Abstract
Refugees have been born in, or have lived in, regions highly endemic for the five main viral hepatitides A, B, C, D, and E, compared to the developed countries where they relocate. National guidelines for medical screening and vaccination, including guidelines for viral hepatitis in newly arrived refugees to the USA, are developed by the Centers for Disease Control and Prevention Division of Global Migration and Quarantine (CDC/DGMQ).
Hepatitis B and C develop chronic infections, which are contagious and mostly silent and cause significant morbidity and mortality. Safe and effective hepatitis B vaccination and medications effective for treatment of hepatitis B and curative for hepatitis C have started to improve the goal of controlling morbidity and reducing new infections.
Routine screening for hepatitis B is recommended for all refugees, and hepatitis C screening is recommended for those with increased risk and from high-prevalence regions and countries.
Hepatitis D requires hepatitis B for replication and adds serious complications to chronic hepatitis B. Hepatitis D has a low prevalence, and asymptomatic screening is not indicated. Screening for acute hepatitis A and E is not needed for newly arrived refugees due to their short infection cycle.
This chapter reviews these screening guidelines as well as characteristics of the different viral hepatitides, serology testing, and vaccination recommendations for refugees.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Refugee
- Viral infection
- Acute hepatitis
- Chronic hepatitis
- Hepatitis A
- Hepatitis E
- Hepatitis D
- Hepatitis B
- Hepatitis C
- Hepatitis serology
- Surface antigen
- Surface antibody
- Core antibody
- Perinatal transmission
- Maternal transmission
- Vaccinations
Viral Hepatitis A, B, C, D, and E in Refugees (Screening and Clinical Considerations)
Where you were born and where you have lived determine most of a refugee’s viral hepatitis risk.
Introduction
Viral hepatitis disproportionately affects refugees, asylees, and immigrants from resource-poor regions. Five main viruses (hepatitis A, B, C, D, and E) that infect the human liver cause liver diseases that are endemic to many parts of the world (see Table 7.1). All of the viral hepatitides have an acute phase that lasts several months with similar symptoms when present (see Table 7.2). Many times, acute hepatitis A, B, and E in children and hepatitis C in adults are very mild or have no symptoms.
Hepatitis A virus is the most widespread and common hepatitis infection. It causes an acute infection that is self-limited and is highly endemic in underdeveloped regions of the world.
Hepatitis B virus has infected an estimated 30% of the world mostly through perinatal transmission and close household contacts of children under age 5. Hepatitis B at birth and in young children <5 years old is seen as self by the early immune system (immune tolerance) leading to chronic hepatitis defined as hepatitis B surface antigen (HBsAg) positive for 6 months or greater. Hepatitis B can persist as a chronic infection, which can be lifelong and is one of the most common chronic infections with a worldwide prevalence of 3.5% [1]. There is an associated 15–40% risk of developing end-stage liver disease from cirrhosis and/or hepatocellular carcinoma (HCC) [2,3,4,5].
Acute hepatitis C infections are caused by infected blood products, medical procedures, self-inflicted injections, and 75–85% become chronic hepatitis C (CHC) which is endemic in both developed and underdeveloped regions, disproportionately affecting those areas with infected blood products and unsafe medical procedures.
Hepatitis D is an incomplete viral particle that requires hepatitis B to replicate and is known to worsen the liver damage and limit effective treatment options for hepatitis B. Hepatitis E virus causes mostly acute self-limited infections (except in pregnant women, immunocompromised, and those with chronic liver disease) and is associated with sporadic epidemics after flooding in areas with lack of water purity and untreated sewage, especially in crowded settings like those encountered by refugees.
Worldwide Transmission and Prevention
Initiatives to prevent and control the widespread and massive amount of hepatitis infections worldwide are led by the World Health Organization (WHO). Significant improvements have been achieved with the very safe and effective hepatitis B vaccine focused on vaccination at birth and in early childhood through the Expanded Vaccination Program.
The new infection rate of hepatitis B reduced from 4.7% in the pre-vaccination era to 0.8% in 2017. However, Africa’s new infection rate is still at 3% [6].
Widespread challenges will need to be addressed in order to achieve the stated WHO goal by 2030 for worldwide reduction of new hepatitis B infections by 90% and mortality by 65%. In 2019, the WHO reported 124 countries have national hepatitis plans in place and only 58% include domestic funding [6].
Effective antiviral medications to control and prevent liver damage from hepatitis B and curative treatment for hepatitis C are now available, and low-cost versions are available according to the WHO. Hepatitis C treatment is increasing worldwide from 1.7 million in 2015 to 5 million in 2017 with the increased use of highly effective (cure rates of 95%), well-tolerated direct-acting antiviral medications [1].
Major challenging features of the viral hepatitides include:
-
1.
Chronic hepatitis B (CHB) and CHC are usually silent conditions until late-stage complications develop. Most people do not know they are infected (only 9% or 22 million are diagnosed with hepatitis B, and 20% or 14 million are diagnosed with hepatitis C); therefore, many are at ongoing risk for spreading infection and will miss the opportunity to get treatment that may help avoid developing end-stage liver conditions [1].
-
2.
In order to reduce hepatitis A and hepatitis E, more municipal resources and efforts toward water purity and sewage control will be required as well as implementation of hepatitis A vaccination and development of a hepatitis E vaccination (a licensed vaccine for hepatitis E genotype 3 is available in China).
-
3.
Persons who inject drugs (PWID) continue to be at risk for spreading hepatitis B, C, and D; safe injection practices and addiction services are not adequate. Hepatitis C infects an estimated 71 million of which 5.6 million (8%) continue to inject drugs [6].
-
4.
Hepatitis D has a much lower prevalence than hepatitis B or C, and hepatitis D data is limited by lack of testing and reporting by some countries. Its spread is controlled by vaccination preventing hepatitis B which is essential for the hepatitis D replication.
Hepatitis Screening and Follow-Up in US Refugees
Newly arrived US refugees are recommended to complete a Domestic Medical Examination with specific guidelines outlined by the Centers for Disease Control and Prevention (CDC):
-
1.
Screening for hepatitis B in all refugees.
-
2.
Screening for hepatitis C in all of those with risk factors or are adults born during 1945–1965 and adults that were born or lived in areas with higher hepatitis C prevalence >2%.
-
3.
Vaccination for hepatitis A for children and hepatitis B vaccination for all susceptible children and adults.
-
4.
Referral of all chronically infected with hepatitis B or C.
-
5.
Asymptomatic testing for hepatitis A and E is not needed due to the self-limiting course with no significant chronic phase, in which exposure and symptoms most times resolve prior to arrival.
-
6.
Hepatitis D screening is not indicated and clinical testing can be considered in those of concern upon referral for hepatitis B [7].
Hepatitis B screening and vaccination may start overseas. The CDC Vaccination Program for US-Bound Refugees is a voluntary program that screens refugees prior to arriving in the USA for HBsAg and attempts to provide at least the first two doses of the hepatitis B vaccination. Clinicians preforming the Domestic Health Screening in the USA for refugees will need to review Department of State screening forms (DS 3025 and 3026) forwarded electronically to state health departments and other designated clinics. The CDC overseas vaccination program is voluntary; not all refugees will have been screened [8, 9]. Asylum seekers and other similar immigrants would start health screening in the USA. Other vaccination records from abroad, if administered at the appropriate time, are acceptable proof of vaccination.
Challenges for the long-term follow-up care for refugees, immigrants, and asylum seekers are:
-
1.
A substantial percentage of those previously admitted to the USA have moved in and out of various health systems, have been lost to follow up, and have no longer recall their hepatitis B or C status, even those initially diagnosed with chronic hepatitis. Since most chronic hepatitis is silent until end-stage disease is present, the risk for spreading hepatitis B and C continues, and opportunities are missed for surveillance, counseling, and treatment even when receiving health care in the USA.
-
2.
Foreign-born travel internationally more often than American-born; thus, a traveler’s hepatitis A and B status needs to be known and reviewed to provide vaccination and counseling before returning to endemic areas. Electronic medical record systems can identify patients from hepatitis B endemic areas based on the country of birth or patients in the birth cohort for hepatitis C born during 1945–1965 and determine if appropriate serology tests are needed and alert the treating provider [10, 11].
Hepatitis B
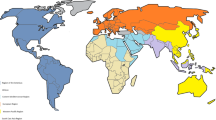
Chronic hepatitis B (CHB) infection is one of the most common chronic infections worldwide and is a high concern for newly arrived refugees due to high prevalence of refugees that are HBV carriers, the long-term health risks, and risk of transmission to household and sexual contacts. Clinicians should be aware of the hepatitis B endemic regions and assess for appropriate testing when caring for patients born or that have lived in those regions (see Fig. 7.1).
Geographic distribution of chronic hepatitis B virus infection. (Source. Viral Hepatitis B in the World. World Health organization. https://www.who.int/hepatitis/news-events/global-hepatitis-report2017-infographic/en/)
Epidemiology
The World Health Organization (WHO) in 2015 estimated the burden worldwide as 3.5% of the world population (257 million people) living with chronic infection, defined as hepatitis B surface antigen (HBsAg) positive for more than 6 months [1]. In 2015, the WHO estimated 887,220 persons died from HBV infection (337,454 due to HCC, 462,690 from cirrhosis, and 87,076 from acute hepatitis) [12, 13]. Most refugees are from countries and regions with intermediate (>2%) or highly endemic (≥8 %) prevalence of CHB infection. In highly endemic regions, most new infections are in infants and young children due to perinatal and household exposure. Lifetime risk of exposure to hepatitis B is about 20 to 60% in intermediate endemic and 60% in highly endemic regions with at least 2% to about 8% developing chronic infection. CHB is mostly asymptomatic until complications occur. The US population has about 1.3–2.2 million infected with CHB, and the overall prevalence of CHB infection is less than 1%, but foreign-born account for about 47–70% of those infected [16]. Antiviral treatment for hepatitis B is available and indicated for those with progressive chronic infection to reduce or postpone the development of end-stage liver disease. Despite the known need for clinical evaluation and monitoring for CHB, in 2010, the Institute of Medicine report highlighted that 65% of all persons with CHB in the USA are undiagnosed and only half of those diagnosed receive appropriate care [17, 18].
Perinatal transmission of CHB is as high as 90% in the highly endemic areas. Good measures are available to prevent transmission of HBV at delivery that dramatically reduce the new CHB infection rate of infants to HBV-infected mothers, but still in the USA, about 1000 babies are born yearly infected with HBV due to lack of pregnancy screening for mothers with CHB [19]. Treatment of newborns born to HBsAg-positive mothers with the hepatitis B immune globulin within 12 hours of birth and the three-dose hepatitis B vaccination series are both highly effective at breaking the chain of perinatal transmission.
The US data on the prevalence of HBsAg in newly arrived refugees between 2006 and 2008 demonstrated 2.8% overall prevalence, ranging 0.6–15.5%, with 95% confidence range of 2.6–3.0%. The highest prevalence was among refugees from Eritrea (15.5%), Liberia (12.2%), Myanmar (12.4%), Ethiopia (9.1%), Somalia (8.3%), and Malaysia (8.8%). Six other countries (Iran, Iraq, Laos, Russia, Thailand, and Vietnam) were noted to have substantially decreased rates when compared with 1991 prevalence data [14]. Arriving refugee populations in the USA from 2011 to 2015 have a reported average prevalence of HBV infection 5.7% with variations depending on the specific population. Overall 42% were nonimmune and still susceptible to exposure [15].
It is important to realize that refugees in the USA live their life and marry in their respective ethnic communities, which have high prevalence rates of CHB. Ensuring serology testing of all refugees for chronic hepatitis B infection and identifying those that lack protective immunity against hepatitis B will yield high-value health information to protect refuges from infection transmission, disease progression, and applying hepatitis B vaccination series.
Clinical Course
Hepatitis B is asymptomatic in infants and infection with the virus seen as self (immunotolerant phase) by the early immune system. After several decades of life, the adult immune system starts to react to the infection, causing liver inflammation and damage during the immunoreactive phase. Unvaccinated adults that are infected acutely with HBV will display acute hepatitis symptoms (Table 7.2) similar to acute hepatitis from other causes for up to 6 months, and almost all will become immune with less than 5% (who are usually immunocompromised) that will develop CHB.
Screening and Vaccination Guidelines
Refugee Hepatitis B Screening National Guidelines [7]
Test
Hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), and hepatitis B core antibody (anti-HBc) in all children and adults (for interpretation, see Tables 7.3 and 7.4).
Vaccinate
Previously unvaccinated and susceptible children 0–18 years of age and susceptible adults.
Refer
All persons with chronic HBV infection for additional ongoing medical evaluation and counseling.
Additional Hepatitis B Refugee Screening Considerations [7]
-
1.
Test children born in the USA, not vaccinated at birth, for HBsAg, if parents are from high HBV endemic regions ≥8%.
-
2.
Any refugee with potential exposure to HBV within the last 60 days of hepatitis B testing should have repeat testing in 3–6 months.
-
3.
Testing for hepatitis B should be done regardless of prior hepatitis B vaccination. CHB infection is mostly silent, and hepatitis B vaccination would not be protective if they are already infected prior to vaccination.
-
4.
Testing for HBsAg should not be done within 1 month of vaccination; it may lead to a false-positive result.
-
5.
Screen all pregnant women and any individual that may develop high-risk conditions and hepatitis symptoms or acquire HBV infection risk factors (see Table 7.5).
Preventive Hepatitis B Vaccination for Refugees [20]
-
1.
Timing: The first vaccination of the series may be done at the time of HBsAg testing. It will not be harmful in HBsAg-positive cases. There are several single-antigen hepatitis B vaccines licensed in the USA including two-dose series and the traditional three-dose series. Combination hepatitis vaccination series are three or four doses.
-
2.
Overseas testing and vaccination: The Overseas Vaccination Program for US-Bound Refugees offers voluntary testing of HBsAg and the first two hepatitis B vaccinations at participating sites for the US-bound refugees. HBsAg-positive people receive counseling, and their household contacts are offered the entire three-dose HBV vaccine series if they are present long enough to receive it before departure.
Overseas medical records document the refugee’s vaccinations on the Form DS-3025 (Vaccination Documentation Worksheet) and the HBsAg result in the Form DS-3026 Medical History Remarks Section. These records are available to state health departments and other designated clinics through the CDC Electronic Disease Notification (EDN) system [8, 9].
New arrivals with a negative overseas HBsAg test that have started HBV vaccinations can forgo further testing for anti-HBs and anti-HBc and complete the remaining HBV vaccinations. Refugee arrivals that are HBsAg negative and no HBV vaccine has been initiated can be offered serologic testing and HBV vaccination series if indicated or just receive the HBV vaccination series.
-
3.
Prior vaccination: Immunizations administered outside the USA are accepted if they come with written documentation. Acceptable written vaccination information (date, type of vaccination, and the location or name of clinic) and administration intervals at the appropriate age can be accepted as valid, if the schedule was similar to the standard US recommendations (inappropriate age at the time of the previous vaccine is unacceptable).
-
4.
Vaccination series: If one or two doses of the hepatitis B vaccine series were given abroad and properly documented, the series should be completed without restarting, following an acceptable US schedule. The minimum intervals are 4 weeks between first and second doses and another 8 weeks between second and third doses.
A positive anti-HBs test after one documented dose of the hepatitis vaccine is not considered protective, and the three-dose series should be completed.
-
5.
Immune response: Severe malnutrition at the time of the vaccination could impair immune response to some vaccines. Consider revaccination after nutritional reconstitution or assessing for immunity by serology.
-
6.
Travel post-arrival: Established refugees frequently will be returning to endemic areas to visit friends and relatives (VFRs). Hepatitis B serology should be reviewed and susceptible patients vaccinated [21].
-
7.
Negative anti-HBs serology despite history of complete hepatitis B vaccination series: Repeat the series one time. Those who have received a hepatitis B vaccination series twice have a low risk of acquired infection; thus, in an immunocompetent host, no follow-up testing or vaccination is required.
Preventive Counseling
Identification of those infected with hepatitis B will lead to increased awareness and the opportunity for counseling to protect health and prevent spreading infection by:
-
1.
Recommending careful hygiene (use barrier protection; do not share razors, toothbrushes, injection equipment, glucose testing equipment; cover cuts and scratches; clean blood spills with bleach; do not donate body fluids).
-
2.
Assessment of hepatitis B status (susceptible, immune, or infected) for all household members and sexual contacts. Vaccinate susceptible household members and contacts.
-
3.
Avoid alcohol (many are unaware of the risk) and optimize weight, lipids, and blood sugar to prevent metabolic syndrome and fatty liver [22].
Management/Referral
For those infected with HBV (HBsAg positive):
-
1.
Assess hepatitis A serology for immunity or need to protect by vaccination.
-
2.
Rule out coinfection with HCV and HIV.
-
3.
Refer to gastroenterology or a liver specialist to assess for chronic liver disease, periodic monitoring for liver cancer, and consideration of antiviral therapy which cannot cure HBV but can reduce liver inflammation, disease progression, and HCC risk for those with active HBV liver disease.
Hepatitis D Coinfection or Superinfection with Hepatitis B
Hepatitis D virus (HDV) is an incomplete virus that requires HBV infection to replicate and infect humans. HDV infection is estimated to occur in about 15–20 million people (5% of the 257 million CHB-infected people are coinfected) [1, 23]. Not all countries test or report HDV infection rates; thus, information is not complete.
Epidemiology
The WHO reports hepatitis D is more common in Africa (Central and West Africa), Asia (Central and Northern Asia, Vietnam, Mongolia, Pakistan, Japan, and Chinese Taipei), Pacific Islands (Kiribati, Nauru), Middle East (all countries), Eastern Europe (Eastern Mediterranean regions, Turkey), South America (Amazonian basin), and Greenland [23]. HDV infection is decreasing in areas of the world where CHB prevalence rates are decreasing due to expansion of global childhood vaccination [23].
Refugees with elevated prevalence rates of hepatitis B infection are susceptible to HDV, but specific refugee prevalence data is rare.
Low prevalence has been reported in past surveys of HBV-infected Albanian refugees (one case was detected from 91 HBsAg positive) and Southeast Asian refugees (no HDV detected) [24, 25].
Transmission risks are the same as HBV (percutaneously, close contacts, sexually, infected blood or blood products; vertical transmission is rare but possible). HDV enhances the severity of acute and chronic hepatitis B.
Clinical Course
Two forms of HDV infection occur in association with hepatitis B virus:
-
1.
Coinfection is the simultaneous acute hepatic infection of both HBV and HDV viruses that is mild and 95% of the time it clears.
-
2.
Superinfection is an HDV infection of a person already chronically infected with the hepatitis B virus (CHB) that presents as a severe acute hepatitis (Table 7.2) and leads to chronic hepatitis D infection in up to 80% of the cases. The rates of cirrhosis, fulminant hepatitis, and mortality are much higher than in CHB infection alone [23, 26].
Screening
Routine testing is not recommended for HDV in newly arrived refugees.
Prevention
There is no HDV vaccine. HBV vaccination will protect those not infected with hepatitis B from HDV infection but cannot protect the estimated 257 million CHB carriers worldwide from HDV infection susceptibility [1]. Preventive measures include promotion of worldwide blood product and injection safety and harm reduction services for PWID.
Management
HDV infection should be suspected in those infected with hepatitis B that have elevated and/or worsening liver function tests. Clinical tests include serologic antibodies (IgM and IgG anti-HDV) and confirmatory serum HDV RNA. Referral to a liver specialist is indicated if HDV is suspected or confirmed. Treatment options are limited to older interferon-based treatments; newer antiviral antinucleos(t)ides do not work well for HDV infection.
Liver transplantation is an option for ESLD and fulminant hepatitis caused by HDV.
Chronic Hepatitis C
Hepatitis C virus (HCV) infection is a slowly progressive and clinically mild chronic liver infection that over 2–3 decades can develop into cirrhosis, and then there is a 1–5% annual risk of developing hepatocellular carcinoma (HCC) [27, 28]. Most with HCV are asymptomatic and may be unaware of their infection until chronic liver disease complications develop.
Epidemiology
The anti-HCV world prevalence was estimated at 2.5% (includes past and current infections) in 2015; the WHO estimates 71 million people (1% of the world’s population) are chronically infected. HCV is found with the highest rates in areas that have non-sterile and unsafe medical procedures from injections, equipment, or blood products (see Fig. 7.2) [1, 29, 30]. In 2015, there was an estimated 1.75 million new HCV infections and about 399,000 preventable HCV-related deaths, and about 843,000 with HCV were cured [1].
World map of prevalence of hepatitis C virus infection. (Source: Centers for Disease Control Health Information for International Travel (Yellow Book chapter 4) 2020. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/hepatitis-c)
HCV prevalence rates vary between and within countries, and the highest country rates for HCV antibody seroprevalence (not confirmed infections) are found in Egypt (14.7%), Cameroon (13.8%), Burundi and Uzbekistan (11.3%), Mongolia (10.7%), and Libya (7.9%) [30, 31]. The high rates in Egypt have been traced to use of contaminated needles during a rural campaign to eradicate schistosomiasis in the Nile River basin [32]. Newly arriving refugee groups are being assumed to have similar HCV prevalence rates similar to the regions and countries that they originated unless specific data after arrival is available. HCV prevalence rates are below 0.9–1.2% in North America, Europe, and Australia, which accept many of the relocated refugees from around the world [1].
HCV transmission in developing countries where most refugees originate is mainly through non-sterile and unsafe medical procedures from injections, equipment, and blood products. WHO global estimates in 2010 that 5% of health-care-related injections remain unsafe (compared with 39% in year 2000) and are still a concern in Southeast Asia, in East Africa, and in the Middle East. Global blood donations are estimated to be screened 97% of the time [1]. In developed countries, current transmission is caused by sharing of needles by people who inject drugs (PWID). Perinatal transmission of HCV occurs at a rate of 5–6%, and health-care needlestick from an HCV-infected patient has a 1.8% infection rate. Although sexual contact (not monogamous) increases risk, it is less efficient and a low rate compared to intravenous drug abuse. HCV is detected in breast milk, but breast-feeding is not associated with increased risk [33]. HCV infection in refugees is not a major communicable disease threat to the US public health compared with tuberculosis, hepatitis B, or syphilis since in the USA, blood supply and most medical procedures and equipment are sterile and safe, and most modes of human contact have low infection rates.
Clinical Course
Hepatitis C infection has an incubation period of 2 weeks to 6 months, and a symptomatic acute hepatitis phase occurs in less than 30% of those infected. The symptoms are indistinguishable from other acute hepatitis syndromes (Table 7.2) and last less than a month. Chronic HCV infection persists in about 70–85% of those infected, and over 2–3 decades of chronic HCV infection, approximately 20% to 40% (depending on age) develop cirrhosis with increased risk of HCC [34]. Chronic HCV infection is known to progress more often to cirrhosis with moderate alcohol intake, infection at older age, coinfection with HIV, or infection with schistosomiasis [35,36,37,38,39].
Screening Guidelines
Screen for HCV infection in those born during 1945–1965 or those of any age with risk factors (Table 7.6) similar to guidelines for the general US population.
The CDC states it is also reasonable to screen all adult and child refugees originating from or have lived in countries with high moderate (2–5%) or high (≥5%) prevalence [7].
Other Countries Hepatitis C Refugee Screening Guidelines
Screen for HCV in refugees with risk factors or high-risk conditions and who were born or have lived in countries with HCV infection prevalence of >3% for Australia, ≥3% for Canada, and ≥2% for the United Kingdom.
HCV screening guidelines will continue to evolve with new prevalence data for specific groups, regions, and counties as well as improved cost-benefit from treatment with new direct-acting antiviral drugs. Prevalence can vary highly in specific groups and locations, probably related to the mechanism of a particular exposure and lack of complete data in many regions; thus, some resettled refugee groups may have higher prevalence than the specific country or region they originated from [40, 41].
Testing for antibody to HCV (anti-HCV) if positive is followed by confirmatory testing with HCV polymerase chain reaction (PCR). The HCV PCR test is needed regardless of HCV antibody results for patients with immunocompromised conditions, children less than 18 months with HCV-positive mothers (due to passive acquired maternal antibodies), and patients with end-stage renal disease (anti-HCV may be false negative).
Prevention
There is no vaccination. Illicit intravenous drug users should be offered treatment referrals. Prevention programs should encourage the use of sterile injection equipment and advise against sharing needles. If HCV infected and not immune to hepatitis A and/or B, protective vaccinations are indicated.
Counseling
Infected people should be advised to avoid alcohol use and to not share personal items contaminated with infectious blood (toothbrushes, razors, or nail clippers). Physicians should discuss limiting drugs that affect the liver. Safe sex practices should be endorsed. Although breast-feeding has not been associated with increased transmission, it should be avoided with cracked or bleeding nipples. Physicians should encourage weight control and healthy diet to reduce risk of fatty liver disease.
Management/Referral
A liver specialist is needed for those infected by HCV to:
-
1.
Evaluate for chronic liver disease
-
2.
Receive consideration for curative treatment with the new direct-acting antiviral medications for hepatitis C infection that are safe, have few side effects, and have cure rates of >95% with 12 weeks of treatment
-
3.
Perform lifelong HCC surveillance screening for those with cirrhosis [1]
Acute Hepatitis A
Hepatitis A virus (HAV) is the most highly prevalent acute viral hepatitis and is endemic in most of the developing world. HAV is shed through feces. The most common transmission is the fecal oral route from the contamination of water to the food supply. It can also be transmitted through direct contact with an infected person.
Epidemiology
In HAV highly endemic areas, 90% have been infected by age 10 years. The infection is mild and self-limited in most children and usually lasts less than 2 months, but in about 10% of cases, prolonged or relapsing symptoms can last 6–9 months [42]. Symptoms and signs of acute hepatitis (Table 7.2) are more likely with increased age (older children and adults). Most adult refugees were infected as children and have lifelong immunity. Unvaccinated children and young adults from areas with good water sanitation may be susceptible to HAV infection, including some urban middle-class individuals from developing countries. In general, lower-income regions (see Fig. 7.3) correlate with intermediate to high hepatitis A endemicity. High-income regions and countries have low prevalence rates of HAV infection and have higher susceptibility [43].
Clinical Course
Incubation usually is 14–28 days, and symptoms of acute hepatitis (Table 7.2) occur in older children (age > 6) and adults. Symptom duration is usually 2 weeks to 2 months, but 3–20% can have prolonged or relapsing symptoms up to 6–9 months. Most can expect complete resolution [44]. Serious complications are rare, except that those immunocompromised or with chronic liver disease may develop fulminant hepatitis. HAV does not have a chronic phase.
Screening
Routine testing for HAV infection in asymptomatic refugees is not recommended at any age.
Prevention
ACIP recommends the two-dose HAV vaccine for all children around age 1 (12–23 months) or catch up to age 18 [44].
Currently, the US-bound refugee populations do not receive predeparture hepatitis A vaccination.
An alternative reasonable approach to vaccination of those older than 2 years of age is to test for immunity with hepatitis A serology (total anti-HAV IgG) [7]. Considering the high prevalence of previous HAV exposure in the US-bound refugee populations and the cost of the test versus the cost of two visits to administer two doses of HAV vaccine, HAV serology testing is known to be cost-effective in adult refugees from regions of HAV prevalence >33% where a two-dose series of HAV vaccination is being considered [45, 46]. For established refugee travelers that are likely to be visiting friends and relatives (VFRs) in highly endemic areas, serology testing or vaccination can be done for unvaccinated VFRs <20 years old. In VFRs age >20, it is cost-effective to check serology and vaccinate if susceptible [21].
Refugees with chronic liver disease (including CHB and CHC) are at risk for developing fulminant hepatitis from HAV infection. Hepatitis A immunity should be checked and susceptible individuals vaccinated.
Other Prevention/Counseling
Access to sanitary water is a key factor in prevention. People should be counseled on avoidance of infected close contacts or careful hygiene measures when in close contact with infected individuals.
Management
In a refugee with signs and symptoms of acute hepatitis, testing of IgM anti-HAV serology can confirm active HAV infection. Treatment would be supportive care, rest and symptom control, and contact precautions.
Acute Sporadic and Epidemic Hepatitis E
Hepatitis E virus (HEV) liver infections occur worldwide through four genotypes (1, 2, 3 and 4). Genotypes 1 and 2 are due to fecal contaminated drinking water and occur in the undeveloped areas of the world. HEV infection usually is an acute mild self-limited infectious hepatitis.
Worldwide Distribution
Geographically, most large outbreaks are caused by genotype 1 in Africa, South America, and Asia and occur after natural disasters like flooding, that cause water contamination in overcrowded situations (temporary housing, refugee camps). Genotype 2 is associated with sporadic cases and smaller outbreaks that occur in endemic areas of Mexico and West Africa [47, 48]. Genotypes 3 (developed countries) and 4 (India, Mainland China, Taiwan, and Japan) are zoonotic and cause occasional transmission to humans. See Fig. 7.4.
Geographic distribution of hepatitis E. (Source: Centers for Disease Control and Prevention. Hepatitis E Questions and Answers for Professionals. https://www.cdc.gov/hepatitis/hev/hevfaq.htm#section1)
Clinical Course
Acute HEV incubation usually ranges from 15 to 60 days with acute hepatitis symptoms (Table 7.2) that are more likely to occur in young adult males (age 15–39) [47]. It is usually asymptomatic in children. Most people recover from HEV infection completely. High risk for complications is seen in preexisting liver disease and pregnant women.
Women in all stages of pregnancy are most likely to experience severe hepatitis symptoms from HEV infection with genotype 1 with serious complications including fulminant hepatitis and stillbirth, and reported mortality rates range from 10% to 50% or more [49]. The high variability in reported mortality of HEV infection with pregnancy in part may be due to prior exposure and resultant immunity.
Chronic HEV infection, due to genotype 3 by zoonotic transmission of eating raw pork or shellfish, has been documented in developed counties in immunosuppressed groups (HIV, solid organ transplant recipients, and others on chronic immunosuppression) [47, 50,51,52]. HEV infection in the USA is most likely to be in returning travelers from endemic areas [47].
Screening
No screening for refugees is recommended for HEV infection due to rare chronic phase and short infection cycle.
Prevention
No vaccination is available, except in China where there is a licensed vaccine for HEV genotype 4 that may be effective against other genotypes, but it is not yet known [48]. Improving water sanitation and sewage systems.
Management
HEV infectious hepatitis should be considered in a new arrival (<3 months) from an HEV endemic area with potential exposure and acute hepatitis symptoms (Table 7.2) and in whom other acute hepatitis syndromes (A, B, and C) have been ruled out. There is no FDA-approved test for HEV in the USA. HEV testing (IgM and IgG antibodies to HEV and PCR assay for HEV RNA) can be requested from the CDC Division of Viral Hepatitis Laboratory for clinical evaluation [53].
Treatment is supportive care with hospitalization for fulminant hepatitis and severe illness in pregnancy.
Summary
Hepatitis screening is an important part of a domestic refugee medical exam. Hepatitis B is a common hepatitis worldwide that causes chronic infection and is preventable by vaccination. It is endemic in many of the countries refugees come from. Thus, it is important to screen refugees, provide follow-up care for those who are chronic carriers, and vaccinate those who are susceptible. Hepatitis C is screened and managed as per recommendations for the US adults. Hepatitis A is a common infection worldwide, but it is an acute and self-limited infection and most refugees have acquired immunity, so screening is not necessary. Hepatitis D and E also do not require routine screening in refugees.
Chronic hepatitis treatment is available and can reduce the risk of developing end-stage liver disease.
Long-term periodic disease surveillance and treatment for hepatitis B and C carriers can be challenging due to problems inherent in the longitudinal primary care for refugees. Detection, preventive vaccination, counseling, and treatment of viral hepatitis are all opportunities that can lead to substantial health benefits for refugees.
References
Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39.
McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–55.
McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381–96.
Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52.
World Health Organization at the International Liver Congress. Vienna Austria April 13, 2019. https://www.who.int/hepatitis/news-events/5million-access-hcv-cure/en/. Accessed 06 May 2019.
Centers for Disease Control and Prevention. Screening for viral hepatitis during the domestic medical examination of newly arrived refugees. November 26, 2018. [Last reviewed: Feb 25, 2019] https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/hepatitis-screening-guidelines.html. Accessed 21 Mar 2019.
Centers for Disease Control and Prevention. Vaccination program for U.S.-bound refugees. [Last reviewed: October 31, 2016] Available at: www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/interventions/immunizations-schedules.html. Accessed 01 May 2019.
Centers for Disease Control and Prevention. Immigrant and refugee health electronic disease notification system. [Last reviewed: Dec 28, 2017]. Available at: www.cdc.gov/immigrantrefugeehealth/Electronic-Disease-Notification-System.html. Accessed 26 April 2019.
Southern WN, Drainoni ML, Smith BD, et al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat. 2010;18(7):474–81. https://doi.org/10.1111/j.1365-2893.2010.01327.x.
de la Torre A, Ahmad M, Ayoub F, et al. Electronic health record year and country of birth testing and patient navigation to increase diagnosis of chronic viral hepatitis. J Viral Hepat. 2019; https://doi.org/10.1111/jvh.13098.
WHO global health estimated for 2015 published in 2016 (Global Health Estimates 2015: deaths by cause, age, sex, country and region, 2000–2015. Available at http//www.who.int/entity/healthinfo/global_burden_disease/GHE2015_Deaths_Global_2000_2015.xls?ua=1. Accessed 21 Mar 2019.
Weekly epidemiological record World Health Organization. Hepatitis B vaccines: WHO position paper-July 2017;92:369–392.
Rein DB, Lesesne SB, O’Fallon A, et al. Prevalence of hepatitis B surface antigen among refugees entering the United States between 2006 and 2008. Hepatology. 2009;51:431–4.
Scott KC, Taylor EM, Mamo B, et al. Hepatitis B screening and prevalence among resettled refugees — United States, 2006–2011. MMWR Morb Mortal Wkly Rep. 2015;64(21):570–3.
Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. https://doi.org/10.1002/hep.24804.
Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010.
Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18:377–83.
Ko SC, Fan L, Smith EA, Fenlon N, Koneru AK, Murphy TV. Estimated annual perinatal hepatitis B virus infections in the United States, 2000–2009. J Pediatr Infect Dis Soc. 2014. piu115.
Centers for Disease Control and Prevention. Evaluating and updating immunizations during the domestic medical examination for newly arrived refugees. Immigrant and refugee health domestic guidelines. 2012 [last reviewed: Mar 6, 2014]. Available at: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/immunizations-guidelines.html. Accessed 21 Mar 2019.
Keystone JS. Chapter 58 Visiting friends and relatives. In: Walker PF, Barnett ED, editors. Immigrant medicine. Philadelphia: Saunders Elsevier; 2007. p. 747.
Terrault NA, Lok AS, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99.
Hepatitis D fact sheet. In: World Health Organization: media centre [website] July 2018 update. http://www.who.int//en/news-room/fact-sheets/detail/hepatitis-dfactsheets/hepatitis-d/en/. Accessed 22 Feb 2019.
Chironna M, Germinario C, Lopalco PL, et al. HBV, HCV and HDV infections in Albanian refugees in Southern Italy (Apulia region). Epidemiol Infect. 2000;125(1):163–7.
Maynard JE, Hadler SC, Fields HA. Delta hepatitis in the Americas: an overview. Prog Clin Biol Res. 1987;234:493–505.
Botelho-Souza LF, Vasconcelos M, Dos Santos AO, et al. Hepatitis delta: virological and clinical aspects. Virol J. 2017;14(1):177. https://doi.org/10.1186/s12985-017-0845-y.
de Oliveria Andrade LJ, D’Oliveira A, Melo RC, et al. Association between hepatitis C and hepatocellular carcinoma. J Global Infect Dis. 2009;1(1):33–7.
Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372(1732). pii: 20160274. https://doi.org/10.1098/rstb.2016.0274.
Hepatitis C fact sheet. In: world Health Organization 18 July 2018. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 28 Feb 2019.
Petruzziello A, Marigliano S, Loquercio G, et al. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40.
Greenaway C, Thu Ma A, Kloda LA, et al. The Seroprevalence of hepatitis C antibodies in immigrants and refugees from intermediate and high endemic countries: a systematic review and meta-analysis. PLoS One. 2015;10(11):e0141715. https://doi.org/10.1371/journal.pone.0141715.
CDC. Progress toward prevention and control of hepatitis C virus infection-Egypt, 2001–2012. MMWR Morb Mortal Wkly Rep. 2012;61(29):545–9.
Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1–39.
Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44(1):20–9.
Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74.
Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5.
Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8.
Kamal SM, Rasenack JW, Bianchi L, et al. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4+ T-cell and cytokine response. Gastroenterology. 2001;121(3):646–56.
Koshy A, al-Nakib B, al-Mufti S, et al. Anti-HCV-positive cirrhosis associated with schistosomiasis. Am J Gastroenterol. 1993;88(9):1428–31.
Mixson-Hayden T, Lee D, Ganova-Raeva L, et al. Hepatitis B virus and hepatitis C virus infections in United States-bound refugees from Asia and Africa. Am J Trop Med Hyg. 2014;90(6):1014–20.
Shire AM, Sandhu DS, Kaiya JK, et al. Viral hepatitis among Somali immigrants in Minnesota: association of hepatitis C with hepatocellular carcinoma. Mayo Clin Proc. 2012;87(1):17–24.
WHO position paper on hepatitis A vaccines. Weekly epidemiological record No. 28–29.2012;87:261–276.
Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–7.
Hollinger FB, Martin A. Hepatitis A virus. In: Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 550–81.
CDC. Prevention of hepatitis A through active or passive immunization. Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2006;55(RR-7):1–23.
Bryan JP, Nelson M. Testing for antibody to hepatitis A to decrease the cost of hepatitis A prophylaxis with immune globulin or hepatitis A vaccines. Arch Intern Med. 1994;154:663–8.
Centers for Disease Control and Prevention Division of Viral Hepatitis. Hepatitis E information. Questions and answers for health professionals. Overview and statistics [last updated: 2018 May 9]. Available at: https://www.cdc.gov/hepatitis/hev/hevfaq.htm. Accessed 12 Feb 2019.
World Health Organization. Hepatitis E vaccine WHO position paper, May 2015. Wkly Epidemiol Rec. 2015;18:185–200.
Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28(9):1190–9. https://doi.org/10.1111/j.1478-3231.2008.01840.x.
Dalton HR, Bendall RP, Keane FE, et al. Persistent carriage of Hepatitis E in patients with HIV infection. N Engl J Med. 2009;361:1025–7.
Ollier L, Tieulie N, Sanderson F, et al. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430–1.
Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358(8):811–7.
Centers for Disease Control and Prevention Division of Viral Hepatitis. Hepatitis E information for health professionals. Laboratory testing requests. 2015 [Last updated: 2015 May 31]. Available at: https://www.cdc.gov/hepatitis/hev/labtestingrequests.htm. Accessed 12 Feb 2019.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pryce, D.J. (2020). Viral Hepatitis. In: Annamalai, A. (eds) Refugee Health Care. Springer, Cham. https://doi.org/10.1007/978-3-030-47668-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-47668-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47667-0
Online ISBN: 978-3-030-47668-7
eBook Packages: MedicineMedicine (R0)