Abstract
Obesity is a global epidemic and obese populations are at a much higher risk of developing diseases such as hypertension, type 2 diabetes, stroke and congestive heart failure. Increased adiposity is the hallmark of this physiological alteration of the body in response to excess intake of energy rich food, and this condition has far reaching health consequences in humans. Adipose dysfunction develops over time leading to increased secretion of inflammatory cytokines that cause inflammation and oxidative stress, which are independent risk factors for cardiovascular disease. Diastolic dysfunction is characteristic of the cardiac pathology associated with obesity. Obesity is a manageable condition and in some cases completely reversible with lifestyle modifications such as increased physical activity and a calorically restricted diet. In other cases, obesity can be reversed with either medications or surgery. In this regard, food derived compounds have been reported to have therapeutic benefits. Resveratrol is one such compound; it belongs to a family of plant compounds called polyphenols. In this chapter, we will review the causes and consequences of obesity, obesity associated cardiovascular disease and the potential of resveratrol in prevention/treatment of obesity and obesity associated cardiovascular disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cardiovascular disease (CVD) and subsequent heart failure claims at least half a billion lives every year, around the globe. According to estimates available through the World Health Organization, around 17.9 million people died of CVD in 2016 [1]. There are a number of risk factors for the development of CVD such as hypertension, diabetes, hyperlipidemia, coronary disease, valvular disease and certain genetic mutations [2,3,4]. In combination with neurohormonal and cellular changes, the heart is capable of acutely compensating for many of the stresses arising from these pathophysiological conditions through a combination of structural and functional remodeling [5]. However, prolonged stress leads to maladaptive remodeling and the permanent loss of function of the heart and culminates in heart failure [6]. There are a number of therapeutic strategies currently being used to prevent, abate or reverse the development of heart failure [7]. For mild cases of CVD, life style modifications is the first step of therapy and pharmaceutical agents are prescribed when the disease has already progressed beyond what is manageable with life style changes [8, 9]. In certain cases such as valvular disease, electrical abnormalities and structural disabilities of the heart at birth, surgeries or devices such as pacemakers are used as the first step in treatment of the disease [7]. However, despite all of the modern biomedical inventions and advanced therapeutic methodologies, heart failure still claims millions of lives every year around the globe. This scenario leads us to think of alternative strategies that may more effectively prevent the development or arrest the progression of CVD into overt heart failure and mortality.
The obesity epidemic is directly and indirectly associated with millions of deaths every year [10]. Obesity is a condition wherein excess fat gets deposited under the skin and in other major organs of the body. This increased adiposity further increases risk of development of diabetes, hypertension, vascular diseases and other independent risk factors of cardiovascular disease [11]. Heart failure is a major cause of death in obese patients and the millions of individuals becoming obese every year are at risk of developing some form of heart disease [12].
Family genetics, gene abnormalities, diet, level of physical activity and other environmental factors are considered to be the major risk factors of obesity [13,14,15,16]. Genetic predisposition, together with diet plays a significant role in the development of obesity [17]. The development and increased availability of energy dense foods has certainly boosted the incidence of obesity around the globe (Fig. 15.1) [18]. High caloric intake accompanied by low physical activity results in increasing lipids stored as fat. After a certain point, cells that store fat (adipocytes) cannot keep up with the demand and become dysfunctional [19]. Healthy, smaller adipocytes secrete cardioprotective beneficial adipokines such as adiponectin. In contrast, dysfunction, enlarged adipocytes secrete pro-inflammatory adipokines such as leptin [20]. This adipocyte dysfunction leads to high levels of circulating lipids which subsequently results in increased uptake of lipids by other organs. Increased lipid deposition contributes to cellular stresses resulting in dysfunction of the organ [21]. The heart is one of the target organs in hyperlipdemic situations and the resulting stress causes cardiac dysfunction that culminates in heart failure [22].
Evolution of obesity. From: https://www.flickr.com/photos/christopherdombres/7350782488
Earlier forms of therapies were all derived from natural products. Different types of plants were used to treat all human ailments [23, 24]. Many of these medications were either eaten as the food by itself or mixed in combination with other foods [25, 26]. Nutraceuticals is the modern term for compounds that are naturally derived that, together with nutrition, delivers medicinal effects [27]. By this definition, resveratrol (RES) can be considered a nutraceutical as it is a polyphenol that is mainly found in grapes and other berries. Over the years, research has shown that RES has strong medicinal properties against a variety of human ailments including cancer, cardiovascular diseases, diabetes and some types of infections [28]. Cardioprotective properties of RES have been well documented and has recently also been shown to be beneficial in human clinical trials [29, 30].
Obesity
Obesity is a condition wherein excess fat accumulates in the body. A way to assess obesity is to classify individuals using the body mass index (BMI) which is calculated by dividing body weight by the square of that individual’s height. For adults the classification of obesity states is as follows (Table 15.1).
However, BMI is not a direct measure of fat stores and hence additional measurements are required to calculate the levels of body fat [31]. Waist circumference, skin fold test, waist to hip ratio calculation and modern techniques such as whole-body air displacement plethysmography (ADP), underwater weighing, dual energy X-ray absorptiometry (DXA) and even ultrasound techniques can be used to accurately determine body fat composition [32,33,34,35,36]. A small percentage of the obese population are completely healthy despite being obese and are often termed as being “healthy-obese” [37]. However, in most cases obesity is accompanied by several comorbidities and obese individuals experience poor quality of life and suffer psychologically from social stigma [38, 39]. Obesity is a manageable condition and in some cases completely reversible with life style changes including increasing physical activity, adopting a caloric restricted diet, taking medications and in some cases surgery [40,41,42]. Irrespective of the status of national development (underdeveloped, developing or developed), the growing incidence of obesity and increased dependence on health care systems has become a major worldwide concern [43,44,45,46,47].
World Statistics
At least 2.8 million deaths that occur every year are associated with being obese or overweight. The prevalence of obesity worldwide has almost tripled since 1975 and as of 2016, a total of more than 1.9 billion adults were considered obese or overweight. Shockingly, more people reside in countries where obesity causes more deaths than being underweight [48]. The prevalence of obesity was highest in the Americas (approximately 62% overweight and 26% obese) and lowest in South East Asia (approximately 14% overweight and 3% obese). Women are reported to be more prone to obesity when compared to men around the world [48, 49]. In some African and Mediterranean countries, the prevalence of obesity in women is almost double that of men. Income is also correlated with the increased prevalence of obesity [18].
Canadian Statistics
According to the latest data published by Statistics Canada, roughly 1 in 4 Canadian adults (27%) are obese [50]. Statistics Canada data from 2018 shows that 69.4% of men and 56.7% of women 18 years and older are overweight or obese [51]. These estimates are higher than from the self-reported data in 2009 which showed 59.2% of Canadian men and 43.9% of women in the overweight or obese category [52]. The number of obese children and youth is also increasing with 30% between the ages of 5 and 17 being obese or overweight [53,54,55]. The geographic distribution of obesity varies within the country. The lowest reported prevalence being 22% in British Columbia, while the highest in New Brunswick along with Newfoundland and Labrador where prevalence is as high as 38% [50]. Obesity is also seen more commonly in certain ethnic populations [56, 57].
Obesity in Children
Childhood obesity is another major area of concern as it possesses several health risks including cardiovascular disease and early mortality in their adulthood [54, 58, 59]. Based on data from Statistics Canada in 2018, 23.7% of children between the ages 12–17 years are overweight or obese [51]. Obesity in children and youth is measured using a different set of BMI cut-offs. According to the International Obesity Task Force (IOTF), a BMI greater than 21.22 and 26.02 kg/m2 for 12 year old boys and girls respectively, is categorized as obese. There are other systems of BMI categories so the estimates of childhood obesity may vary accordingly. For example, in the 2004 Canadian Community Health Survey based on the IOTF system, obesity rates were reported to be 8.2% among children and youth (2–17 years). However, based on the Centers for Disease Control system, the estimate increased to 12.7%, while based on the WHO system it was estimated to be 12.5% [60].
Health Costs
Obesity is associated with a number of co-morbidities. The risk of type 2 diabetes, hypertension, cardiovascular diseases and cancers increases significantly with being obese. Due to social stigma around obese individuals, a number of psychological conditions are also prevalent among the obese [61, 62]. Premature mortality rates are also reported to increase alongside the severity of obesity [10]. All these factors contribute to an increased life time dependence on the health care system by these individuals when compared to the non-obese. This directly results in higher than normal health expenditure per obese individual and puts a burden on the health care system. Estimates based on 2008 data put health care costs related to obesity between $4.6 billion to $7.1 billion per year [63]. These cost figures show how important it is to ramp up the awareness and fight against obesity, especially among those who are in the higher risk categories [64].
Overall, the increasing prevalence of obesity is a major global health concern and needs immediate attention to protect future generations. Race, sex, income and other socioeconomic factors have been seen to be associated with increased risk for obesity [65]. Childhood obesity is also increasing which is particularly alarming given that the risk of obesity increases with age. Childhood obesity also results in early onset of comorbidities such as diabetes, hypertension and reduced life expectancy [66].
Pathology of Obesity
Adiposity/obesity and Adipose Dysfunction
Obesity is a condition that develops due to excess fat deposition in the body overtime. Generally, excess energy in the body is stored as fat. Accordingly, the amount of fat stored is the difference between food (energy) intake and energy expenditure (Fig. 15.2).
Human body stores excess circulating lipids in adipocytes [67, 68]. These stored fats are kept as a reserve energy bank that is used during a state of fasting [69]. Adipocytes are normally an integral part of the human body and are required for normal physiology. However, when circulating lipids are chronically higher than normal, the amount of fat deposits also increases. Overtime, the increased adiposity results in pathological changes of obesity [70]. There are many factors such as energy dense diet, physical inactivity, gene mutations and/or other pathophysiological conditions that contribute to increased circulating lipids [71, 72].
Adiposity could be increased in two different ways, either by increasing the number (hyperplasia) or the size (hypertrophy) of adipocytes [73, 74]. The first is considered to be physiological; while the second, wherein the adipocyte enlarges, is considered pathological [75]. Earlier, adipose tissues were considered as just a store of fat. However, later it was discovered that adipose tissues are also an endocrine organ and adipocytes secrete hormones (adiponectin, leptin and resistin), cytokines (TNF-α, IL-6) and proteins (cholesteryl ester treansfer protein, angiotensin II, plasminogen activator inhibitor 1) involved in the metabolism and functions of the liver, muscles, vasculature, brain and other organs of the body [76]. Cytokine secretions from adipocytes are generally known as adipokines [77]. Some adipokines are beneficial while others cause unhealthy effects on biological functions [20]. In normal or healthy conditions, adipocytes secrete more adipokines which are beneficial, while in pathophysiological conditions where adipocyte dysfunction occurs, the balance will be shifted to an increased release of detrimental adipokines [75, 77]. The origin of adipocyte dysfunction is mainly associated to the physiological demands of storing very high levels of fat. Resident macrophages are also present among the adipocytes and are involved in fat storage and secretion of cytokines. The adipocytes and macrophages are highly involved in the genesis of chronic inflammation in the adipose tissue and release of pro-inflammatory factors into the blood [78]. Consequently, there is also increased lipolysis and release of free fatty acids into circulation. Accordingly, adipose tissue is considered to be the major source of the pathophysiological effects in obesity [19, 79]. This also makes adipocytes a potential drug target to ameliorate the metabolic disarray in obese conditions. To some extent, targeting adipocyte dysfunction has shown promise in preventing or improving metabolic imbalances in obesity [80].
Obesity and Cardiovascular Disease
According to seminal Framingham Heart Study, the risk of developing heart failure in obese individuals was 2 times that of normal weight subjects [81]. It has been found that 32−49% of heart failure patients are considered obese; furthermore, obese patients develop heart failure up to 10 years earlier than their normal BMI counterparts [82]. Cardiovascular complications are one of the major contributors to poor health and lower life expectancy among obese populations [83, 84]. Obesity, especially abdominal obesity, is an independent risk factor for CVD [85, 86]. Higher BMI is directly associated with adipocyte dysfunction, increased release of adipokines, insulin resistance, hypertension, increased inflammation and oxidative stress that promotes the development of cardiovascular disease [87]. Although not unanimously accepted, the ‘obese paradox’ theory claims that obese individuals have a better prognosis to CVD when compared to normal weight individuals [82]. A possible explanation for this paradoxical theory is that the BMI measurements are insufficient to accurately assess the state of obesity and adipocyte dysfunction in an individual [88]. Additional measurements such as waist circumference and waist to hip ratio would better classify the subjects based on the levels of fat deposition. A study on the Monza population has shown that with every 1 kg/m2 increase in BMI, the risk of developing left ventricular (LV) hypertrophy increases by 5.1%, and for every 1 cm increase in waist circumference the risk increase by 2.5% [89]. Visceral adiposity and subcutaneous fat deposits contribute to increased waist circumference which has been found to be an independent risk factor for developing heart disease [90]. For increased risk of CVD, the WHO’s cut-off values for waist circumference are 102 cm in men and 88 cm in women [91].
Obesity exerts stress on the heart by increasing the blood volume and cardiac output simultaneously, placing a larger workload on the heart [10]. This results in adverse changes to hemodynamics, cardiovascular structure and function. Obesity increases total body area and volume by additional fat tissue and the changes in cardiovascular system are aimed at maintaining sufficient blood supply to the whole body [10]. Adipose tissue contains a large volume of fluid which is present in the interstitial spaces of the tissue. The interstitial space adds up to approximately 10% of the total adipose tissue weight. Obesity also increases lean body mass which independently elevates cardiac output [92]. A combination of increase in lean and fat mass could account for a large increase in stroke volume and cardiac output. The expansion in volume of blood increases the preload on the heart and shifts the Frank-Starling curve to the left. A significant change in vascular structure and function is also observed in obesity. Obesity causes arterial stiffness [93], increased intima-media thickness [94, 95] and increased calcification [96]. All these vascular changes are also independent predictors of CVD. Further, these vascular changes may also contribute to the development of hypertension in obese individuals [97].
These changes in hemodynamics increases wall tension and induces LV dilation and hypertrophy [82]. Prolonged exposure to these stressful conditions reduces LV wall compliance and then diastolic dysfunction ensues. Initial adaptations by the LV help preserve LV systolic function in the early stages of cardiac remodeling. Overtime, impairment in systolic function will develop and heart failure will be initiated [98]. It was also found that the fatty heart, as a result of increased fat deposits is more prone to cardiomyopathy [99]. Damage to heart muscles by fat accumulation happens in two ways, metaplasia and lipotoxicity [100]. In metaplasia, some cells (epithelial or mesenchymal) are replaced by fat cells, disrupting the cardiac electroconduction. In lipotoxicity, free fatty acid accumulation in cardiomyocytes induces cell death in the myocardium. In either case, damage to cells results in myocardial weakening, resulting in the development of cardiomyopathy [10]. The obesity associated increase in blood volume also induces left atrial enlargement, which increases the risk of developing atrial fibrillation. Based on the findings from Women Health Study, obesity was associated with increased risk for atrial fibrillation [101]. Other types of arrhythmias and sudden cardiac death are also found at higher rates in obese populations [82, 102]. Obesity and metabolic dysfunction increases the risk of coronary artery disease. The incidence of coronary atherosclerosis is very high in adult obesity and is a major risk factor for heart disease [103]. Obesity is also directly linked to increased incidence of stroke. The INTESTROKE study has found that waist to hip ratio was strongly associated with increased risk for stroke [104]. Obstructive sleep apnea is another risk factor for hypertension and CVD [105]. Obesity is one of the major risk factors for obstructive sleep apnea, and in many sleep apnea patients are undiagnosed which increases the risk of heart disease.
Adipose tissue is also an endocrine organ releasing a number of molecules into the blood stream. TNF-α, IL-6, leptin, angiotensinogen, resistin and plasminogen activator inhibitor-1 are released from adipose tissues and have direct or indirect effect on promoting development or progression of heart disease [106]. A significant proportion of the circulating concentrations of these molecules have originated from adipose tissue. Most of these are mediators of the inflammatory response and may be involved in progression of coronary artery diseases [107].
Indirect effect of obesity on cardiovascular pathology involves impairments of kidney structure and function. Glomerular hyperfiltration, increased albumin loss, glomerulosclerosis and progressive loss of kidney function are associated with obesity-induced kidney damages [108]. Population studies, PREVEND [109] and Framingham Heart Study [110] have found direct correlation between kidney damage and obesity.
Lipids and Heart
Fatty acids are the primary energy source of the heart. In normal physiological conditions, approximately 70% of energy is derived from the oxidation of fatty acids [111]. The remaining energy is derived from glucose, lactate and ketones. Generation of ATP from fatty acid oxidation is a comparatively more oxygen demanding process than generating ATP from glucose. The heart has the ability to switch to glucose as the major energy source during oxygen deficient conditions such as ischemia, hypertension and other pathological conditions [112, 113]. This allows the heart to adapt to difficult conditions, preserve available oxygen and minimize the damage to the tissue. Fetal hearts also depend more on glucose and lactate for energy, while adult hearts shift to fatty acid oxidation to meet their energy needs [112]. Diet, hepatic fatty acid synthesis and lipolysis in adipose tissue are the major sources of lipid for the heart. Heart tissues can use both non-esterified (free fatty acids) and esterified (bound to lipoproteins) fatty acids. Circulating triglycerides undergo lipolysis mediated by endothelium-bound lipoprotein lipase and are then internalized via membrane receptors, transporters or simply by diffusion [114]. Internalized free fatty acids are then converted to fatty acyl-CoAs and then either stored as acyl glycerides (mono, di or tri) or transported to mitochondria for ATP generation [115]. Triglycerides stored intracellularly are processed to free fatty acids by hormone sensitive lipase and adipose triglyceride lipase [116].
Lipotoxicity
The balancing act of energy homeostasis is much more complex than the earlier mentioned equation (Fig. 15.1). For example, there is a significant difference between white adipose tissue (WAT) and brown adipose tissue (BAT). While both are fat deposits, their physiological roles are different. WAT is associated with the genesis of metabolic syndrome while, BAT contributes to thermogenesis [117]. Diet is the major source of fatty acids (FA); it is also synthesized from other sources through de novo lipogenesis [118]. Depending on physiologic demands FAs are released into circulation from adipose tissue by lipolysis and will be used by other organs. FAs can also be transported into the cells by different protein transport mechanisms [115, 119]. These FAs are then used for a variety of cellular mechanisms involving synthesis of membrane, signaling molecules, post-translational protein modification, transcriptional regulation and more importantly for energy production through beta oxidation [120]. Normally, a balance is maintained between lipid uptake and oxidation thereby preventing lipid accumulation. Metabolic disturbances often results in increased lipid levels in the circulation. Higher circulating FAs in obesity and type 2 diabetes causes excess deposition of FAs in non-adipose tissues such as kidney, liver, skeletal muscles and heart [121]. The excess lipid accumulating inside the cell may cause cellular dysfunction through ER stress, mitochondrial dysfunction, oxidative stress and ultimately results in cell death [122]. This process of lipid induced cellular damage and death is known as lipotoxicity [123,124,125].
Lipotoxicity in the Heart
The heart is one of the major organs affected by lipid accumulation [21]. Lipid accumulation is also observed in cardiac pathologies wherein the myocardium reverts to glucose as the primary source of energy [112]. Lipotoxic effects leads to cardiomyocyte dysfunction, contractile abnormalities, cell death and pathogenesis of heart failure [111, 126]. Cardiomyopathies observed in in the setting of type 2 diabetes and obesity are often a result of lipotoxic damage to the myocardium [116]. Long chain fatty acids like palmitate have been found to induce lipotoxicity in the heart muscle cells when compared to short chain fatty acids [127, 128]. Some pathological cellular changes associated with lipid accumulation are ER stress, mitochondrial dysfunction and oxidative stress. Increased ceramide accumulation has been observed as a contributor towards cell death in the heart [129]. Insulin is involved in regulation of glucose metabolism, activation of survival pathways in ischemia and also in intracellular Ca2+ handling. Lipotoxicity induces insulin resistance and thereby causes cardiomyocyte dysfunction. Activation of protein kinase C (PKC), mitogen activated protein kinases and reduced peroxisome proliferator-activated receptors are also considered to be involved in the process of lipid accumulation and cellular responses in the cell [21]. Lipid accumulation also induces contractile abnormalities through the degeneration of myofibrils [130].
Resveratrol
RES is a phytoalexin compound produced by plants mainly in response to fungal infections, UV radiation and other environmental stresses such as cold temperatures [131]. RES is present in significant amounts in grapes, peanuts, soy beans, pomegranates, mulberry and bilberry [132] and to a lesser extend in pine, eucalyptus and spruce trees, and in a few flowering plants, such as Veratrum grandiflorum and Veratrum formosanum [133, 134].
RES was discovered in the roots of white hellebore plants [135]. Later it was also found in roots of Polyganum cuspidatum, a Japaneese knotweed which was also called Ko-jo-kon and is the richest known source of RES. It was used in the preparation of Japanese and Chinese herbal medicines against skin infections like warts, dermatitis and athletes foot [136]. This was followed by reports on presence of RES in eucalyptus and pine [137, 138]. In 1976 Langcake and Price reported the presence of RES in grape vines for the first time [139]. During this period RES was mainly investigated for its anti-fungal properties and used as a screening marker for disease resistant grape cultivars [140, 141]. The first report linking RES to potential cardiovascular benefits was from a Japanese group which showed that RES administration reduced triglyceride synthesis in mice [142]. Later in 1992, moderate red wine consumption was linked to the reduced incidence of cardiovascular disease among the French population; this theory is known as the ‘French Paradox’ [143]. At the same time, Siemann and Creasy reported that RES might be one of the bioactive ingredients in wine [144]. Further, Frankel et al. showed that the phenolic component of red wine inhibited LDL oxidation which is a risk factor for atherogenesis and thereby ischemic heart disease [145]. The association of RES to the French Paradox generated a greater interest in RES research wherein either purified RES or food containing significant amount of RES were tested on a wide range of research models of human disease [131]. The highlights of RES research outcomes are its beneficial effects against different types of cancers, cardiovascular diseases and also against metabolic diseases such as diabetes and obesity [146,147,148].
Resveratrol Chemistry
RES is a stilbene derivative, produced in plants by stilbene synthase. Stilbene synthase catalyze the synthesis of RES from one molecule of p-coumaroyl CoA and three molecules of malonyl CoA. RES exists in two structural isomeric forms, cis- and trans-RES (Fig. 15.3) (molecular weight: 228.24); both isomers are lipophilic in nature [149]. RES has a melting point around 260 °C. It is insoluble in water but soluble in ethanol and DMSO. The trans-RES isomer is relatively more stable as compared to the cis-RES isomer; however, the trans form can get converted to the cis for when exposed to heat or UV radiation [150]. The trans-RES in the powder form is stable in normal atmosphere at room temperature and undergoes negligible oxidation in these conditions. RES is susceptible to photolysis if exposed to direct sunlight. Due to its structural similarity to the synthetic estrogen diethylstilbestrol, RES is also considered to be a phytoestrogen [133].
Cis-resveratrol (a) and trans-resveratrol (b). From: https://en.wikipedia.org/wiki/File:Cis_and_trans_resveratrol_notext.svg
Cardioprotection with Resveratrol
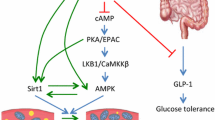
RES is present at high amounts in grape skins and subsequently in red wines (Fig. 15.4). This led to the ‘French Paradox’ theory in which lower incidence of cardiovascular disease in the French population was associated with a higher consumption of red wine [150]. Subsequent research confirmed the cardioprotective properties of RES [151]. RES was reported to exhibit cardioprotective properties by reducing cardiac abnormalities in hypertension, ischemic heart disease, obesity, diabetes and cardiomyopathies; RES has been shown to improve heart and blood vessel structure and function in animal models of cardiovascular disease [30, 152, 153]. Major cellular mechanisms that mediate effects of RES range from improving cardiovascular risk factors such as hyperlipidemia and insulin resistance to reducing oxidative stress and inflammation. Among the many molecules identified as RES targets in the heart, AMPK, SIRT1 and nitric oxide (NO) are most frequently reported [152]. RES is found to enhance AMPK activity and thereby its downstream signaling pathway which indirectly results in increased NO production [154, 155]. AMPK activation could also be involved in RES-mediated decrease in fibrosis [156]. An increase in SIRT1 expression is associated with RES administration. SIRT1 could improve cardiac function by increasing SERCA2A expression and thereby improving Ca2+ handling. SIRT1 could also induce AMPK activation and improve mitochondrial function [157]. Anti-inflammatory and antioxidant activities were the first properties to be identified and associated to health benefits of RES. RES inhibits NFkB activation and translocation into the nucleus, preventing the transcription of a variety of genes detrimental to the cell [158]. RES also helps preserve major antioxidant enzyme activities such as superoxide dismutase, catalase, and glutathione peroxidase, while reducing NADPH oxidase activity. Nuclear factor erythroid 2-related factor 2 (Nrf2) is involved in maintaining an antioxidant environment inside the cells and RES is found to promote Nrf2 activation [159]. Modulation of L-type calcium channel is also a potential mechanism by which RES could improve Ca2+ irregularities in cardiac cells [160].
Major sources of resveratrol: grapes and red wine. From: https://www.publicdomainfiles.com/show_file.php?id=13534675212806 (wine glass). https://www.publicdomainpictures.net/en/view-image.php?image=299825&picture=grapesvintage-illustration (grapes)
Resveratrol in Obesity-Induced Heart Disease
There is sufficient evidence showing that RES improves metabolic abnormalities in animals [161]. However, there are only a few studies that have explored the cardioprotective property of RES in obesity. The first study showed that RES administration in rats for 2 weeks reduced both infarct size and cardiac apoptosis in ex-vivo ischemic-reperfused hearts [162]. In another study RES prevented an increase in blood pressure and preserved vascular function in an animal model of diet-induced obesity, the high fat fed rats [163]. Louis et al. reported that RES reversed diastolic heart dysfunction in high fat fed rats [164] and Qin et al. showed a significant decrease in cardiac hypertrophy and improvement in diastolic heart function in obese mice exhibiting characteristics of early stage type II diabetes [165]. Cardiomyopathy is the major form of heart disease affecting the obese and overweight population. Yingjie et al. showed that RES attenuated high fat diet-induced cardiomyopathy in a mouse model [166]. This effect was associated with upregulation of estrogen receptor alpha which has been proposed to mediate RES actions in vivo. As discussed earlier, obesity pathologically affects vascular function. NO is an endogenous vasodilator and an established target of RES [167]. Huang et al. reported that RES treatment mitigated vascular dysfunction in high fat fed mice through upregulating eNOS/NO mechanism [168].
To date, no study has examined the impact of resveratrol in preventing obesity induced deterioration of heart function in humans. However, there are a few clinical studies which have examined the potential of resveratrol in reducing obesity. A recent clinical trial reported significant reductions in weight loss in obese patients who were on a combination of resveratrol and orlistat (a standard anti-obesity medication) [169]. Similarly a combination of resveratrol and hesperetin (a bioactive compound) was reported to reduce glucose levels and improved vascular function in overweight and obese patients [170]. A combination of epigallocatechin-3-gallate (a bioactive compound) and resveratrol was also shown to increase mitochondrial capacity and stimulate fat oxidation in overweight and obese patients [171]. Earlier studies reported that resveratrol supplementation reduced glucose, triglycerides and markers of inflammation in obese men [172], improved cerebral blood flow in obese subjects [173], and reduced intestinal and hepatic lipoprotein production in obese individuals with mild hypertriglyceridemia [174].
Overall, there is some evidence that RES improves heart structure and function in animal models of obesity. However, there is no information on impact of RES on heart structure and function in humans with obesity, therefore future studies should examine this aspect. Given that diet and genetic predisposition are major contributors towards the development of obesity, future research is necessary to examine if RES can protect the human heart in the settings of diet induced obesity. It is also important to know if RES can improve diastolic heart dysfunction in obese animals and thereby prevent the progression to heart failure. Finally, more research exploring cellular mechanisms involved in the cardioprotective action of RES is needed. Given the success of RES in combination with standard medication or other bioactive compounds in improving metabolic parameters in overweight and obese subjects, it would be interesting to examine the potential of combination therapy in preventing heart dysfunction in these subjects.
References
WHO (2017) Cardiovascular diseases. WHO
Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM (2012) Lifetime risks of cardiovascular disease. N Engl J Med 366:321–329. https://doi.org/10.1056/NEJMoa1012848
Lloyd-Jones DM, Nam BH, D’Agostino RB Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ (2004) Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA 291:2204–2211. https://doi.org/10.1001/jama.291.18.2204
Murabito JM, Nam BH, D’Agostino RB Sr, Lloyd-Jones DM, O’Donnell CJ, Wilson PW (2004) Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med 140:434–440
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582
Grossman W, Paulus WJ (2013) Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling. J Clin Invest 123:3701–3703. https://doi.org/10.1172/JCI69830
Liu SS, Monti J, Kargbo HM, Athar MW, Parakh K (2013) Frontiers of therapy for patients with heart failure. Am J Med 126(6–12):e6. https://doi.org/10.1016/j.amjmed.2012.04.033
Wexler R, Pleister A, Raman SV, Borchers JR (2012) Therapeutic lifestyle changes for cardiovascular disease. Phys Sportsmed 40:109–115. https://doi.org/10.3810/psm.2012.02.1957
Guyatt GH, Devereaux PJ (2004) A review of heart failure treatment. Mt Sinai J Med 71:47–54
Bastien M, Poirier P, Lemieux I, Despres JP (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56:369–381
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH (2009) The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9:88
Artham SM, Lavie CJ, Patel HM, Ventura HO (2008) Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr 3:155–161. https://doi.org/10.1111/j.1559-4572.2008.00001.x
Walley AJ, Blakemore AI and Froguel P (2006) Genetics of obesity and the prediction of risk for health. Hum Mol Genet 15 Spec No 2:R124–R130. doi: https://doi.org/10.1093/hmg/ddl215
Hebebrand J, Hinney A (2009) Environmental and genetic risk factors in obesity. Child Adolesc Psychiatr Clin N Am 18:83–94. https://doi.org/10.1016/j.chc.2008.07.006
Dasouki MJ, Youngs EL, Hovanes K (2011) Structural chromosome abnormalities associated with obesity: report of four new subjects and review of literature. Curr Genomics 12:190–203. https://doi.org/10.2174/138920211795677930
Youngson NA, Whitelaw E (2011) The effects of acquired paternal obesity on the next generation. Asian J Androl 13:195–196. https://doi.org/10.1038/aja.2010.163
Drewnowski A (2004) Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med 27:154–162. https://doi.org/10.1016/j.amepre.2004.06.011
Drewnowski A, Darmon N (2005) The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 82:265S-273S
Bluher M (2013) Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 27:163–177. https://doi.org/10.1016/j.beem.2013.02.005
Northcott JM, Yeganeh A, Taylor CG, Zahradka P, Wigle JT (2012) Adipokines and the cardiovascular system: mechanisms mediating health and disease. Can J Physiol Pharmacol 90:1029–1059. https://doi.org/10.1139/y2012-053
Drosatos K, Schulze PC (2013) Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 10:109–121. https://doi.org/10.1007/s11897-013-0133-0
Ussher JR (2014) The role of cardiac lipotoxicity in the pathogenesis of diabetic cardiomyopathy. Expert Rev Cardiovasc Ther 12:345–358. https://doi.org/10.1586/14779072.2014.891939
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109(Suppl 1):69–75
Mosihuzzaman M (2012) Herbal medicine in healthcare–an overview. Nat Prod Commun 7:807–812
Santos AK, Costa JG, Menezes IR, Cansancao IF, Santos KK, Matias EF, Coutinho HD (2010) Antioxidant activity of five Brazilian plants used as traditional medicines and food in Brazil. Pharmacogn Mag 6:335–338. https://doi.org/10.4103/0973-1296.71789
Bhamarapravati S, Pendland SL, Mahady GB (2003) Extracts of spice and food plants from Thai traditional medicine inhibit the growth of the human carcinogen Helicobacter pylori. Vivo 17:541–544
Brower V (2005) A nutraceutical a day may keep the doctor away. Consumers are turning increasingly to food supplements to improve well-being when pharmaceuticals fail. EMBO Rep 6:708–711. https://doi.org/10.1038/sj.embor.7400498
Raederstorff D, Kunz I, Schwager J (2013) Resveratrol, from experimental data to nutritional evidence: the emergence of a new food ingredient. Ann N Y Acad Sci 1290:136–141. https://doi.org/10.1111/nyas.12147
Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC (2013) Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 19:6064–6093
Raj P, Louis XL, Thandapilly SJ, Movahed A, Zieroth S, Netticadan T (2014) Potential of resveratrol in the treatment of heart failure. Life Sci 95:63–71
Rothman KJ (2008) BMI-related errors in the measurement of obesity. Int J Obes (Lond) 32(Suppl 3):S56–S59. https://doi.org/10.1038/ijo.2008.87
Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, Dorr M, Felix S, Lehnert H, Pittrow D, Silber S, Volzke H, Stalla GK, Wallaschofski H, Wittchen HU (2010) The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab 95:1777–1785. https://doi.org/10.1210/jc.2009-1584
Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, Gensichen J (2013) General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr 67:573–585. https://doi.org/10.1038/ejcn.2013.61
Das SK (2005) Body composition measurement in severe obesity. Curr Opin Clin Nutr Metab Care 8:602–606
Deurenberg P, Deurenberg-Yap M (2002) Validation of skinfold thickness and hand-held impedance measurements for estimation of body fat percentage among Singaporean Chinese, Malay and Indian subjects. Asia Pac J Clin Nutr 11:1–7
de Koning L, Merchant AT, Pogue J, Anand SS (2007) Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 28:850–856. https://doi.org/10.1093/eurheartj/ehm026
Hamer M, Stamatakis E (2012) Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 97:2482–2488. https://doi.org/10.1210/jc.2011-3475
Wang J, Sereika SM, Styn MA, Burke LE (2013) Factors associated with health-related quality of life among overweight or obese adults. J Clin Nurs 22:2172–2182. https://doi.org/10.1111/jocn.12280
Gouveia MJ, Frontini R, Canavarro MC, Moreira H (2014) Quality of life and psychological functioning in pediatric obesity: the role of body image dissatisfaction between girls and boys of different ages. Qual Life Res. https://doi.org/10.1007/s11136-014-0711-y
Dalle Grave R, Calugi S, El Ghoch M (2013) Lifestyle modification in the management of obesity: achievements and challenges. Eat Weight Disord 18:339–349. https://doi.org/10.1007/s40519-013-0049-4
Lagerros YT, Rossner S (2013) Obesity management: what brings success? Therap Adv Gastroenterol 6:77–88. https://doi.org/10.1177/1756283X12459413
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L and Clegg AJ (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13:1–190, 215–357, iii–iv. doi: https://doi.org/10.3310/hta13410
Pawloski LR, Ruchiwit M, Markham SM (2011) The growing burden of obesity in Thailand: a review of current trends and policies. Pediatr Nurs 37:256–261
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378:815–825. https://doi.org/10.1016/S0140-6736(11)60814-3
Tsai AG, Abbo ED, Ogden LG (2011) The time burden of overweight and obesity in primary care. BMC Health Serv Res 11:191. https://doi.org/10.1186/1472-6963-11-191
Ramachandran A, Snehalatha C (2010) Rising burden of obesity in Asia. J Obes. https://doi.org/10.1155/2010/868573
Withrow D, Alter DA (2011) The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 12:131–141. https://doi.org/10.1111/j.1467-789X.2009.00712.x
WHO (2018) Obesity and overweight.
Borders TF, Rohrer JE, Cardarelli KM (2006) Gender-specific disparities in obesity. J Community Health 31:57–68
Canada S (2018) Obesity in Canadian Adults, 2016 and 2017.
Canada S (2018) Health characteristics, annual estimates. June 25
Canada S (2009) Overweight and obese adults (self-reported)
Karen C, Roberts MS, de Groh M, Aziz A, Gilbert J-A (2012) Overweight and obesity in children and adolescents: results from the 2009 to 2011 Canadian Health Measures Survey. Health Reports
Roberts KC, Shields M, de Groh M, Aziz A, Gilbert JA (2012) Overweight and obesity in children and adolescents: results from the 2009 to 2011 Canadian Health Measures Survey. Health Rep 23:37–41
Canada PHAo (2017) Tackling obesity in Canada: childhood obesity and excess weight rates in Canada
Ng C, Corey PN, Young TK (2011) Socio-economic patterns of obesity among aboriginal and non-Aboriginal Canadians. Can J Public Health 102:264–268
Bruce SG, Riediger ND, Zacharias JM, Young TK (2011) Obesity and obesity-related comorbidities in a Canadian First Nation population. Prev Chronic Dis 8:A03
Must A (1996) Morbidity and mortality associated with elevated body weight in children and adolescents. Am J Clin Nutr 63:445S-447S
Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC (2010) Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 362:485–493. https://doi.org/10.1056/NEJMoa0904130
PHAC C (2013) Obesity in Canada
Dalrymple KL, Galione J, Hrabosky J, Chelminski I, Young D, O’Brien E, Zimmerman M (2011) Diagnosing social anxiety disorder in the presence of obesity: implications for a proposed change in DSM-5. Depress Anxiety 28:377–382. https://doi.org/10.1002/da.20794
Zimmerman M, Hrabosky JI, Francione C, Young D, Chelminski I, Dalrymple K and Galione JN (2011) Impact of obesity on the psychometric properties of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for major depressive disorder. Compr Psychiatry 52:146–150. doi: https://doi.org/10.1016/j.comppsych.2010.05.001
Canada PHAo (2011) Obesity in Canada—Health and economic implications.
Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W (2012) Obesity and severe obesity forecasts through 2030. Am J Prev Med 42:563–570. https://doi.org/10.1016/j.amepre.2011.10.026
Rengma MS, Sen J, Mondal N (2015) Socio-economic, demographic and lifestyle determinants of overweight and obesity among adults of Northeast India. Ethiop J Health Sci 25:199–208. https://doi.org/10.4314/ejhs.v25i3.2
Lee EY, Yoon KH (2018) Epidemic obesity in children and adolescents: risk factors and prevention. Front Med 12:658–666. https://doi.org/10.1007/s11684-018-0640-1
Fischer-Posovszky P, Wabitsch M, Hochberg Z (2007) Endocrinology of adipose tissue: an update. Horm Metab Res 39:314–321. https://doi.org/10.1055/s-2007-976539
Lapid K, Graff JM (2017) Form(ul)ation of adipocytes by lipids. Adipocyte 6:176–186. https://doi.org/10.1080/21623945.2017.1299298
Viscarra JA, Ortiz RM (2013) Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism 62:889–897. https://doi.org/10.1016/j.metabol.2012.12.014
Trayhurn P (2014) Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr. https://doi.org/10.1146/annurev-nutr-071812-161156
Lin JD, Wu TY, Lin LP, Hsu SW, Liu CT, Wu CL (2013) An exploratory study of health behaviors and the risks for triple H (hypertension, hyperlipidemia, and hyperglycemia) in young adults with disabilities between 20 and 39 years of age. Res Dev Disabil 34:3211–3217. https://doi.org/10.1016/j.ridd.2013.06.044
Nestruck AC, Davignon J (1986) Risks for hyperlipidemia. Cardiol Clin 4:47–56
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324. https://doi.org/10.1371/journal.pcbi.1000324
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C (2019) Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. https://doi.org/10.3390/ijms20092358
Skurk T, Alberti-Huber C, Herder C, Hauner H (2007) Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033. https://doi.org/10.1210/jc.2006-1055
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971. https://doi.org/10.1093/eurheartj/ehn387
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9:191–200. https://doi.org/10.5114/aoms.2013.33181
Suganami T, Nishida J, Ogawa Y (2005) A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 25:2062–2068. https://doi.org/10.1161/01.ATV.0000183883.72263.13
Bluher M (2009) Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117:241–250. https://doi.org/10.1055/s-0029-1192044
Wang GX, Cho KW, Uhm M, Hu CR, Li S, Cozacov Z, Xu AE, Cheng JX, Saltiel AR, Lumeng CN, Lin JD (2014) Otopetrin 1 protects mice from obesity-associated metabolic dysfunction through attenuating adipose tissue inflammation. Diabetes 63:1340–1352. https://doi.org/10.2337/db13-1139
Hubert HB, Feinleib M, McNamara PM, Castelli WP (1983) Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67:968–977
Csige I, Ujvarosy D, Szabo Z, Lorincz I, Paragh G, Harangi M, Somodi S (2018) The impact of obesity on the cardiovascular system. J Diabetes Res 2018:3407306. https://doi.org/10.1155/2018/3407306
Jiang J, Ahn J, Huang WY, Hayes RB (2013) Association of obesity with cardiovascular disease mortality in the PLCO trial. Prev Med 57:60–64. https://doi.org/10.1016/j.ypmed.2013.04.014
Flegal KM, Graubard BI, Williamson DF, Gail MH (2007) Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298:2028–2037. https://doi.org/10.1001/jama.298.17.2028
Shields M, Tremblay MS, Connor Gorber S, Janssen I (2012) Abdominal obesity and cardiovascular disease risk factors within body mass index categories. Health Rep 23:7–15
Ortega-Loubon C, Fernandez-Molina M, Singh G, Correa R (2019) Obesity and its cardiovascular effects. Diabetes Metab Res Rev 35:e3135. https://doi.org/10.1002/dmrr.3135
Taube A, Schlich R, Sell H, Eckardt K, Eckel J (2012) Inflammation and metabolic dysfunction: links to cardiovascular diseases. American journal of physiology. Heart Circulatory Physiol 302:H2148–H2165
Chrysant SG, Chrysant GS (2013) New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens 7:85–94. https://doi.org/10.1016/j.jash.2012.11.008
Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, Giannattasio C, Grassi G, Mancia G (2011) Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension 58:1029–1035. https://doi.org/10.1161/HYPERTENSIONAHA.111.175125
Britton KA, Fox CS (2011) Ectopic fat depots and cardiovascular disease. Circulation 124:e837–e841. https://doi.org/10.1161/CIRCULATIONAHA.111.077602
Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight M, Obesity P, Naaso TOS, American Society for N and American Diabetes A (2007) Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 85:1197–1202
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart A, Obesity Committee of the Council on Nutrition PA and Metabolism (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113:898–918. https://doi.org/10.1161/CIRCULATIONAHA.106.171016
Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA (2005) Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 23:1839–1846
Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M (2004) Increased carotid intima-media thickness and stiffness in obese children. Diab Care 27:2506–2508
Ozcetin M, Celikyay ZR, Celik A, Yilmaz R, Yerli Y, Erkorkmaz U (2012) The importance of carotid artery stiffness and increased intima-media thickness in obese children. S Afr Med J 102:295–299
Lee CD, Jacobs DR Jr, Schreiner PJ, Iribarren C, Hankinson A (2007) Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 86:48–54
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G (2010) Mechanisms of obesity-induced hypertension. Hypertens Res 33:386–393. https://doi.org/10.1038/hr.2010.9
Aurigemma GP, de Simone G, Fitzgibbons TP (2013) Cardiac remodeling in obesity. Circ Cardiovasc Imaging 6:142–152. https://doi.org/10.1161/CIRCIMAGING.111.964627
Goldberg IJ, Trent CM, Schulze PC (2012) Lipid metabolism and toxicity in the heart. Cell Metab 15:805–812. https://doi.org/10.1016/j.cmet.2012.04.006
Carpenter HM (1962) Myocardial fat infiltration. Am Heart J 63:491–496
Karasoy D, Bo Jensen T, Hansen ML, Schmiegelow M, Lamberts M, Gislason GH, Hansen J, Torp-Pedersen C, Olesen JB (2013) Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace 15:781–786. https://doi.org/10.1093/europace/eus422
Chrostowska M, Szyndler A, Hoffmann M, Narkiewicz K (2013) Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab 27:147–156. https://doi.org/10.1016/j.beem.2013.01.004
Steyn K, Sliwa K, Hawken S, Commerford P, Onen C, Damasceno A, Ounpuu S, Yusuf S, Africa IIi (2005) Risk factors associated with myocardial infarction in Africa: the INTERHEART Africa study. Circulation 112:3554–3561. https://doi.org/10.1161/CIRCULATIONAHA.105.563452
O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S, Investigators I (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376:112–123. https://doi.org/10.1016/S0140-6736(10)60834-3
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G (2013) Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 62:569–576. https://doi.org/10.1016/j.jacc.2013.05.045
Van de Voorde J, Pauwels B, Boydens C, Decaluwe K (2013) Adipocytokines in relation to cardiovascular disease. Metabolism 62:1513–1521. https://doi.org/10.1016/j.metabol.2013.06.004
Luc G, Empana JP, Morange P, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Evans A, Kee F, Bingham A, Machez E, Ducimetiere P (2010) Adipocytokines and the risk of coronary heart disease in healthy middle aged men: the PRIME Study. Int J Obes (Lond) 34:118–126. https://doi.org/10.1038/ijo.2009.204
Wickman C, Kramer H (2013) Obesity and kidney disease: potential mechanisms. Semin Nephrol 33:14–22. https://doi.org/10.1016/j.semnephrol.2012.12.006
de Jong PE, Verhave JC, Pinto-Sietsma SJ (2002) Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord 26(Suppl 4):S21–S24. https://doi.org/10.1038/sj.ijo.0802213
Foster MC, Hwang SJ, Massaro JM, Hoffmann U, DeBoer IH, Robins SJ, Vasan RS, Fox CS (2011) Association of subcutaneous and visceral adiposity with albuminuria: the Framingham Heart Study. Obesity (Silver Spring) 19:1284–1289. https://doi.org/10.1038/oby.2010.308
Schulze PC, Drosatos K, Goldberg IJ (2016) Lipid use and misuse by the heart. Circ Res 118:1736–1751. https://doi.org/10.1161/CIRCRESAHA.116.306842
Taegtmeyer H, Sen S, Vela D (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 1188:191–198. https://doi.org/10.1111/j.1749-6632.2009.05100.x
Pascual F, Coleman RA (2016) Fuel availability and fate in cardiac metabolism: a tale of two substrates. Biochim Biophys Acta 1861:1425–1433. https://doi.org/10.1016/j.bbalip.2016.03.014
Trent CM, Yu S, Hu Y, Skoller N, Huggins LA, Homma S, Goldberg IJ (2014) Lipoprotein lipase activity is required for cardiac lipid droplet production. J Lipid Res 55:645–658. https://doi.org/10.1194/jlr.M043471
van der Vusse GJ, van Bilsen M, Glatz JF (2000) Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res 45:279–293
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258. https://doi.org/10.1152/physrev.00015.2009
Saely CH, Geiger K, Drexel H (2012) Brown versus white adipose tissue: a mini-review. Gerontology 58:15–23. https://doi.org/10.1159/000321319
Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N (2014) De novo lipogenesis in health and disease. Metabolism. https://doi.org/10.1016/j.metabol.2014.04.003
Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF (2010) Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 82:149–154. https://doi.org/10.1016/j.plefa.2010.02.029
Das UN (2006) Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J 1:420–439. https://doi.org/10.1002/biot.200600012
Nishi H, Higashihara T, Inagi R (2019) Lipotoxicity in kidney, heart, and skeletal muscle dysfunction. Nutrients. https://doi.org/10.3390/nu11071664
Turkieh A, Caubere C, Barutaut M, Desmoulin F, Harmancey R, Galinier M, Berry M, Dambrin C, Polidori C, Casteilla L, Koukoui F, Rouet P, Smih F (2014) Apolipoprotein O is mitochondrial and promotes lipotoxicity in heart. J Clin Invest 124:2277–2286. https://doi.org/10.1172/JCI74668
Nolan CJ (2014) Lipotoxicity, beta cell dysfunction, and gestational diabetes. Cell Metab 19:553–554. https://doi.org/10.1016/j.cmet.2014.03.020
Eguchi K, Manabe I (2014) Toll-like receptor, lipotoxicity and chronic inflammation: the pathological link between obesity and cardiometabolic disease. J Atheroscler Thromb
Lake AD, Novak P, Hardwick RN, Flores-Keown B, Zhao F, Klimecki WT, Cherrington NJ (2014) The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol Sci 137:26–35. https://doi.org/10.1093/toxsci/kft230
Schulze PC (2009) Myocardial lipid accumulation and lipotoxicity in heart failure. J Lipid Res 50:2137–2138
de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M (1997) Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38:1384–1394
Hickson-Bick DL, Buja LM, McMillin JB (2000) Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J Mol Cell Cardiol 32:511–519. https://doi.org/10.1006/jmcc.1999.1098
Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49:2101–2112. https://doi.org/10.1194/jlr.M800147-JLR200
Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Eppenberger HM, Spinas GA, Donath MY (2001) Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes 50:2105–2113
Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A (2010) Resveratrol: a multifunctional cytoprotective molecule. Curr Pharm Biotechnol 11:810–818
Giovinazzo G, Ingrosso I, Paradiso A, De Gara L, Santino A (2012) Resveratrol biosynthesis: plant metabolic engineering for nutritional improvement of food. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 67:191–199
Pervaiz S (2003) Resveratrol: from grapevines to mammalian biology. FASEB J 17:1975–1985. https://doi.org/10.1096/fj.03-0168rev17/14/1975
de Lorgeril M, Salen P, Guiraud A, Boucher F, de Leiris J (2003) Resveratrol and non-ethanolic components of wine in experimental cardiology. Nutr Metab Cardiovasc Dis 13:100–103
Takaoka MJ (1940) Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.). J Faculty Sci Hokkaido Imperial University 3:1–16
Tsai WH, Yang CC, Li PC, Chen WC, Chien CT (2013) Therapeutic potential of traditional chinese medicine on inflammatory diseases. J Tradit Complement Med 3:142–151. https://doi.org/10.4103/2225-4110.114898
Hillis WE, Isoi K (1965) Variation in the chemical composition of Eucalyptus sideroxylon. Phytochemistry 4:541–550
Tyukavkina NA, Gromova AS, Lutskii VI, Voronov VK (1974) Hydroxystilbenes from the bark of Pinus sibirica. Chem Nat Compd 8:570–572
Langcake P, Pryce RJ (1976) The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol 9:77–86
Pool RM, Creasy LL, Frackelton AS (1981) Resveratrol and the viniferins, their application to screening for disease resistance in grape breeding programs. Vitis 20:136–145
Kubo M, Shin H, Haneda T, Tani T, Namba K (1981) Studies on the antifungal substance of crude drug: 2. On the roots of Polygonum cuspidatum (Polygonaceae). Shoyakugaku Zasshi 35:58–61
Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S (1982) Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull 30:1766–1770
Renaud S, de Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339:1523–1526
Creasy EHSaLL (1992) Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic 43:49–52
Frankel EN, Kanner J, German JB, Parks E, Kinsella JE (1993) Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 341:454–457
Xu Q, Si LY (2012) Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action. Nutrition research (New York, N.Y.) 32:648–658.
Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC (2013) Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective. Ann N Y Acad Sci 1290:37–51
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS (2017) The role of resveratrol in cancer therapy. Int J Mol Sci. https://doi.org/10.3390/ijms18122589
Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U (2002) Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106:1652–1658
Opie LH, Lecour S (2007) The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J 28:1683–1693. https://doi.org/10.1093/eurheartj/ehm149
Li H, Forstermann U (2012) Red wine and cardiovascular health. Circ Res 111:959–961. https://doi.org/10.1161/CIRCRESAHA.112.278705
Yu W, Fu YC, Wang W (2012) Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem 113:752–759. https://doi.org/10.1002/jcb.23431
Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li J, Long R, Zhou Y (2013) Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2013.10.034
Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR (2008) Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem 283:24194–24201. https://doi.org/10.1074/jbc.M802869200
Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, Anderson HD, Netticadan T (2011) Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol 668:217–224. https://doi.org/10.1016/j.ejphar.2011.06.042
Beauloye C, Bertrand L, Horman S, Hue L (2011) AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res 90:224–233. https://doi.org/10.1093/cvr/cvr034
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Penumathsa SV, Maulik N (2009) Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol 87:275–286. https://doi.org/10.1139/Y09-013
Li H, Xia N, Forstermann U (2012) Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26:102–110. https://doi.org/10.1016/j.niox.2011.12.006
Zhang LP, Yin JX, Liu Z, Zhang Y, Wang QS, Zhao J (2006) Effect of resveratrol on L-type calcium current in rat ventricular myocytes. Acta Pharmacol Sin 27:179–183. https://doi.org/10.1111/j.1745-7254.2006.00250.x
Poulsen MM, Jorgensen JO, Jessen N, Richelsen B, Pedersen SB (2013) Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann N Y Acad Sci 1290:74–82. https://doi.org/10.1111/nyas.12141
Lekli I, Szabo G, Juhasz B, Das S, Das M, Varga E, Szendrei L, Gesztelyi R, Varadi J, Bak I, Das DK, Tosaki A (2008) Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol 294:H859–H866. https://doi.org/10.1152/ajpheart.01048.2007
Aubin MC, Lajoie C, Clement R, Gosselin H, Calderone A, Perrault LP (2008) Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther 325:961–968. https://doi.org/10.1124/jpet.107.135061
Louis XL, Thandapilly SJ, MohanKumar SK, Yu L, Taylor CG, Zahradka P, Netticadan T (2012) Treatment with low-dose resveratrol reverses cardiac impairment in obese prone but not in obese resistant rats. J Nutr Biochem 23:1163–1169. https://doi.org/10.1016/j.jnutbio.2011.06.010
Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS (2012) The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation 125:1757–1764. https://doi.org/10.1161/circulationaha.111.067801
Lu Y, Lu X, Wang L, Yang W (2019) Resveratrol attenuates high fat diet-induced mouse cardiomyopathy through upregulation of estrogen related receptor-alpha. Eur J Pharmacol 843:88–95. https://doi.org/10.1016/j.ejphar.2018.10.018
Xia N, Forstermann U, Li H (2014) Resveratrol and endothelial nitric oxide. Molecules 19:16102–16121. https://doi.org/10.3390/molecules191016102
Huang JP, Hsu SC, Li DE, Chen KH, Kuo CY, Hung LM (2018) Resveratrol mitigates high-fat diet-induced vascular dysfunction by activating the Akt/eNOS/NO and Sirt1/ER pathway. J Cardiovasc Pharmacol 72:231–241. https://doi.org/10.1097/FJC.0000000000000621
Arzola-Paniagua MA, Garcia-Salgado Lopez ER, Calvo-Vargas CG, Guevara-Cruz M (2016) Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: a randomized controlled trial. Obesity (Silver Spring) 24:1454–1463. https://doi.org/10.1002/oby.21523
Xue M, Weickert MO, Qureshi S, Kandala NB, Anwar A, Waldron M, Shafie A, Messenger D, Fowler M, Jenkins G, Rabbani N, Thornalley PJ (2016) Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 65:2282–2294. https://doi.org/10.2337/db16-0153
Most J, Timmers S, Warnke I, Jocken JW, van Boekschoten M, de Groot P, Bendik I, Schrauwen P, Goossens GH, Blaak EE (2016) Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am J Clin Nutr 104:215–227. https://doi.org/10.3945/ajcn.115.122937
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14:612–622. https://doi.org/10.1016/j.cmet.2011.10.002
Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR (2013) Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens 31:1819–1827. https://doi.org/10.1097/HJH.0b013e328362b9d6
Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF (2013) High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol 33:2895–2901. https://doi.org/10.1161/ATVBAHA.113.302342
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lieben Louis, X., Krishnan, S., Wigle, J.T., Netticadan, T. (2020). Obesity and Cardiovascular Disease: Impact of Resveratrol as a Therapeutic. In: Tappia, P.S., Bhullar, S.K., Dhalla, N.S. (eds) Biochemistry of Cardiovascular Dysfunction in Obesity. Advances in Biochemistry in Health and Disease, vol 20. Springer, Cham. https://doi.org/10.1007/978-3-030-47336-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-47336-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47335-8
Online ISBN: 978-3-030-47336-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)