Abstract
Purpose of the review: Most of our blood volume is contained in the venous compartment. The so-called “compliant veins” are an adjustable blood reservoir that is playing a paramount role in maintaining hemodynamic stability. Several autonomous reflexes govern the capacity of this reservoir. The mean systemic filling pressure (Pmsf) is the pressure in the cardiovascular system when there is no blood flow, and is pressure that can describe the capacitance of the venous reservoir. This pressure can be measured in human patients by both non-invasive or minimally invasive methods. However, the significance of this hemodynamic variable is still not fully understood. The purpose of this review is to summarize what is known about the venous reservoir and the Pmsf and how we can use this information to assess the cardiovascular state of critically ill patients.
Findings: The venous tone is governed by sympathetic reflex, mainly related to barocerectors via α(alpha)-adrenergic stimulation and to chemoreceptors. The vasoconstriction affects significantly the capacitance of the system by shifting blood between the stress and non-stress volume compartments. The Pmsf is the pivot pressure of the circulation, and a quantitative index of intravascular volume, and it is also governed by the mechanisms that affect the venous tone. Pmsf can be measured at bedside by three methods described in critically ill patients. This pressure can be also modified by fluid therapy and vasoactive medications.
Pmsf along with other haemodynamic variables can provide valuable information to correctly understand the cardiovascular status of critically ill patients and better managing fluid therapy and cardiovascular support. Future studies using Pmsf will show its usefulness for fluid administration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mean systemic filling pressure
- Pmsf
- Venous system
- Arterial baroreceptor reflex
- Chemoreceptor reflex
- Capacitance vessels
- Venous tone
-
1.
The venous system serves a blood reservoir adjustable to the blood flow requirements.
-
2.
The venous tone is governed by the sympathetic activity via baroreceptors (using α[alpha]-adrenergic receptors) and chemoreceptors.
-
3.
The Pmsf is a quantitative measurement of the volume status and represents a measurement of the venous reservoir tone.
-
4.
Pmsf can be measured at bedside by using inspiratory hold maneuvers, by using a stop-flow arterial-venous equilibrium pressure or can be estimated using a computerized mathematic algorithm.
-
5.
Pmsf monitoring can provide important information when a clinician wants to challenge the system using a bolus of fluids or a passive leg raising (PLR) test. It can also guide decisions regarding the use of further fluid or vasoconstrictors.
Introduction
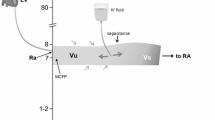
The assessment of the intravascular volume status in critically ill patients is crucially important and enormously challenging. The importance is based on the evidence that both hypovolemia and fluid overload are dangerous situations in critical illness [1,2,3,4]. The challenge consists in finding a parameter able to provide information about the intravascular filling independently from other confounders such as cardiac function, vascular tone or preload reserve. Cardiac preload is defined as the end-diastolic myocardial stretch (sarcomere tension), which in clinical practice is impossible to measure. Hence, some indicators of preload have been suggested: right atrial pressure (RAP) and its surrogate central venous pressure (CVP) are considered static measurements of right ventricular preload. The problem with this pragmatic approach is that clinical values of CVP do not accurately reflect preload. For example, when cardiac function decreases, CVP increases immediately without changes in intravascular volume. The mean circulatory filling pressure can quantify the intravascular filling independently from the cardiac function: it is the mean pressure that would be measured at all points in the cardiovascular system if the heart were stopped suddenly and the blood were redistributed rapidly between the arterial and venous territory [5]. This pressure is equal to the pressure at the pivotal point of the circulation, which is assumed to be located in the capacitance vessels. This pressure depends on the stressed volume and the capacitance of the system (Fig. 8.1). Therefore, in order to better understand hemodynamics at the bedside, it is essential to know the factors that affect the capacitance vessels, which are basically the venous system. In this chapter, we review some basic concepts of venous physiology that provide useful tools to manage patients in intensive care.
Model of the systemic circulation. Circulatory model with two reservoirs: the big venous reservoir, mainly located in the splanchnic venous territory and the small reservoir, just before the pump: the right atrium. The bigger size of the venous segments suggest a bigger volume and also a greater distensibility, compared with the lower compliances of the arterial segments. The segments between compartments represent the resistances, which are more variable at the arterial segments. The separation between the stressed and unstressed volume represents the adjustable capacitance of the venous reservoir
The Venous System
The venous system is not merely a conduct of blood to the heart. It works as an adjustable blood reservoir, able to modify blood flow according to changing metabolic demands. Veins contain 70% of total blood volume, whereas arteries contain only 13–18%, and capillaries 7% [6, 7]. Venous walls have a much larger compliance compared to arterial walls. Let us imagine this “blood reservoir” as a distensible compartment. The volume required to fill a distensible tube, such as a tire or a blood vessel, with no pressure rise is called the “unstressed” volume (Vo) . At this point, the volume depends on the total capacity (or capacitance) of the reservoir, since the pressure is zero. Further volume expansion will imply necessarily a pressure rise and an elastic distension of the tube wall. He relationship between pressure and volume defines the compliance (C) of the reservoir walls. This volume is the “stressed” volume (Vs) and is related to the pressure (P) in the equation:
As with other parts of the vascular system, the vein’s walls are composed of three basic histological layers: the tunica intima, the tunica media, and the tunica adventitia. The tunica media contains a variable thick layer of vascular smooth muscle cells. These cells can be stimulated to contract by multiple mechanisms: nervous reflex signals, hormonal stimulation, by stretching the smooth muscle, and several other ways. We are going to examine some of these mechanisms.
Arterial Baroreceptor Reflex Influence
Arterial hypotension reduces baroreceptor activity and provokes an increased sympathetic discharge, which causes venoconstriction, arterial vasoconstriction, and increased cardiac contractility and heart rate. The classic studies from Heymans and colleagues [8] demonstrated the influence of the carotid sinus baroreceptors on the blood volume of the mesentery, spleen, liver, and intestine. Most of the change observed in vascular capacitance takes place in the splanchnic bed.
Shoukas et al. [9] studied the reflex control of the total systemic vascular capacity in vagotomized dogs, measuring blood volume shifts caused by the carotid sinus reflex by diverting venous return into a reservoir while cardiac output and central venous pressure were maintained at a constant level. The isolated carotid sinus pressure (ISP) was lowered or raised in 25 mm Hg steps between 75 to 200 mm Hg. This procedure mobilized blood into (when decreasing ISP) or out (when increasing ISP) of the reservoir indicating a decrease or an increase in total vascular capacity, respectively. They observed that the total volume shift was approximately 7.5 mL/Kg for ISP changes from 75 to 200 mm Hg, whereas when the arterial blood pressure was controlled at 75 mm Hg, the total volume shift was 260 ml for the same change in ISP. This greater change in reservoir volume with fixed mean arterial pressure demonstrated the total influence of the carotid reflex system on the total capacity of the systemic vascular bed. The volume change with uncontrolled mean arterial pressure indicates the actual blood volume that the overall reflex system mobilizes as it changes vascular resistance and capacity. As the reflex did not affect the total systemic and arterial compliances, the authors concluded that the reflex controls the total systemic venous capacity to a degree that changes cardiac output potentially by 30–40% per 25-mm Hg change in ISP. Similar results were reported by Hainsworth [10] in an experiment where the aortic arch was stimulated and a hind limb of a dog was vascularly isolated with blood pumped at constant flow. They observed that the large superficial veins of the dog’s hind limb participate in the baroreceptor reflex. Likewise, Shigemi et al. [11] showed in an elegant study that α(alpha)-adrenergic mechanisms contribute significantly to active changes in systemic venous capacity, whereas the β(beta)-adrenergic system has very little effect on the active changes in venous vessels, but does contribute to the overall capacity by reducing the venous (hepatic) outflow resistance when the carotid sinus baroreflex system is activated. The active changes are those due to changes in vascular compliance (the slope of the pressure-volume relationship) or changes in unstressed vascular volume or vascular capacity in contrast with passive (physical) changes in the vascular capacity, defined as movements along the same pressure-volume curve secondary to a change in the blood flow and concomitant changes in vascular distending pressures. In this study, 14 dogs were vagotomized and anesthetized, and the carotid sinus were isolated. A constant flow, constant central venous pressure cardiopulmonary bypass was used to determine changes in vascular capacity. The changes in unstressed vascular volume were calculated when carotid sinus pressure was reduce from 200 to 50 mm Hg first without any adrenergic receptor antagonist, then with either an α(alpha)-(phentolamine) or a β(beta)-(propranolol) antagonist, and then with both. The change in unstressed volume in the systemic circulation was reduced by 72% with phentolamine, by 35% with propranolol, and by 73% with both antagonists. This suggests that the α(alpha)-adrenergic mechanism predominates over β(beta)-adrenergic mechanism in the active control of venous capacity by the carotid sinus baroreflex system [12]. The β(beta)-adrenergic mechanism may play a role in passive capacity changes. Both active and passive changes in vascular capacity contribute to the regulation of cardiac filling and therefore cardiac output. The β(beta)-adrenergic stimulation effect on the venous system is controversial; some authors reported a venodilation effect [13, 14] while others concluded that β(beta)-receptor stimulation induces blood redistribution from the periphery to the heart by reducing general venous resistance [15, 16] or hepatic outflow resistance [11, 17].
During hypovolemia these reflexes cause venoconstriction, sending blood back to the central circulation. Actually, even after 20% of the total blood volume has been lost, the circulatory system functions almost normally because of this variable reservoir function of veins [6]. Similarly, when a person is standing absolutely still, the pressure in the veins of the feet is about 90 mm Hg simply because of the gravitational effect of the blood in veins. This effect could actually be life threatening if there was no compensatory reflex. Hainsworth [18] pointed out that almost all the possible venoconstriction reflex is used to maintain cardiac output (CO). Venous tone is thus very important in hemodynamic hemostasis.
Chemoreceptor Reflex Influence
Studies by Kahler and colleagues [19] showed in a preparation where dogs were pump perfused from a blood reservoir and oxygenator to which venous blood returned, during hypoxia (SaO2 50%) the volume of the reservoir increased by 16 ± 2.8 mL/Kg. After splenectomy and bilateral adrenalectomy, the change was only 10.9 mL/Kg. They concluded that hypoxia generates venoconstriction, but circulating catecholamines are also necessary for the full response. Breathing 5% of carbon dioxide in air causes an increase in CO in people, while changes in arterial pressure and heart rate are relatively small [20]. The change in CO can be also related to the hyperventilation, but Price et al. concluded that an increase in sympathetic activity was the primary reason.
Smith and Crowell [21] tested the response of the mean circulatory filling pressure (MCFP) and CO to hypoxia by ventilating dogs with 8% oxygen in nitrogen. MCFP increased 27% while RAP decreased; there was a 15% increase in arterial pressure and 45% increase in CO, suggesting a significant venoconstriction with increased cardiac contractility. With reflexes blocked with spinal anesthesia, the MCFP resistance for venous return and arterial pressure fell during hypoxia while CO and RAP did not change.
Moderate hypercapnea and hypoxia have little direct non-reflex effect on CO and Pmsf [22]. Severe hypercapnea (PaCO2 to114 mm Hg [15.2 KPa]) caused an increase in Pmsf by 5.5 mm Hg, whereas a PaO2 of 34 mmHg (4.5 KPa) caused an increase in Pmsf by 2.5 mmHg [23].
The venoconstrictive effect of the veins of the limbs in mammals in response to chemoreceptor stimulation is not completely established, except under extreme conditions. Mild chemoreceptor stimulation has little effect on the capacitance system: It has little constrictive effect or even a dilating influence on skin and skeletal muscle veins of dogs [24,25,26,27]. The saphenous vein does not respond to either carotid or aortic chemoreceptor stimulation [26,27,28,29].
The Capacitance Vessels
Veins cannot be considered a pharmacologically homogeneous system [30, 31] and their overall response to stimulus is very difficult to predict. Certain parts of the venous system are particularly compliant: these include the spleen, the liver, the large abdominal veins and the venous plexus beneath the skin. Splanchnic and cutaneous veins have a high population of α(alpha)1- and α(alpha)2-adrenergic receptors, so they are very sensitive to adrenergic stimulation, contrary to skeletal and muscle veins [32]. There are nerve terminations in the proximity of many small vein smooth muscles [33] but not in the veins of skeletal muscle [34]. However, circulating catecholamines can induce contraction of venules and veins of skeletal muscle and mesentery [33, 34]. Thus, probably catecholamines released from the sympathetic nerve termination of the arterial side may pass through the capillary bed and affect the venous system.
Some authors consider it reasonable to assume that all parts of the capacitance system would act as a unit in cardiovascular homeostasis [7, 35] although lack of response of one part of the system (i.e., limb veins of people) should not be considered as evidence that others parts of the system (i.e., the splanchnic bed) are also nonreactive to stimulation. Cutaneous veins respond vigorously to temperature regulation reflexes [35, 36] whereas the splanchnic veins are more involved in the reflex system for cardiovascular homeostasis. Moreover, the effect of changes in sympathetic activity with baroreceptor stimulation is not uniform on the venous tone in different organs such as the spleen, kidney, or heart [37, 38] and likewise the pharmacological response of veins from different organs [39].
Smooth muscle of the veins and arteries do not respond necessarily in the same way to chemical signals. Dihydroergotamine can activate the veins but not the arteries [40]. The venous system primarily has α(alpha)-adrenergic receptors [41,42,43,44]. Stimulation of the β(beta)-adrenergic receptors of arterioles cause vasodilation but has little effect on the veins [45, 46]. Angiotensin can increase Pmsf [45, 47]. Isoprotenerol, a β(beta)-adrenergic agonist, causes a decrease in Pmsf when veins are constricted with angiotensin. On the other hand, vasopressin has very little effect on Pmsf [48] or on vascular capacity once reflex blockade [49] and similar results were reported regarding natriuretic peptides [50]. Nitroglycerin and nitroprusside decrease Pmsf and increase unstressed blood volume but do not change vascular compliance in ganglion-blockade dogs [51]. Verapamil and nifedipine increase venous return by reducing the resistance to venous return without changing the Pmsf, whereas nitroglycerin in small doses can reduce Pmsf without changes in resistance to venous return [52]. Diltiazem reduces both resistance and Pmsf increasing CO [52].
The splanchnic veins seem to be the major site of capacitance activity. Price et al. [53] observed that the splanchnic blood volume decreased by 500 mL after 1 l of hemorrhage in healthy male volunteers, while mean arterial pressure, heart rate, CO, and splanchnic vascular resistance did not change significantly from baseline. The authors point out active venoconstriction without simultaneous arteriolar vasoconstriction as an explanation of the results. Hainsworht et al. [54] studied the response of splanchnic vascular capacitance to changes in carotid sinus pressure in anesthetised dogs, perfused at constant blood flow and at constant pressure from the inferior vena cava. Vascular resistance responses were expressed as the changes in perfusion pressure and capacitance responses were determined by integrating changes in vena cava outflow. Decreasing the pressure in the isolated carotid sinuses over the whole baroreceptor sensitivity range increased mean perfusion pressure from 91 to 149 mm Hg (a 67% increase in resistance) and decreased mean capacitance by 111 ml. (5 ml kg−1). However, the range of carotid sinus pressures over which capacitance responses occurred was at a significantly higher level than the corresponding range for resistance responses. Comparison of the reflex responses with the responses to direct stimulation of efferent sympathetic nerves shows that quantitatively similar responses of resistance and capacitance to those induced by a large step decrease in carotid pressure could be produced by stimulating maximally the efferent sympathetic nerves at 5 Hz. These results suggest that at all levels of carotid sinus pressure there is no difference in the impulse traffic to resistance and capacitance vessels. The difference in the ranges of carotid pressure for resistance and capacitance responses is due to the greater sensitivity of the capacitance vessels to sympathetic nerve activity.
The Mean Systemic Filling Pressure
When the heart pumps blood continuously into the aorta, the mean pressure in the aorta remains high, averaging 80–100 mm Hg. As the blood flows into the systemic circulation, the mean pressure falls progressively as low as the level of the right atrial pressure (RAP). When the heart stops, the arterial pressure falls down and the RAP progressively increases. At a certain point, blood will not be flowing, and the pressure will be the same in all parts of the circulatory system. This was called the mean circulatory filling pressure (MCFP). When the pulmonary circulation is excluded, we call this the mean systemic filling pressure (Pmsf). This pressure was described by Bayliss and Starling [55], and they figured out that somewhere in the circulation there must be a point where the pressure is not changing when the heart stops. Actually, during a cardiac arrest, the pressure in the small veins (<1 mm) and venules do not change substantially, they are the “pivoting point” of the system [56]. This pressure is less than the capillary pressure, close to the portal venous pressure and greater than the RAP. Its anatomic location is not necessarily at the same venous branching level in different organs. The importance of this pressure, rather than its anatomical location, is that it provides a quantitative measurement of the intravascular filling status independent from cardiac function: its value is equal to the Pmsf.
Later in 1952, Guyton [5] studied the central venous resistance by observing what was called the “static blood pressure.” This pressure was measured in a preparation of anesthetized dogs after fibrillating the heart and once the arterial and venous pressure achieved equilibrium (30–50 s). In some cases he used a roller propulsion pump to bring blood from the arteries to the veins and to achieve the equilibrium in less than 20 s. A special external venous circuit was used in 37 experiments on open-chest dogs for the study of progressive resistance to the return of blood to the heart. They measured blood flow, arterial pressure, peripheral venous pressure, and RAP. As the blood flow decreased progressively to zero, the arterial pressure fell and the peripheral venous pressure rose slightly to approach arterial pressure. The extrapolated point of approach of these two pressures correlated with static blood pressures measured by heart fibrillation. Guyton described several values for the static blood pressure, and consequently the upper limit of venous pressure, under several conditions:
-
5.96 mm Hg within a few seconds after the hearts of normal dogs were fibrillated and before vasomotor reflexes could develop
-
17.0 mm Hg after developing the most powerful vasomotor constriction that could be attained by a Cushing reflex
-
Unlimited values after giving infusions of fluid immediately before fibrillation of the heart, depending on the amount of fluid and how long before fibrillation it was administered
Later, Guyton [57] introduce the term mean circulatory filling pressure (MCFP) to refer to this static pressure. This term was chosen to make a distinction between the systemic circulation (excluding the pulmonary circulation) and the entire circulatory system, but it is actually the same concept described previously by Starling. Guyton realized that the MCFP is clearly affected by the vasomotor reflexes: The MCFP measured within the first few seconds after the heart stops beating is only about one-half the same pressure measured 30 s or more after the heart stops beating. In normal dogs, the MCFP was about 6.3 mm Hg, while in a dog under total spinal anesthesia the MCFP fell to 5 mm Hg. Increasing the vasomotor tone by giving a continuous infusion of epinephrine from minimal to maximal doses caused a maximal increase in MCFP up to 16 mm Hg. At very high rates of epinephrine infusion the MCFP was still raising slightly while the MAP was not increasing anymore.
Guyton [57] also observed that when massive volumes of fluids are given to a dog, the MCFP rises immediately and then falls along a negative exponential curve to approach almost the baseline value. Changes in hematocrit were also measured and he initially thought that as long as the fluid volume is excessive after the infusion, active leakage of fluid from the circulation occurs but this leakage ceases as soon as the MCFP approaches the baseline values. This was further studied later by Prather et al. [58] in an experiment: blood volume was expanded rapidly in 36 dogs using 500 ml of whole blood, 6% dextran-saline solution, or Tyrode’s solution. The Tyrode’s group returned to normal blood volume within 80 min whereas the blood and dextran groups showed 25% and 70% retention, respectively, 2 h later; both MCFP and CO increased from 2 to 3 times immediately after infusion in all groups and returned to baseline levels within 90–120 min. Indeed, these factors returned to normal even in the dextran and blood groups although the blood volumes were still elevated; since the MCFP returned to normal in 2 h despite continued elevation of blood volume it was concluded that considerable stress-relaxation of the circulation occurred. The increase in intrinsic vascular volume due to stress-relaxation was estimated to be 13% in the blood group and 32% in the dextran group.
Guyton [59, 60] also observed that it is actually the difference in pressure between two points, not any single pressure at any point of the cardiovascular system that determines the rate of flow. Given that most of blood is in the venous territory, the pressure at this point is particularly interesting. Guyton suggested that venous return must be defined by three parameters: MCFP, the right atrial pressure (RAP), and the resistance to venous return (RVR). This can be also mathematically represented as follows:
Guyton [61] proposed this concept after drawing venous return curves in recently dead dogs. He replaced the heart with a pump and controlled the right atrial pressure (RAP) by changing the minute capacity of the pump (adjusting the height of a Starling resistor). He also controlled the MCFP by increasing or decreasing the total quantity of blood. From these curves one can spot that for any given RAP, the greater the MCFP, the greater the venous return is. And importantly, under isovolumetric conditions, the greater is the RAP, the lower is the venous return. As during steady conditions, cardiac output (CO) and venous return are equal, MCFP plays an important role on the regulation of CO.
Guyton concluded that MCFP is the driving pressure for the venous return and the RAP is the backpressure against the MCFP, but this concept has not been exempted from controversy. Brengelmann [62,63,64] pointed out that in Guyton experiments flow was controlled to obtain a desired level of RAP; in other words, the independent variable was blood flow instead of RAP. From his point of view, what venous return curves really show is the steady-state relationship between the blood flow through the systemic vasculature and the RAP. The equation of the venous return proposed by Guyton follow the Poiseuille’s equation structure that relates a pressure gradient with the magnitude of flow through a fixed conduct segment. Logically, pressure gradient and flow are a consequence of pumping, and that is why Brengelmann concludes that the driving force of venous return is the same as the one for cardiac output: the pump. Brengelmann made a fair point by criticizing the role of the Pmsf or RAP as independent variables, which has been also pointed out by other authors [56]. In a closed loop system such as the cardiovascular system, no pressure is really independent of flow, except the PMSF. However, from a physiological perspective it does not make any sense that the heart (the pump) governs the level of blood flow. Quite the opposite, the normal heart finely matches the metabolic demand with the oxygen delivery. That is why in this maybe over-simplified model, the Pmsf, which is complexly regulated by the sympathetic system, governs blood flow.
Measurement of the PMSF in Humans with Intact Circulation
The challenge of measuring the venous tone is that Pmsf is not easy to measure in patients with an intact circulation. Schipke et al. [65] performed a fibrillation-defibrillation sequence in 82 patients to measure the Pmsf over 13 s. A true equilibrium pressure was not achieved, and the arterial-central venous pressure difference was 13.2 ± 6.2 mm Hg.
Pinsky [66] proposed a model in animals with an intact circulation to construct venous return curves observing the relationship between instantaneous changes in right ventricular CO and RAP during intermittent positive pressure recruitment maneuvers and then extrapolating the RAP value to zero CO. Pmsf calculated were similar to Pmsf measured during circulatory arrest. Other studies [67,68,69] have confirmed this linear relationship between VR and CVP and derived Pmsf from the regression equation in animal models with intact circulation. Maas and colleagues [70] applied the same rationale to study the effect of a 12-s inspiratory hold maneuver to three different steady-state levels on central venous pressure (CVP) and blood flow (CO) measured via the pulse contour method during the last 3 s in mechanically ventilated postoperative cardiac patients. This interesting study showed again a linear relationship between CVP and CO, and, importantly, Pmsf could be estimated in intensive care patients with an intact circulation. Obviously this technique is only feasible in fully sedated patients under mechanical ventilation. This method was also used by Keller and colleagues [71] to assess the changes of passive leg raising (PLR) on venous return: They observed nine postoperative cardiac patients at baseline, during PLR and after volume expansion (500 ml of hydroxyethyl starch) . They reported a Pmsf at baseline of 19.7 mm Hg. This only increased to 22 mm Hg after PLR and to 26.9 mm Hg after volume expansion (VE). Although CO increased after PLR and VE, the gradient of pressure of venous return (difference between Pmsf and CVP) increased by 2 mm Hg after PLR and by 5.8 mm Hg after VE. This could explain why a PLR test does not systematically increase CO in fluid responsive patients [72], or even for a fluid challenge, the increase in Pmsf is an essential condition to effectively test the cardiac response.
The main problem of this method is the potential interaction of the hold-inspiratory maneuver with the values of Pmsf. An increase in the intrathoracic pressure increases the RAP and the pressure backward in the venous territory. In a recent study, Berger et al. found that the inspiratory hold maneuver overestimate Pmsf by a mean difference of 3 mmHg [73]. On the other hand, it reduces cardiac output and arterial pressures, which can trigger baroreceptor reflexes from the aortic and carotid territories and generate venoconstriction.
Parkin and Wright [74] described a method for estimating a mean systemic filling pressure analogue (Pmsa) using the mean arterial pressure (MAP), RAP, CO, and anthropometric data. The calculation of Pmsa was fully described in other publications [75]. In essence, they used a mathematical algorithm to build a cardiovascular model using the patient’s data. The clinical validity of this approach was tested in ten patients in acute renal failure receiving continuous vein-venous hemofiltration [76]. Fluid replacement therapy was electro-mechanically controlled to a target value of Pmsa. Despite some limitations of this study, this approach supports the concept of using Pmsa as a quantitative parameter of the intravascular volume status. This method was used to analyze hemodynamic changes after a fluid challenge (250 ml of colloids or crystalloids in 5 min) in patients admitted to intensive care [77]: Pmsa increased similarly in responders and non-responders, as expected but interestingly CVP increased more in non-responders, neutralizing the changes in the gradient of pressure of venous return as described by Guyton.
Recently, Gupta et al. [78] used Pmsa to investigate the performance of cardiac power (defined as the product of arterial pressure and cardiac output) relative to Pmsa (CPvol). CPvol represents a measurement of cardiac performance adjusted to the vascular tone. According to the authors, values below 0.047 of CPvol have a high sensitivity (97%) and not so high specificity (57.5%) to predict fluid responsiveness.
Anderson [79] proposed a noninvasive technique to measure Pmsf by a rapid occlusion of the circulation in the arm (Pmsf-arm). Once the arterial (Pa) and venous pressures (Pv) in the arm equilibrate, the pressure measured would be Pmsf. The precision of this technique has been recently studied [80]. Four repeated measurements were performed in 20 patients after cardiac surgery. Pa and Pv equalized after 60 s of cuff inflation. For a single measurement, the coefficient error (CE) was 5% (± 2%) and the least significant change (LSC) was 14% (±5%). Averaging two measurements, the CE improves to 4% (±1%), and the LSC was reduced to 10% (±4%).
Maas et al. [81] compared these 3 methods in 11 postoperative cardiac surgery patients. Bland-Altman analysis for the difference between Pmsf-arm and Pmsf showed a bias of −1.0 (±3.1) mm Hg (p = 0.06) and a coefficient of variation (CV) of 15%. Although there was a non-significant bias, one may think that this is actually quite significant considering the small sample size of this study. Regarding the difference between Pmsf and Pmsa there was a bias of −6.0 (± 3.1) mm Hg (p < 0.001) and a CV of 17%. The three methods were useful to track changes after volume expansion.
The higher values of Pmsf observed in critically ill humans compared with the values reported from animal studies is still a focus of research. Repessé and colleagues [82] observed the Pmsf in 202 patients who died in the intensive care unit (ICU). The Pmsf was measured 1 min after the cardiac arrest, having disconnected the ventilator and then recorded the equilibrium pressure in the arterial and/or central venous line. This was called “one-minute Pmsf,” which had a mean value of 12.8 ± 5.6 mm Hg. Although the values reported in this study are closer to those previously described in animal models, the methodology proposed raised several questions. The cause of death and the process of dying was not described in this study. A critically ill patient may suffer a sudden cardiac arrest or may suffer a progressive deterioration that may take minutes or hours. By the time the heart stops, central nervous system hypoxia could be stabilized and a denervation process, with its consequences on the vascular tone, might be fully in place. This is crucial because the effect of an intact sympathetic system is essential to determine the real value of the Pmsf, as discussed earlier [9, 83, 84]. Given these limitations, the values reported in this study may not actually represent the real measurement of Pmsf. It should be viewed, instead, as an estimation of the Pmsf in patients with low vascular tone and no sympathetic activity. Therefore, the mean value reported cannot be compared with those reported in previous studies in humans with intact circulation [77, 80, 81].
Should the Venous Tone be Monitored at Bedside? Practical Implications
Despite the importance of venous tone on the maintenance of cardiovascular stability, there is still little evidence about the impact of this information on the management of critically ill patients.
Rangapa et al. [85] investigated the potential of a computerized decision-support system (Navigator™, Applied Physiology, Sidney, Australia) to improve consistency of hemodynamic evaluation and treatment decisions by ICU clinical staff with different levels of expertise and experience in 20 patients admitted after elective cardiac surgery. The study showed that Pmsa was commonly underestimated by all categories of ICU staff, and that this system may improve consistency in decision-making.
Sondergaard et al. [86] carried out a small pilot clinical trial in 27 postoperative patients requiring goal-directed therapy to evaluate the efficiency of the Navigator™ system in achieving hemodynamic targets (measuring the percentage time in target zone and the averaged standardized distance from the centre of the target [87] and time to achieve targets) and the level of concordance between the therapy suggested by the system and an expert clinician. The mean percentage time in the target zone was 36.7% for control and 36.5% for intervention, and the ASD was 1.5 in control and 1.6 in intervention (no p value was reported). There was a high level of concordance between decision support recommendation and anesthetist action (84.3%). The authors concluded that the treatment recommended by the Navigator system mirrored that of a senior anesthetist in the achievement of therapeutic goals. Unfortunately, this study is probably underpowered to show differences in the efficiency measurements, fluid balance, or vasoactive medications. In addition, it is quite interesting that in both cases the percentage of time in the target zone was so low.
In a small study, Yastrebov et al. [88] investigated the relationship between Pmsf-arm and echocardiographic variables of left ventricular filling such as left ventricular end diastolic area, volume and inferior vena cava (IVC) diameter in 13 healthy patients before surgery. Only a weak correlation was found between Pmsf-arm and the IVC diameter.
Some interesting studies demonstrated that some useful information could be obtained by observing the mean systemic filling pressure. The current consensus on circulatory shock and hemodynamic monitoring states that even in the context of fluid responsive patients fluid management should be carefully titrated, especially in the presence of elevated intravascular filling pressures [89]. However, a fluid challenge should increase Pmsf in order to challenge the cardiovascular system. Otherwise the test would not be valid. In a recent clinical trial [90], 80 patients after cardiac surgery were randomized to difference doses of crystalloids (from 1 to 4 mL/kg) infused over 5 min. Pmsf was measured with the arterial-venous occlusion method, and the effective dose was defined as the one that achieve and increase in Pmsf at least by 14% from baseline. In this study, 4 mL/kg was the dose that effectively increases Pmsf, and it was also found a significant difference in the proportion of fluid responders between the dose-groups: from 20% in the group of 1 ml/Kg to 65% in the group of 4 mL/kg.
In addition, a fluid challenge can be used not only to test fluid responsiveness but also, as spotted by Maas and colleagues [91], to assess systemic compliance. Given that Pmsf is the pressure at the pivot point, this may represent an estimation of the venous reservoir compliance. In this study, systemic compliance is reported from 15 postoperative cardiac surgery patients around 64 mL/mm Hg. Systemic venous compliance could be very useful information to prioritize treatment: A high compliance after a fluid challenge may indicate the use of vasopressors instead of infusion of a large amount of fluids. Another study [92] showed that administration of noradrenaline increased CO in preload responsive patients. Noradrenaline increased Pmsf either by reducing venous compliance or by venoconstriction (reduction of venous capacity and shifting unstressed volume to stressed compartment, see Fig. 8.2). Unfortunately, the authors did not assess the effect of noradrenaline on venous compliance. In the rest of the patients, noradrenaline had predominantly an arterial vasoconstrictive effect, increasing cardiac afterload. This study stressed the importance of monitoring venous tone and CO when using vasopressors.
Volume-pressure relationship in the venous compartment. The point 1 represents the total blood volume at the mean systemic filling pressure P1. For this point, the volume at 0 pressure is the unstressed volume (V0) and the difference between the total volume (V1) and V0 is the stress volume (Vs). The continuous black line represents the baseline compliance. The point 2 represents a change in pressure induced by a change in intravascular volume. When a certain amount of blood is removed from the venous system, point 1 can move forward. Point 3: the system now contains less blood (V3) at the same pressure given that some unstressed volume (now V30) was recruited into the stressed volume. However, the system can maintain the pressure-volume relationship (parallel dashed line). This means that the capacity of the system was reduced but not the compliance. When the system suffers an increased compliance, the same total volume is displaced from point 1 to point 4, as it is not able to generate the same amount of pressure (P4). To return to P1, volume must be expanded (V5) unless compliance is corrected
Conclusion
The venous system plays an important role in the hemodynamic stability. Most of blood volume is stored and regulated in the venous territory by sympathetic reflexes that can modify the capacitance of the venous reservoir. The mean systemic filling pressure can be now measured and it is the pressure of the pivot point of the circulation, where the pressure is independent of blood flow. This pressure is the driving pressure of the venous return, and the reflexes that affect the venous reservoir affect Pmsf. Three methods have been described to measure Pmsf at bedside in patients with intact circulation. This variable can be now integrated to evaluate the intravascular filling status, to assess the efficacy of cardiovascular interventions and to explain the pathophysiology of the states of shock at the bedside.
References
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33(2):315–22.
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014;46(5):361–80.
McArdle GT, Price G, Lewis A, Hood JM, McKinley A, Blair PH, et al. Positive fluid balance is associated with complications after elective open infrarenal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2007;34(5):522–7.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74.
Guyton AC, Satterfield JH, Harris JW. Dynamics of central venous resistance with observations on static blood pressure. Am J Phys. 1952;169(3):691–9.
Guyton AC. Textbook of medical physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006.
Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;63(4):1281–342.
Heymans C. Reflexogenic areas of the cardiovascular system. Perspect Biol Med. 1960;3:409–17.
Shoukas AA, Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res. 1973;33(1):22–33.
Hainsworth R, Karim F, Stoker JB. The influence of aortic baroreceptors on venous tone in the perfused hind limb of the dog. J Physiol. 1975;244(2):337–51.
Shigemi K, Brunner MJ, Shoukas AA. Alpha- and beta-adrenergic mechanisms in the control of vascular capacitance by the carotid sinus baroreflex system. Am J Phys. 1994;267(1 Pt 2):H201–10.
Muller-Ruchholtz ER, Losch HM, Grund E, Lochner W. Effect of alpha adrenergic receptor stimulation on integrated systemic venous bed. Pflugers Arch. 1977;370(3):241–6.
Altura BM. Pharmacology of venular smooth muscle: new insights. Microvasc Res. 1978;16(1):91–117.
Muller-Ruchholtz ER, Losch HM, Grund E, Lochner W. Effect of beta adrenergic receptor stimulation on integrated systemic venous bed. Pflugers Arch. 1977;370(3):247–51.
Green JF. Mechanism of action of isoproterenol on venous return. Am J Phys. 1977;232(2):H152–6.
Imai Y, Satoh K, Taira N. Role of the peripheral vasculature in changes in venous return caused by isoproterenol, norepinephrine, and methoxamine in anesthetized dogs. Circ Res. 1978;43(4):553–61.
Rutlen DL, Supple EW, Powell WJ Jr. The role of the liver in the adrenergic regulation of blood flow from the splanchnic to the central circulation. Yale J Biol Med. 1979;52(1):99–106.
Hainsworth R. Vascular capacitance: its control and importance. Rev Physiol Biochem Pharmacol. 1986;105:101–73.
Kahler RL, Goldblatt A, Braunwald E. The effects of acute hypoxia on the systemic venous and arterial systems and on myocardial contractile force. J Clin Invest. 1962;41:1553–63.
Price HL. Effects of carbon dioxide on the cardiovascular system. Anesthesiology. 1960;21:652–63.
Smith EE, Crowell JW. Influence of hypoxia on mean circulatory pressure and cardiac output. Am J Phys. 1967;212(5):1067–9.
Rothe CF, Flanagan AD, Maass-Moreno R. Reflex control of vascular capacitance during hypoxia, hypercapnia, or hypoxic hypercapnia. Can J Physiol Pharmacol. 1990;68(3):384–91.
Rothe CF, Stein PM, MacAnespie CL, Gaddis ML. Vascular capacitance responses to severe systemic hypercapnia and hypoxia in dogs. Am J Phys. 1985;249(6 Pt 2):H1061–9.
Browse NL, Shepherd JT. Response of veins of canine limb to aortic and carotid chemoreceptor stimulation. Am J Phys. 1966;210(6):1435–41.
Calvelo MG, Abboud FM, Ballard DR, Abdel-Sayed W. Reflex vascular responses to stimulation of chemoreceptors with nicotine and cyanide. Activation of adrenergic constriction in muscle and noncholinergic dilatation in dog’s paw. Circ Res. 1970;27(2):259–76.
Eckstein JW, Mark AL, Schmid PG, Iizuka T, Wendling MG. Responses of capacitance vessels to physiologic stimuli. Trans Am Clin Climatol Assoc. 1970;81:57–64.
Mancia G. Influence of carotid baroreceptors on vascular responses to carotid chemoreceptor stimulation in the dog. Circ Res. 1975;36(2):270–6.
Iizuka T, Mark AL, Wendling MG, Schmid PG, Eckstein JW. Differences in responses of saphenous and mesenteric veins to reflex stimuli. Am J Phys. 1970;219(4):1066–70.
Pelletier CL, Shepherd JT. Venous responses to stimulation of carotid chemoreceptors by hypoxia and hypercapnia. Am J Phys. 1972;223(1):97–103.
Ishikawa N, Ichikawa T, Shigei T. Possible embryogenetical differences of the dog venous system in sensitivity to vasoactive substances. Jpn J Pharmacol. 1980;30(6):807–18.
Zimmerman BG, Abboud FM, Eckstein JW. Comparison of the effects of sympathomimetic amines upon venous and total vascular resistance in the foreleg of the dog. J Pharmacol Exp Ther. 1963;139:290–5.
Rowell LB. Human Cardiovascular Control, vol. 25. New York: Oxford University Press; 1993. p. 1432.
Furness JB, Marshall JM. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974;239(1):75–88.
Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982;332:169–86.
Folkow B, Mellander S. Veins and venous tone. Am Heart J. 1964;68:397–408.
Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159.
Ninomiya I, Nisimaru N, Irisawa H. Sympathetic nerve activity to the spleen, kidney, and heart in response to baroceptor input. Am J Phys. 1971;221(5):1346–51.
Ninomiya I, Irisawa A, Nisimaru N. Nonuniformity of sympathetic nerve activity to the skin and kidney. Am J Phys. 1973;224(2):256–64.
Sutter MC. The pharmacology of isolated veins. Br J Pharmacol Chemother. 1965;24:742–51.
Mellander S, Nordenfelt I. Comparative effects of dihydroergotamine and noradrenaline on resistance, exchange and capacitance functions in the peripheral circulation. Clin Sci. 1970;39(2):183–201.
Appleton CP, Lee RW, Martin GV, Olajos M, Goldman S. Alpha 1- and alpha 2-adrenoceptor stimulation: changes in venous capacitance in intact dogs. Am J Phys. 1986;250(6 Pt 2):H1071–8.
Patel P, Bose D, Greenway C. Effects of prazosin and phenoxybenzamine on alpha- and beta-receptor-mediated responses in intestinal resistance and capacitance vessels. J Cardiovasc Pharmacol. 1981;3(5):1050–9.
Ruffolo RR Jr. Distribution and function of peripheral alpha-adrenoceptors in the cardiovascular system. Pharmacol Biochem Behav. 1985;22(5):827–33.
Shi AG, Ahmad S, Kwan CY, Daniel EE. Characterization of alpha-adrenoceptor subtypes by [3H]prazosin and [3H]rauwolscine binding to canine venous smooth muscle membranes. Can J Physiol Pharmacol. 1989;67(9):1067–73.
Hirakawa S, Itoh H, Kotoo Y, Abe C, Endo T, Takada N, et al. The role of alpha and beta adrenergic receptors in constriction and dilation of the systemic capacitance vessels: a study with measurements of the mean circulatory pressure in dogs. Jpn Circ J. 1984;48(7):620–32.
Rothe CF, Flanagan AD, Maass-Moreno R. Role of beta-adrenergic agonists in the control of vascular capacitance. Can J Physiol Pharmacol. 1990;68(5):575–85.
Lee RW, Lancaster LD, Buckley D, Goldman S. Peripheral circulatory control of preload-afterload mismatch with angiotensin in dogs. Am J Phys. 1987;253(1 Pt 2):H126–32.
Pang CC, Tabrizchi R. The effects of noradrenaline, B-HT 920, methoxamine, angiotensin II and vasopressin on mean circulatory filling pressure in conscious rats. Br J Pharmacol. 1986;89(2):389–94.
Martin DS, McNeill JR. Whole body vascular capacitance response to vasopressin is mediated by autonomic function. Am J Phys. 1991;261(2 Pt 2):H493–9.
Chien Y, Pegram BL, Kardon MB, Frohlich ED. ANF does not increase total body venous compliance in conscious rats with myocardial infarction. Am J Phys. 1992;262(2 Pt 2):H432–6.
Ogilvie RI, Zborowska-Sluis D. Effects of nitroglycerin and nitroprusside on vascular capacitance of anesthetized ganglion-blocked dogs. J Cardiovasc Pharmacol. 1991;18(4):574–80.
Ito H, Hirakawa S. Effects of vasodilators on the systemic capacitance vessels, a study with the measurement of the mean circulatory pressure in dogs. Jpn Circ J. 1984;48(4):388–404.
Price HL, Deutsch S, Marshall BE, Stephen GW, Behar MG, Neufeld GR. Hemodynamic and metabolic effects of hemorrhage in man, with particular reference to the splanchnic circulation. Circ Res. 1966;18(5):469–74.
Hainsworth R, Karim F. Responses of abdominal vascular capacitance in the anaesthetized dog to changes in carotid sinus pressure. J Physiol. 1976;262(3):659–77.
Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16(3–4):159–31. 7.
Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol. 1985;74(2):499–509.
Guyton AC, Polizo D, Armstrong GG. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Phys. 1954;179(2):261–7.
Prather JW, Taylor AE, Guyton AC. Effect of blood volume, mean circulatory pressure, and stress relaxation on cardiac output. Am J Phys. 1969;216(3):467–72.
Guyton AC, Lindsey AW, Kaufmann BN, Abernathy JB. Effect of blood transfusion and hemorrhage on cardiac output and on the venous return curve. Am J Phys. 1958;194(2):263–7.
Guyton AC, Lindsey AW, Kaufmann BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Phys. 1955;180(3):463–8.
Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35(1):123–9.
Brengelmann GL. Letter to the editor: comments on value and determinants of the mean systemic filling pressure in critically ill patients. Am J Physiol Heart Circ Physiol. 2015;309(8):H1370–1.
Brengelmann GL. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol. 2006;101(5):1532.
Brengelmann GL. Counterpoint: the classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is not correct. J Appl Physiol. 2006;101(5):1525–6. discussion 6–7
Schipke JD, Heusch G, Sanii AP, Gams E, Winter J. Static filling pressure in patients during induced ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2003;285(6):H2510–5.
Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(3):765–71.
Versprille A, Jansen JR. Mean systemic filling pressure as a characteristic pressure for venous return. Pflugers Arch. 1985;405(3):226–33.
Den Hartog EA, Versprille A, Jansen JR. Systemic filling pressure in intact circulation determined on basis of aortic vs. central venous pressure relationships. Am J Phys. 1994;267(6 Pt 2):H2255–8.
Hiesmayr M, Jansen JR, Versprille A. Effects of endotoxin infusion on mean systemic filling pressure and flow resistance to venous return. Pflugers Arch. 1996;431(5):741–7.
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med. 2009;37(3):912–8.
Keller G, Desebbe O, Benard M, Bouchet JB, Lehot JJ. Bedside assessment of passive leg raising effects on venous return. J Clin Monit Comput. 2011;25(4):257–63.
Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38(9):1824–9.
Berger D, Moller PW, Weber A, Bloch A, Bloechlinger S, Haenggi M, et al. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol. 2016;311(3):H794–806.
Parkin WG, Wright CA. Three dimensional closed loop control of the human circulation. Int J Clin Monit Comput. 1991;8(1):35–42.
Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput. 2008;22(6):391–400.
Parkin G, Wright C, Bellomo R, Boyce N. Use of a mean systemic filling pressure analogue during the closed-loop control of fluid replacement in continuous hemodiafiltration. J Crit Care. 1994;9(2):124–33.
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39(7):1299–305.
Gupta K, Sondergaard S, Parkin G, Leaning M, Aneman A. Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med. 2015;41(2):265–72.
Anderson RM. The gross physiology of the cardiovascular system. 2012th ed. Tucson, AZ: Racquet Press; 1993.
Aya HD, Rhodes A, Fletcher N, Grounds RM, Cecconi M. Transient stop-flow arm arterial-venous equilibrium pressure measurement: determination of precision of the technique. J Clin Monit Comput. 2016;30(1):55–61.
Maas JJ, Pinsky MR, Geerts BF, de Wilde RB, Jansen JR. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med. 2012;38(9):1452–60.
Repesse X, Charron C, Fink J, Beauchet A, Deleu F, Slama M, et al. Value and determinants of the mean systemic filling pressure in critically ill patients. Am J Physiol Heart Circ Physiol. 2015;309(5):H1003–7.
Deschamps A, Magder S. Baroreflex control of regional capacitance and blood flow distribution with or without alpha-adrenergic blockade. Am J Phys. 1992;263(6 Pt 2):H1755–63.
Drees JA, Rothe CF. Reflex venoconstriction and capacity vessel pressure-volume relationships in dogs. Circ Res. 1974;34(3):360–73.
Rangappa R, Sondergaard S, Aneman A. Improved consistency in interpretation and management of cardiovascular variables by intensive care staff using a computerised decision-support system. Crit Care Resusc. 2014;16(1):48–53.
Sondergaard S, Wall P, Cocks K, Parkin WG, Leaning MS. High concordance between expert anaesthetists’ actions and advice of decision support system in achieving oxygen delivery targets in high-risk surgery patients. Br J Anaesth. 2012;108(6):966–72.
Smorenberg A, Lust EJ, Beishuizen A, Meijer JH, Verdaasdonk RM, Groeneveld AB. Systolic time intervals vs invasive predictors of fluid responsiveness after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2013;44(5):891–7.
Yastrebov K, Aneman A, Slama M, Kokhno V, Luchansky V, Orde S, et al. The stop-flow arm equilibrium pressure in preoperative patients: stressed volume and correlations with echocardiography. Acta Anaesthesiol Scand. 2019;63:594–600.
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–815.
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45(2):e161–e8.
Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg. 2012;115(4):880–7.
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–50.
Conflict of Interest
Hollmann D. Aya received financial support for educational programs and for attending symposia from Applied Physiology and LiDCO.
Maurizio Cecconi received honoraria for speaking at symposia, financial support for educational programs, and honoraria for advisory board from Edwards Lifesciences, LiDCO, Deltex, Applied Physiology, Massimo, Bmeye, Cheetah, and Imacor.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aya, H.D., Cecconi, M. (2020). Mean Systemic Filling Pressure Is an Old Concept but a New Tool for Fluid Management. In: Farag, E., Kurz, A., Troianos, C. (eds) Perioperative Fluid Management. Springer, Cham. https://doi.org/10.1007/978-3-030-48374-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-48374-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48373-9
Online ISBN: 978-3-030-48374-6
eBook Packages: MedicineMedicine (R0)