect of Shwachman-Diamond syndrome leukocytes. Cell Motil Cytoskeleton. 2004;57(3):158–74.
Donadieu J, et al. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica. 2012;97(9):1312–9.
Dror Y, et al. Clonal evolution in marrows of patients with Shwachman-Diamond syndrome: a prospective 5-year follow-up study. Exp Hematol. 2002;30(7):659–69.
Smith A, et al. Intermittent 20q- and consistent i(7q) in a patient with Shwachman-Diamond syndrome. Pediatr Hematol Oncol. 2002;19(7):525–8.
Valli R, et al. Shwachman-Diamond syndrome with clonal interstitial deletion of the long arm of chromosome 20 in bone marrow: haematological features, prognosis and genomic instability. Br J Haematol. 2019;184(6):974–81.
Donadieu J, et al. Hematopoietic stem cell transplantation for Shwachman-Diamond syndrome: experience of the French neutropenia registry. Bone Marrow Transplant. 2005;36(9):787–92.
Burroughs LM, et al. Allogeneic hematopoietic cell transplantation using treosulfan-based conditioning for treatment of marrow failure disorders. Biol Blood Marrow Transplant. 2017;23(10):1669–77.
Da Costa L, Narla A, Mohandas N. An update on the pathogenesis and diagnosis of Diamond-Blackfan anemia. F1000Res. 2018;7
Vlachos A, et al. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119(16):3815–9.
Vlachos A, et al. Increased risk of colon cancer and osteogenic sarcoma in Diamond-Blackfan anemia. Blood. 2018;132(20):2205–8.
Vlachos A, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142(6):859–76.
Ulirsch JC, et al. The genetic landscape of Diamond-Blackfan anemia. Am J Hum Genet. 2018;103(6):930–47.
Narla A, Vlachos A, Nathan DG. Diamond Blackfan anemia treatment: past, present, and future. Semin Hematol. 2011;48(2):117–23.
Sjogren SE, et al. Glucocorticoids improve erythroid progenitor maintenance and dampen Trp53 response in a mouse model of Diamond-Blackfan anaemia. Br J Haematol. 2015;171(4):517–29.
Flygare J, et al. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117(12):3435–44.
Lipton JM, et al. Improving clinical care and elucidating the pathophysiology of Diamond Blackfan anemia: an update from the Diamond Blackfan Anemia Registry. Pediatr Blood Cancer. 2006;46(5):558–64.
Willig TN, et al. Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. DBA group of Societe d’Hematologie et d’Immunologie Pediatrique (SHIP), Gesellshaft fur Padiatrische Onkologie und Hamatologie (GPOH), and the European Society for Pediatric Hematology and Immunology (ESPHI). Pediatr Res. 1999;46(5):553–61.
Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116(19):3715–23.
Tufano AM, et al. Deleterious consequences of Diamond Blackfan anemia on reproductive health and pregnancy outcomes: a report from the Diamond Blackfan Anemia Registry (DBAR). Blood. 2014;124(21):4399.
Lahoti A, et al. Endocrine dysfunction in Diamond-Blackfan Anemia (DBA): a report from the DBA Registry (DBAR). Pediatr Blood Cancer. 2016;63(2):306–12.
Bartels M, Bierings M. How I manage children with Diamond-Blackfan anaemia. Br J Haematol. 2019;184(2):123–33.
Faivre L, et al. High-risk pregnancies in Diamond-Blackfan anemia: a survey of 64 pregnancies from the French and German registries. Haematologica. 2006;91(4):530–3.
Camitta BM, Storb R, Thomas ED. Aplastic anemia (second of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306(12):712–8.
Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76–81.
Young NS. Aplastic Anemia. N Engl J Med. 2018;379(17):1643–56.
Weinberger M, et al. Patterns of infection in patients with aplastic anemia and the emergence of Aspergillus as a major cause of death. Medicine (Baltimore). 1992;71(1):24–43.
Torres HA, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98(1):86–93.
Valdez JM, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52(6):726–35.
Quarello P, et al. Epidemiology of infections in children with acquired aplastic anaemia: a retrospective multicenter study in Italy. Eur J Haematol. 2012;88(6):526–34.
Williams DA, et al. Diagnosis and treatment of pediatric acquired aplastic anemia (AAA): an initial survey of the North American Pediatric Aplastic Anemia Consortium (NAPAAC). Pediatr Blood Cancer. 2014;61(5):869–74.
Marsh J, et al. Should irradiated blood products be given routinely to all patients with aplastic anaemia undergoing immunosuppressive therapy with antithymocyte globulin (ATG)? A survey from the European Group for Blood and Marrow Transplantation Severe Aplastic Anaemia Working Party. Br J Haematol. 2010;150(3):377–9.
DeZern AE, Guinan EC. Aplastic anemia in adolescents and young adults. Acta Haematol. 2014;132(3–4):331–9.
Marsh JC, Kulasekararaj AG. Management of the refractory aplastic anemia patient: what are the options? Blood. 2013;122(22):3561–7.
Locasciulli A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–8.
Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120(6):1185–96.
Frickhofen N, et al. Antithymocyte globulin with or without cyclosporin a: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101(4):1236–42.
Rosenfeld S, et al. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–5.
Fuhrer M, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106(6):2102–4.
Fuhrer M, et al. Relapse and clonal disease in children with aplastic anemia (AA) after immunosuppressive therapy (IST): the SAA 94 experience. German/Austrian Pediatric Aplastic Anemia Working Group. Klin Padiatr. 1998;210(4):173–9.
Scheinberg P, et al. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr. 2008;153(6):814–9.
Dufour C, et al. Outcome of aplastic anemia in adolescence: a survey of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99(10):1574–81.
Marsh JC, Kulasekararaj AG. Management of the refractory aplastic anemia patient: what are the options? Hematology Am Soc Hematol Educ Program. 2013;2013:87–94.
Rogers ZR, et al. Immunosuppressive therapy for pediatric aplastic anemia: a North American Pediatric Aplastic Anemia Consortium study. Haematologica. 2019;104(10):1974–83.
Scheinberg P, et al. Prolonged cyclosporine administration after antithymocyte globulin delays but does not prevent relapse in severe aplastic anemia. Am J Hematol. 2014;89(6):571–4.
Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma. 2004;45(3):433–40.
Scheinberg P, et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94(3):348–54.
Scheinberg P, et al. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133(6):606–11.
Bacigalupo A, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100(5):696–702.
Davies JK, Guinan EC. An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol. 2007;136(4):549–64.
Dufour C, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171(4):585–94.
DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32(4):629–42.
Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2(15):2020–8.
Gluckman E, et al. Results and follow-up of a phase III randomized study of recombinant human-granulocyte stimulating factor as support for immunosuppressive therapy in patients with severe aplastic anaemia. Br J Haematol. 2002;119(4):1075–82.
Tichelli A, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117(17):4434–41.
Marsh JC, Ganser A, Stadler M. Hematopoietic growth factors in the treatment of acquired bone marrow failure states. Semin Hematol. 2007;44(3):138–47.
Olnes MJ, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–9.
Townsley DM, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–50.
Takeshima M, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150.
McGowan KE, et al. Aplastic anaemia in pregnancy – a single centre, North American series. Br J Haematol. 2019;184(3):436–9.
DeZern AE, et al. Detection of paroxysmal nocturnal hemoglobinuria clones to exclude inherited bone marrow failure syndromes. Eur J Haematol. 2014;92(6):467–70.
Nelson A, Myers K. Shwachman-Diamond Syndrome. 2008 Jul 17 [Updated 2018 Oct 18]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1756/.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sasa, G.S., Vlachos, A. (2020). Bone Marrow Failure Disorders in the Adolescent Female. In: Srivaths, L. (eds) Hematology in the Adolescent Female. Springer, Cham. https://doi.org/10.1007/978-3-030-48446-0_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-48446-0_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48445-3
Online ISBN: 978-3-030-48446-0
eBook Packages: MedicineMedicine (R0)