Abstract
Hydatid disease is one of the major zoonotic diseases carrying significant burden on public health and economy, caused by the tapeworm Echinococcus which belongs to the family Taeniidae. Humans are considered the incidental intermediate host. The liver is the most commonly affected organ, followed by the lungs. However, any other human organ can potentially be involved. Primary extrahepatic hydatid disease has been reported in the abdominal cavity, retroperitoneum, spleen, kidneys, adrenals, and even myocardium. Parasites spread through portal circulation. Other courses of spread include lymphatic invasion by the parasite. The spread of hydatid disease in the abdomen can be along peritoneal fluid circulation or intra-abdominal recesses, which may explain intraperitoneal seeding. Treatment alternatives mainly involve anthelminthic therapy and interventional procedures including PAIR, PPDC, conservative surgical approach, and radical surgical resection (absolute pericystectomy or hepatectomy). Palliative treatment comprises of drainage of infected cysts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Hydatid disease is a common disease in agricultural and animal raising societies and is endemic in the Middle East, Far East, Mediterranean, South America, Australia, and Turkey [1, 2]. It is one of the major zoonotic diseases carrying significant burden on public health and economy [3]. Hydatidosis is the most pathogenic zoonoses in humans in the northern hemisphere with an annual incidence of up to 1.2 per 100,000 [4]. It is caused by the infection with the tapeworm Echinococcus which belongs to the family Taeniidae. Four species of Echinococcus potentially infect humans, with E. granulosus and E. multilocularis being the most common. The two other species are E. vogeli and E. oligarthrus, and these have only rarely been associated with human infection [5]. The chief morphological difference between the species of Echinococcus is the length of the tapeworm, where E. granulosus can reach up to 7 mm in length. The type of disease that occurs in humans depends on the species of Echinococcus involved. Three forms of Echinococcus infection have been identified: cystic, alveolar, and polycystic. The most common form is cystic echinococcosis which is caused by E. granulosus. The other forms, alveolar and polycystic echinococcosis, are rarely encountered in humans and are not as widespread as cystic echinococcosis. Infection with E. multilocularis is rare; however, it is the most virulent species and can cause alveolar echinococcosis [3].
The clinical course of echinococcal infection is highly variable and depends on the localization, extent, and proliferation of the larval tissue as well as on the host reaction. The clinical presentation of Echinococcus infection depends on the site of the cysts and their sizes. Small and/or calcified cysts may remain asymptomatic indefinitely. However, symptoms due to mass effect within organs, obstruction of blood or lymphatic flow, or complications such as rupture or secondary bacterial infections can occur [6]. Many infections are acquired in childhood but do not cause clinical manifestations until adulthood. Also, latent periods before symptoms arise have been reported. While approximately 50% of detected cases occur in asymptomatic patients, many more cases remain undiagnosed or are found incidentally at autopsy. Humans are considered the incidental intermediate host. Upon ingestion, the larval stage will emerge in the bowels, invade into the blood vessels, and migrate into almost every organ of the body [7] The liver is the most commonly affected organ with Echinococcus infection, followed by the lungs. However, any other human organ can potentially be involved. Primary extrahepatic hydatid disease has been reported in the abdominal cavity, retroperitoneum, spleen, kidneys, adrenals, and even myocardium [8, 9].
Normally parasites spread through portal circulation. Other courses of spread include lymphatic invasion by the parasite. The spread of hydatid disease in the abdomen can be along peritoneal fluid circulation or intra-abdominal recesses, which may explain intraperitoneal seeding. After infection with this disease, patients can remain asymptomatic for long time before presenting with symptoms. Most patients with abdominal extrahepatic hydatid disease present with abdominal discomfort, some may present with anaphylaxis or fever. However, a sudden abdominal pain in these patients may indicate intracystic bleed, rupture, or infection, with intracystic bleeding being the most dangerous complication [10]. Diagnostic challenges are imposed on clinicians due to the non-specific clinical presentation of this disease, especially in extra-abdominal hydatid disease. Ultrasound (US) and computed tomography (CT) scan usually confirm the diagnosis with a sensitivity of 93–98% and 97%, respectively. Most patients have a single organ involvement (most commonly the liver), and a single cyst is present in more than 70% of cases [11].
Treatment of small hydatid calcified cysts doesn’t always require surgical treatment and can be managed conservatively. Treatment alternatives mainly involve anthelminthic therapy and interventional procedures including PAIR (puncture, aspiration, injection, respiration), PPDC (percutaneous puncture with drainage and curettage), conservative surgical approach (open cystectomy with or without omentoplasty), and radical surgical resection (absolute pericystectomy or hepatectomy). Palliative treatment comprises of drainage of infected cysts. Laparoscopic or open drainage procedures include aspiration, injection of scolicidal agent, deroofing, evacuation, and conversion of the cyst into a non-dependent cavity [12].
Life Cycle
The life cycle of Echinococcus infection was first described by Haubner [12]. The disease is transmitted when food/water containing parasitic eggs is eaten, or through a close contact with an infected animal. The eggs are released in the stool of carnivores that are infected by the parasite. Commonly infected animals include dogs, foxes, and wolves. An adult worm resides in the small intestine of a definitive host. The eggs get released from gravid proglottids that are passed in the feces that get ingested by an intermediate host. The eggs hatch in the intestines of the intermediate host and release oncospheres that penetrate through the intestinal wall and target organs such as the liver and lungs developing into a cyst(s). The membranes and capsule provides the parasite with immunity against destruction. The cyst gets enlarged, creating protoscolices with daughter cyst. These cysts may contain thousands of protoscolices, and this fast reproductive potential poses a problem in the intermediate host. The definitive host then becomes infected after ingesting the infected intermediate host. After ingestion, the protoscolices attach and then develop into adult worms, and the cycle starts all over again. Humans get infected when they get in contact with infected animal hosts [13].
Extrahepatic Anatomical Locations of Disease
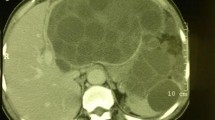
Splenic Hydatidosis
The first case report of splenic hydatid disease discovered on autopsy was published by Berthelot in 1790 [14]. Splenic hydatidosis accounts for around 0.5% of all patients with echinococcosis and up to 4% of the abdominal hydatid disease [9]. Patients are classified as either primary or secondary (more common) with other organs involved. The rare isolated contamination of the spleen by echinococcus is related to the anatomy of the portal vein system and the flow of the embryos of the tapeworms via blood. Sometimes, the eggs may penetrate the circulation without forming cysts in the liver, thus allowing them to settle in other organs via the blood stream. However, it is also possible that when a human host has a fissured cyst, scolices or daughter hydatid cysts can migrate through the wall of the cyst. They can be released to the body and may form new cysts in nearby or distant organs, such as the spleen, and this is known as secondary echinococcosis [7, 11]. Other hypotheses also attempted to explain the spread of this disease directly to the spleen, including a portal vein retrograde extension from the liver to the spleen or via systemic dissemination. The long-term outcome is variable and many patients remain asymptomatic. The splenic hydatid cyst exhibits a variety of clinical features, requiring a high index of suspicion for diagnosis [13]. The rarity of splenic hydatid disease imposes a diagnostic challenge for clinicians, especially in non-endemic areas. Splenic hydatid disease is quite a slowly progressing disease rendering patients completely asymptomatic for years [15]. Accordingly, diagnosis is made either as an incidental finding or as part of workup for non-specific abdominal complaints. Today, the diagnosis of splenic hydatid disease is greatly aided by ultrasonography (US) and computed tomography (CT) [15, 16]. Differential diagnosis of splenic cysts includes cystic neoplasms, abscesses, and benign cysts, which are categorized into true cysts and pseudocysts. True cysts include parasitic (hydatid) and non-parasitic cysts (epidermoid, dermoid, hemangiomas, and lymphangiomas). Pseudocysts are mainly traumatic (hematoma). Several therapeutic options are present. However, the most effective methods comprise excision of the infected tissues, either surgically or the through less invasive procedures such as puncture-aspiration-injection-reaspiration (PAIR) approach. Treatment options are usually individualized based on presentation and medical status of patients. Nevertheless, it is still controversial if performing total splenectomy is more beneficial than performing spleen sparing surgical approaches [14]. Literature that focuses on splenic hydatid disease is still based on individual cases with weak evidence to support splenectomy, spleen-preserving surgery, or interventional treatment options. It has been described that large or multiple splenic cysts are best managed surgically eliminating the higher risk of rupture and its complications including anaphylaxis, septic shock, and bleeding. A spleen sparing approach is advisable when the cysts are peripheral at the surface of the spleen [17]. However this approach potentially carries a higher risk of blood loss compared to unroofing and evacuation of hydatid cysts. Meimarakis et al. have advocated, based on their case series of ten patients, for total splenectomy as the best treatment option for complete removal of infected tissues and avoiding potentially infected remnant cavity, bearing in mind post-splenectomy complications [17]. Prophylactic antiparasitic therapy may reduce recurrence rates. In addition, preoperative or early postoperative vaccination is important for surgical patients in order to decrease the risk of post-splenectomy sepsis which can be life-threatening [17].
Pulmonary Hydatidosis
Hydatid disease of the lung can be primary or secondary. Initially, patients are asymptomatic. Of patients with pulmonary hydatid disease, around 20–40% also have liver involvement. Pulmonary involvement appears to be more common in pediatric population [18]. Usually, the evolution of a hydatid cyst in any sold organ is affected by several factors including the texture of the organ itself, and the elasticity of surrounding tissues and organs. The rate of growth of hydatid disease in the lungs is faster than that of the liver since lungs are softer in consistency compared to the liver. Accordingly, the growth rate of pulmonary hydatidosis is faster in children compared to adults, since children have more elastic tissues. In addition, the location of the lungs in the thorax puts it under negative pressure which may also accelerate the growth rate of hydatid cysts [18]. Imaging is the principal investigational modality for pulmonary hydatid cyst including chest X-ray, CT, or magnetic resonance imaging (MRI) of the lungs. Radiological presentation of hydatid disease varies based on the presence or absence of complications [18]. What is special about pulmonary hydatid disease is that it does not lead to calcification of cyst wall and rarely causes formation of daughter cysts, thus making the radiological diagnosis more challenging. The role of ultrasound is limited due to the ribcage, except when lesions are peripheral. Complications of pulmonary hydatidosis include rupture, secondary bacterial infection, pneumothorax, and bronchial fistulization, all of which are serious complications [18]. Rupture of cyst is the most frequent complication occurring in almost half of patients. It can occur intrabronchially or into the pleural cavity. Intrabronchial rupture can cause hypersensitivity reactions and hydatoptysis which is the expectoration of cyst membranes and even parasites. Hydatid cysts can even erode into major vessels such as the aorta [19]. Echinococcosis can involve the pleural cavity, mediastinum, or chest wall. Secondary involvement of the diaphragm and thoracic cavity with hydatid disease occurs in 1–16% of cases of hepatic hydatid disease [20]. Surgical intervention in the treatment of pulmonary hydatid disease is the treatment of choice; however pharmacotherapy is helpful as an adjunct therapy. Surgery is indicated in most cases as operative mortality is low (1–2%), morbidity rates are acceptable, and the recurrence rate is also low (1–3%) [21]. Indications for surgical intervention include large cysts that are peripheral with risk of rupture, infection, anatomical location, and presence of substantial mass effect. The most important technical point is to remove the entire cyst and preserve as much lung parenchyma as possible. Surgical techniques include enucleation, pericystectomy, cystostomy, open aspiration, and lung resection [19]. Enucleation is suitable only for small cysts less than 5 cm in diameter. Surgical intervention for pulmonary hydatid disease is a safe intervention with good outcomes, very low morbidity, and mortality. A meta-analysis by Athanassiadi et al. of 4255 patients with pulmonary hydatid disease showed that surgical intervention had only a mortality rate of 1.45% and a morbidity rate up to 17% with excellent cure rate [22]. Video-assisted thoracoscopic surgery (VATS) is becoming a very useful technique in the field of thoracic surgery. It has been employed for the removal of peripheral and small cysts with lower morbidity compared to conventional surgery [19].
Renal Hydatidosis
Renal hydatid cyst is one of the rare presentations of hydatid disease and constitutes up to 3% of all cases. Patients may not report any symptoms for years [23]. Disease involving the kidney may progress slowly with vague complaints, leading to progressive damage of the kidney putting it at risk of loss of function. Early diagnosis is mandatory using a combination of modalities including proper clinical history, imaging, and laboratory testing. Cyst rupture into the urinary collecting system leading to hydatiduria is one of the complications although it is seen in only 10–20% of cases and is usually microscopic rupture only. Gross rupture and passage of cyst contents is uncommon, but if it is present, it makes the diagnosis much easier. Renal ultrasound helps in the diagnosis when daughter cysts and floating membranes are visualized; however the accuracy of ultrasound remains operator dependent. A CT scan remains the gold standard for proper diagnosis with an accuracy of 98% and extremely high sensitivity to visualize daughter cysts [24] PAIR is usually successful for renal hydatid cysts. However, surgery remains the best modality of treatment and disease control via renal sparing cystectomy avoiding renal parenchymal destruction [23, 24]. Nephrectomy must be reserved for extreme cases of destroyed kidneys only.
Rare Locations
Rare locations for hydatid disease have been reported and include the myocardium, brain, spine, adrenal gland, pancreas, gallbladder, ovaries, and bones.
Brain involvement with hydatid disease is rare and has been reported in up to 2% of all cases [25]. Patent ductus arteriosus and patent foramen ovale are recognized risk factors. Primary hydatid disease of the brain can present clinically as intracranial space occupying lesion with clinical manifestations based on the location of the cyst. This is more common in children. Surgery remains the best therapeutic option with acceptable rates of morbidity and low mortality [26].
Primary adrenal hydatid disease accounts for only 0.5% of all reported cases [27]. One of the reported cases included a case of adrenal hydatidosis complicated by fistulization to small bowels that was managed through laparotomy and en bloc resection of the mass.
The incidence of cardiac echinococcosis is about 0.03–1.1% of all cases of Echinococcosis. Surgery is mandatory in most cases due to the risk of rupture and anaphylactic shock. However, early detection of cardiac echinococcosis is challenging, since symptoms manifest only when the cyst is large enough to compress adjacent structures or to rupture. Cardiac hydatid cysts are characterized by multiple thin walls with a high tendency to invade the myocardium [28]. Cardiac magnetic resonance (CMR) imaging is a useful tool for the diagnosis and presurgical planning .
Hydatid disease can manifest initially in the pancreas in a small proportion of patients. The head of the pancreas is more commonly involved. It can be misdiagnosed as a pancreatic pseudocyst or cystic neoplasms of the pancreas. Thus, it should be thought of as part of the differential diagnosis in patients with cystic lesions of the pancreas in endemic regions [29]. Surgery remains the best definitive diagnostic and therapeutic tool available. Management options include laparoscopic cyst excision, percutaneous drainage, or cystectomy with deroofing and drainage [29].
The gallbladder is one of the least common anatomical locations for primary hydatid disease. So far, only five cases have been reported in the medical literature [30]. One of the hypotheses to explain the pathogenesis of gallbladder hydatid disease is the contamination of gallbladder via biliary route. Proper diagnosis can be difficult in terms of localization of primary gallbladder hydatid cysts, which is not always easy due the anatomical proximity of the gallbladder to the liver. Total pericystectomy with cholecystectomy is the classical surgical approach, with careful dissection at the pericystic wall to avoid any biliary injuries or radical liver resection [30, 31].
Hydatid disease can affect the spine in 0.2–1% of reported cases. Unless there is a clinical suspicion of hydatid disease of the spine to consider performing neuroimaging, this curable disease will be missed by most clinicians. It may manifest through compression symptoms, most commonly presenting with radiculopathy [32]. Magnetic resonance imaging (MRI) is the preferred imaging modality in the diagnosis of spinal hydatid disease. The initial treatment option is surgical excision for neural decompression. The type of surgical intervention, extent of resection, and the need for spinal stabilization depend on the location and extent of destruction leading to spinal instability [33].
Role of Serological Testing
Laboratory tests for Echinococcus infection include Casoni intradermal skin test, Weinberg complement fixation (CF) test, hemagglutination inhibition (HAI), enzyme-linked immunosorbent assay (ELISA), and IgE testing. These tests help in establishing the diagnosis; however false-negative rates are concerning especially in cases of intact hydatid cysts whereby the antigens are sequestered [27, 34]. In addition, it has been shown that the rates of seropositivity are generally higher in liver hydatid disease compared to other organ involvement. Hepatic hydatid disease has been confirmed in 80–94% of the patients via immunologic tests, but extrahepatic hydatidosis was only confirmed in 65% of cases, even if multiple tests were applied.
Role of Anthelminthic Therapy
In most cases, pre- and postoperative courses of albendazole ought to be considered to sterilize the cyst, to diminish the opportunity of anaphylaxis, to diminish the pressure in the cyst wall, and to decrease the recurrence rate. Albendazole inhibits tubulin, causing blockage of glucose absorption, leading to glycogen consumption and degenerative alterations in the endoplasmic reticulum and mitochondria of the germinal layer, in this way enhancing lysosomal activity inducing cell autolysis [35]. A study demonstrated that surgery combined with antiscolicidal agents such as albendazole showed better results in terms of treatment and recurrence rates compared to surgery alone [36]. Albendazole therapy requires a minimum period of 11 days to have an incremental response, with a recommended dosage of 10–15 mg/kg/day, twice daily. However, there are several contraindications for anthelminthic therapy, including risk of rupture especially for large cysts, bone marrow suppression, and pregnancy especially in the first trimester [37].
Conclusion: Hydatid disease is a common disease in agricultural and animal raising societies and is considered endemic in the Middle East. It carries significant burden on public health and economy. Extrahepatic hydatid disease has been reported in several anatomical locations including the abdominal cavity, retroperitoneum, spleen, kidneys, adrenals, and even myocardium. Clinicians shall keep a low threshold for proper diagnosis of hydatid disease and consider the best curative approach whether surgical or conservative based on the clinical presentation, location, and extent of hydatid disease.
References
Rasheed K, Zargar SA, Telwani AA. Hydatid cyst of spleen: a diagnostic challenge. N Am J Med Sci. 2013;5(1):10–20.
Ramia-Angel JM, Gasz A, de la Plaza-Llamas R, Quinones-Sampedro J, Sancho E, Garcia PJ. Hidatidosis of the spleen. Pol Przegl Chir. 2011;83(5):271–5.
Sarkar M, Pathania R, Jhobta A, Thakur BR, Chopra R. Cystic pulmonary hydatidosis. Lung India. 2016;33(2):179–91.
Neil M, Burgkart RH, Guntmar G, Von Eisenhart-Rothe R. Primary extrahepatic alveolar echinococcosis of the lumbar spine and the psoas muscle. Ann Clin Microbiol Antimicrob. 2011;10:13.
Ameur HB, Affes N, Abdelhedi C, Kchaou A, Boujelbene S, Beyrouti MI. Hydatid cyst of the spleen: Tunisian series of 21 cases. Indian J Surg. 2015;77(Suppl 2):515–9.
Bourée P. Hydatidosis: dynamics of transmission. World J Surg. 2001;25:4–993.
Manouras AJ, Nikolaou CC, Katergiannakis VA, et al. Spleen-sparing surgical treatment for echinococcosis of the spleen. Br J Surg. 1997;84:1162.
Romig T. Epidemiology of echinococcosis. Langenbeck's Arch Surg. 2003;388:209–17.
Magistrelli P, Masetti R, Coppola R, Messia A, Nuzzo G, Picciochi A. Surgical treatment of hydatid disease of the liver. World J Surg. 1991;126:518–23.
Prousalidid J, Tzardinoglou K, Sgouradis L, Katsohis C, Aletras H. Uncommon sites of hydatid disease. World J Surg. 1998;22:17–22.
Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–35.
Brunt LM, Langer JC, Quasebarth MA, et al. Comparative analysis of laparoscopic versus open splenectomy. Am J Surg. 1996;172:596–601.
Mokhtari MAM, Spoutin A. Splenic hydatid cyst and relevation with anaphylaxis. Res J Med Sci. 2008;2:248–50.
Eris C, Akbulut S, Yildiz MK, et al. Surgical approach to splenic hydatid cyst: single center experience. Int Surg. 2013;98(4):346–53.
Caremani M, Benci A, Maestrini R, Accorsi A, Caremani D, Lapini L. Ultrasound imaging in cystic echinococcosis. Proposal of a new sonographic classification. Acta Trop. 1997;67:91–105.
Gossios KJ, Kontoyiannis DS, Dascalogiannaki M, Gourtsoyiannis NC. Uncommon locations of hydatid disease: CT appearances. Eur Radiol. 1997;7:1303–8.
Meimarakis G, Grigolia G, Loehe F, Jauch KW, Schauer RJ. Surgical management of splenic echinococcal disease. Eur J Med Res. 2009;14(4):165–70.
Sarkar M, Pathania R, Jhobta A, Thakur BR, Chopra R. Cystic pulmonary hydatidosis. Lung India. 2016;33(2):179–91.
Harris DG, Van Vuuren WM, Augustyn J, Rossouw GJ. Hydatid cyst fistula into the aorta presenting with massive hemoptysis. Case report and literature review. J Cardiovasc Surg. 2001;42:565–7.
Gomez R, Moreno E, Loinaz C, et al. Diaphragmatic or transdiaphragmatic thoracic involvement in hepatic hydatid disease: surgical trends and classification. World J Surg. 1995;19:714–9.
Dogan R, Yuksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1055 patients. Thorax. 1989;4(4):192–1999.
Athanassiadi K, Kalavrouziotis G, Loutsidis A, Bellenis I, Exarchos N. Surgical treatment of echinococcosis by a transthoracic approach: a review of 85 cases. Eur J Cardiothorac Surg. 1998;14:134–40.
Zmerli S, Ayed M, Horchani A, Chami I. El ouakdi M, Ben slama MR. Hydatid cyst of the kidney: diagnosis and treatment. World J Surg. 2001;25(1):68–74.
Reza HAM, Rreza G, Nastaran B, Mousa M. Renal hydatid cyst; a rare infectious disease. Oxf Med Case Reports. 2019;2019(3):omz011.
Senapati SB, Parida DK, Pattajoshi AS, Gouda AK, Patnaik A. Primary hydatid cyst of brain: two cases report. Asian J Neurosurg. 2015;10(2):175–6.
Ali M, Mahmood K, Khan P. Hydatid cysts of the brain. J Ayub Med Coll Abbottabad. 2009;21(3):152–4.
Ruiz-rabelo JF, Gomez-alvarez M, Sanchez-rodriguez J. Rufian peña S. complications of extrahepatic echinococcosis: fistulization of an adrenal hydatid cyst into the intestine. World J Gastroenterol. 2008;14(9):1467–9.
Su L, Yu J, Dai C, Liu Y, Peng L. Echinococcosis in left ventricle: a case report. Medicine (Baltimore). 2019;98(16):e15267.
Ahmed Z, Chhabra S, Massey A, et al. Primary hydatid cyst of pancreas: case report and review of literature. Int J Surg Case Rep. 2016;27:74–7.
Krasniqi A, Limani D, Gashi-luci L, Spahija G, Dreshaj IA. Primary hydatid cyst of the gallbladder: a case report. J Med Case Rep. 2010;4:29.
Noomene R, Ben Maamer A, Bouhafa A, Haoues N, Oueslati A, Cherif A. Primary hydatid cyst of the gallbladder: an unusual localization diagnosed by magnetic resonance imaging (MRI). Pan Afr Med J. 2013;14:15.
Bhake A, Agrawal A. Hydatid disease of the spine. J Neurosci Rural Pract. 2010;1(2):61–2.
Khazim RM. Spinal hydatid disease. South Med J. 2006;99:114.
Illuri HR, et al. Hydatid disease: a 2 years retrospective study in a tertiary care center in South India. Inte Surg J. 2018;5(2):602–7.
Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37:1679–84.
Karavias DD, Vagianos CE, Bouboulis N, et al. Improved techniques in the surgical treatment of hepatic hydatidosis. Surg Gynecol Obstet. 1992;174:176–80.
Stamatakis J. Hepatic hydatid disease. In: Johnson CD, Taylor I, editors. Recent advances in surgery (17). London: Churchill Livingstone; 1994. p. 35–48.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faraj, W., Nassar, H., Zaghal, A., Khalife, M. (2020). Management of Extrahepatic Abdominal Echinococcal Disease and Its Complications. In: Tsoulfas, G., Hoballah, J., Velmahos, G., Ho, YH. (eds) The Surgical Management of Parasitic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-47948-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-47948-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47947-3
Online ISBN: 978-3-030-47948-0
eBook Packages: MedicineMedicine (R0)