Abstract
Extremophilic microorganisms usually inhabit extreme ecosystems and show optimal growth under extreme conditions. Extreme conditions are defined as those far from the optimal conditions for human life. Thus, ecosystems that present very low or high temperature, pH values, or radiation index, habitats containing a NaCl concentration higher than 1.0 M and deep sea, among others, are considered extreme ecosystems. Derived from these physicochemical conditions, extreme ecosystems have an interesting biodiversity that could represent an attractive source of novel extremozymes. The use of many methods to ensure the bioprospection is needed to find robust enzymes with industrial applications. In this sense, dependent and independent culture approaches are successfully used to identify and characterize novel biocatalysts as laccases. Laccases are copper-containing oxides enzymes found in many organisms (plants, bacteria, fungi, insects, oysters), and they have a key role in the lignin deconstruction. These enzymes catalyze the monoelectronic oxidation of a huge range of substrates. Especially, microbial laccases have been extensively studied since structural and functional views. These oxidoreductases are frequently extracellular proteins, and the interest in their applications has significantly grown in recent years. Currently, laccase-based industrial processes as green-clean bioprocesses are very attractive for the biotechnology. Thus, the search for new genes encoding laccases is an important objective to supply the increasing demand of the bioprocess-based industry. In this chapter, a discussion of the structure and application of laccases will be presented, with an emphasis in extremophilic laccases. Also, a revision related to biotechnological applications using extremophilic laccases will be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One of the main concerns in current biotechnology industries is to increase efficiency and competitiveness in the face of challenges imposed by the well-established chemistry industry. In some industrial fields, shifting the framework toward biotechnological processes is not yet affordable (Chen and Jiang 2018). Even though biotechnological solutions promise to be a “green” alternative, the truth is that current methods have still many shortcomings that should be addressed to effectively introduce biological treatments in the market. Among others, biotechnological processes:
-
• Rely on high-energy consumption (for sterilization, optimal temperature stabilization, aeration, agitation).
-
• Have a considerable production of wastewater.
-
• Are prone to microbial contamination.
-
• Are more time-consuming due to constraints imposed by microorganism growth rates.
-
• Are not easily adapted to continuous flow as many fermentations are best held on batch.
-
• Are not easily automated as many variables require expert assessment.
-
• Broth media is difficult to recycle.
-
• Product separation and purification are lengthy and expensive.
-
• Require high infrastructure investment.
-
• Require highly trained personnel.
In recent years, extremophiles have arisen as a promising tool to overcome many of these difficulties. Imposing extreme conditions such as high salinity, low or high pH, low temperatures, or high solute concentration can significantly reduce the possibilities of microbial contamination in the processes. Using extreme conditions would eliminate the requirement of sterility and the associated cost (Chen and Jiang 2018). Also, since there is a low risk of contamination, batch fermentations are no longer a necessity, and continuous flow processes could be designed. Altogether, these changes could considerably reduce the cost of infrastructure. Then, the main challenge becomes screening for extremophile microorganisms and their enzymes and understanding their mechanisms to thrive in extreme environments.
Extreme ecosystems (i.e., salterns, stromatolites, acidic hot springs, hydrothermal vents, alkaliphilic lakes, and acid water of mines) could harbor an enormous diversity of unknown laccase-like enzymes. Because our knowledge about laccase genes in extreme ecosystems is still very limited, we even cannot visualize the great biotechnological potential that extremophile laccase-like enzymes could represent. Considering that industrial applications demand large-scale enzyme productions, laccases isolated from extremophiles need to be heterologously expressed in different hosts. Currently, the heterologous overexpression is considered the main bottleneck for the laccase industry. There are serious restrictions with the expression levels of many extremophile laccases in classical industrial hosts such as Escherichia coli, Pichia pastoris, Saccharomyces cerevisiae, and Aspergillus niger. Particularly “rare laccase genes” isolated from extremophile microorganisms impose serious limitations for the protein heterologous expression, because the codon usage and the promoter regulation can negatively impacting on recombinant laccase production in different hosts. In addition, in many cases posttranslational modifications are also needed to obtain functional extremophile laccases. Thus, new industrial heterologous systems with a particular focus on extremophile enzymes (i.e., laccases) are needed to increase the chances of producing sufficient levels of extremozymes. As we later discuss, after recombinant laccases are successfully produced, they are useful for a huge range of biotechnological applications. The biochemical properties of the extremophile laccases allow their application under critical conditions such as high or low temperature, huge range of pH, among others.
2 Extremophile Microorganisms
Extremophiles are a heterogeneous group of microorganisms growing under extreme conditions. Extreme environments are characterized by very particular physicochemical conditions; for example, very high or low temperature, over 1 atm hydrostatic pressure, extremely acid or alkaline pH values, high radiation index, severe nutrient limitations, hypersalinity and low water activity, among others.
According to the environmental requirements for these microorganisms to grow optimally, they can be classified as thermophiles or hyperthermophiles (when >40 °C is required), psychrophile (when <15 °C is required), acidophiles or alkaliphiles (when acid or alkaline pH values are required), halophiles (when >0.5 M NaCl is required), and barophiles (when >1 atm is required). Other classifications include the term xerophiles for microorganisms requiring low water activity (<0.8) at optimum growth. Organisms that tolerate these adverse conditions but have their optimal growth rate under standard conditions are defined as extremotolerant.
Interestingly, many environments show a combination of these conditions. For example, deep oceans and deep-sea sediments combine high pressure, high salinity, and low temperature; hydrothermal vents have high temperature and high pressure with a low content of organic matter; acid mine drainages are characterized by low pH values (<3), high heavy metal concentrations, and low nutrient availability. All these habitats are classified as poly-extreme, and their endogenous microbiota is considered poly-extremophilic.
Extreme environments are widely distributed around the world. In addition to those previously mentioned, these include volcanoes, arid and hyperarid zones, hypersaline soils, salterns, deserts, alkaline or acid lakes, and frozen habitats, among others. Another type of extreme environments has attracted our attention in recent years. Certain habitats are classified as extreme due to high levels of environmental pollution, which imposes several restrictions for life. These environments are derived from anthropogenic activities such as industrialization and are also colonized by a huge microbial biodiversity that can withstand high levels of toxic compounds.
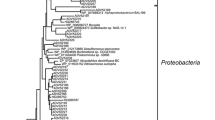
Extremophiles include microorganisms belonging to the three domains of life, Archaea, Bacteria, and Eukarya. Figure 1 displays a phylogeny representing extremophilic microorganisms of different genera and their extremophilic characteristics. Historically, Archaea and Bacteria have been largely studied as extremophile microorganisms. However, the study of extremophilic fungi has been recently addressed (since the last 20 years) (Gunde-Cimerman et al. 2003). Extremophiles have emerged as a unique opportunity to analyze the biology in the limits of life in a planetary context (Merino et al. 2019). Frequently, the study of extremophilic microorganisms has been related to the origin of life, as well as with the potential search of life in alternative biospaces. Over the past several decades, the search for life in planets or celestial bodies different than Earth has been a human obsession. Extremophiles are biological systems that offer a great opportunity to analyze biological behaviors in conditions that could simulate other planetary bodies. In this context, the Atacama Desert has been the classical environment since its soil composition mimics the Mars’ surface. This is one of the oldest, arid, and most hostile deserts on Earth with <1 mm of rain per year. For many years it was considered as uninhabited territory due to its poly-extreme features. However, the study of its autochthone microbiota that includes archaeal, bacteria, fungi, protozoa, algae, and viruses has allowed establishing “the basis for a new perspective for life at this poly-extreme territory” (cited from Gómez-Silva 2018).
Phylogenetic tree showing the extremophiles and the resistant characteristics that appear in at least one species of each genera, identified with the color code. Reprinted from Leal G, Ferreira D, and Vermelho A, Marine extremophiles. A source of hydrolases for biotechnological applications. Mar Drugs 2015, 13(4), 1925–1965. Copyright © 2015 the authors; licensee MDPI, Basel, Switzerland. Open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/)
For modern science, extremophiles are fascinating microbes because they can successfully live under conditions in which human life would be remotely imaginable. In contrast with our anthropocentric point of view, the Earth harbors many niches that represent no conventional habitable ecosystems. However, all of these are absolutely colonize by microorganisms with a huge diversity of molecular and cellular adaptations to survive under extreme conditions (Singh et al. 2019a, b). These microbes usually exhibit strong metabolic plasticity at the genomic level that reflects their potentialities to implement biochemical strategies to maintain the metabolic homeostasis under extreme conditions.
3 Structural Basis for Extremotolerance
An important aspect of using extremophilic strains for biotechnological processes is not only profiting from their optimal growth rate at extreme conditions but also ensuring the maximal activity of the desired enzymatic treatment. A strain which grows in acidic pH is of no use if the target enzyme required for the process is not produced or has suboptimal kinetic parameters under these conditions. Therefore, the characterization of enzyme activity at extreme conditions is also critical.
In general, halophilic enzymes that are stable at high salt concentrations can be used after or concurrently with chemical processes that use salts, acids, or bases. Psychrophilic enzymes can be applied in situations where the components of a reaction are heat sensitive. Thermophilic enzymes not only are stable under high temperatures but in many cases are also highly resistant to proteases, chaotropic agents, detergents, and organic solvents. All these adaptations of extremozymes could give a more versatile toolbox to couple biotechnological processes with the already efficient and established physicochemical processes in the industry.
The factors that influence the stability of extremozymes include changes in the distribution of hydrophobic patches and charges in the solvent-exposed surface of the protein, reduction in the size of loops and number of cavities, increase in the extent of secondary structure, and more extensive networks of ionic interactions stabilizing the tertiary structure as well as multimeric protein conformation.
For example, comparison of crystallographic structure between thermophilic and mesophilic proteins has revealed an increase of networks of ionic interactions in the former, which can have a stabilizing effect over a much longer range than hydrophobic interactions. Also, site-directed mutagenesis studies have shown that introducing mutations to achieve a more rigid core protein can also increase the stability at high temperatures. Finally, thermophilic enzymes have a lower occurrence of labile residues such as cysteine, asparagine, and aspartate.
Among laccases, several characteristics are proposed to contribute to thermal stability. One of the first identified characteristics is the high glycosylation content in some thermostable laccases, e.g., from Botrytis cinerea (49% glycosylation) (Slomczynski et al. 1995), Ceriporiopsis subvermispora (Fukushima and Kirk 1995), Postia placenta (40% glycosylation) (An et al. 2015), and Pycnoporus sanguineus (Dantán-González et al. 2008). For example, six and four N-glycosylation positions were predicted in the two laccases (PPLCC1 and PPLCC2, respectively) of Postia placenta (An et al. 2015). While PPLCC1 showed a high thermostability, PPLCC2 was drastically sensible at the temperature. Thus, the glycosylation extent of PPLCC1 (40%) vs 4.7% presents in PPLCC2 might partially explain the higher thermostability of PPLCC1 than PPLCC2 (An et al. 2015). Also, directed evolution of laccases from P. cinnabarinus (PcL) (Camarero et al. 2012) and M. thermophila (MtL) (Bulter et al. 2003; Zumárraga et al. 2007) showed that mutants with increased stability were hyperglycosylated, with sugar moieties contributing around 50% of their molecular weight. However, other studies suggest that the thermal stability of laccases is not explained exclusively by glycosylation (Koschorreck et al. 2008; Marques De Souza and Peralta 2003). In fact, as with other thermophilic proteins, the crystal structure of the B. subtilis CotA shows higher hydrophobic interaction between domains when compared with other monomeric LMCO (laccase-like multicopper oxidase) and laccases and reduced flexibility due to the increased content of proline residues (Enguita et al. 2003).
Contrary to proteins from thermophiles, psychrophilic enzyme adaptations must ensure the required flexibility for the catalytic mechanisms to occur. Therefore, the activity of cold-active enzymes often relies on decreasing the hydrophobicity in the core of the protein and decreasing the number of hydrogen bonds, the ion interaction networks or salt bridges, and the overall charge of the protein. Also, these enzymes must remain active at low water activity, i.e., when the water molecules are less available to interact with proteins as they are forming structured ice lattices. In these conditions, the enzymes must bind more tightly the water to ensure that there are multiple hydration layers supporting enzyme function.
At high salinity, water is sequestered in hydrated ionic structures, similarly as happens in ice structures, limiting the availability of free water molecules for protein hydration. Halophilic enzymes generally have numerous acidic residues located on the surface which increases the negative surface charge and therefore the hydration of the protein. The acidic residues on the surface of halophilic enzymes also reduce the hydrophobicity and promote electrostatic repulsion of the proteins. Additionally, genome-wide analysis of proteins from halophiles has shown a reduction in the number of lysine or larger hydrophobic residues (phenylalanine, isoleucine, and leucine) at the protein surface, consistent with a reduction in surface hydrophobicity and increased flexibility. These properties prevent the “salting-out” effect, i.e., the aggregation of proteins at high salt concentrations.
4 Extremophiles as a Source of Laccases: Some Examples
Extremophile microbes are an attractive source of enzymes for metabolizing recalcitrant compounds such as polycyclic aromatic hydrocarbons, pesticides, pharmaceutical compounds, polysaccharides, lignocellulose, and resins, among others. Enzymes produced by extremophiles are usually extremozymes as they display robust biochemical properties and are functionally active in extreme conditions (Singh et al. 2019a, b). From a biotechnological point of view, these enzymes are very attractive for industries due to their downstream applications as biocatalysts to produce fine chemicals such as biopolymers, biomaterials, and biofuels (Raddadi et al. 2015).
Extreme environments have been routinely explored to discover extremozymes. One example is the bioprospection of thermophiles and hyperthermophiles as a novel source of polysaccharide-degrading enzymes such as amylases, pullulanases, cellulases, hemicellulases, esterases, and pectinases. Some of these lignocellulolytic proteins displayed stability at temperatures higher than 70 °C (Elleuche et al. 2015). High-temperature biotechnological processes demand thermozymes, which should be functional at high temperatures (>60 °C). Functional-based and sequencing-based metagenomics have been successfully driven to describe new thermostable enzymes. Figure 2 shows a schematic representation to identify genes encoding thermozymes.
Schematic representation of high-throughput screenings for genes encoding thermozymes, cloning, and expression followed by biochemical characterization of biocatalysts and subsequent large-scale production for suitable industrial applications. Reprinted from Curr Opin Microbiol 25, Elleuche S, Schäfers C, Blank S, Schröder C, and Antranikian G, Exploration of extremophiles for high-temperature biotechnological processes, 113–119, copyright © 2015, with permission from Elsevier
Microorganisms inhabiting marine ecosystems, especially those found in deep sea, also constitute an interesting source of enzymes. Marine extremophiles include psychrophiles, halophiles, and piezophiles, and their enzymes are functionally active under harsh physical-chemical conditions (Leal et al. 2015). According to this, hydrolases from prokaryotes isolated from these environments have been largely investigated resulting in the characterization of several glycoside hydrolases useful for industrial applications.
The biobased industry is considered one of the best solutions to face global challenges: climate change, agriculture production, generation of new antibiotics and biomaterials, etc. The progress of this industry will positively impact on bioprocesses and will allow developing biological processes to convert biomass to high-value products (Krüger et al. 2018). Biobased economy, as also known, requires sustainable and high efficient eco-friendly technologies based on enzymes. Extreme habitats represent an excellent niche for this industry, since various sectors could be benefited with biocatalysts derived from ecosystems such as hot spring, salt lakes, polar sea ice, etc. Figure 3 shows the contribution of extremophilic microorganisms and their enzymes to biobased industry.
Contributions of extremophiles and their enzymes to bioeconomy. Cross-sectional technologies and other factors influencing a new emerging bioeconomy. Reprinted from New Biotechnol 40, Krüger A, Schaefers C, Schroeder C, and Antranikian G, Toward a sustainable biobased industry – highlighting the impact of extremophiles, 144–153, copyright © 2018, with permission from Elsevier
In the search for laccases from extremophiles, many efforts have been made. Miyazaki (2005) purified and characterized a hyperthermophilic laccase obtained from the thermophilic bacterium Thermus thermophilus HB27. This enzyme displayed an optimum temperature of 92 °C and a high thermostability (>14 h at 80 °C). The proteins showed activity on the canonical laccase substrates: guaiacol and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate). This laccase was ranked in 2005 as the most thermophilic laccase reported.
Recently, a new thermophile laccase was reported from Thermus sp., and its capacity to delignify lignocellulosic biomass was investigated. This enzyme shows activity on different laccase substrates such as 2,2′-azino-di-(3-ethylbenzothiazoline sulfonate) (ABTS) and 2,6-dimethoxyphenol (DMP). Also, the protein exhibited a high thermostability because retained 80% of its activity was exposed 16 h at 70 °C and revealed a copper-dependence activity (Navas et al. 2019). Moreover, this laccase evidenced biotechnological potentialities for delignification of Eucalyptus biomass.
A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum able to degrade a lignin model compound was described (Ghatge et al. 2018). This enzyme showed its optimum activity under alkaline conditions (pH = 8) and high temperature (70 °C). In addition, retained 80% of its activity after 24 h under thermoalkaliphilic conditions and its half-life was 12 h at 90 °C. This poly-extremophilic laccase displayed dependence of copper and manganese, while other metal ions did not affect the enzymatic activity. To investigate the properties as a biocatalyst for biotechnological applications, the enzyme catalyzed the conversion of guaiacylglycerol-B-guaiacyl ether (GGGE) to C5-C5 biphenyl tetramer, and the mechanisms for dimerization of a dimeric lignin model compound GGGE were proposed as Fig. 4 represents. The laccase of C. thermarum oxidized a wide range of substrates (2,6-DMP, ABTS, catechol, caffeic acid, ferulic acid, and phloroglucinol), which indicates that this laccase is a versatile enzyme.
Proposed mechanism of laccase for dimerization of a dimeric lignin model compound GGGE. Reprinted by permission from Springer Nature: Springer, Appl Microbiol Biotechnol 102(9), 4075–4086, A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum strain TA2.A1 able to catalyze dimerization of a lignin model compound, Ghatge S, Yang Y, Song WY, Kim TY, and Hur HG, copyright © Springer-Verlag GmbH Germany, part of Springer Nature 2018
Other thermotolerant bacterial laccases have been also characterized. For example, McoA was described as a thermostable laccase from the bacterium Aquifex aeolicus because it showed thermostability at 90 °C for 5 h (Fernándes et al. 2007). McoA was annotated as a multicopper oxidase and presents higher specificity for cuprous and ferrous ions than for aromatic compounds. According to this, McoA was classified as a metallo-oxidase. Bacillus has been also described as a bacteria genus with high potential to produce thermotolerant enzymes. Bacillus sp. PC-3 was reported as a novel thermophilic bacterium that excretes a thermostable laccase with a significant activity at a wider pH range (Sharma et al. 2018). In addition, Anoxybacillus sp. was also reported as a new thermophilic bacterium with hyperthermostable alkaline laccase activity (Al-balawi et al. 2017).
But not only thermophilic laccases have been studied. Halophilic bacterial laccases are also very attractive for biotechnological applications. With this in mind, a laccase produced by Aquisalibacillus elongatus was investigated resulting that the purified enzymes showed high stability against organic solvents, salts, metals, inhibitors, and surfactants. The enzyme is active in a huge range of pH (from 5 to 10) and revealed to be useful for industrial applications related to lignin deconstruction (Rezaei et al. 2017).
From Arctic marine environments, 13 laccase-positive Psychrobacter species were identified (Moghadam et al. 2016). It was demonstrated that some laccases are located on plasmids while others in the chromosomes of these psychrophilic bacteria. The laccase multicopper oxide exhibited activity on ABTS and guaiacol, and the enzymes also operated by Mg2+ and Ca2+ addiction. In addition, Cladosporium tenuissimum was classified as psychrotolerant bacterium that produces extracellular laccase activity at low temperature (Dhakar and Pandey 2016).
Also, laccases with high pH tolerance range have been also studied in the extremophile bacteria Thioalkalivibrio sp. (Ausec et al. 2015). This genus seems to be a potential source of robust bacterial laccases with a high tolerance to temperature and pH (Ausec et al. 2015). On other hand, Geobacillus thermopakistaniensis isolated from hot spring in Pakistan produces extracellular laccase isoforms highly stable to several halides (NaF, NaCl, NaBr) and organic solvents (methanol, ethanol, propanol, isopropanol) (Basheer et al. 2017).
5 Metagenomics as a Powerful Method for Laccase Bioprospection
Over the last two decades, omic technologies have changed the paradigm of enzyme bioprospection from extreme environments. Metagenomic-based approaches have allowed to explore a wide variety of different ecosystems and exploit their microbial biodiversity. Laccase bioprospection from extreme environments has gained attention since novel oxidoreductases could be discovered using both dependent and independent culture approaches. Figure 5 displays a general approach that could be useful for laccase bioprospection. Transcriptomic and proteomic can be considered as powerful strategies to discover laccases from extremophiles. On the other hand, functional metagenomics allows exploring genomes to identify genes encoding laccases. Independent culture methods could result in the discovery of new laccases with robust properties over a wider range of environmental conditions. However, from our point of view, one of the limitations for functional-based metagenomic screening is the activity-based method. Typically, these are based on the degradation of a specific substrate, and generally, a color change around the metagenomic clone is used to identify the positive clone screened. For laccases, substrates should be carefully selected to increase the success rate. PCR-based strategies also are an alternative to amplify laccase genes from a metagenome isolated from extreme environments. To do so, three different strategies could be used as Fig. 6 displays. PCR-based methods have been poorly employed to detect laccases from extreme ecosystems.
Culture-dependent and culture-independent approaches for biocatalytic screenings. Reprinted from Batista RA, del Rayo M, Talia P, Jackson SA, O’Leary ND, Dobson AD, and Folch-Mallol JL, From lignocellulosic metagenomes to lignocellulolytic genes: trends, challenges, and future prospects, Biofuel Bioprod Bior 10(6), 864–882, with permission from John Wiley & Sons (copyright © 2016 Society of Chemical Industry and John Wiley & Sons, Ltd)
Consensus, degenerate, and CODEHOPs primers for functional screenings. Reprinted from Batista RA, del Rayo M, Talia P, Jackson SA, O’Leary ND, Dobson AD, and Folch-Mallol JL, From lignocellulosic metagenomes to lignocellulolytic genes: trends, challenges, and future prospects, Biofuel Bioprod Bior 10(6), 864–882, with permission from John Wiley & Sons (copyright © 2016 Society of Chemical Industry and John Wiley & Sons, Ltd)
A novel polyphenol oxidase actives on a huge range of substrates (syringaldazine, 2,6 dimethoxyphenol, veratryl alcohol, guaiacol, tetramethylbenzidine, 4-methoxybenzyl alcohol, 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, phenol red) were characterized from metagenomic libraries obtained from bovine rumen microbiota (Beloqui et al. 2006). This enzyme showed activity in pH from 3.5 to 9.0 and was characterized as a new family of polyphenol oxidases. Other alkaline laccase was identified from a metagenomic library obtained from mangrove soil (Ye et al. 2010). However, laccase detection from functional-based screening is very difficult. In this sense, during the functional analysis of 52,000 metagenomic clones obtained from Antarctic DNA, any laccase activity was detected, while lipases, esterases, and cellulases were detected (Ferrés et al. 2015).
In addition to activity-based screening, sequence-screening strategies have been used for laccase detection. Using this methodology a new bacterial laccase was characterized from marine environments in South China Sea. This enzyme was overexpressed in E. coli and is active on syringaldazine and in high temperature. Moreover, this marine-derived laccase showed an alkaline activity, which is enhanced by chloride addiction (Fang et al. 2012). Genome mining is also useful for laccase bioprospection. It has demonstrated when an alkaline and thermostable laccase Ssl1 from Streptomyces sviceus was characterized (Gunne and Urlacher 2012). This phenol oxidase is a small laccase, and its activity is not affected by addiction of detergents and organic solvents. Metagenomic approaches have also derived in the crystallization of laccase. For example, a putative two-domain-type laccase detected from a metagenomic screening was characterized and structurally studied by X-ray analysis (Komori et al. 2009).
6 Pushing the Boundaries of Natural Laccases
As previously seen, there is great potential in screening microorganisms from diverse environments to obtain novel catalysts for industrial applications. Nevertheless, natural enzymes are not specifically suited to perform in settings with all the requirements of industrial processes, as the evolutionary pressures that have driven their changes were oriented to ensure fitness in a biological environment. As our understanding of the molecular dynamics and behavior of protein increases, new and innovative approaches to improve enzyme function and properties are gaining relevance. The engineering of protein structure and function can increase and expand enzyme applications in the industry. Obtaining engineered enzymes is generally achieved by two different strategies: directed evolution or rational design. Other strategies are the result of the combination of these methods.
Directed evolution is one of the most powerful approaches for improvement of enzyme function. The rationale of this method is to mimic the effect of evolution by applying a selective pressure through several generations of a protein population. In each generation, mutations are (randomly or selectively) introduced in the gene and evaluated to assess if the resulting protein can pass to the next generation. The major advantage of this method is that prior knowledge of the protein structure or molecular interactions is not required. The limitations though are the technical availability of a high-throughput screening method that can help select the proteins with the desired enhanced property and the high combinatorial number of mutations that must be screened if the sequence space is not constrained.
The method of directed evolution has been implemented both in vitro and in silico with very good results. In vitro strategies generally rely on the construction of gene libraries that are expressed in an appropriate host. Mutations can be introduced randomly by using physical or chemical mutagenic treatments or specifically by using several molecular biology techniques. To simulate the process in silico, an initial protein structure must be obtained or modeled, and the screening methods rely on evaluating protein structural properties that are previously known to correlate with protein function.
Many of the factors that determine enzyme stability and extremophile performance have been studied and are partially understood. Therefore, several attempts have been made to apply that knowledge for obtaining engineered proteins that display desirable characteristics. In the case of laccases, many efforts have been oriented to increase the redox potential of the enzymes in order to improve their catalytic properties, but some studies have been also pursued an increase in optimal temperature and stability. For example, Mateljak et al. (2019) screened a library of laccase variants derived by multiple strategies of recombination and mutation of an original gene from a basidiomycete strain (PM1). The natural laccase produced by this strain had an optimal enzymatic activity at 80 °C (Coll et al. 1993a, b), ensuring a good starting point for improvement. The library was subjected to sequential rounds of computationally guided mutagenesis and directed evolution, and the resulting laccase product not only showed increased redox potential but also had increased stability to thermal denaturation (near 3-fold increase in the halftime of the enzyme activity at 70 °C) and pH (around 18-fold increase in stability at pH 2 after 96 h) when compared to a previously obtained mutant. One interesting finding in this study was that the introduction of hydrophobic residues that “sheltered” the copper catalytic site from solvent exposure increased the stability of the enzymatic activity. This phenomenon was also observed in some natural laccase with high thermal stability, suggesting this could be a structural feature of thermophilic/thermotolerant laccases (Mateljak et al. 2019). In another example, Sheng et al. (2017) used the methodology of Bacillus subtilis spore display to screen laccase mutants with increased pH stability, reaching a 62-fold increase in half lifetime at pH 4 when compared with the wild-type laccase.
In contrast to directed evolution, the rational design method is based on the understanding of the enzyme structure-function relationship. In this case, targeted mutations are oriented to change a structural property of the protein that is already known to be involved in its function. In the case of laccases, an example of site-directed mutagenesis showed that changes in a loop of a native laccase from Bacillus HR03 increased thermal stability and indirectly improved kinetic parameters, apparently by the stabilizing effect of the mutations (Mollania et al. 2011).
Figure 7 illustrates all the functional properties of laccases that have been engineered with particular industrial purposes in mind. All these examples show that improving the function and stability of laccases is a promising field of research that will enable obtaining more efficient biocatalysts with improved stability to be used in industrial processes.
Designer laccases. Modified, mass-produced laccases with improved stability, activity, and specificity will emerge tailor-made for disparate industrial purpose. Reprinted from Trends Biotechnol 28(2), Rodgers CJ, Blanford CF, Giddens SR, Skamnioti P, Armstrong FA, and Gurr SJ, Designer laccases: a vogue for high-potential fungal enzymes? 63–72, copyright © 2010, with permission from Elsevier
7 Biotechnological Applications of Extremophilic Laccases
Extremophilic enzymes usually withstand harsh conditions (pH, salinity, high or low temperatures, etc.), so many have been proven to show advantages for biotechnological processes, which mesophilic enzymes cannot withstand (Chandra et al. 2017). These enzymes could perform better in several applications in environmental, industrial, medical, chemical, pharmaceutical, textile, agriculture, food or animal feed, and biofuels industries (Ibrahim and Ma 2017). Besides, an important increase in the global market demand of enzymes has been registered, and it was estimated to be USD 4.61 billion in 2016 and is projected to reach USD 6.30 billion by 2022 (Industrial Enzymes Market 2019).
Here we will review some aspects of the biotechnological uses of extremophilic laccases.
7.1 Plant Biomass Decomposition for Biorefinery Processes
Petrol in the world is becoming scarce or found at deep-sea wells, so every time is more difficult to acquire. Furthermore, oil handling provokes pollution derived from spills, accidents, and use of oil-derived products such as combustion of gasolines and diesel, machinery wasted oil, etc. (Thomas 2017). For these reasons, biorefinery technology has been developing to obtain biofuels (mostly bioethanol and biodiesel) and other substitutes for oil-derived products such as waterproof compounds, cement additives, battery components, etc. (Parajuli et al. 2015). These compounds are all carbon-based molecules that come from the hydrocarbons found in petrol. An interesting alternative carbon source to manufacture current substitutes for oil-derived compounds is lignocellulose, a complex composite found in vegetal biomass.
The cell wall in plants and algae is composed mainly of four carbon-based polymers: cellulose, hemicellulose, pectin, and lignin. The first three are polysaccharides, which are imbibed in a lignin matrix. Cellulose is a uniform polymer composed of glucose molecules linked in beta-(1–4) bonds which can be hydrolyzed by cellulases; hemicellulose is more complex since it is a branched polymer of different sugars being xylose the most abundant one, but other sugars such as arabinose, and glucose, or organic acids like acetic or ferulic acid can be linked to the structure. Different enzymes are then needed to obtain the monomeric sugars or acids from hemicellulose. Pectin is mostly composed of linear chains of alpha-(1–4) linked D-galacturonic acid (Toushik et al. 2017). As can be known, lignocellulose is a great feedstock from which many different carbohydrates can be obtained (Toushik et al. 2017; Guo et al. 2018; Lange 2018).
However, lignin is a polyphenolic polymer that hampers the access of enzymes to the polysaccharides found in plant biomass. This polymer contains different types of bonding among its aromatic rings, mainly C–O–C bonds (β-O-4/4′, α-O-4/4′, α/γ-O-γ, 4-O-5/5′, etc.) and C–C bonds (5–5/5′, β-β, β-1, β-5, etc.), which constitute complex linkages difficult to degrade by enzymes (Zakzeski et al. 2010; Zhou et al. 2011).
Fortunately, a certain type of microorganisms (bacteria and fungi) is able to oxidize and thus depolymerize lignin to gain access to the polysaccharide matrix. This process is achieved by the secretion of oxidative enzymes such as peroxidases, monooxygenases, and most importantly laccases (Roth and Spiess 2015).
In biorefinery applications are necessary to pre-treat the biomass to separate the components. Currently, pre-treatments for biomass are carried out using physical (steam explosion, high temperatures) or chemical (acid or alkaline treatments, ozonolysis, etc.) procedures (Barrera et al. 2016). An alternative is to use enzymes, and laccase has been used to depolymerize lignin in order to get access to the polysaccharides, which are then saccharified, by cellulases and hemicellulases to obtain sugar-rich syrups that can be fermented by yeasts or bacteria to obtain bioethanol (Molina et al. 2014). Most laccases are optimally active at low pHs and high temperatures, but this provokes that activity is lost rapidly due to enzyme inactivation (see Table 1 in Baldrian 2006). It is then important that these enzymes can withstand extreme conditions because then pre-treatments using laccase in combination with chemical or physical pre-treatments could result in a beneficial synergistic mode of obtaining biomass components. Several reports have shown promising results on the use of extremophilic laccases to enhance carbohydrate recovery from biomass (Brander et al. 2014; Chen et al. 2012; Rezaie et al. 2017; Guerriero et al. 2015).
7.2 Pulp and Paper Industry
Paper is produced from wood and consists mainly of cellulose, so it is necessary to remove the lignin from the wood pulp. Currently, oxygen chlorine-based delignification, oxygen delignification, or bleaching procedures are used to treat the wood pulp in order to manufacture paper (Carter et al. 1997; Ramesh et al. 2017). However, these methods cause environmental concerns because of the chemical wastes that are generated (e.g., hydrogen peroxide or chlorine derivatives). A more environmentally friendly alternative is the use of enzymes, which besides maintain better integrity of cellulose due to its specificity (Yang et al. 2018a, b). However, there are still challenges regarding costs of production (Ramesh et al. 2017). So a cost-effective, environmentally friendly and specific process could be achieved using extremophilic enzymes, especially laccase, which specifically removes lignin, preserving the carbohydrate structure of wood pulp (Rezaie et al. 2017; Navas et al. 2019).
7.3 Textile Industry
The dyes used in the textile industry are synthetic and thus many times toxic and recalcitrant due to its chemical structure, which renders them resistant to decolorization by chemicals such as detergents, high temperatures, and reactive oxidative species such as H2O2 or light (Chandra et al. 2017). Besides, the dying process itself is carried out in conditions in which mesophilic enzymes would denature (high temperatures, acidic pH, high NaCl concentrations, organic products, etc.). So, wastewaters from the textile industry are a problem because they can have catastrophic effects in rivers, lakes, and other water bodies because they reduce light income to the system and reduce oxygen solubility, and many of them are toxic due to heavy metals in their structure, or they can generate amines which can cause mutations and are carcinogenic.
For this reason, wastewater from textile industries must be treated before discharging, although currently most of it goes untreated to the ecosystems. Traditionally, chemical treatments have been used for dye degradation such as ozonification, photocatalytic degradation, oxidation by Fenton’s reagent, etc. Also, physical methods such as adsorption or filtration are used. However, a large hope is placed in biodegradation of industrial dyes by extremophilic microorganisms, especially those that can produce laccases (Amoozegar et al. 2015). Since extremophilic laccases can withstand harsh conditions, they can be combined with other methods that require low pH values and high temperatures or metal ions in coexistence of inorganic solvents, for example (Hayat et al. 2015; Zhuo et al. 2015; Singh et al. 2019a, b). Being unspecific oxidases, extremophilic laccases are promising enzymes for synthetic dye decolorization (Rodríguez-Couto 2019). For example, a thermostable laccase from the basidiomycete fungus Pycnoporus sanguineus was heterologously expressed in Trichoderma atroviride achieving a substantial amount of azo dyes decolorization (Balcázar et al. 2016).
7.4 Bioremediation
Currently, a plethora of xenobiotic compounds is released into the biosphere both from natural and artificial sources. Natural xenobiotic compounds are mainly heavy metals (Ortiz et al. 2018) and polycyclic aromatic hydrocarbons (PAHs) from incomplete wood combustion or volcanic emanations (Hussein and Mansour 2016). Anthropogenic sources of xenobiotics also include PHAs from the oil and gas industry, together with pesticides, industrial dyes, explosives, endocrine disruptors, and pharmaceutical products. These compounds are found in air, water, and soils, so their impact in living forms is disastrous (Giuliani et al. 2015; Hussein and Mansour 2016). Laccase promiscuity toward its substrates has been widely used to treat xenobiotic pollution, especially in soil and water, but also in the air (Sharma et al. 2018; Vergara et al. 2018). An advantage of laccases is that they can use low-molecular-weight mediators to oxidize compounds with nonoptimal redox potential for the enzyme, so they have proved to degrade a huge variety of xenobiotic compounds (Sharma et al. 2018).
For the treatment of xenobiotic compounds, enzyme immobilization is a method that has resulted in a more efficient process to cope with enzyme stability and re-usage. Several substrates have been used to immobilize enzymes that can now be used in columns or aqueous beds to treat polluted waters (Zhong et al. 2017; Zheng et al. 2016). An advantage of this technique is that natural substrates as porous rocks or natural fibers can be used to immobilize the laccases, and then recycling results in low-cost strategies (Abdel et al. 2013).
A problem to consider in which extremophile laccases could help is that many of the aforementioned xenobiotic compounds have very limited solubility in water. This fact can reduce the concentration at which many PAHs, pesticides endocrine disruptors, etc. can be oxidized by these kinds of enzymes. To overcome this difficulty, organic solvents could be used to prepare solutions in which the xenobiotic compound could be more concentrated, but to achieve this, the laccase must be robust (Fig. 8). Extremophilic laccases fulfill this criterion (Lončar et al. 2016; Yang et al. 2018a, b).
Biochemical properties and substrates exhibited for several laccases. Reprinted from J Mol Catal B Enzym 134, Lončar N, Božić N, and Vujčić Z, Expression and characterization of a thermostable organic solvent-tolerant laccase from Bacillus licheniformis ATCC 9945a, 390–395, copyright © 2016, with permission from Elsevier
8 Application: Perspectives
Stability, half-time life, amount of activity, and cost are factors that still hamper the use of enzymes for biotechnological purposes. To look for commercial applications which are cost rendering, the temperature-dependent catalytic properties of laccases are most important. Extremophilic organisms have been selected to produce enzymes with robust characteristics regarding thermal stability, organic solvent resistance, performance in the presence of metals or high salt conditions, etc. Thus, these organisms represent an invaluable source of enzymes which, if properly isolated and characterized, could bridge the cost-limiting effect of denaturation of mesophilic enzymes. Metagenomic prospection is also a most promising tool for the isolation of extremophilic laccases without the need of culturing extremophile organisms, which precisely by their nature could be an impairment (Batista et al. 2016). An important bottleneck for obtaining industrially cost-effective enzymes is laccase expression. Usually, yeasts have been a choice for laccase expression since they are single-celled organisms that proliferate in simple media and have a rapid doubling time. However, although some promising reports are arising using Pichia pastoris (Zerva et al. 2019), best results are obtained with filamentous fungi as host for the heterologous laccase genes (Wikee et al. 2019; Wang et al. 2017). Although laccases were originally discovered in plants, expression in these organisms has proved to be difficult (De-Jesús et al. 2019). Despite some limitations, extremophilic laccases still represent the best option to use for biotechnological applications.
References
Abdel S, Abdel M, Folch JL, Perezgasga L, Sánchez E, Castrejón ML, Ortiz ML (2013) Optimization of methyl parathion biodegradation and detoxification by cells with the opd gene in suspension or immobilized on tezontle. J Environ Sci Health B 48(6):449–461
Al-balawi T, Wood A, Solis A, Cooper T, Barabote R (2017) Anoxybacillus sp. strain UARK-01, a new thermophilic soil bacterium with hyperthermostable alkaline laccase activity. Curr Microbiol 74:762–771
Amoozegar MA, Mehrshad M, Akhoondi H (2015) Application of extremophilic microorganisms in decolorization and biodegradation of textile wastewater. In: Microbial degradation of synthetic dyes in wastewaters. Springer, Cham, pp 267–295
An H, Xiao T, Fan H, Wei D (2015) Molecular characterization of a novel thermostable laccase PPLCC2 from the brown rot fungus Postia placenta MAD-698-R. Electron J Biotechnol 18:451–458. https://doi.org/10.1016/j.ejbt.2015.09.004
Ausec L, Crnigoj M, Snajder M, Poklar N, Mandic I (2015) Characterization of a novel high-pH-tolerant laccase-like multicopper oxidase and its sequence diversity in Thioalkalivibrio sp. Appl Microbiol Biotechnol 99:9987–9999
Balcázar E, Méndez LH, Batista RA, Esquivel U, Ayala M, Kumar VV, Savary O, Cabana H, Herrera A, Folch JL (2016) Xenobiotic compounds degradation by heterologous expression of a Trametes sanguineus laccase in Trichoderma atroviride. PLoS One 11(2):e0147997. https://doi.org/10.1371/journal.pone.0147997
Baldrian P (2006) Fungal laccases–occurrence and properties. FEMS Microbiol Rev 30(2):215–242. https://doi.org/10.1111/j.1574-4976.2005.00010.x
Barrera I, Guzmán N, Peña E, Vázquez T, Cerón R, Folch J, Salazar J, Aburto J (2016) Ozonolysis of alkaline lignin and sugarcane bagasse: structural changes and their effect on saccharification. Biomass Bioenergy 94:67–172
Basheer S, Rashdi N, Ashraf R, Sohail M, Siddiqui M, Imanaka T, Aktar R (2017) Identification of a novel copper-activated and halide tolerant laccase in Geobacillus thermopakistaniensis. Extremophiles 21:563–571
Batista RA, del Rayo M, Talia P, Jackson SA, O’Leary ND, Dobson AD, Folch-Mallol JL (2016) From lignocellulosic metagenomes to lignocellulolytic genes: trends, challenges and future prospects. Biofuels Bioprod Biorefin 10(6):864–882
Beloqui A, Pita M, Polaina J, Martínez-Arias A, Golyshina O, Zumárraga M, Yakimov M, García-Arellano H, Alcalde M, Fernández V, Elborough K, Andreu J, Ballesteros A, Plou F, Timmis K, Ferrer M, Golyshin P (2006) Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen. Biochemical properties, structural analysis, and phylogenetic relationships. J Biol Chem 281:22933–22942
Brander S, Mikkelsen JD, Kepp KP (2014) Characterization of an alkali- and halide-resistant laccase expressed in E. coli: cot a from Bacillus clausii. PLoS One 9(6):e99402. https://doi.org/10.1371/journal.pone.0099402
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH (2003) Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microbiol 69:987–995. https://doi.org/10.1128/AEM.69.2.987
Camarero S, Pardo I, Cañas AI, Molina P, Record E, Martínez AT, Martínez MJ, Alcalde M (2012) Engineering platforms for directed evolution of laccase from Pycnoporus cinnabarinus. Appl Environ Microbiol 78:1370–1384. https://doi.org/10.1128/aem.07530-11
Carter DN, McKenzie DG, Johnson AP, Idner K (1997) Performance parameters of oxygen delignification. Tappi J (USA), Erratum 81(3):12
Chandra R, Kumar V, Yadav S (2017) Extremophilic ligninolytic enzymes. In: Extremophilic enzymatic processing of lignocellulosic feedstocks to bioenergy. Springer, Cham, pp 115–154
Chen GQ, Jiang XR (2018) Next generation industrial biotechnology based on extremophilic bacteria. Curr Opin Biotechnol 50:94–100. https://doi.org/10.1016/j.copbio.2017.11.016
Chen SC, Wu PH, Su YC, Wen TN, Wei YS, Wang NC, Hsu CA, Wang HJA, Shyur LF (2012) Biochemical characterization of a novel laccase from the basidiomycete fungus Cerrena sp. WR1. Protein Eng Des Sel 25(11):761–769. https://doi.org/10.1093/protein/gzs082
Coll PM, Fernandez-Abalos JM, Villanueva JR, Santamaria R, Perez P (1993a) Purification and characterization of a phenoloxidase (laccase) from the lignin-degrading basidiomycete PM1 (CECT 2971). Appl Environ Microbiol 59(8):2607–2613
Coll PM, Tabernero CARLOS, Santamaría RAMON, Pérez PILAR (1993b) Characterization and structural analysis of the laccase I gene from the newly isolated ligninolytic basidiomycete PM1 (CECT 2971). Appl Environ Microbiol 59(12):4129–4135
Dantán-González E, Vite-Vallejo O, Martínez-Anaya C, Méndez-Sánchez M, González MC, Palomares LA, Folch-Mallol J (2008) Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int Microbiol 11:163–169. https://doi.org/10.2436/20.1501.01.56
De-Jesús R, Folch JL, Dubrovsky JG (2019) Transgenic callus of Nicotiana glauca stably expressing a fungal laccase maintains its growth in presence of organic contaminants. Plant Cell Tiss Org 138:311–324. https://doi.org/10.1007/s11240-019-01626-2
Dhakar K, Pandey A (2016) Extracellular laccase from a newly isolated psychrotolerant strain of Cladosporium tenuissimum (NFCCI 2608). Proc Natl Acad Sci India 83:685–690
Elleuche S, Schäfers C, Blank S, Schröder C, Antranikian G (2015) Exploration of extremophiles for high temperature biotechnological processes. Curr Opin Microb 25:113–119
Enguita FJ, Martins LO, Henriques AO, Carrondo MA (2003) Crystal structure of a bacterial endospore coat component. J Biol Chem 278:19416–19425. https://doi.org/10.1074/jbc.m301251200
Fang Z, Li T, Wang Q, Zhang X, Peng H, Fang W, Hong Y, Ge H, Xiao Y (2012) A bacterial laccase from marine microbial metagenome exhibiting chloride tolerance and dye decolorization ability. Appl Microbiol Biotechnol 89:1103–1110
Fernándes AT, Soares CM, Pereira MM, Huber R, Grass G, Martins LO (2007) A robust metallo-oxidase from the hyperthermophilic bacterium Aquifex aeolicus. FEBS J 274(11):2683–2694
Ferrés I, Amarelle V, Noya F, Fabiano E (2015) Construction and screening of a functional metagenomic library to identify novel enzymes produced by Antarctic bacteria. Adv Pol Sci 26:96–101
Fukushima Y, Kirk TK (1995) Laccase component of the Ceriporiopsis subvermispora lignin-degrading system. Appl Environ Microbiol 61:872–876
Ghatge S, Yang Y, Song WY, Kim TY, Hur HG (2018) A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum strain TA2. A1 able to catalyze dimerization of a lignin model compound. Appl Microb Biotech 102(9):4075–4086
Giuliani S, Piazza R, El Moumni B, Polo FP, Vecchiato M, Romano S, Zambon S, Frignani M, Bellucci LG (2015) Recognizing different impacts of human and natural sources on the spatial distribution and temporal trends of PAHs and PCBs (including PCB-11) in sediments of the Nador lagoon (Morocco). Sci Tot Environ 526:346–357
Gómez-Silva B (2018) Lithobiontic life: “atacama rocks are well and alive”. Antonie Van Leeuwenhoek 111(8):1333–1343
Guerriero G, Hausman JF, Strauss J, Ertan H, Siddiqui KS (2015) Destructuring plant biomass: focus on fungal and extremophilic cell wall hydrolases. Plant Sci 234:180–193
Gunde-Cimerman N, Zalar P, Petrovic U, Plemenitas A (2003) Fungi in salterns. In: Ventosa A (ed) Halophilic microorganisms. Springer, Berlin, Heidelberg, pp 103–113. https://doi.org/10.1007/978-3-662-07656-9_7
Gunne M, Urlacher V (2012) Characterization of the alkaline laccase Ssl1 from Streptomyces sviceus with unusual properties discovered by genome mining. PLoS One 7(12):e52360
Guo H, Wang XD, Lee DJ (2018) Proteomic researches for lignocellulose-degrading enzymes: a mini-review. Bioresour Technol 265:532–541
Hayat H, Mahmood Q, Pervez A, Bhatti ZA, Baig SA (2015) Comparative decolorization of dyes in textile wastewater using biological and chemical treatment. Sep Purif Technol 154:149–153
Hussein AS, Mansour MS (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyp J Petrol 25(1):107–123
Ibrahim N, Ma K (2017) Industrial applications of thermostable enzymes from extremophilic microorganisms. Curr Biochem Engin 4(2):75–98
Industrial Enzymes Market (2019). Available from: www.marketsandmarkets.com/MarketRep-orts/IndustrilEnzyme. Accessed:29 May 2019
Komori H, Miyazaki K, Higuchi Y (2009) Crystallization and preliminary X-ray diffraction analysis of a putative two-domain-type laccase from a metagenome. Cryst Com 65:264–266
Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79:217–224. https://doi.org/10.1007/s00253-008-1417-2
Krüger A, Schaefers C, Schroeder C, Antranikian G (2018) Towards a sustainable biobased industry–highlighting the impact of extremophiles. New Biotechnol 40:144–153
Lange JP (2018) Lignocellulose liquefaction to biocrude: a tutorial review. ChemSusChem 11(6):997–1014
Leal G, Ferreira D, Vermelho A (2015) Marine extremophiles. A source of hydrolases for biotechnological applications. Mar Drugs 13:1925–1965. https://doi.org/10.3390/md13041925
Lončar N, Božić N, Vujčić Z (2016) Expression and characterization of a thermostable organic solvent-tolerant laccase from Bacillus licheniformis ATCC 9945a. J Mol Catal B: Enzymatic 134:390–395
Marques De Souza CG, Peralta RM (2003) Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J Basic Microbiol 43:278–286. https://doi.org/10.1002/jobm.200390031
Mateljak I, Monza E, Lucas MF, Guallar V, Aleksejeva O, Ludwig R, Leech D, Shleev S, Alcalde M (2019) Increasing redox potential, redox mediator activity, and stability in a fungal laccase by computer-guided mutagenesis and directed evolution. ACS Catal 9:4561–4572. https://doi.org/10.1021/acscatal.9b00531
Merino N, Aronson HS, Bojanova DP, Feyhl-Buska J, Wong ML, Zhang S, Giovannelli D (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol 10:780
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 9(6):415–425
Moghadam M, Albersmeier A, Winkler A, Cimmino L, Rise K, Frank M, Kalinowski J, Ruckert C, Wentxel A, Lale R (2016) Isolation and genome sequencing of four artic marine Psychrobacter strains exhibiting multicopper oxidase activity. BMC Gen 17:117–129
Molina C, Sánchez A, Serafin-Muñoz A, Folch-Mallol J (2014) Optimization of enzymatic saccharification of wheat straw in a micro-scale system by response surface methodology. Rev Mex de Ingen Quím 13(3):765–768
Mollania N, Khajeh K, Ranjbar B, Hosseinkhani S (2011) Enhancement of a bacterial laccase thermostability through directed mutagenesis of a surface loop. Enzym Microb Technol 49:446–452. https://doi.org/10.1016/j.enzmictec.2011.08.001
Navas LE, Martínez FD, Taverna ME, Fetherolf MM, Eltis LD, Nicolau V, Estenoz D, Campos E, Benintende GB, Berretta FB (2019) A thermostable laccase from Thermus sp. 2.9 and its potential for delignification of eucalyptus biomass. AMB Express 9(1):24
Ortiz ML, Mussali P, Sánchez-Salinas E, Tovar-Sánchez E (2018) Mining and mine tailings: characterization, impacts, ecology and bioremediation strategies. In: Fuentes MS, Colin VL, Saez JM (eds) Strategies for bioremediation of organic and inorganic pollutants. CRC Press, Boca Raton, pp 190–214
Parajuli R, Dalgaard T, Jørgensen U, Adamsen APS, Knudsen MT, Birkved M, Gylling M, Schjørring JK (2015) Biorefining in the prevailing energy and materials crisis: a review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies. Renew Sustain Energy Rev 43:244–263
Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99(19):7907–7913
Ramesh P, Krishna Prasad R, Baskar R (2017) Enzymatic and chemical delignification of Kraft wood pulp: optimization & sequential studies. Environ Eng Manag J 6(11):2497–2504
Rezaei S, Shahverdi AR, Faramarzi MA (2017) Isolation, one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresour Technol 230:67–75
Rezaie R, Rezaei S, Jafari N, Forootanfar H, Khoshayand MR, Faramarzi MA (2017) Delignification and detoxification of peanut shell bio-waste using an extremely halophilic laccase from an Aquisalibacillus elongatus isolate. Extremophiles 21(6):993–1004. https://doi.org/10.1007/s00792-017-0958-7
Rodríguez-Couto S (2019) Fungal Laccase: a versatile enzyme for biotechnological applications. In: Recent advancement in white biotechnology through fungi. Springer, Cham, pp 429–457
Roth S, Spiess AC (2015) Laccases for biorefinery applications: a critical review on challenges and perspectives. Bioproc Biosyst Engin 38(12):2285–2313. https://doi.org/10.1007/s00449-015-1475-7
Sharma V, Ayothiraman S, Dhakshinamoorthy V (2018) Production of highly thermo-tolerant laccase from novel thermophilic bacterium Bacillus sp. PC-3 and its application in functionalization of chitosan film. J Biosci Bioeng 127(6):672–678
Sheng S, Jia H, Topiol S, Farinas ET (2017) Engineering cot a laccase for acidic pH stability using Bacillus subtilis spore display. J Microbiol Biotechnol 27:507–513. https://doi.org/10.4014/jmb.1608.08026
Singh P, Jain K, Desai C, Tiwari O, Madamwar D (2019b) Microbial community dynamics of extremophiles/extreme environment. In: Microbial diversity in the genomic era. Academic Press, pp 323–332
Singh G, Singh S, Kaur K, Kumar S, Sharma A, Sharma P (2019a) Thermo and halo tolerant laccase from Bacillus sp. SS4: evaluation for its industrial usefulness. J Gen Appl Microbiol 65(1):26–33. https://doi.org/10.2323/jgam.2018.04.002
Slomczynski D, Nakas JP, Tanenbaum SW (1995) Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol 61:907–912
Thomas CS (2017) Petroleum and coal proven reserves: the case for coal and the demise of OPEC. In: Stopping climate change: the case for hydrogen and coal. Springer, Cham, pp 35–40
Toushik SH, Lee KT, Lee JS, Kim KS (2017) Functional applications of lignocellulolytic enzymes in the fruit and vegetable processing industries. J Food Sci 82(3):585–593
Vergara A, Revah S, Moreno P, Scott F (2018) Biofiltration of volatile organic compounds using fungi and its conceptual and mathematical modeling. Biotechnol Adv 36(4):1079–1093
Wang R, Yang J, Zhang G, Chao Y, Li Z, Ye Q, Qian S (2017) Co-expression of beta-glucosidase and laccase in Trichoderma reesei by random insertion with enhanced filter paper activity. Mol Biotechnol 59(8):353–364. https://doi.org/10.1007/s12033-017-0018-7
Wikee S, Hatton J, Turbé D, Yann A, Yann M, Daou M, Lomascolo A, Kumar A, Lumyong S, Sciara G, Faulds CB, Record E (2019) Characterization and dye decolorization potential of two laccases from the marine-derived fungus Pestalotiopsis sp. Intern J Mol Sci 20(8):1864
Yang X, Berthold F, Berglund LA (2018a) Preserving cellulose structure: delignified wood fibers for paper structures of high strength and transparency. Biomacromolecules 19(7):3020–3029. https://doi.org/10.1021/acs.biomac.8b00585
Yang Q, Zhang M, Zhang M, Wang C, Liu Y, Fan X, Li H (2018b) Characterization of a novel, cold-adapted, and thermostable laccase-like enzyme with high tolerance for organic solvents and salt and potent dye decolorization ability, derived from a marine metagenomic library. Front Microbiol 9:2998. https://doi.org/10.3389/fmicb.2018.02998
Ye M, Li G, Liang W, Liu Y (2010) Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and high soluble expression. Appl Microbiol Biotechnol 87:1023–1031
Zakzeski J, Bruijnincx PC, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110(6):3552–3599
Zerva A, Koutroufini E, Kostopoulou I, Detsi A, Topakas E (2019) A novel thermophilic laccase-like multicopper oxidase from Thermothelomyces thermophila and its application in the oxidative cyclization of 2′, 3, 4-trihydroxychalcone. New Biotechnol 49:10–18
Zheng F, Cui BK, Wu XJ, Meng G, Liu HX, Si J (2016) Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int Biodeterior Biodegradation 110:69–78
Zhong Z, Pang S, Wu Y, Jiang S, Ouyang J (2017) Synthesis and characterization of mesoporous cu–MOF for laccase immobilization. J Chem Technol Biotechnol 92(7):1841–1847. https://doi.org/10.1002/jctb.5189
Zhou CH, Xia X, Lin CX, Tong DS, Beltramini J (2011) Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem Soc Rev 40(11):5588–5617
Zhuo R, He F, Zhang X, Yang Y (2015) Characterization of a yeast recombinant laccase rLAC-EN3-1 and its application in decolorizing synthetic dye with the coexistence of metal ions and organic solvents. Biochem Eng J 93:63–72
Zumárraga M, Bulter T, Shleev S, Polaina J, Martínez-Arias A, Plou FJ, Ballesteros A, Alcalde M (2007) In vitro evolution of a fungal laccase in high concentrations of organic cosolvents. Chem Biol 14:1052–1064. https://doi.org/10.1016/j.chembiol.2007.08.010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pérez-Llano, Y., Soler, H.P., Olivano, A.R., Folch-Mallol, J.L., Cabana, H., Batista-García, R.A. (2020). Laccases from Extremophiles. In: Schlosser, D. (eds) Laccases in Bioremediation and Waste Valorisation. Microbiology Monographs, vol 33. Springer, Cham. https://doi.org/10.1007/978-3-030-47906-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-47906-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47905-3
Online ISBN: 978-3-030-47906-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)