Abstract
The endothelial glycocalyx establishes the osmotic pressure difference of the plasma proteins across the vascular wall and plays a major role to determine the distribution of infused fluids in both normal and clinical settings. Loss of the glycocalyx compromises the retention of infused fluid in the plasma volume. On the basis of results from improved approaches to preserve and image glycocalyx structures, and quantitative evaluations of water and red cell interactions with glycocalyx components, the glycocalyx is now best understood as fibrous networks with varying composition within a three dimensional structure: a quasi-periodic inner matrix associated with the endothelial cell membrane that forms the permeability barrier, and a more porous outer region whose composition varies with distance from the endothelial membrane and which determines red cell hemodynamics. This chapter evaluates the strength and weakness of experimental and theoretical investigations that have led to this understanding and points to the emerging methods to investigate glycocalyx structure using advanced imaging methods. The increasing awareness of the importance of changes in the glycocalyx as an early indicator of microvascular dysfunction in both acute and chronic disease requires methods to evaluate the glycocalyx in the clinical setting. The methods to measure whole body glycocalyx volume from the difference between the distribution volumes of red cells and macromolecular tracers overestimate glycocalyx volume and are likely of limited usefulness. The common concept that the changes in the thickness of the glycocalyx layers extending more than 0.5 μm from the endothelial surface can be used as reliable biomarkers of the function of the glycocalyx as a permeability barrier must also be carefully evaluated. While direct visualization of changes in the penetration of red cells into the cell-free layer at the walls of sublingual microvessels appears to characterize some glycocalyx dysfunction, the approach ignores differences in porosity between inner and outer layers of the glycocalyx, and the role of changes in red cell mechanics, independent of the glycocalyx, to influence penetration into the cell free layer. By identifying these limitations, the chapter should provide a basis to re-evaluate ideas about the distribution of infused fluids within and across the glycocalyx during perioperative fluid therapy, encourage further improvements of these and similar methods, and enable comparisons with analytical approaches to measure the accumulation of specific glycocalyx components in plasma and urine as biomarkers of glycocalyx function. On the basis of the principles outlined in this Chapter, the final summary addresses some of the frequently asked questions about glycocalyx function and fluid balance that are likely to arise during perioperative fluid therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glycocalyx
- Glycocalyx structure-function
- Glycocalyx volume
- 3D glycocalyx reconstruction
- Side stream dark field imaging
- Revised Starling principle
- Glycocalyx composition
- Electron microscopy of glycocalyx
-
1.
The glycocalyx establishes the osmotic pressure difference of the plasma proteins across the vascular wall and plays a major role to determine the distribution of infused fluids in both normal and clinical settings. One of the most important modern concepts in perioperative fluid therapy is that loss of glycocalyx components is an early step in microvascular dysfunction, leading to disturbances of plasma volume and transvascular fluid distribution.
-
2.
The glycocalyx is extremely difficult to preserve and visualize in its normal state. The glycocalyx is best understood as a fibrous network with varying composition within a three-dimensional structure. A quasi-periodic inner matrix associated with the endothelial cell membrane forms the permeability barrier and a more porous outer layer determines red cell hemodynamics. The common concept that the changes in the thickness of the glycocalyx layers extending more than 0.5 microns from the endothelial surface can be used as biomarkers of glycocalyx function in model systems and patients must be carefully evaluated.
-
3.
Preservation of the glycocalyx requires suppression of matrix metalloproteinase activity and free radical generation and avoidance of conditions of both hypovolemia and hypervolemia. The potent glycolipid antiinflammatory agent sphingosine-1-phosphate (S1P) plays a critical role to maintain glycocalyx and endothelial barrier integrity. S1P is carried in the circulation by albumin and the Apolipoprotein-M fraction of high density lipoprotein. Conditions that limit S1P availability (low Apo-M levels and decreased synthesis of S1P) likely contribute to loss of endothelial glycocalyx.
-
4.
The most direct evidence of damage to the glycocalyx comes from increased concentrations of glycocalyx components in the circulation or urine. New analytical methods based on mass spectroscopy may measure organ specific changes in glycocalyx injury. Measurements of a glycocalyx volume as a difference between the distribution volumes of red cells and macromolecular tracers such as dextran and albumin always overestimate the volume of plasma within the glycocalyx and are not a reliable index of the way the glycocalyx modifies intravascular fluid distribution.
-
5.
Direct visualization of changes in the penetration of red cells into the cell free layer at the walls of sublingual microvessels of patients using side stream dark field imaging is currently being actively evaluated as a biomarker of changes in the glycocalyx. While there is increased penetration of red cells towards the vessel wall in microvessels vessels up to 50 μm in diameter in some disease states, the claim that such changes are a reliable biomarker of the function of the glycocalyx requires much more careful evaluation, particularly with regard to changes in the glycocalyx that are important for peri-operative fluid therapy.
Introduction
Glycocalyx is a term that describes the sugar based extracellular matrix present on all cells. The endothelial glycocalyx is a specialized version and forms the first contact surface between blood and tissue. It is involved in physiological responses that determine tissue homeostasis including fluid, nutrient, and large molecule transport between blood and tissue. Preclinical investigations have demonstrated that the glycocalyx forms part of the barrier that regulates water and large molecules movement through vascular endothelium, senses the magnitude of local blood flow and regulates local nitric oxide production. The glycocalyx also senses the direction of local blood flow and modulates endothelial remodeling, and forms the layer over which red cells transit through microvessels. By limiting access of leukocytes and other vascular cells, including platelets, to the endothelial surface, the glycocalyx also plays a key role in inflammation and the coagulation system [1,2,3,4,5,6,7,8]. These homeostatic functions are compromised when all or part of the glycocalyx is lost or damaged [9,10,11,12]. Mounting evidence also indicates that a clear understanding of the functions of the glycocalyx has the potential for improved clinical outcomes in both acute and chronic disease states including interventions involving perioperative fluid management [13,14,15]. The glycocalyx is the primary determinant of fluid flows and plasma protein concentration differences between circulating blood and the body tissue, and the consequences of this for fluid therapies is described in accompanying Chap. 2 on the Revised Starling Principle.
In this Chapter the focus is on the evolving understanding of the glycocalyx as a 3-dimensional layered structure close to the endothelial surface. Figure 3.1 shows a state-of-the art view of the glycocalyx on a glomerular capillary built up from images obtained by the process of focused ion beam scanning electron microscopy in which layers of fixed tissue 10 nm thick are successively removed [16]. The image is from the most recent of a series of investigations reported by the authors of this chapter that have provided new understanding of structure-function relations in the glycocalyx in both fenestrated and non-fenestrated microvessels fixed under a variety of conditions and using both conventional as well as newer ways to stain glycocalyx components ([16, 17] and see Sections “Imaging the Glycocalyx and Structure-Function Relationships” and “Background: Imaging the Glycocalyx: More Detailed Technical Issues” below). An evaluation of such a 3D structure is important because: (1) the regulation of the different physiological functions of the glycocalyx described above (e.g. permeability barrier versus lubrication layer for red cell movement through microvessels) depends of different properties of its substructure; (2) measurements of biomarkers for glycocalyx function in clinical settings (distribution space for red cells in microvessels or the volume of the glycocalyx) can result in misleading estimates of changes in the glycocalyx structure when its 3D organization is not taken into account; (3) understanding the mechanisms that degrade the glycocalyx or that contribute to its protection and stability require knowledge of the heterogeneity of the glycocalyx and its internal organization; and (4) new approaches to the investigation of the glycocalyx at a molecular level require better understanding of the limitations of current approaches which often assume that the glycocalyx is a relatively uniform structure.
The endothelial glycocalyx of a renal glomerular filtration capillary. The original data (from [16] with permission) was: (a) 3D series built from a scanning electron microscope sequence with 10 nm of material milled away between images by a focused ion beam. The front edge of glycocalyx is 4 μm wide. (b) 3D reconstruction of a transmission electron tomogram into a 1.2 μm by 0.73 μm by 0.16 μm cuboid. The glycocalyx was stained with the LaDy GAGa technique. The figure with parts of the glycocalyx (yellow), endothelium (blue) and podocyte foot processes (red) colored for emphasis is adapted from [16]. Details of the glycocalyx staining and 3D scanning electron microscopy of the glycocalyx are in Sect. 10
We discuss these topics below, beginning with a brief overview of glycocalyx composition in relation to 3D structure, and how current knowledge provides some insight into ways to protect the glycocalyx. In the second part of the review we evaluate approaches to measurement of changes in the glycocalyx both in clinical settings and in basic research. Because of the layered structure we generally use the term glycocalyx to describe the molecular components that form a physical structure directly and indirectly attached to the endothelial cell surface. The term endothelial surface layer (ESL) is also often used as a less specific term to describe the region next to the blood vessel wall, and is generally assumed to also refer to the whole glycocalyx but it is likely that the thickness of the ESL is modulated by mechanisms in addition to the glycocalyx, including the micromechanics of red cell movement through blood vessels.
Composition in Relation to a Layered Structure
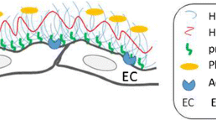
Overall the glycocalyx is a complex fibrous network of molecules that extends from the endothelial cell membrane for distances that range from about 0.5 to possibly several microns. The mechanisms determining the overall organization of the glycocalyx are still poorly understood but include the anchoring of core proteins to the endothelial cell membrane and its underlying cytoskeleton, charge interactions between the side chains of these core proteins, which carry a net negative charge, and other electrostatic and weak chemical interactions with plasma constituents including hyaluronan, plasma proteins, and small electrolytes. The structure is maintained by a dynamic balance between mechanisms to degrade the layer and mechanisms for its restoration by synthesis of components. This is suggested by the top panel of Fig. 3.2. There are several detailed reviews of glycocalyx structure [2, 4, 5, 8]. Here we briefly review the properties of known components that can be used to place constraints of glycocalyx structure. As illustrated in the lower panel of Fig. 3.2, these include:
-
(a)
The syndecan family of core proteins: Endothelial cells (ECs) express several forms of this family which have glycosaminoglycan (GAG) attachment sites close to their N-terminus substituted by heparan sulfate (HS) [19]. Syndecan-1 contains two additional sites closer to the membrane for chondroitin sulfate (CS) [20]. Syndecans have many biochemical roles and often dimerize with cytoplasmic tails associated with the cytoskeleton. These attachments are assumed to play a role in the organization of the glycocalyx and contribute to signal transduction mechanisms [21,22,23,24,25]. Because the molecular weights of GAGs and core proteins suggest molecular lengths from the endothelial surface of the order of 100 nm, i.e. ~0.4 nm per disaccharide residue and >200 residues, it is likely that syndecans form part of the inner layers of the glycocalyx [26].
-
(b)
The glypicans: Glypican-1(64 kDa), a member of the Glypican family of core proteins, is expressed on ECs. Glypican-1 is bound directly to the plasma membrane through a C-terminal glycosylphosphatidylinositol (GPI) anchor [27]. The GPI anchor localizes this proteoglycan to specialized membrane microdomains, often termed lipid rafts, that include the endothelial surface caveolae. The GAG attachment sites in Glypican-1 are exclusively substituted with heparan sulfate.
-
(c)
Hyaluronan: In contrast to these core proteins, hyaluronan or hyaluronic acid (HA) is a much longer disaccharide polymer, of the order of 1000–10,000 kDa (lengths can be of the order of several microns), synthesized on the cell surface and not covalently attached to a core protein [23]. HA associates with the glycocalyx through its interaction with surface receptors, such as the transmembrane glycoprotein CD44, and CS chains [28,29,30]. Because of its large molecular dimension and cable forming property, HA side chains can extend well beyond the core proteins and thereby form part of the scaffold for the glycocalyx [31, 32]. HA is not sulfated but obtains its negative fixed charge density from carboxyl groups that endow it with exceptional hydration properties.
-
(d)
Plasma Components: The interaction of many plasma proteins with the endothelial surface is regulated by charge and chemical binding with the side chains of the glycosaminoglycans (see for example [33]). Of particular importance to the organization of the glycocalyx is albumin, which not only binds to the glycocalyx via positively charged arginine and lysine groups to contribute to stability and organization, but also is part of a signaling cascade that regulates matrix metalloproteinase release to degrade the glycocalyx (see details in Section “Restoration and Preservation of the Glycocalyx”).
-
(e)
Loss of Glycocalyx components: One of the most important modern concepts in vascular physiology is that loss of glycocalyx components is an early step in vascular dysfunction [6, 9, 34]. Similarly, loss of glycocalyx components indicates that the integrity of the endothelial barrier as an osmotic and permeability barrier has been compromised. The most direct evidence of damage to the glycocalyx comes from increased concentrations of glycocalyx components in the circulation or urine, above that due to the normal breakdown and continual re-constitution of the glycocalyx at the vascular interface. Examples of conditions that have been associated with glycocalyx damage include hypovolemia, leading to poor tissue perfusion and subsequent ischemia/reperfusion-injury [35], diabetes [36], sepsis [37,38,39], and exposure to a range of inflammatory agents including tumor necrosis factor-(α), cytokines, proteases, heparinase, and viruses such as the Dengue virus [40, 41]. Most current analytical techniques to measure circulating glycocalyx components rely mainly on ELISA based assays, which, though of varying specificity, do provide clear evidence of loss of glycocalyx components after injury. Examples include increased syndecan-1 (42-fold from a baseline of 12 ng/ml) and heparan sulphate (ten-fold from a baseline of 5 mg/ml) in patients undergoing major vascular surgery [42] and increased syndecan-1 (27.1–110 ng/ml) and HA (16.8–35.0 ng/ml) in dialysis patients [43]. Similarly increased levels of plasma HA in type 1 diabetes are associated with increased circulating level of hyaluronidase (170–236 U/ml). All these methods require extensive and relatively slow analytic procedures. This limitation is a major driver of attempts to develop more direct approaches to evaluate the glycocalyx as will be discuss later. Nevertheless it is likely that detailed analyses of the chemical composition of glycosaminoglycan products and core proteins in circulating plasma using sophisticated mass spectroscopy and other spectrographic methods will become an important part of future analyses. This is especially the case if the origin of fragments from different vascular locations can be identified. For example, using these more sophisticated methods a 23 fold increases in the amount of HS fragments after lung injury has been reported [44].
One of the earliest attempts to illustrate the endothelial glycocalyx as a complex three-dimensional structure is shown in the top panel. The hypothetical model emphasizes the presence of an inner region consisting of glycoproteins and proteoglycans associated with the endothelial cell membrane and an outer layer with structure and composition varying with distance from the endothelial surface and in the plane of the endothelial surface. Hyalunonan forms part of the scaffold for the outer layer, which also includes adsorbed plasma proteins and solubilized glycosaminoglycans. A more detailed recent illustration of some of the inner structure components is shown in the lower panel. The physical and chemical properties of components of the inner layer have guided recent attempts to quantify the functions of glycocalyx as a permeability barrier and to form part of the lubrication layer for red cells. The section to the right of the red dashed line shows the consequence of glycocalyx degradation resulting in exposure of endothelial cell surface receptors to circulating plasma components. Top panel from [3] with permission. Lower panel modified from [18] with permission
Restoration and Preservation of the Glycocalyx
The glycocalyx is a dynamic structure whose structure and function is determined by the balance between synthesis and degradation of glycocalyx components [9, 11, 45]. In both animal models and clinical studies, direct restoration of glycocalyx components to circulating plasma (e.g. HA and CS [29]) or the infusion of glycosaminoglycan precursors, are reported to restore some function [46]. However the mechanisms to stimulate synthesis and reassembly of the glycocalyx remain to be investigated in much more detail.
Useful insight into the balance between stabilization of the glycocalyx and its degradation came from the observation that albumin, previously understood as an essential structural component of the glycocalyx, was part of a homeostatic mechanism that regulates glycocalyx degradation. Specifically, Zeng and colleagues [47] demonstrated that heparan sulphate, chondroitin sulphate, and the ectodomain of syndecan-1 were shed from the endothelial cell surface after removal of plasma proteins, but were retained in the presence of the potent glycolipid antiinflammatory agent sphingosine-1-phosphate (S1P) at concentrations greater than 100 nM. S1P1 receptor antagonism abolished this protection of the glycocalyx by S1P and plasma proteins. The action of S1P to preserve glycocalyx components was shown to involve suppression of matrix metalloproteinase (MMP) activity by S1P. Specific inhibition of MMP-9 and MMP-13 also protected against glycocalyx loss [47]. These results are consistent with observation in other animal experiments that activated MMPs lead to loss of glycocalyx and increased leukocyte attachment. Further, agents such as doxycycline that protect the glycocalyx act, at least in part, by inhibiting MMPs [48].
Another key observation was that, after red cells were removed from the perfusate, albumin alone was not sufficient to maintain the normal permeability of rat mesenteric microvessels. However when both albumin and red cells were present, or when albumin solutions were pre-conditioned by exposure to red cells, permeability was maintained and S1P concentrations in the perfusate exceeded 100 nM [49]. These observations established that albumin not only binds S1P but also facilitates the release of S1P from red cells and carries it to the endothelium. Normally albumin carries 40% of the circulating S1P [50].
The actions of S1P and abumin-bound-S1P to regulate glycocalyx degradation are now recognized as part of a wider range of actions of S1P to stabilize the endothelial barrier. Previous investigations demonstrated that S1P and S1P analogs maintained the barrier by stabilizing adhesion complexes between adjacent endothelial cells, focal adhesion to extracellular matrix, and the actin cytoskeleton [45]. Equally important is the growing understanding of the delivery of S1P to the endothelial surface receptors by Apolipoprotein-M, a component of high-density lipoproteins (HDLs), which binds S1P and carries up to 60% of the circulating S1P [50, 51]. The Apo-M fraction of HDLs likely contributes to the vasoprotective actions of HDLs. Both albumin bound S1P and Apo-M bound S1P modulate the stability of intercellular junction complexes, but investigations by Hla and coworkers demonstrate that there is preferential activation of S1P receptors and signaling pathways in endothelial cells depending on the carrier [50, 52]. For Apo-M this may involve co-operative interaction of S1P and HDL binding sites on the endothelium and glycocalyx. In in vitro investigations, Apo-M delivery is more effective than albumin delivery to modify junctional complexes but the actions of Apo-M dependent delivery to stabilize the glycocalyx and to modulate release of S1P from circulating sources have not been investigated. A recent report also describes a separate pathway for S1P to protect the glycocalyx and junction complexes via mechanisms to inhibit mitochondrial dysfunction [53].
Some of the SIP-dependent mechanisms regulating the glycocalyx are illustrated in Fig. 3.3. The clinical significance of these new areas of investigation is that S1P delivery may be limited in a number of disease states. For example, reduced plasma Apo-M levels have been associated with acute myocardial infarction, endotoxemia, diabetes, and metabolic syndrome [52]. It is possible that the capacity to carry S1P may also be limited by oxidation of HDL or albumin glycosylation. Further, although it is known that activated platelets secrete S1P, the primary source of S1P in normal plasma is red cells that synthesize and store high levels of SIP [54]. There may be reduced synthesis of S1P in diseased or infected red cells. Also the increased mortality and morbidity described in patients transfused with red cells older than about I4 days may be explained, in part, by reduced S1P synthesis and corresponding loss of glycocalyx protection [54].
The roles of sphingosine-1-phosphate (S1P) and albumin to stabilize the glycocalyx. S1P is stored in circulating red cells, released into the circulation bound to albumin and Apolipoprotein-M in HDLs. Ligation to the S1P1 receptor on endothelium activates signaling pathways that regulate the stability of the inter-endothelial cell junctions, inhibits MMP activation, and abolishes MMP dependent syndecan-1 ectodomain shedding. Conditions that reduce S1P availability (e.g. low plasma protein) have been demonstrated to attenuate the inhibition of MMP9 and MMP13 and result in loss of the glycocalyx. S1P also stabilizes the inter-endothelial junctions. Adapted from [45] with permission
Other strategies to protect the glycocalyx involving inhibition of proteases and hyaluronidase are being explored, but the regulation of these processes remains an area for investigation [9]. Perhaps the most direct strategy to preserve the glycocalyx is avoidance of conditions likely to damage the glycocalyx including hypovolemia and associated reperfusion injury. Thus fluid therapy that aims to maintain tissue perfusion is likely to be protective of the glycocalyx. On the other hand there is evidence that excessive fluid infusion leading to hypervolemia results in damage to the glycocalyx due to the release of atrial natriuretic peptide [55] although the mechanism of action is not clear because ANP can also have vasoprotective actions [56,57,58].
As these and other approaches are evaluated in more detail in different clinical settings there is a need for new strategies to assess the integrity of the glycocalyx. In addition to the development of better assays for glycocalyx components in the plasma as suggested above, more direct methods are currently being actively promoted. One of the approaches involves attempts to measure the volume of the glycocalyx by comparing the volume available to tracers assumed to penetrate the glycocalyx from the volume of circulating plasma. The second approach uses direct visualization of the small vessels in the sublingual microcirculation to measure changes in the penetration of red cells into the endothelial surface layer as a marker of glycocalyx loss. To enable evaluation of these new approaches it is useful to review current understanding of the 3D structure of the glycocalyx, based on investigations using electronmicroscopic methods, a range of optical techniques, and 3D image reconstruction approaches.
Imaging the Glycocalyx and Structure-Function Relationships
Of the vast number of imaging techniques available, the most useful for imaging the glycocalyx are optical microscopy (OP) with its dynamic and fluorescent capabilities, and electron microscopy (EM) with its molecular resolution and newer 3D structural capabilities. Other imaging approaches such as atomic force microscopy and magnetic resonance imaging have been less useful. Both OP and EM approaches have significant limitations. The dimensions of the glycocalyx are on the limit of the resolution of optical microscopy that has a theoretical value close to 200 nm. This level of resolution is not reached when the glycocalyx is examined in wet biological samples. Similarly, the theoretical resolution of electron microscopy (<0.01 nm) is not reached in biological samples prepared by fixation and imbedding in plastic resins. Furthermore, sample preparation for electron microscopic analysis can result in loss of key components depending on the fixative and subsequent processing. What is actually visualized after such additional sample processing depends on the degree to which key components are retained and the chemical interaction of specific stains with these components. Further details of imaging methods are in Section “Background: Imaging the Glycocalyx: More Detailed Technical Issues”, and we have included some Top Tips boxes to help follow the interpretation of the evidence discussed.



When our understanding of the permeability properties of the vascular wall was first put on a sound quantitative basis using the pore theory of capillary permeability [59, 60] it was suggested that the physical structures that were described in terms of water and solute exchange though “pores” were actually the interstices in the intercellular cement. At the level of light microscopy the “cement-like” substance appeared to be associated with the endothelial cell surface and parts of the junctions between endothelial cells. Early electron microscopists rejected the idea of this intercellular cement because none was found in the early investigations of junctions of microvessels in tissue fixed for electron microscopy. Instead they focused attention on barriers within the junctions and on specialized transport pathways associated with the endothelial caveolae. It required the systemic studies of investigators like John H Luft to demonstrate that chemical reactions between fixatives such as glutaraldehyde, contrast agents such as osmium tetroxide, and complex compounds such as ruthenium red could be used to demonstrate a dense endocapillary layer that extended several 10s of nanometers into the vessel lumen and then faded in a fluffy indeterminate boundary [61]. This endocapillary layer was seen adhering to the outer leaflets of the luminal endothelial cell membranes and also extended into the luminal aspect of some junctions and openings of luminal vesicles. Botanists had previously used compounds such a ruthenium red to stain the acid mucopolysaccharides on the surface of plant cells so it was concluded that similar compounds as well as other plasma components formed the endocapillary layer.
As indicated above these early observations of a surface glycocalyx did not significantly modify the idea, current in the late 1960s, and well into the 1980s, that the size limiting structures in the endothelial barrier, corresponding to the small pores of classical pore theory, resided within the narrow constrictions of the junctions between adjacent endothelial cells. However, by the mid-1980s two independent lines of investigation refocused attention of the glycocalyx at the microvessel level (some of the extensive background to these studies is reviewed in the first Handbook of Microcirculation, published in 1984 [62,63,64,65]). One was the formal development of a quantitative framework to evaluate the permeability properties of a fiber matrix on the endothelial surface. Curry and Michel [66] demonstrated that a matrix with fiber dimensions similar to the side chains of glycosaminoglycans would offer little resistance to low molecular weight nutrient solutes as they crossed via the interendothelial junctions, but would form the primary barrier to plasma protein at the endothelial cell surface. Equally important were renewed experiments to understand the mechanisms whereby vascular permeability to water and macromolecular solutes was increased when plasma proteins such as albumin were removed from perfusates. In the absence of albumin or plasma, the permeability of the endothelial barrier to water and large molecules was increased without significant change in the structure in the junctions. When the cationic arginine groups on albumin were chemically shielded, albumin no longer maintained normal low permeability consistent with the theory that electrostatic interactions of albumin with the negative charged glycosaminoglycan side chains contributed to glycocalyx stability and organization (See [67]). An additional “proof of concept” result was that cationic ferritin, a large protein that could be visualized in EM sections when bound to the endothelial surface as the result of its large positive charge, formed a layer on the endothelial surface similar in thickness to the observed glycocalyx structure, and restored permeability after albumin was removed.
The second line of investigations focused on the mechanism determining red cell and plasma flows within microvessels, and the suggestion that a red cell free plasma layer close to 0.5 μm thickness must be present near the walls of cremaster muscle microvessels to account for the measured microvessel hematocrit [68, 69].
A series of key observations that still form the basis for many current investigations and clinical approaches to investigate the glycocalyx were made by Brian Duling and his colleagues in small blood perfused vessels in situ [70]. As shown in Fig. 3.4 they demonstrated that large Dextran molecules (MW greater than 70 kDa) were excluded from a plasma layer of thickness (0.3–0.5 μm) close to the microvessel walls. The dimension of this plasma layer was similar to the layer from which red cells were excluded. Smaller neutral and cationic test probes slowly penetrated the barrier. Injury to the glycocalyx (e.g. by exposure to additional epi-illumination) decreased the region from which dextran and red cells were excluded. The idea that an extended glycocalyx structure might determine both the red cell exclusion gap by forming part of a lubrication layer and also a porous barrier that excluded circulating macromolecules was established after it was demonstrated that enzymes that degraded HS increased the penetration of both red cells and large dextrans (70 kDa MW) into the endothelial surface layer [29, 68, 70]. It was soon common to assume that an endothelial surface layer measured using these light microscopy approaches (up to 0.5 μm thick in capillaries) measured the full extent of the membrane-attached glycocalyx, and to dismiss the less extensive glycocalyx structures observed in electron microscopy as the collapsed forms of this more extended glycocalyx.
The endothelial glycocalyx excludes large macromolecules such as 70 kDa dextran and circulating red cells. Parts A and B show digitized images of a capillary segment in a normal vessel. The width of both the blood cell column (A) and the column of high molecular weight FITC-dextran (70 kDa) (B) were significantly smaller than the anatomic capillary diameter. Damage to the glycocalyx (exposure of the capillary to epi-illumination with generation of free radicals) increased the width of RBC (C) and the FITC-dextran columns (D), without a significant effect on the anatomic capillary diameter. Scale bar represents 5 μm. Reproduced with permission [70]
These observations also led to the idea that movement of test molecules from circulating plasma into the glycocalyx was a restricted diffusion process largely dependent of their size and charge. This ignores the fact that the time constants for penetration of albumin and dextrans (>40 kDa, <70 kDa) from the plasma into the endothelial surface layer were of the order of tens of minutes, far longer than expected due only to electrostatic exclusion and restricted diffusion. For example if the restricted diffusion coefficient of albumin was only 1% of its free value, the half time for penetration into a 1 μm thick layer would be about 1 s, two orders of magnitude faster than observed. Further, a large molecule such as 70 kDa dextran, which did not normally penetrate the layer, when linked to albumin entered the layer despite the increased molecular size of the complex. Together with more recent evidence that different enzyme treatment results in differing degrees of penetration of tracer molecules into the glycocalyx [71], these observations indicate that complex diffusion, binding and chemical interactions compromise simple interpretations of tracer penetration and distribution in the glycocalyx (see Section “Glycocalyx Volume Measurement in Human Subjects” on the measurement of glycocalyx volume).
In summary, by the end of the 1990s there was a clear understanding that the glycocalyx was a key regulator of microvascular function because it played a major role to regulate plasma and red cell flows through microvessels and to form the main molecular sieve determining the exchanges on plasma constitutes and particularly the plasma proteins between circulating blood and the body tissues. At the same time it was apparent that the advances that had enabled visualization of this endocapillary layer provided only a low-resolution image of the structure of the glycocalyx and pointed to the difficulties that investigators still face today to quantify changes in the glycocalyx structure.
The Glycocalyx as a 3 Dimensional Layered Structure in Microvessels
As explained in more detail in Section “Background: Imaging the Glycocalyx: More Detailed Technical Issues” and in the adjacent side bars there have been important technical developments that not only improve the preservation of the glycocalyx, but enable more detailed investigations of the glycocalyx as a 3D quasi-periodic structure within a region 200–400 nm of the endothelial surface. Figure 3.5 based on samples from Rostgaard and Qvortrop [72] demonstrates bush-like structures in the glycocalyx that project 100 nm from the fenestrated endothelium of rat small interstine and post-glomerular microvessels. These structures were preserved using a novel perfluorocarbon perfusion technique. Figure 3.6 summarises results from the first application of computer autocorrelation functions and Fourier transforms of EM images from frog mesentery capillaries fixed by both conventional fixation and rapidly frozen, deep-etched preparations of glycocalyx. The analyses demonstrated a network of fibrous molecules with a characteristic interfiber spacing of 20 nm and fiber diameter of 12 nm. Additional investigations on these preparations showed that the regularly distributed fibers occurred in clusters with a common intercluster spacing of ~100 nm. The result that similar measurements of fiber size, spacing, and arrangement were made in preparations made without exposing the tissues to chemical fixatives as well as those prepared using conventional fixation and staining techniques indicated that there was a common quasi-periodic structure to the inner glycocalyx. Using the regular bush-like structure observed in the original Rostgaard and Qvortrup study [72] as a guide, Squire and colleagues [17] proposed a structural model of the glycocalyx in which the available space for macromolecule movement between the fibers in a cluster was close to 8 nm. This dimension was determined within bush-like bundles by fibers (12 nm in diameter) arranged at 20 nm mean interfiber spacing. They also suggested that the 100 nm intercluster spacing reflected attachments by transmembrane proteins to a quasi-regular submembrane actin network. These investigations gave, for the first time, evidence of a more organized structure of the glycocalyx within about 200 nm of the endothelial surface with anchoring foci that appear to emanate from the underlying actin cortical cytoskeleton, a concept supported by biochemical evidence [73,74,75] . A sketch of this ultrastructural model is shown in the left panels of Fig. 3.7.
Examples of bush-like structures in the glycocalyx first described in electron micrographs of fenestrated capillaries from the lamina propria of rat small intestine (a and b) and glomerulus (c). The tissue was processed using perfusion fixation with a perflurocarbon perfusate, glutaraldehyde as fixative, and tannic acid for staining. Part A shows the bushes projecting into the capillary lumen about 150 nm. Part B is a tangential section showing filamentous molecules (5–10 nm thick) forming the bushes with 20–40 molecules per bush. Part C is similar to A from rat glomerulus. Scale bar in A and C is 0.5 μm, in B is 0.1 μm. These bush like structures observed in TEM have guided the interpretation of autocorrelation analyses of images of the glycocalyx to reveal the quasi-periodicity of molecular structures of the inner glycocalyx. Reproduced with permission from [72]
The top panel shows regions of a normal capillary from frog mesentery prepared using conventional fixations. Individual microvessels were perfused with Ringers solution containing 4% bovine serum albumin and ruthenium red prior to fixation with 1% osmium tetroxide (a). The boxed region is shown enlarged in b and the results of auto-correlation analyses in c. The analysis indicates periodicities in directions parallel to the luminal endothelial surface and normal to the surface. The lower panel shows images from a frog mesentery microvessel rapidly frozen, and freeze fractured to show the inner region of the glycocalyx. The autocorrelation analysis of the boxed region demonstrates hexagonal arrangement of the spacing of larger structures 80–120 nm apart (see lines and arrows). Smaller periodicities of around 20 nm are seen within these major repeats. The similarity of autocorrelation functions from tissues after different fixation and staining protocols, and from both frog and mammalian vessels suggest a common quasi-periodic structure of the inner glycocalyx. Scale bars 200 nm in a, 60 nm in b and 110 nm in c. Scale bars are 200 nm in d–f. Modified with permission from [17]
Model molecular configuration of the inner glycocalyx is shown in the sketches on the left showing fibrous strands projecting from the endothelial surface with some periodicity along the strands. Adjacent tufts are separated by 80–100 nm in a hexagonal array. A more formalized schematic is shown on the right with the geometric arrangements assumed for detailed quantitative analysis of transendothelial water and solute exchange through the glycocalyx and intercellular junctions, and red cell movement over a lubricating layer formed by the glycocalyx. Adapted from [8, 17]
A similar approach performed on archival tissue samples from Rostgaard and Qvortrup’s laboratories [76] also found an interfiber spacing of 19.5 nm to be common within fiber clusters in many different tissues from rats and rabbits. Taken together with the results in frog vessels, these analyses based on autocorrelation methods suggested that an interfiber spacing close to 20 nm was likely to be a common characteristic of regions of the glycocalyx that were organized into quasi-periodic arrays. However though spacings over 80 nm were observed the 100 nm previously observed was not one of them, perhaps due to amalgamating the fenestrated and unfenestrated tissues into one result. Interpretation of the longer distances remains inconclusive, and to definitely demonstrate it solely by this method, without additional specific labeling, may not be possible. Nevertheless in the sketch in Fig. 3.7 it is assumed to be characteristic of the inner regions of the glycocalyx in both non-fenestrated and fenestrated microvessels and measurements and conclusions based on these studies are quoted in several review papers and used in mathematical models [1, 5, 8]. As indicated above, the evidence is still quite limited and there are technical limitations to the interpretation of autocorrelation analyses based on projections through thick specimens. Furthermore, a more useful “en face” view of the glycocalyx cannot be obtained from these approaches which give mainly a sideview of the glycocalyx (See Section “Background: Imaging the Glycocalyx: More Detailed Technical Issues”).
As a step towards better 3D imaging of the glycocalyx a recent study used electron tomographic methods to examine the glycocalyx [77]. Here images from thicker 300 nm plastic sections were taken at many different angles enabling reconstructing into a 3D dataset. The resulting reconstructions not only allow comparison with the autocorrelation approach described above, but also allow the image to be rotated to enable en face observation of the lateral spacing of the anchoring foci on the endothelial surface in mammalian microvessels (both continuous and fenestrated). The method can therefore offer further evaluation of the periodic structure of 10–12 nm diameter fibers and any characteristic longer spacing of around 100 nm. As new analyses become available there is a growing consensus that a quasi-periodic structure that projects at least 50 nm and up to 400 nm from the endothelial membrane is present and can, to a first approximation, be characterized by the idealized mathematical model with hexagonal symmetry shown on the right of Fig. 3.7. For further details of the strengths and limitations of these methods see Section “Background: Imaging the Glycocalyx: More Detailed Technical Issues”.
Quantitative Investigations of Glycocalyx Structure-Function
A key question is to understand how there can be, on one hand, an endothelial surface layer equal to or greater than 0.5 μm in thickness from which red cells are excluded, yet on the other hand ultrastructural analyses describe structures closer to the endothelial surface that have the properties of a molecular sieve. The hypothesis that a layered structure consistent with the inner semi-periodic structure and an outer less porous layer, likely stabilized by hyaluronic acid and adsorbed plasma proteins, can be tested using the model in Fig. 3.7. The approach with an emphasis on the inner structure has been reviewed in detail elsewhere [5, 6, 8, 45], and the main conclusions relevant to this chapter are summarized below.
With respect to trans-vascular exchange, the water filled spaces between the fibers in the quasi-periodic arrays forming the periodic array in Fig. 3.6 have similar molecular dimension to plasma proteins such as albumin. The combined effect of steric exclusion and electrostatic interactions between albumin and the fibers results in high resistance to plasma protein exchange across the glycocalyx and a large concentration difference of plasma protein between the circulating blood and the space beneath the glycocalyx. The main colloid osmotic pressure difference, which opposes the hydrostatic pressure difference between blood and tissue, is therefore across the glycocalyx. At high filtration rates (when microvessel pressure exceeds the effective colloid osmotic pressure of the plasma proteins) the ultrafiltrate of plasma (with a low plasma protein concentration) flows into the intercellular junction below the glycocalyx from where it is funneled through infrequent breaks in the strands of tight junction proteins that effectively seal off most of the perimeter of the endothelial cells. This maintains a plasma protein concentration difference across the glycocalyx that is larger than that between blood and the interstitial space. At lower pressures some of the plasma protein in the interstitial space does diffuse back into the space below the glycocalyx, but plasma protein concentration in the protected space below the glycocalyx always remains less than in the interstitial space as it is diluted by the ultrafiltrate [78,79,80] The result is a steady state balance between the hydrostatic and colloid osmotic pressures across the glycocalyx to maintain slow filtration as described in Chap. 2 on the Revised Starling Principle.
An example of the gradients of albumin concentration at high and low filtration rates is Fig. 3.8 (From [81]). The figure emphasises the importance of maintaining the integrity of the glycocalyx. The effectiveness of the colloid osmotic pressure difference across the glycocalyx to oppose the hydrostatic pressure difference between blood and tissue and maintain low filtration is reduced when the glycocalyx is damaged or lost. Furthermore, it is important to stress that the primary pathways for water exchange are the intercellular junctions in the endothelium of continuous capillaries and the fenestrae in fenestrated capillaries. In continuous capillaries (lung, heart, skin, muscle) less than 1% of the area of glycocalyx lies over the line of contact between adjacent endothelial cells and even less is associated with the infrequent breaks in the junctional strands within the junctions. A critical assumption in the models of Figs. 3.7 and 3.8 is that the composition and structure of the glycocalyx in the region of the primary water pathways is the same as that observed over the other 99% of the endothelial surface. In fenestrated capillaries, the fraction of the endothelial surface that forms the transcellular water pathway is larger, but the glycocalyx structure in the region of fenestrae is assumed the same as that observed over at other sites on the endothelium (as in Fig. 3.7).
Model demonstrating that the osmotic pressure of the subglycocalyx region is always lower than that in the mixed interstitial fluid in a filtering microvessel. The ultrafiltrate crossing the normal glycocalyx has a low plasma protein content. When funneled into breaks in the junctional strands this ultrafiltrate reduces diffusion of plasma proteins from the interstitium. This maintains a larger effective plasma protein osmotic pressure difference across the glycocalyx than that across the whole endothelial barrier (the blood to tissue protein concentration difference). From [81] with permission
In parallel with the use of the model in Fig. 3.7 to evaluate water and solute exchange, the role of the glycocalyx to modify plasma and red cell flows along microvessels has been extensively investigated by a number of investigators. Of particular importance is the idea that the movement of water within the glycocalyx in the direction of blood flow is also restricted by the fibers. Via this mechanism, the glycocalyx forms a “lubricating layer” which reduces the friction between red cells and the microvessel wall. This lubricating layer must cover the whole endothelial surface to be effective. Thus one way to test whether properties of the glycocalyx characteristic of the whole endothelial surface also describe fluid exchange through the more localized junctions or fenestrae is to use the results of calculations by Weinbaum et al. and others [80, 82, 83] to test whether estimates of resistance to water flow across the glycocalyx (i.e. during trans-vascular exchange) can also account for the properties of a functional lubricating layer. For example, the Darcy coefficient (Kw, a measure of conductance to water flow) has values in the range 10−13 to 10−14 cm2 associated with the periodic structures close to the membrane of Fig. 3.8 (within 200 nm of the surface). According to Weinbaum et al. [83], layers 200 nm thick or greater are sufficient to describe an effective lubrication layer mechanism for red cells. This mechanism would be similar to the way a skier using a hard flat ski crosses powdered snow.
The results of this analyses [1, 8, 78, 81] demonstrate a consistent description of the glycocalyx as both a permeability barrier and an effective lubricating layer with glycocalyx thicknesses in the range of 200–400 nm. The structure has a quasi-periodic structure imposed by direct or indirect association of glycocalyx components with the endothelial cell cytoskeleton and cell membrane and binding of plasma protein components including albumin (Figs. 3.7 and 3.2). To a first approximation this seems a reasonable view of the glycocalyx in mammalian microvessels less than 5 μm in diameter such as those investigated by Vink and Duling (Fig. 3.4). However, this analysis requires further evaluation. For example the “skiing” analogy does not take into account the contribution of the red cell glycocalyx. In model systems the dual glycocalyx interaction has been demonstrated to be important, so the criterion based only on the endothelial glycocalyx properties may need to be modified [84, 85]. Further, in continuous endothelium, it will be important to understand how changes in adhesion complexes that regulate the stability of intercellular junction [45] may also modify the quasi-periodic structure of glycocalyx components attached to the cytoskeleton in the region of the junctions.
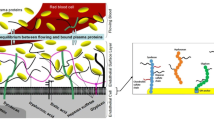
Direct and indirect measurements that suggest a glycocalyx thickness greater than 0.5 μm in larger micovessels (see Sections “Measurement of Red Cell Gap in Human Subjects” and “Glycocalyx Volume Measurement in Human Subjects”) also highlight fundamental gaps in our knowledge about the uniformity of glycocalyx structure and composition. While a two to five fold increase in thickness of a glycocalyx layer with the same properties as described above would support red cell flows, and reduce leukocyte interactions with the endothelial cell, the same increase in thickness predicts a reduction in trans-vascular water movement to well below all measured values in microvessels [65]. There are several ways to resolve this problem, all involving assumptions about the composition and distribution of the glycocalyx components at a distance greater than 300–400 μm from the endothelial surface. One of the simplest assumptions is that the outer layers of the glycocalyx (thickness L) have less resistance to water flows than the inner layers. Curry and Michel [86] have evaluated the functional properties of the bi-layer model shown in Fig. 3.9. It has an inner structure with permeability properties similar to those associated with the glycocalyx in Fig. 3.2, but with an outer layer with increased porosity.
Cartoon to illustrate a bi-layer model of the endothelial glycocalyx. The inner layer is a quasi-periodic matrix associated with bush-like structures with spacings between fibers similar to the dimensions of albumin. as in Fig. 3.7. This layer forms the primary molecular sieve at the microvascular wall. As drawn the bush like structures that are anchored to the cell cytoskeleton represent syndecan molecules which have heparan sulfate and chondroitin sulfate attached as side chains and extend up to 200 nm from theendothelial surface. The outer gel-like layer is more porous and assumed to have some of the characteristics of a hyaluronic acid solution . This layer forms a “lubrication layer” for the movement of red cells . Red cells and endothelial cell dimensions are not to scale. In a small capillary the red cell would be curled in on itself and would apply pressure to the outer layer as part of its functions as a mechanotransducer. From [87] with permission
For example, the criterion for an effective lubrication layer (L/Kw0.5 > 100) is met with an outer glycocalyx thickness up to 1 μm but with a hydraulic resistance more that an order of magnitude less than the inner core. In such a bi-layer the normal range of permeability properties is accounted for if the outer layer also has permeability to albumin more than 20 times higher than that of the inner layer. This property ensures that the tendency for albumin to accumulate at the boundary between the outer layer and the more restrictive inner layer is reduced by rapid diffusion of accumulated albumin back into the circulation. The composition of outer glycocalyx layers that extend more than 5 μm from the endothelial surface is not well understood. Curry and Michel [86] assumed that hyaluronic acid was a principal component because individual molecules can be more that 1 micron in length. However as suggested by the top panel of Fig. 3.2 the composition of the outer layer is likely be determined by a dynamic balance between loss of glycocalyx components, binding of circulating plasma components and ongoing synthesis of key components by the endothelial cells.
It is important to evaluate such models, not only to further understand fundamental glycocalyx structure-function relations in larger microvessels, but also because these models suggest that changes in the thickness and distribution of the outer layer may not be representative of changes in the inner layer. Thus the methods developed for application in the clinic (such as measurements of glycocalyx volume and visualization of the layer from which red cells are excluded in sublingual microvessels) which often suggest a glycocalyx layer several microns in thickness may not measure changes in the glycocalyx that are characteristic of the core structure of the glycocalyx. By focussing on glycocalyx composition as a function of glycocalyx thickness, the bi-layer model is a specific example of a way to evaluate the assumption that the glycocalyx close to the main transvascular exchange pathways (both intercellular and transcellular, that together occupy less than 1% of total endothelial surface) has the same structure and composition as that measured over the rest of the endothelial surface. The bi-layer model places limits on the permeability properties of the outer layer that are compatible with measured transvascular exchange and thereby may be a useful guide for further evaluation of the layered structure of the glycocalyx. Promising approaches include the use of super resolution imaging methods such as those described by Fu and colleagues (see below) [88] when combined with fluorescence probes that can penetrate into the inner layers of the glycocalyx.
Measurement of Red Cell Gap in Human Subjects
To overcome some of the limitation of analyses of glycocalyx function that require either chemical analyses of glycocalyx components including proteoglycans, hyaluronan, or heparan sulfates (HS) in the plasma, and invasive and time-consuming methods using the distribution of labelled red blood cells (RBCs) and low-molecular weight dextrans, there has been a renewed focus on non-invasive tools for analysing the endothelial surface layers using methods such as sidestream–dark field imaging. This method is the development of methods introduced in 1990 to improve the contrast between red cells, the vessel wall and the background known as orthogonal polarization spectral imaging [89]. The orthogonal polarization spectral method was not developed initially for imaging the glycocalyx, but has been widely used to measure other characteristics of the microvasculature including capillary densities and an index of local microvascular flow [90]. However, the enhanced contrast between the boundary of the red cell column and the vessel wall has led to its application in the sublingual microcirculation of human patients. For clinical settings the aim has been to measure changes in the extent that red cells penetrate the normal cell free layer near the wall of microvessels. A critical assumption, based on the observations in small vessels such as those in Fig. 3.4 is that an increased width of the red cell column (or decreased cell free layer thickness) indicates loss of glycocalyx. There has been a succession of reports describing the adaptation of the orthogonal polarization spectral approach as sidestream dark field imaging.
In some reports technical details are minimal but the approach appears to have been used in at least three forms [36, 43, 91, 92]. Version 1 measures an ESL thickness in small blood vessels (diameter <15 μm) as the space between the red cell column and the image of the vessel wall whose position is enhanced by computer image processing. Version 2 (Fig. 3.10), abandons measurements of the position of the vessel wall altogether and measures only the red cell column width at multiple sites (20 or more) along a microvessel segment. A statistical analysis of the distribution of red cell column widths is used as a measure of the access of RBCs into the cell free boundary layer. A perfused boundary region (PBR) thickness is defined as the difference between the mean red cell column width and a characteristic diameter of the region into which red cells penetrate, apparently derived from a statistical analysis based on the 25 and 75 percentiles of the red cell width distribution. This version is fully automated using software developed at the University of Amsterdam and blinded to the investigator. A third version, first described in detail in 2008 [36], compares the space available to red cells in the absence of rolling leukocytes to the transient increase in space available to red cells after leukocyte passage. The method is based on the observation that leukocytes, which are more rigid than red cells compress the capillary endothelial glycocalyx during their passage through the lumen, thus allowing a transient “widening” of the erythrocyte column [83]. The change in erythrocyte column diameter observed during such widening divided by two is related to the dimension of the microvascular glycocalyx.
The idea that red cells penetrate closer to the vascular wall when the glycocalyx is damaged in a capillary (as in Fig. 3.4) is being tested as a basis for sidestream dark field imaging in microvessels up to 50 μm diameter in the sublingual vessels of human patients. The dimensions of a perfused boundary region (shown as a hatched region, and defined as the region between the yellow bands) in the microvessel is estimated from the distribution of diameters of the region accessible to red cells at many sites along a microvessel, and the median red cell column width during sidestream dark field imaging. Based on the description in [43]
There have been several reports describing one or more of these approaches. In an initial paper [27], Nieuwdorp et al., using Version 3 in hamster muscle, reported the gap between red cells and the compressed glycocalyx to vary from 0.4 to 0.8 μm in vessels 4–8 μm diameter. These gap dimensions were smaller than the gap between erythrocyte and a measure of the position of the vessel edge (Version 1) suggesting the leukocyte did not penetrate right up to the vessel wall. They do fall in the same range as the measurements by other investigators in small blood vessels. The authors concluded that the technology provides values of the ESL that correlate well with the invasive technique based on tracer dilution in animal experiments (see below). Furthermore in the same report, PRB thickness evaluated in sublingual microvessels with mean diameter of 5.37 ± 0.45 μm in human subjects was 0.58 ± 0.16 μm. In a brief preliminary study it was also reported that the PRB layer was reduced in thickness in human patients in a variety of disease states.
The strategy of using Version 3 does not appear to have been followed up in any detail in spite of its being an interesting idea. Instead, application of version 2 to measure only changes in the penetration of red cells into the boundary region (PBR) has been reported in vessels less than 50 μm diameter. Some reported values are as follows: In vessels larger than 5 μm but less than 50 μm diameter, Vlahu et al. measured an increase in the perfused red cell column width from 16.4 μm in control subjects to 17.7 μm in dialysis patients [43]. There was no change in the reference red cell column width (10.5 in control vs 10.1 μm). The difference between perfused red cell column width and reference red cell column width indicated a statistically significant increase PBR (3.6 ± 0.4 μm compared with 3.3 ± 0.4 μm in healthy controls [43]. It was argued that the method was providing an index of glycocalyx loss; because the increase in PRB was measured in the same patients as where increased circulating levels of glycocalyx breakdown products were measured (e.g. the circulating HA and sydecan-1 levels reported in Section “Composition in Relation to a Layered Structure” were from the same patients). In other reports, patients with evidence of cardiovascular disease, PBR values were 2.2 ± 0.3 μm compared with 2.0 ± 0.3 μm in controls [69], and in chronically ill patients PBR was 2.7 μm (range 2.6–2.9) compared with 2.5 (range 2.4–2.7) μm in controls [70]. On the other hand Amraoui et al. found no correlation of PBR with cardiovascular risk [93]. The relatively small changes in PBR described in these studies raise questions about the power of the technique to discriminate diseased states from more healthy states, In spite of these concerns clinical evaluation of the non-invasive sublingual imaging has demonstrated inter-operator reproducibility and linked systemic vascular dysfunction with a variety of diseases including diabetes, sepis, systemic sclerosis and fevers [94, see also 95] As with all such developments, improvement can only be made when the key questions about the approach are addressed.
Sidestream dark field imaging methods assume that the change in the width of flowing red cells measures a real change in glycocalyx. Although there are independent estimates of endothelial surface layers extending several microns from the endothelial surface in large vessels (see below) there are reasons to question such estimates as representative primarily of changes in the glycocalyx. For example, the well-known Fåhraeus effect describes the formation of cell free layers in microvessels in terms of the micromechanics of red cells, independent of interactions with a glycocalyx. Measurements independent of a surface layer (in glass microtubes), as well as theoretical models, demonstrate that a cell free layer several microns in thickness is expected in microvessels up to 50 μm diameter [96,97,98,99]. Furthermore, independent of changes in the glycocalyx, it is reasonable to propose mechanisms that may change the micromechanics of red cell movement within microvessels under inflammatory conditions (e.g. increased rolling and attachment of inflammatory cells, and the release and/or uptake of microparticles at the endothelial cell surface). In summary, the use of new optical techniques such as sidestream dark field imaging provides interesting new ways to evaluate changes in microvascular function. However claims that the sidestream dark field imaging approach, as currently applied, provides a specific biomarker of glycocalyx function particularly with regard to properties of the glycocalyx important in relation to peri-operative fluid therapy cannot be justified at this time. Nevertheless the idea that measurements of changes in red cell flow in easily visualized microvessels in human patients can be an aid to the diagnosis and treatment of chronic disease is an important area for further research, and further understanding of ways to evaluate the contribution of changes in glycocalyx structure and composition must be actively pursued. Examples of new investigations include comparison of results with other measurement methods on a vessel by vessel approach whilst modifying local red cell flows.
Glycocalyx Volume Measurement in Human Subjects
An alternate way to evaluate changes in glycocalyx function in human subjects has been to use measurement of a glycocalyx volume as a biomarker of glycocalyx. Two methods for estimating the volume of glycocalyx of the entire cardiovascular system have been described. Both depend on measurements of volume by the dilution of a tracer substance. It is important to revise the general principle and underlying assumptions of this approach in order to assess the value of these methods (see also Chap. 2).
The principle of estimating the volume of a fluid filled compartment from the dilution of a tracer is very simple: a known mass of a solute, m, is dissolved and mixed into the fluid whose volume is to be measured. After a short period of time, which is believed to be sufficient for complete mixing of the marker solute throughout the entire volume to be measured, the concentration of the solute in the fluid, C, is measured. Then since C = m/V,
The method should give the volume of the fluid in the compartment if two important conditions are met. These are: (1) all the tracer molecules are retained within the compartment at the time the concentration is measured and (2) at equilibrium, all the tracer molecules added to the system at zero time are at the same concentration in all regions of the compartment.
It is very rare for one to be able to assume with confidence that all the tracer molecules initially injected into a fluid compartment have all been retained there by the time its solution has mixed sufficiently in all regions to reach a uniform concentration. This is certainly not true when the tracer has been injected into the circulation and there is a standard way of dealing with it. Once it is thought that mixing within the circulation is complete, losses from the blood (or plasma) may be detected by serial measurements of tracer concentration. Providing the loss of tracer from the circulation is slow, it is reasonable to assume that initially it should follow an exponential decline and the logarithm of the concentration when plotted against time should describe a linear relation. This may then be back-extrapolated to zero time, when the concentration would be equal to that which would have been present if mixing throughout the compartment had been instantaneous. This estimate of concentration at zero time is then used with the total amount of tracer injected into the compartment to calculate its volume.
The second condition is too often neglected yet may easily be compromised for a range of reasons. For the entire tracer initially added to the compartment to be at the same concentration throughout the compartment, the tracer must not bind to any structures within the compartment. This would remove a fraction of its mass from solution and reduce the value of m in eq. (3.1).
Also, the tracer should be an uncharged molecule and have the same molecular size as water. If the tracer carries a negative charge, it may be excluded from regions of fluid closely bounded by surfaces or extracellular molecules which are themselves negatively charged. Alternatively, it may be concentrated in regions where bounding molecules carry a positive charge. Also, if the molecule is larger than water it will be excluded from regions of the compartment where the fluid filled spaces are comparable with or less than its own diameter to a greater degree than water molecules. Consequently its concentration in these regions will be lower than in more open regions. These phenomena are well known by investigators of interstitial fluids [100,101,102] and 60 years ago it was recognized that estimates of extracellular fluid volumes by tracer dilution were inversely proportional to the molecular size of the tracer (inulin giving the lower values and thiocyanate the higher ones). The condition of the tracer being the same molecular size as water is probably only approached when isotopes of water (either D2O or HTO) are used as labels to estimate total body water.
The problem with estimating the volume of the glycocalyx is that the glycocalyx is a barrier to the diffusion of macromolecules. Therefore, even if a tracer, considerably larger than water, is able to penetrate the glycocalyx, it will be excluded from a significant fraction of the fluid phase within the matrix of fibrous molecules. To estimate the volume of the fluid within the glycocalyx, the partition coefficient for the tracer between the plasma and the glycocalyx must be known.
It may seem logical, therefore, to estimate the fluid volume within the glycocalyx using a tracer that is a small molecule and has a partition coefficient between glycocalyx and plasma close to one. Whilst small tracers may enter the glycocalyx from the plasma and distribute themselves rapidly within its fluid phase, they also diffuse rapidly out of it through the channels between the endothelial cells or through the fenestrae into the much larger volume of interstitial fluid. In most, if not all, organs and tissues, the volume of the fluid phase of the glycocalyx is considerably less than the volume of the interstitial fluid (ISF) and because the rates of disappearance of small molecule tracers from the plasma and into the ISF are rapid, the transit time of tracer through the glycocalyx is too short to provide information of glycocalyx volume. If, however, once a small molecule had entered the glycocalyx, it was bound avidly to glycocalyx components and did not dissociate significantly and enter the interstitial space, it could be used as an indicator of glycocalyx volume. To estimate this quantitatively, once again one would have to know the partition coefficient of the tracer between the plasma and the glycocalyx. Then, the volume that one might calculate would be a virtual volume representing the number of binding sites on the glycocalyx on the luminal endothelia of the circulation and not the volume of the fluid phase within the glycocalyx.
Two methods for estimating glycocalyx volume have been published and both of them are open to criticism. The first of these methods was described by Nieuwdorp et al. and consists of measuring the initial volume of distribution of 40 kDa dextran (D40) [103], which it thought to enter the glycocalyx from the plasma giving a volume for the sum of the plasma volume and the glycocalyx volume. The plasma volume, estimated from the red cell volume and the large vessel hematocrit, is then subtracted from the distribution volume of D40, to give a value for the volume of the glycocalyx. Using this method in healthy volunteers, they obtained a mean value of 1.7 litres and a considerably lower value in patients with Type 2 diabetes. As pointed out [104], the method fails to meet the conditions of the dilution principle in several ways. First, the tracer, fluorescently labeled D40, appears to be a mixture of dextran polymers with an average molecular weight of 40 kDa. Consequently after its injection into the circulation, its disappearance from the plasma cannot be described by a simple exponential function over the first 10 min as dextran polymers of differing molecular weight leave the circulation at rates which are roughly inversely proportion to their molecular size. This gives rise to a considerable under-estimate of tracer concentration at zero time. Second, their use of the large vessel hematocrit to estimate plasma volume is uncritical and may lead to further errors (see below). Third, the authors assume the partition coefficient of D40 between the plasma and fluid phase of the glycocalyx to be one. This cannot be so because even the smaller D40 molecules are large compared with water molecules and should be excluded from the water surrounding the fibrous molecules within the glycocalyx. The larger D40 molecules should be severely excluded from the water surrounding the glycocalyx structures by a distance at least equal to the Stokes-Einstein radius of the D40 molecule. Since the glycocalyx acts as a molecular filter to macromolecules, the water within it is confined to spaces with widths comparable to those of the dextran molecules.
The importance of knowing the partition coefficient of the tracer molecule between the plasma and the glycocalyx can be illustrated by some simple algebra. If Cp and Cg are the equilibrium concentrations of a tracer in the plasma and the glycocalyx water and λ is the partition coefficient between glycocalyx and plasma. Then:
If m moles of tracer are added to the plasma when time = 0, and all the tracer is confined to the plasma (volume = Vp) and the fluid phase of the glycocalyx (volume = Vg), at equilibrium (i.e. when mixing is complete):
When eq. (3.1) is used to calculate the volume of distribution of the tracer, the desired result of estimating the sum of the volumes of the plasma and the glycocalyx water is obtained only if λ = 1, i.e.
Since λ is unknown, only λVg can be estimated by subtracting Vp from m/Cp. Furthermore, changes in the value of m/Cp are open to misinterpretation. If the thickness of the glycocalyx is reduced without change of λ, apparent glycocalyx volume, λVg, will be reduced and may be accompanied by increases of both hydraulic and solute permeabilities of vascular walls. Hydraulic and solute permeability might also increase if the glycocalyx were to expand, opening the water filled spaces within it and increasing λ for the tracer molecules and so amplifying the increase in apparent glycocalyx volume, λVg. The important lesson for the clinician is to regard conclusions reached by this method critically and wait until a sounder theoretical basis for the method has been established.
A second, more carefully argued, method for estimating glycocalyx volume is also open to criticism and misunderstanding [105, 106]. This method arose from measurements of red cell and plasma volume of patients subjected to pre-operative volume loading with colloid solutions, which were either 5% albumin or 6% hetastarch solutions [106]. It is argued that plasma volume estimated from the distribution volume of indocyanine green (ICG) can be divided into a circulating component and a non-circulating component. The non-circulating component is thought to be the glycocalyx or at least the plasma proteins (labeled with ICG) within the glycocalyx. By using fluorescently labeled red cells to estimate the total volume of red cells in the blood, the hematocrit, measured in blood taken from large vessels, is used to determine the circulating component. The volume of the non-circulating component of the plasma is calculated by subtracting the circulating component from the volume of distribution of the ICG. Rehm and colleagues [106] estimated mean values for non-circulating component in the range of 700 ml in patients before volume loading and 300 ml afterwards.
ICG is used routinely for estimating cardiac output, assessing liver function and for estimating plasma volume. After its injection into the circulation it binds rapidly but not covalently to plasma proteins (particularly albumin and lipoproteins). It is an anionic dye and there is a very low concentration of free dye present in the plasma in equilibrium with the bound ICG. It seems likely that this free dye rather than the dye-protein complex enters the glycocalyx and binds to structural molecules within it. Although the authors (of the method) speak of a dynamic equilibrium between proteins in the glycocalyx and those of the plasma, the relevant in vivo observations made in hamster muscle capillaries [107] indicate that rates of penetration of labelled albumin and fibrinogen that would be negligible 2 min after their injection into the circulation. By contrast, a relatively small molecule, ICG could diffuse rapidly into the glycocalyx and if its affinity for sites there were high, near equilibrium concentrations could be rapidly established. This is a minor criticism.
Somewhat more questionable is the assumption that the large vessel hematocrit reflects the fractional volumes of cells and plasma in the circulating component of the blood. It has been known for over 80 years that the hematocrit measured on samples of blood taken from arteries or veins differs from that calculated from estimates of the total volumes of red cells and plasma [108]. Most of the earlier measurements of plasma volume were made using the dye Evans Blue (T1824), which like ICG is an anionic dye that binds rapidly but non-covalently to plasma protein. It is fair to assume that, like ICG, Evans Blue also binds to the glycocalyx and so that its distribution includes the non-circulating component. Further the Evans Blue (in both its bound and unbound forms) may leak from the vascular space (this is the basis for Miles assays, variations of which are used as a (limited) measure of vascular permeability [109]). It was appreciated by the 1950’s that a major contribution to the discrepancy between large vessel and whole body hematocrit was made by the differing velocities of red cells and plasma particularly as blood flows through microvessels. Thus, the large vessel hematocrit, HLV, is the ratio of the flow of red cells to the flow of blood through the circulation (see Revised Starling Chap. 2). The flow of cells, Fc, or plasma, Fp, through the circulation of an organ or tissue can be thought of as the volume of cells divided by the mean transit time of the cells, tc, through the vessels. Similarly the flow of plasma is equal to the volume of plasma Vp divided by its mean transit time, tp. Measurements of the mean transit times of labelled red cells and plasma show considerable variation from organ to organ and from time to time in the same organ. While reported measurements of the ratio of tc/tp through the pulmonary circulation are in the range of 0.98–0.99, the ratio for the liver is closer to 0.5 [110]. If plasma volume is estimated from red cell volume and large vessel hematocrit, then since:
If the whole body hematocrit were substituted for HLV, the term tp/tc would not be part of the expression. Rehm et al. and Jacobs et al. minimise the errors introduced by assuming tp/tc = 1 by using the hematocrit of arterial blood [105, 106]. Providing the fractions of cells and plasma in the blood of the right ventricle approximate to the ratio of their total volumes and the ratio tc/tp through the pulmonary circulation remains close to one, considerable error may be avoided. If hematocrit is determined from the venous blood however, fluctuations in tc/tp through peripheral circulatory beds may suggest several 100 ml of fluid entering or leaving the plasma.
Perhaps the most serious misunderstanding of the Rehm et al. and Jacob et al. interpretation is the failure to recognize that the non-circulating component of plasma represents a virtual volume, not a real one. Changes in its value represent changes in the amount of tracer that is attached to circulating plasma proteins. If m is the amount of tracer injected into the circulation and mb is the amount of tracer that is bound within the glycocalyx, eq. (3.4) can be extended as:
If the affinity of the binding sites for tracer within the glycocalyx is greater than the affinity of plasma proteins for tracer, the ratio of mb/m may increase as Cp falls as circulating tracer is removed by the liver. This could be mistaken for an increase in the non-circulating component of plasma volume. Alternatively, if repeated estimates of plasma volume are made, the failure of tracer to dissociate from a fraction of its binding sites in the glycocalyx could be incorrectly interpreted as a decrease in glycocalyx volume and an expansion of plasma volume.eh.
It is also noted that in a detailed review of the ways that the new understanding of the fluid exchange through the glycocalyx helps clinicians to evaluate fluid therapy, Woodcock and Woodcock [14] also include in discussion the idea that fluid therapies change the amount of the plasma volume that is trapped in the glycocalyx (see Chap. 2 on Revised Starling Principle for details.) . This idea needs much further evaluation because the amount of plasma trapped in the glycocalyx may not be as large as the current measurements of glycocalyx volume suggest.
The foregoing reflects the authors’ critical view of whole body glycocalyx volume measurements, which, we hope, will draw attention to the difficulties of measuring and interpreting plasma volume (and particularly changes in plasma volume) using dilution techniques. As noted at the beginning of this section, the principle is simple but meeting the conditions under which it can be rigorously applied are not.
The Glycocalyx in Large Vessels
It is noted that estimates of glycocalyx volume of the order of 0.7 to more than 1.5 L imply a glycocalyx thickness, averaged over the surface area of the human vasculature of 2–3 μm (assuming surface area of 350 m2 [75]). Part of the confidence in the side stream imaging method and glycocalyx volume estimates is that some measurement of glycocalyx thickness in larger blood vessel approaches these values. For example in a follow-up to EM studies suggesting the glycocalyx was thinner in areas of the carotid artery in mice that were known to be more susceptible to arteriosclerosis, Van der Berg et al. reported an ESL thickness of 4.3 μm in the common carotid region and 2.2 ± 0.7 μm in the sinus region [111]. In addition to lending support to the hypothesis that thinning of the glycocalyx might be a pre-condition for the development of atherosclerosis, these and related measurements using two photon microscopy [112] and FITC-Dextran exclusion [113] have led to broad acceptance that glycocalyx regions several microns thick may be common in larger vessels. As a step towards further evaluation of such large glycocalyx thickness, it is noted that optical methods are even more difficult to apply in larger vessels with thicker walls than in microvessels such as in Fig. 3.4 and the method used to make such measurements may bias values. To apply sophisticated imaging in the larger vessels described above (150 μm in diameter) each vessel was dissected, mounted in a perfusion chamber, and perfused with an electrolyte solution containing 0.1% albumin. It is now known (see Section 2 “Composition in Relation to a Layered Structure” SIP and glycocalyx stability) that in mammalian vessels such low albumin concentrations do not maintain S1P concentrations in the range where they stabilize the endothelial barrier and suppress MMP release [47]. Thus at least some glycocalyx components are likely to be disturbed during vessel dissection and perfusion leading to the possibility of a more diffuse, and relatively thick surface layer, similar to those present under inflammatory conditions [17].
Background: Imaging the Glycocalyx: More Detailed Technical Issues
Light vs Electron Microscopy
The theoretical limit for detection of separate objects with light imaging is the diffraction limiting resolution of around 200 nm, but in the wet tissue, near a curved interface this level of resolution is not reached. This means the dimensions of interest for the glycocalyx may often lie below the resolution of light microscopy. Super-resolution light microscopy can, in ideal circumstances, have resolutions circa 30 nm, but as suggested above, this ideal limit is not reached in samples containing the glycocalyx in tissue. It has been used in some cell culture experiments for HS and HA [88]. The indication was that the HA (in cables) was along the membrane underneath the HS, but further investigation in the microvasculature of perfused tissues would be needed to confirm these findings. A further confounder is that antibodies for GAG chains are large and Fab fragments are not yet available making steric exclusion a problem (for all forms of tagged imaging). Electron beams have a wavelength 100,000 times shorter than normal optics and can, in theory, have 100,000 times the resolution of visible light but because electron optics are far less efficient, the resolution is limited currently to 0.05 nm. Below we review additional limitations imposed by the requirement to prepare biological wet biological samples to be processed for sample analysis in a vacuum and to improve contrast by additional staining of selected glycocalyx components [114, 115].
Tissue Preparation
Since the late 1940’s, water in biological samples has been replaced with plastic as part of sample preparation. Most biological electron microscopy on tissues is achieved through the following simplified protocol: Fixation with glutaraldehyde; staining/fixation with osmium tetroxide; dehydration with grades of ethanol; replacing the ethanol with a plastic resin; ultra-thin sectioning to obtain sections with about 300 μm sides and 80 nm thickness; and finally staining with uranium acetate. These are then further stained with more heavy metals and imaged in a transmission electron microscope. These plastic sections give a resin-limited resolution of the order of 0.5–2 nm in the plane of the section and 80 nm in the depth direction.
It is important to emphasize that in transmission electron microscopy the actual substance of interest is not observed, only the material used to stain the objects. The resultant EM images therefore depend on what is preserved to be stained after glutaraldehyde fixation and ethanol washing steps. Sugars, of which proteoglycans are made, do not chemically fix very well and therefore are very often removed during tissue processing. This is one of a number of problems that make the glycocalyx difficult to study. Another is that it resides at an interface where differences between the hardness of resin replacing the vascular contents and the hardness of resin-embedded tissue compromise imaging in electron microscopy.
Advances in Staining Technology
Because of the variety and complexity of the compounds that form when a stain used to enhance contrast binds to a target site, the relation between deposit density and the amount and location of the muco-polysaccharides can be far from straight forward. For example, after accounting for the various oxidation states of ruthenium atoms in the ruthenium-ammonium compound which is the ruthenium red first used to label the glycocalyx, Luft determined that the products seen in electron microscope deposits are the result not only of an initial linking of ruthenium red to acid polysaccharides, but further reactions where the mucopolysaccharide is oxidized and ruthenium red then acts as a catalyst to promote a series of cyclic reactions to successively build up osmium tetroxide-ruthenium red derived compounds in the region. Much more work is needed to understand the complex chemistry of the many additional stains that have been introduced to label the negative charges on the GAG side chains as the binding point. The most common in use is Alcian blue, which in a way is similar to cupromeronic blue, binds relatively selectively to proteoglycans. The advantage of such copper based stains is they can also be seen easily with optical histology helping the selection of areas of interest to section for electron microscopy [116, 117]. Another common stain is lanthanum containing dense highly positively charged ions favoured by the Chappel group and others [118,119,120,121]. Other stains from the lanthanoid series of elements have also become cheaper, due to improved purification, including terbium, and combinations such as the Lanthanum/Dysprosium GlycosAminoGlycan adhesion technique (LaDy GAGa) [16, 77]. Also thorium dioxide (an actinoid), a compound previously used for vascular radiography, has been shown recently to give similar patterns of staining of the glycocalyx in the glomerulus to LaDy GAGa [122, 123]. The longer spacings observed between periodic densities are broadly in agreement with previous data. Still, the adage of “correlation does not mean causation” makes it difficult to determine if what is observed is physiological, especially with so few examples and with limited knowledge of the mechanisms of binding and structure preservation. An important area for further research is an understanding of the way the composition of the glycocalyx, its rate of synthesis and degradation, and its preservation in sections of different thickness are preserved. There may be vast amounts of archived material to re-examine with such new knowledge.
Future Directions in Glycocalyx Imaging
One way to help resolve difficulties of comparing images of the glycocalyx from optical and EM studies is to develop approaches that enable both methods to be applied to the same sample of glycocalyx. For example, fluorescently labeled probes can be used to separately label the endothelial cell membrane and specific components on the glycocalyx before the same tissue is prepared for electron microscopic study. Using this approach, Betteridge and colleagues measured the membrane to outer glycocalyx distance (close to 400 nm) to be similar to the thickness in EM images of the same vessels of the glycocalyx stained with Alcian blue [124], and importantly in this context the optical and electron measurements correlated with each other, hydraulic conductivity and albumin solute permeability.
Biological electron microscopy has broadened, particularly in the last decade. Cryo-electron microscopy makes use of a non-crystalline form of ice formed by very rapidly freezing water to below −135 °C so that a glass-like ice is formed. When applied to studies of protein structure, this has enabled, with modern computing, imaging of molecular structures comparable to x-ray crystallography, but in an aqueous state, the technique winning a Nobel prize in 2017. It is also possible to replace the ice with acetone at around −90 °C and then add a plastic resin. This process should result in far less loss of content than ethanol dehydration and has been attempted, although full interpretation was not clear on the limited samples. Preliminary application of cryo-fixation methods to glycocalyx structure in frog mesentery has yielded some useful results as described in Section “The Glycocalyx as a 3 Dimensional Layered Structure in Microvessels”, but other applications (e.g. images that appear to show intracellular material leaking across the endothelial cell membrane as in [125]) have as yet been less satisfactory.
Until recently reconstruction of 3D images of endothelial structures on scales greater than one micron has involved the time consuming process of cutting serial sections for TEM and then manually loading and aligning the area of interest for each section. Separate from the tomographic imaging methods introduced above, 3D scanning electron microscopy gets around this problem by detecting an electron beam’s backscattered electrons to build up an image of the surface of a sample. Moreover, once the surface has been imaged, a diamond knife or an ion beam can remove a layer and the new surface imaged. When a diamond knife is used the method is called serial block face scanning electron microscopy and when an ion beam is used the method is called focused ion beam scanning electron microscopy. If additional heavy metals are added, to improve contrast, the images are stunning and resolution can be <10 nm in the plane of the scan and 30 nm in depth for thousands of sections. Preliminary application of this approach to glomerular glycocalyx stained with LaDyGAGa technique showed glycocalyx tufts over the surface of a glomerular filtration vessel as well as gaps through the glycocalyx to the membrane (See Fig. 3.1) [16]. These images leave many fundamental physiology questions unresolved, but the techniques are available and it is not long before they replace transmission electron microscopy for routine use in plastic sections.
Summary. Frequently Asked Questions and some Answers Based on Sections “Introduction” to “Background: Imaging the Glycocalyx: More Detailed Technical Issues”
The following section summarizes some of the main conclusions in this chapter with a particular focus on questions likely to arise about the role of glycocalyx function and infused fluid composition that effect peri-operative fluid management. Some key issues include:
-
1.
The use of albumin solutions in restoring the glycocalyx during the perioperative period.
-
2.
The role of the glycocalyx when infusion fluids having similar colloid osmotic pressures (COP) are not equally effective in reducing edema formation.
-
3.
Ways to protect the glycocalyx during peri-operative fluid therapy.
-
4.
The role of the glycocalyx in reducing the immune response and coagulation by preventing the adhesion of leukocytes to the endothelium and by binding anticoagulants.
-
5.
The effect of negative charges on the glycocalyx and red cells on the movement of red cells in microvessels and the action of high sodium loads to neutralize those negative charges.
-
6.
Measurement of glycocalyx thickness or volume.
It is important to emphasize that the role of the glycocalyx as a major determinant of the effectiveness of peri-operative fluid therapy has been widely recognized only recently, and it is still early in the process of evaluating the contribution that new insights make to better clinical outcomes. However as explained in this Chapter and the accompanying Chapter on the revised Starling Principle, there are sound arguments derived from a knowledge of glycocalyx and endothelial barrier structure and function that can be applied to guide the clinician in the management of fluid therapy. By far the most important and well understood is the role of the inner glycocalyx as the primary molecular filter, retaining most of the plasma colloids within the vascular volume. This function requires maintenance of the spacing between the fibers of the glycocalyx at dimensions close to the molecular diameters of plasma proteins, particularly albumin. Albumin itself may play a direct role in this organization of the glycocalyx by electrostatic binding that regulates the spacing fibers. Albumin in the circulation and within the glycocalyx also contributes to the delivery of sphingosine-1-phosphate (S1P) stored in red cells to the endothelial cells. S1P acting via the S1P1 receptor regulates signaling pathways that both suppress glycocalyx loss by matrix metalloproteinases, and stabilizes inter-endothelial cell adhesion mechanisms. However, with respect to the role of albumin containing fluids to restore or maintain the glycocalyx during perioperative fluid therapy (1), it is not clear that addition of albumin to infusion fluids would improve the stability the glycocalyx because albumin concentrations required for glycocalyx stabilization and S1Pdelivery are low. However, it is now recognized that the Apolipoprotein-M fraction of circulating HDLs carries at least 60% of the plasma S1P content. Conditions where Apo-M levels are low may contribute to glycocalyx loss [51].
The first aspect of (2) to consider is the COP of albumin solutions versus saline alone. When the glycocalyx is intact and the capillary pressure in an organ is less than or close to the normal plasma colloid osmotic pressure, infused fluid escapes very slowly into the extravascular space of the organ, avoiding edema (e.g. the lung) because the transvascular hydrostatic pressure difference is always closely balanced by the colloid osmotic pressure difference of plasma proteins across the glycocalyx. This illustrates the application of the Revised Starling Principle as explained in more detail in Chap. 2. Thus in the absence of glycocalyx damage, and any large loss of plasma protein, an effective strategy involving saline infusion sufficient to balance renal and evaporative fluid loss, avoiding over-hydration appears consistent with both clinical observation and current understanding of glycocalyx function. This strategy protects the glycocalyx and guards against edema by avoiding local reperfusion damage due to hypovolemia and poor perfusion on one hand, and dilution of the plasma proteins (hypervolemia) on the other. This conclusion does not apply when plasma protein levels are low and the plasma colloid osmotic pressure is insufficient to maintain the low filtration states indicated above. Then, the effectiveness of albumin in infusion fluid is determined by its contribution to plasma oncotic pressures across the glycocalyx.
Further to the question of the relative effectiveness of albumin containing solution versus alternate colloids in solution (2), present knowledge limits detailed comment. In principle all high molecular weight colloid solutions that are primarily excluded by the glycocalyx should be similarly effective. However, issues arise from observations in animal experiments that an albumin solution may be more effective than a colloid solution containing hydroxyethyl starch (HES) with a higher COP. Leaving aside issues about the relative safety of HES in certain patient populations, it is important to emphasize that macromolecules penetrate the glycocalyx at different rates. In animal model experiments, albumin penetrates the glycocalyx far more slowly than other macromolecules (eg dextran). If the subtle interaction of HES transport by diffusion and coupled water flows leads to HES accumulation in the sub-glycocalyx region, the colloid osmotic pressure difference across the glycocalyx due to infused HES may be much smaller than expected from the COP of the infused fluid. The relative effectiveness of albumin versus other colloids may be modified further when key components of the glycocalyx are lost (as measured by increased plasma composition of heparin sulphate or hyaluronan) causing loss of plasma fluid to the extravascular space because of the trans-vascular leak of plasma macromolecules.
With respect to questions about the restoration of the glycocalyx under conditions where key components are lost (3), the following points can be emphasized: Matrix metalloproteinases are a common mechanism to damage the glycocalyx, so strategies such as the use of MMP inhibitors may be important. Also, because damage to the underlying endothelium (e.g. inflammatory gap formation) disrupts the glycocalyx in the region of intercellular junctions, stabilization of the endothelium (using anti-inflammatory strategies) goes hand-in-hand with glycocalyx stability. On the other hand, alternate actions of HES including the possibility that some high molecular weight components may help plug gaps in the glycocalyx or intercellular gaps under inflammatory conditions cannot be excluded.
With respect to other barrier and binding actions of the glycocalyx that are important during the peri-operative period (4), the idea that the glycocalyx acts as a barrier to leukocyte interaction with the endothelial surface is consistent with known glycocalyx structures forming a deformable mechanical barrier at the endothelial surface. Nevertheless the observation the leukocytes can penetrate closer to the endothelial surface than red cells suggests that other factors such as upregulation of adhesion proteins to increase leukocyte adhesion, and chemokine cofactors, in the inflammatory cascade may be just as important as the mechanical forces in determining leukocyte interactions when the glycocalyx is damaged [126]. Similarly the capacity of the glycocalyx to bind a range of circulating plasma proteins is well established. This includes various plasma components regulating coagulation. The sites for such protein binding are lost or disrupted when the glycocalyx is damaged.
With respect to the contribution of negative charge on the glycocalyx to red cell movement through the smallest microvessels (5), it is noted that under static conditions charge plays an important role in the spacing of the glycocalyx matrix fibers, and it is also important in opposing red cell aggregation, and adhesion of red cells to endothelium. At normal microvascular flow rates, however, red cell movement is dominated by the compressibility of the outer layers of the matrix and resistances to water. This does not rule out more complex electrostatic interactions at very low or intermittent flows, but it is not clear that changes in the glycocalyx under high salt loads can be attributed simply to charge shielding. Recent evidence that salt loading results in sodium accumulation in skin interstitium and that this may play a role in immune responses in the vasculature suggests that high salt loading may modulate vascular structure via an immune response [127].
With respect to biomarkers of glycocalyx function in patients, recent clinical evaluations indicate that increased penetration of red cells into the plasma free layer on the walls of microvessels with microvascular dysfunction but the relative contributions of changes in the glycocalyx and the mechanics of red cell flows independent of the glycocalyx to these measurements remains to be evaluated in different disease states. A recent critical review (Michel et al. [128]) addresses some published misunderstanding of the colloid osmotic pressure difference across the endothelial glycocalyx as a determinant of blood to tissue fluid exchange. Also estimates of infused saline distribution volumes in the vascular space based on volume kinetic analysis [129] are sensitive to the same potential errors in measurements of hematocrit and Hb concentrations as the estimates of whole body glycocalyx volume described above (see also Chap. 2).
References
Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40(4):828–39.
Dane MJ, et al. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol. 2015;308(9):F956–66.
Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653–66.
Reitsma S, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–59.
Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014;16:505–32.
van den Berg BM, et al. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58(Suppl):75–80.
Van Teeffelen JW, et al. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17(3):101–5.
Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67.
Becker BF, et al. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80(3):389–402.
Broekhuizen LN, et al. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol. 2009;20(1):57–62.
Lipowsky HH. Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models. 2011;8(1):57–62.
VanTeeffelen JW, Brands J, Vink H. Agonist-induced impairment of glycocalyx exclusion properties: contribution to coronary effects of adenosine. Cardiovasc Res. 2010;87(2):311–9.
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(13):1243–51.
Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94.
Woodcock TM, Woodcock TE. Revised Starling equation predicts pulmonary edema formation during fluid loading in the critically ill with presumed hypovolemia. Crit Care Med. 2012;40(9):2741–2742. author reply 2742
Arkill KP, et al. Resolution of the three dimensional structure of components of the glomerular filtration barrier. BMC Nephrol. 2014;15:24.
Squire JM, et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136(3):239–55.
Rabelink TJ, de Zeeuw D. The glycocalyx--linking albuminuria with renal and cardiovasculardisease. Nat Rev Nephrol. 2015;11:667–76.
Bernfield M, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–93.
Kokenyesi R, Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994;269(16):12304–9.
Simons M, Horowitz A. Syndecan-4-mediated signalling. Cell Signal. 2001;13(12):855–62.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277(19):3876–89.
Yoneda A, Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003;22(1):25–33.
Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4(12):926.
Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. In: International review of cytology, vol. 207. Amsterdam: Academic; 2001. p. 113–50.
Pomin VH, Mulloy B. Glycosaminoglycans and proteoglycans. Pharmaceuticals. 2018;11(1):27.
Fransson LA, et al. Novel aspects of glypican glycobiology. Cell Mol Life Sci. 2004;61(9):1016–24.
Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6(7):2397–404.
Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Phys. 1999;277(2 Pt 2):H508–14.
Singleton PA, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281(45):34381–93.
Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277(7):4585–8.
de la Motte, et al. Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer. J Histochem Cytochem. 2011;59(3):252–7.
Gallagher J. Fell-Muir lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra. Int J Exp Pathol. 2015;96(4):203–31.
Nieuwdorp M, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol (1985). 2008;104(3):845–52.
Rehm M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–906.
Nieuwdorp M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55(2):480–6.
Hofmann-Kiefer KF, et al. Serum heparan sulfate l levels are elevated in endotoxemia. Eur J Med Res. 2009;14:526–31.
Nelson A, et al. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30(6):623–7.
Uchimido R, et al. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical Care. 2019;23(1):16.
Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279(6):H2815–23.
Puerta-Guardo H, et al. Dengue virus NS1 disrupts the endothelial glycocalyx. Leading to hyperpermeability. PLoS Pathog. 2016;12(7):e1005738.
Yacoub S, et al. Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: an observational study. J infect Disease. 2016;214(5):697–706.
Vlahu CA, et al. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23(11):1900–8.
Schmidt EP, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289(12):8194–202.
Curry FR, Adamson RH. Tonic regulation of vascular permeability. Acta Physiol (Oxf). 2013;207(4):628–49.
Broekhuizen LN, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–55.
Zeng Y, et al. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306(3):H363–72.
Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–5.
Curry FE, et al. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am J Physiol Heart Circ Physiol. 2012;303(7):H825–34.
Christensen PN, et al. Impaired endothelial barrier function in apolipoprotein M–deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30(6):2351–9.
Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108(23):9613–8.
Swendeman, et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal. 2017;10(492):eaal2722.
Alves, et al. Sphingosine-1-phosphate reduces hemorrhagic shock and resuscitation-induced microvascular leakage by protecting endothelial mitochondrial integrity. Shock. 2019;52:423–33.
Selim S, et al. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (Lond). 2011;121(12):565–72.
Chappell D, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):538–40.
Chen W, et al. Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial Ca2+ and permeability response to histamine involves cGMP-dependent protein kinase I and TRPC6 channels. Arterioscler Thromb Vasc Biol. 2013;33(9):2121–9.
Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides. Br J Pharmacol. 2012;166(2):522–31.
Morikis VA. Atrial natriuretic peptide down-regulates neutrophil recruitment on inflamed endothelium by reducing cell deformability and resistance to detachment force. Biorheology. 2015;52(5–6):447–63.
Landis EM, Pappenheimer JR. Exchange of substances through the capillary walls. In: Handbook of physiology, Circulation. Washington, DC: American Physiological Society; 1963. p. 961–1034.
Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Phys. 1951;167(1):13–46.
Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25(6):1773–83.
Crone C, Levitt DG. Capillary permeability to small molecules. In: Renkin EM, Michel CC, editors. Handbook of physiology, Section 2: The Cardiovascular System. Bethesda, MD: Am Physiol Society; 1984. p. 411–66.
Curry FE. Mechanics and thermodynamics of transcapillary exchange. In: Renkin EM, Michel CC, editors. Handbook of physiology. Section 2: The cardiovascular system. Bethesda, MD: Am Physiol Society; 1984. p. 309–74.
Michel CC. Fluid movements through capillary walls. In: Renkin EM, Michel CC, editors. Handbook of physiology. Section 2: The cardiovascular system. Bethesda, MD: Am Physiol Society; 1984. p. 375–409.
Renkin EM. Control of microcirculation and blood-tissue exchange. In: Renkin EM, Michel CC, editors. Handbook of physiology. Section 2: The cardiovascular system. Bethesda, MD: Am Physiol Society; 1984. p. 627–87.
Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res. 1980;20(1):96–9.
Michel CC. Capillary permeability and how it may change. J Physiol. 1988;404:1–29.
Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Phys. 1990;258(3 Pt 2):H647–54.
Duling BR, Desjardins C. Capillary hematocrit-what does it mean? News Physiol Sci. 1987;2:66–9.
Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79(3):581–9.
Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80(3):394–401.
Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53(1):1–13.
Carey DJ, et al. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994 Jan;124(1–2):161–70.
Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 2004;5:183–92.
Li W, Wang W. Structural alteration of the endothelial glycocalyx: contribution of the actin cytoskeleton. Biomechanics Model Mechanobiol. 2018;17(1):147–58.
Arkill KP, et al. Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism? Biophys J. 2011;101(5):1046–56.
Arkill KP, et al. 3D reconstruction of the glycocalyx structure in mammalian capillaries using electron tomography. Microcirculation. 2012;19(4):343–51.
Hu X, Weinbaum S. A new view of Starling’s hypothesis at the microstructural level. Microvasc Res. 1999;58(3):281–304.
Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87(2):198–210.
Feng J, Weinbaum S. Lubrication theory in highly compressible porous media: the mechanics of skiing, from red cells to humans. J Fluid Mech. 2000;422:281–317.
Adamson RH, et al. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557(Pt 3):889–907.
Secomb TW, Hsu R, Pries AR. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am J Physiol Heart Circ Physiol. 2001;281(2):H629–36.
Weinbaum S, et al. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100(13):7988–95.
Davies HS, et al. Elastohydrodynamic lift at a soft wall. Phys Rev Lett. 2018;120(19):198001.
Davies HS, et al. An integrated assay to probe endothelial glycocalyx-blood cell interactions under flow in.mechanically and biochemically well-defined environments. Matrix Biol. 2019;78-79:47–59.
Curry FE, Michel CC. The endothelial glycocalyx: barrier functions versus red cell hemodynamics: a model of steady state ultrafiltration through a bi-layer formed by a porous outer layer and more selective membrane-associated inner layer. Biorheology. 2019;56:113–30.
Curry FE. Layer upon layer: the functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc Res. 2017;113:559–61.
Xia Y, Fu BM. Investigation of endothelial surface glycocalyx components and ultrastructure by single molecule localization microscopy: stochastic optical reconstruction microscopy (STORM). Yale J Biol Med. 2018;91(3):257–66.
Groner W, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999;5(10):1209–12.
De Backer D, et al. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med. 2010;36(11):1813–25.
Dane MJ, et al. Association of kidney function with changes in the endothelial surface layer. Clin J Am Soc Nephrol. 2014;9(4):698–704.
Mulders TA, et al. Non-invasive assessment of microvascular dysfunction in families with premature coronary artery disease. Int J Cardiol. 2013;168(5):5026–8.
Amraoui F, et al. Microvascular glycocalyx dimension estimated by automated SDF imaging is not related to cardiovascular disease. Microcirculation. 2014;21(6):499–505.
Rovas A, et al. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit–the GlycoNurse study. Scand J Trauma Resusc Emerg Med. 2018;26(1):16.
Djaberi R, et al. Non-invasive assessment of microcirculation by sidestream dark field imaging as a marker of coronary artery disease in diabetes. Diabet Vasc Dis Res. 2013;10:123–34.
Fahraeus R. The suspension stability of the blood. Physiol Rev. 1929;9(2):241–74.
Fahraeus R, Lindqvist T. Viscosity of blood in narrow capillary tubes. Am J Phys. 1931;96:562–8.
Fedosov DA, et al. Blood flow and cell-free layer in microvessels. Microcirculation. 2010;17(8):615–28.
Pries AR, Secomb TW. Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol. 2005;289(6):H2657–64.
Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78.
Ogston AG, Phelps CF. The partition of solutes between buffer solutions and solutions containing hyaluronic acid. Biochem J. 1961;78:827–33.
Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92(3):1005–60.
Nieuwdorp M, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–32.
Michel CC, Curry FR. Glycocalyx volume: a critical review of tracer dilution methods for its measurement. Microcirculation. 2009;16(3):213–9.
Jacob M, et al. Technical and physiological background of plasma volume measurement with indocyanine green: a clarification of misunderstandings. J Appl Physiol (1985). 2007;102(3):1235–42.
Rehm M, et al. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95(4):849–56.
Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278(1):H285–9.
Lawson HC. The volume of blood-a crititcal examination of the methods for its measurement. In: Handbook of physiology. Washington, DC: Am Physiol Soc; 1962. p. 23–49.
Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of Guinea-pigs. J Physiology. 1952;118(2):228–57.
Goresky CA, Rose CP. Blood-tissue exchange in liver and heart: the influence of heterogeneity of capillary transit times. Fed Proc. 1977;36(12):2629–34.
van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457(6):1199–206.
Megens RT, et al. Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J Vasc Res. 2007;44(2):87–98.
van Haaren PM, et al. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285(6):H2848–56.
Muller-Reichert T, Verkade P. Introduction to correlative light and electron microscopy. Methods Cell Biol. 2012;111:xvii–xix.
Muller-Reichert T, Verkade P. Preface. Correlative light and electron microscopy II. Methods Cell Biol. 2014;124:xvii–xviii.
Bancroft JD, Gamble M. Theory and practice of histological techniques. Philadelphia, PA: Curchill Livingstone/Elsevier; 2008.
Scott JE, Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965;5(3):221–33.
Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell an impressive structure ex vivo but not in culture. Circulation Res. 2009;104(11):1313–7.
Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstaedt H, Oberleithner H, Kuempers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. Plos One. 2013;8(11):e80905.
Okada H, et al. Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit Care. 2017;21:261.
Ando Y, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8(1):17523.
Wagner RC, Chen SC. Ultrastructural distribution of terbium across capillary endothelium: detection by electron spectroscopic imaging and electron energy loss spectroscopy. J Histochem Cytochem. 1990;38(2):275–82.
Hegermann J, et al. Visualization of the glomerular endothelial glycocalyx by electron microscopy using cationic colloidal thorium dioxide. Histochem Cell Biol. 2016;145(1):41–51.
Betteridge KB, et al. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595(15):5015–35.
Ebong EE, et al. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol. 2011;31(8):1908–15.
Dyer DP, et al. Differential structural remodelling of heparan sulfate by chemokines: the role of chemokine oligomerization. Open Biol. 2017;7(1):160286.
Wiig H, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123(7):2803–15.
Michel CC, Woodcock TE, Curry FE. Understanding and extending the starling principle. Acta Anaesthesiol Scand. 2020. https://doi.org/10.1111/aas.13603.
Hahn RG. Understanding volume kinetics. Acta Anaesthesiol Scand. 2020;64(5):570–8. https://doi.org/10.1111/aas.13533.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Curry, F.R.E., Arkill, K.P., Michel, C.C. (2020). The Functions of Endothelial Glycocalyx and Their Effects on Patient Outcomes During the Perioperative Period: A Review of Current Methods to Evaluate Structure-Function Relations in the Glycocalyx in Both Basic Research and Clinical Settings. In: Farag, E., Kurz, A., Troianos, C. (eds) Perioperative Fluid Management. Springer, Cham. https://doi.org/10.1007/978-3-030-48374-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-48374-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48373-9
Online ISBN: 978-3-030-48374-6
eBook Packages: MedicineMedicine (R0)