Abstract
Herein we emphasise how environment, palaeoecology and palaeobiogeography play key roles in the evolution of organisms. Nineteenth-century ammonoid biochronology led to the definition of the Mesozoic stages. Their beginning and end are bound by the biggest mass extinctions of Earth history. This study deals with the initial Triassic stages that needed a remarkably short biotic recovery time. The Lower Triassic stages, all named after nineteenth-century researchers of the Himalayas, are the Griesbachian, Dienerian, Smithian and Spathian.
If the twentieth century saw an increasing use of alternative biotic groups, among which were those conodonts and radiolarians that permit the relative timing of open marine sequences, one can say that twenty-first century isotopic excursion events became increasingly dominant. For the Early Triassic alone, the succession of positive and negative excursions determines the following five events:
-
(I)
Late Permian-Early Triassic boundary
-
(II)
Late Griesbachian
-
(III)
Dienerian-Smithian boundary
-
(IV)
Smithian-Spathian boundary
-
(V)
Middle Spathian-Early Aegean interval
These events comprise three negative events (I, III and IV) and two positive events (II and V). The nature of these events, in control of environmental instabilities and system tracts (sea-level changes), is the key for understanding biological morphogenesis and evolution.
This contribution aims to analyse the phenomena during one of the five largest mass extinctions of Earth history and the fast recovery that followed in its aftermath.
Our study of the conodont subfamily Neogondolellinae suggests that stress-generating phenomena and events appear to have paced successive lineages, alternating from Darwinian anagenesis to atavistic reversal and rediversification.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Evolution during the Early Triassic can be viewed from a new perspective. The Triassic, one of the warmest periods of Earth history, was the theatre for the dismantling of Pangea, the widening of Tethys, the main equatorial seaway that opened up Pangea and the initial opening of the Atlantic. Following the Late Permian-Early Triassic extinction caused by the Siberian Traps (Burgess et al. 2017), conodonts radiated into new Triassic niches that opened with the transgressive action of Tethys. Panthalassan currents spread taxa worldwide and new geographic, oceanic and climatic barriers isolated new faunal provinces. Soon after, conodonts were diminished by events in the Central Atlantic Magmatic Province (CAMP) that led to their extinction at the end of the Triassic.
The multi-element apparatus of the family Gondolellidae evolved from the Permian septimembrate mesogondolellin apparatus to the Late Permian octomembrate neogondolellin apparatus that ranged to the Late Carnian, and then retrograded to an epigondolellin septimembrate apparatus during the Norian and Rhaetian. Bio-environments, from shallow shelf to deeper open marine, vary in salinity and temperature. The Spathian-Julian octomembrate apparatus of the family Gladigondolellidae is linked to the deeper equatorial provinces. The successive clades not only define the rate of evolution, but also show their dependence from environmental conditions and their response to ecological stress.
The Early Triassic is especially rich in retrograde evolutionary events during a very short duration of merely 5 Ma. This is the reason for its particular interest.
5.2 Part One
5.2.1 Environmental Instabilities
Environmental instabilities are controlled by the behaviour of our planet and the sun. In this regard disciplines from tectonics to atmospheric physics, astronomy and climatology all depend in large part on cyclic patterns, e.g. Milankovitch’s cycles. Cycles are by definition repetitive, in opposition to catastrophes, like the collision of the Earth with an asteroid. All instabilities are reflected in the life cycles of living organisms. Some of these can be lethal with no return, such as global extinctions due to meteoritic impacts (Clutson et al. 2018) or volcanism that may be part of incompletely understood tectonic or astronomic cycles .
5.2.2 Morphogenesis of the Bios
Environmental instabilities control morphogenesis. The generation of anatomic shapes is the result of evolution that includes variations in size.
As we analyse the development of conodonts, a protovertebrate that lived during the first half of Phanerozoic times, we recognise that halfway through this period, a major extinction marked the limit between Paleozoic and Mesozoic eras. But conodonts, having survived several intervals of stress during the Late Permian mass extinction, somehow “knew” how to respond, and endured for another 50 Ma, through the Triassic. This was a time when abnormal morphological development under sublethal stress prevailed and this period is the subject of our present essay. Survival under such conditions was partly possible due to retrograde evolution during major extinction periods.
5.2.3 The Triassic as a Period of Time
It must be emphasised that since most research started in the eighteenth century, the Triassic Period was conceived in continental Europe where it consisted of the three units established by Alberti in 1864: Buntsandstein, Muschelkalk and Keuper. Soon these became equivalent to the Lower, Middle and Upper Triassic, yet, not without considerable correlation conflicts. The Lower Triassic became also known as the Scythian which in Europe remains dominated by variegated sandstones of continental origin, the so-called Bunter facies. In the course of the nineteenth century, ammonoid research in the Himalayas resulted in the subdivision of the Lower Triassic or Scythian into the Brahmanian and Jakutian stages. During the twentieth century the detailed ammonoid studies in Arctic Canada led to the distinction of four stages, named after nineteenth-century researchers in the Himalayas: the Griesbachian, Dienerian, Smithian and Spathian. This geological chapter is unfortunately not yet fully settled, since the twofold subdivision of the Induan and Olenekian stages appears rather inadequate. Half a century ago, the geological timescale of Harland et al. (1964) admitted a duration of 47 Ma for the Triassic. At that time Tozer (1967) estimated that the probable duration of an average ammonite species was 1.5 Ma. This led to the overestimation of the Early Triassic (Scythian) time span that, having the highest speciation rate of all Triassic subdivisions, only represents 10% of the total Triassic 52 Ma long time span (e.g. Gradstein et al. 2005). Moreover, following the so-called Late Smithian event that took place less than 1.5 Ma after the PTB mass extinction, Brayard et al. (2017) found that the so-called Paris Biota fulfilled all conditions of full recovery.
After long deliberations in the frame of UNESCO, scientists of many specialities selected the single fossil that determines the base of the Triassic in one chosen locality. They subsequently marked this Global Stratotype Section and Point (GSSP) where this fossil appears in the field with a so-called golden spike, of which Lucas (2018) recently accurately analysed the modalities. The 150-year-long quest by the most eminent specialists to determine that rare zonal beast, supposed to mark the dawn of the Triassic Period, and in this case of the Mesozoic era as well, did not end in the discovery of a large healthy-looking ammonoid from a solid family in full radiation. Instead, the choice fell on a tiny conodont, which was not from the most promising family, Gondolellidae, but a poorly defined variation within a species of the family Anchignathodontidae. Long acquainted to hostile conditions, it also survived the PTB. But the Griesbachian environment, quickly regaining a certain normality of temperature, salinity and sea level, became hostile for this family that soon became extinct. Knell (2012), in “The Great Fossil Enigma, the Search of the Conodont Animal”, does not spare his criticism of the metaphorical “Golden Spikes”, calling it an artificial scheme.
The passage from Paleozoic to Mesozoic was surrounded by extremely life-hostile palaeoenvironments of lower than normal sea levels and abnormal temperatures. The basins that hosted the transitory sequence were unevenly distributed, many were desert-like and fossils were rare. First came the Himalayas where Waagen and Diener (in Von Mojsisovics et al. 1895) compared their Mediterranean with the Indian provinces. They subdivided the Scythian into the Brahmanian and Jakutian stages with the former further divided into the Gangetian and Gandarian substages. These subdivisions alternating between Himalayas and Salt Range were defined by seven pelagic ammonoid zones compared to only one in the Mediterranean Werfen beds. For Shevyrev (2005) there were at least seven regions next to Himalayan Kashmir and Spiti, China (Meishan, Selong) and the high north (Canada, Greenland and Verkhoyansk). The boundary in the northern regions, accommodating an additional Otoceras concavum zone, meant that an ammonoid zone was missing in the rest of the world between the Late Permian Changsingensis zone and the Induan Fissiselatum zone. As a result, it was believed that a continuous P/T boundary section existed nowhere on Earth, not even in the boreal region, where it appeared that the youngest Permian was missing. However, since Zakharov et al. (2014), the Upper Permian Changsinghian stage in Siberia is equivalent to the range of the Otoceras concavum zone. Thus, the characteristic δ13C isotope curve that makes a bulge around 252 Ma appears as the only reliable indication of the PTB level.
Kozur (2006) remarked that Kiparisova and Popov (1956) introduced the Induan and Olenekian stages for the Lower Triassic despite the fact that Von Mojsisovics et al. (1895) had already erected the Brahmanian and Jakutian stages for this time interval. The Olenekian was defined in the Boreal Realm of Russia, while the Induan stage, named after the Indus River (Salt Range), yields rich ammonoid and conodont faunas characteristic of the Tethyan Realm. In reality, however, the Induan was defined with Boreal Otoceras faunas (O. concavum zone), as equivalent to the Salt Range. Kiparisova and Popov (1956) assigned the largest part of the Lower Olenekian to the original Induan at the time that the Boreal ammonoids were still unknown. Later, Kiparisova and Popov (1964) removed the lower part of the Olenekian from the original Induan, bringing a drastic change to the scope of the Induan .
5.2.4 Stages
The distribution of taxa is not uniform as faunas depend on conditions of provincial and environmental nature . Zapfe (1983) was of the opinion that there were three main faunal realms during the Triassic: the tropical Tethyan, moderate Canadian and boreal Russian, with few common ammonoids and each with its particular fauna. Subdivisions were either Himalayan threefold, Canadian fourfold and Russian bifold either coexisting or separated. As an example, the Gangetian and Griesbachian were equal as stages, and the Nammalian stage comprised the Dienerian and Smithian as substages. While the Induan stage encompasses the Griesbachian and Dienerian as substages, the Olenekian contains the Smithian and Spathian as substages. For Tozer the Griesbachian, Dienerian, Smithian and Spathian stages replace all preceding subdivisions and should not be downgraded to substages of the ill-defined Induan and Olenekian stages. Thus, it is important to remember that Tozer (1994) established these four stages (Fig. 5.1) after his life-long study of the Early Triassic ammonoids in Canada, going as far as to get the nameless creeks exposing his stages officially named after the Himalayan stratigraphy pioneers, Griesbach, Diener, Smith and Spath, recognised as Canadian topographic map entities. While keeping ill-defined old names is obsolete, the introduction of the names “Induan” and “Olenekian” as stages was redundant. As there is no doubt that in order to accommodate facial realms, in the sense of Zapfe (1983), each realm when conflicting with a neighbour is to be defined by typical zones, proper for its conditions, so as for Tethyan versus Russian, or local Spathian zones within North America (Orchard 2007).
Tozer’s (1994) Early Triassic stages, based on ammonoids in Canada
Since Harland et al. (1964), the calculated duration for the Lower Triassic has become shorter over the years. Lately, Cohen et al. (2018) published 251.902 ± 0.024 Ma for the PTB, which is less than the 252.6 ± 0.2 Ma for the PTB proposed in Schneebeli-Hermann et al. (2013). These authors adopted 251.22 ± 0.2 Ma for the Dienerian-Smithian boundary and 250.55 Ma for the Early Spathian (Paris Biota of Brayard et al. 2017).
5.2.5 Environmental Changes
Zakharov (1974) subdivided the palaeobiogeography of the Early Triassic using water temperature where boreal freshened waters averaged 14.5 °C versus 23–27 °C for normal Tethyan saline waters. More recently, Garbelli et al. (2015) analysed Tethyan seawater chemistry and temperature from the dawn to the end of the Permian mass extinction, whereas Petsios et al. (2017) studied the impact of temperature across the end-Permian extinction modelling extinction and recovery dynamics. Oxygen being essential for animal life, geochemical proxies are instrumental in determining the broad evolutionary history of oxygen on Earth. Showing the stepwise oxygenation of the Paleozoic atmosphere , Krause et al. (2018) produced a new model that is consistent with available proxy data and independently supports a Paleozoic Oxygenation Event, which likely contributed to the observed radiation of a complex, diverse fauna at this time. Silva-Tamayo et al. (2018) studied the global perturbation of the marine calcium cycle during the Permian-Triassic transition finding a globally distributed negative, globally distributed Changsinghian–Griesbachian δ44/40Ca anomaly in carbonate successions.
Payne and Van de Schootbrugge (2007) produced the geochemical tools that provide the reasons for the coming and going of organisms; among them are the crucial criteria for the appearance or absence of reef organisms. At the base of the Griesbachian, not only a substantial δ13C excursion occurs, but also 87/86Sr and δ18O trends suggest a catastrophic temperature rise, the impact of which inflicted small size on the conodonts. Schaal (2014) found strontium isotopic constraints on Permian-Triassic global change with a new high-resolution seawater 87/86Sr record and a numerical model of the strontium cycle. Strontium isotope data reveal that a rapid radiogenic excursion occurred during the first 2 million years of Early Triassic time. Model results show that the magnitude of CO2 release during Siberian Traps volcanism is sufficient to account for much of the observed increase in seawater 87/86Sr through CO2 enhancement of continental weathering rates. The small size of Early Triassic marine organisms has important implications for the ecological and environmental pressures operating during and after the end-Permian mass extinction. Leu et al. (2018), investigating the size response in conodonts to environmental changes during the late Smithian extinction, found that body size reduction is a common evolutionary response to heavy environmental stress. These changes are not fortuitous as they obey trends that can be seen to be repetitive over time.
For Raup (in Barash 2006), the PTB caused a 52% drop in diversity. Carbon cycle perturbations in the organic carbon isotope chemo-stratigraphy are statistically indistinguishable, despite great variations of sedimentation rates (Retallack 2013). Since Baud and Magaritz (1989), isotopes have added a totally new dimension, that of the climate.
In the aftermath of the end-Permian mass extinction, some of the largest Phanerozoic carbon isotopic excursions are recorded in the Early Triassic Thaynes Group of Utah, USA (Thomazo et al. 2016). Among these a global Smithian-negative carbon isotope excursion has been identified, followed by an abrupt increase across the Smithian-Spathian boundary (SSB ; ~250.8 Ma ago). This chemo-stratigraphic evolution is associated with palaeontological evidence that indicates a major collapse of terrestrial and marine ecosystems during the Late Smithian. It is commonly assumed that Smithian and Spathian isotopic variations are intimately linked to major perturbations in the exogenic carbon reservoir. Using oxygen isotope compositions of conodont apatite (δ18Ophos) Trotter et al. (2015) found three major warming cycles in the Sicani basin. The oxygen isotope (δ18O) record derived from conodont apatite reveals variable long-term climate trends throughout the Triassic Period. This record shows several major first-order negative shifts reflecting intense warming episodes, not only the well-known extreme PTB-Early Triassic event (∼5 ‰), but also two large cycles of similar magnitude (∼1.5, ∼1.7 ‰) and duration (∼7 Ma) during the Late Carnian and late Norian. Between the PTB-Early Triassic and Carnian major episodes, three rapid shorter term warming events of decreasing magnitude punctuate the Mid-Late Anisian, Early Ladinian and latest Ladinian with distinct cooler (i.e. favourable) intervals characterising the early Anisian and early Carnian, indicating a fluctuating but ameliorating Middle Triassic climate trend. Two long periods of sustained cooler conditions occurred during the Late Triassic for much of the Norian and Rhaetian. The five humid events previously recognised from the geological record, including the Carnian Pluvial Episode, are associated with the low δ18O warming phases. The magnitudes of these first-order warming cycles together with widespread geological and palaeontological evidence suggest that they were at least Tethyan-wide events.
Korngreen and Zilberman (2017) summarised δ18Ocarbexcursions during the P-T transition and considered the Early Triassic as the hottest record of marine temperatures (~40 °C). Sun et al. (2012) (see also Payne and Clapham 2012) associated these with the extension of the desert belts into the mid latitudes and the palaeo-mid-high latitudes (50–55°), characterised by temperate/warm multi-exchanges of climates (Péron et al. 2005; Sellwood and Valdes 2006).
Accordingly, two warming and two coolingperiods took place during the Late Permian–Early Triassic interval in intra-basin sediments analysed for δ18O excursions (Sun et al. 2012).
-
(I)
First warming period: Interpretation of δ18Ocarb profiles revealed during the Late Permian, PTB and Griesbachian indicated, by continued worldwide decline in values, a drop of 1.5‰ up to 5‰ (Holser et al. 1989; Heydari et al. 2000; Rampino and Eshet 2017; Haas et al. 2007; Horacek et al. 2009; Richoz et al. 2010; Joachimski et al. 2012; Chen et al. 2013a, b, 2015).
-
(II)
First cooling: Using δ18Ocarb profiles, cooling developed during the uppermost Griesbachian and persisted during the entire Dienerian (Haas et al. 2007; Richoz et al. 2010).
-
(III)
Second warming: Began at the Dienerian/Smithian boundary, lasted throughout the entire Smithian and reached its peak at the Smithian/Spathian boundary (Galfetti et al. 2007) that Sun et al. (2012) called the “Late Smithian Thermal Maximum”.
-
(IV)
Second cooling period: Recognised by Sun et al. (2012) and Romano et al. (2013) in the early Spathian. This was followed by climate stabilisation during most of the Spathian (see also Galfetti et al. 2007; Hochuli et al. 2010; Stefani et al. 2010; Hermann et al. 2011). The uppermost Spathian was considered by Sun et al. (2012) as the third cooling event, retaining a relatively cool climate through the early Anisian (Aegean-Bithynian).
In three southern Israel boreholes, Korngreen and Zilberman (2017) found the δ18O profiles to exhibit values in the –5 to –7.5‰ average range in the Levant region, also typical of Western Tethys. The described changes in the major sources of terrestrial material to the three successions require climate changes that would expand the precipitation zone further to the southern hinterland and conversely. The succession was recorded in the general southward extension of the precipitation zone during the Late Permian (hinterland humidification), northward contraction (hinterland aridisation) from the lattermost Late Permian through the most of the Early Triassic, then re-expanding gradually during the late Early Triassic, reaching a peak during the mid-Anisian (hinterland re-humidification).
The tropical (~10°S) location of the studied successions attempts to attribute the changes of the precipitation zone to the long-term Intertropical Convergence Zone (ITCZ) expansion/contraction that severely affected the precipitation, erosion and vegetation type in the low-latitude hinterlands.
The δ18O Profiles in the Levant Region
The δ18O composite profile (Fig. 5.2) is based on event correlations: Comparison of the three δ18O profiles of the studied successions exhibiting similar trends of decrease/increase trends correlated well with worldwide climate change events: two warming phases at the P-T transition, the first during the Late Permian to the PTB and the second at the Dienerian/Smithian boundary and through the Smithian. An additional warming period is related to the early Middle Triassic (Early Anisian; EAE) warming towards the Middle Anisian (MAE) and after. A cooling period characterises the Late Spathian (LSE-SAB). Note the relatively extreme negative and positive values of the proximal succession and the abrupt shifts .
Composite δ18O profiles of three boreholes in southern Israel. The average range of values (–5 to –7.5‰) is typical of western Tethys climate trends. There are three major warming periods: (I) Late Permian to the PTB; (III) Late Dienerian—most of the Smithian; (V) Early-Middle Anisian, and the two relatively cool periods: (II) Griesbachian-Dienerian and (IV) Late Smithian-Spathian. Each of the periods may exhibit short respites with the opposite trend (after Korngreen and Zilberman 2017)
Late Permian-Early Triassic Negative Shifts
δ18O values generally vary between ~–4.0 and –8.5‰ and are much higher than those of Meishan GSSP and other sections from China with –9.5 to –13‰, 0–20°N (after Joachimski et al. 2012; Sun et al. 2012). However, the range of δ18O values is highly compatible with those from Neotethyan successions (equatorial to 10° S, 25–30° S; Haas et al. 2007; Richoz et al. 2010), and is also compatible with the lighter part surrounded by land Muschelkalk sea range (–6.2 to –2‰, Korte et al. 2005). They are also lighter than marine carbonates deposited in modern equatorial range (0 to –3‰, Carpenter and Lohmann 1995). The two negative shifts of about 1–2‰ were viewed during the Late Permian, followed by a sharp and distinct low δ18O peak at the PTB with an amplitude of up to –3‰, reaching a δ18O value of ~–11‰. These were attributed to a significant warming trend and may be assigned to the first recognised extreme global warming of the Paleozoic-Mesozoic transition (Holser et al. 1989; Heydari et al. 2000; Haas et al. 2007; Horacek et al. 2009; Richoz et al. 2010; Joachimski et al. 2012; Sun et al. 2012). Some stabilisation of the δ18O values associated with relatively short warming and cooling changes along the Griesbachian and most of the Dienerian, attributing the changes to a relatively cooling period recognised in this time interval in other parts of the world (Haas et al. 2007; Richoz et al. 2010; Sun et al. 2012), is defined as relatively constant warm conditions (Fig. 5.2).
Dienerian-Smithian Boundary
The next noticeable negative shifts occurred near the Dienerian-Smithian boundary and throughout the Smithian. Considered as a warming period, the Smithian was assigned to an equivalent global warming event (Galfetti et al. 2007; Sun et al. 2012).
Smithian-Spathian Warming Event (SSB)
For Chen et al. (2013a, b), recovery from the end-Permian mass extinction took several million years. This was partly due to an inhospitable environment and the three episodes of further extinction occurred in late Griesbachian near the Smithian-Spathian boundary (SSB) and in the late Spathian. A short, but extreme warming event precedes the Smithian-Spathian boundary. Mercury concentrations are possibly connected to the SSB volcanism of the Siberian Traps (after Hammer et al. 2019). However, shortly after the Late Smithian event full recovery had been achieved as shown by the Paris Biota (Brayard et al. 2017) (Fig. 5.3).
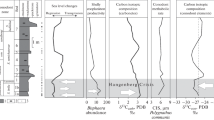
Early Triassic Isotopic Events (modified after Orchard 2007). (I) Late Permian-Early Triassic boundary; (II) Late Griesbachian; (III) Dienerian-Smithian boundary; (IV) Smithian-Spathian boundary; (V) Middle Spathian-Early Aegean interval
Base sea levels and δ13C trends control a succession of nine conodont events: (1) Gradual decline from five families in the Changsinghian to three by the PTB, and to two in the Latest Griesbachian. Single neogondolellin multielement apparatus during this interval. (2) PTB: Siberian Traps caused anoxia and global cooling; Boreal stocks replaced Tethyan warm-water ones. (3) Late Griesbachian δ13C minimum, global warming, local regression; extinction of Anchignathodontidae; cool-water segminiplanate Neogondolellinae retrograde and new ones initialise. (4) Dienerian radiation of segminate proteromorphs (Neospathodus, Kashmirella), reinitialisations concurrent with returning normal marine conditions. (5) Major Basal Smithian transgression: Early-Middle Smithian explosive radiation, Triassic’s strongest. (6) Late Smithian δ13C minimum: low-standand possible renewed volcanism; biggest Triassic extinction, affecting more taxa than both PTB and final end-Triassic extinctions. Mercury concentrations are possibly connected to the SSB volcanism of the Siberian Traps (after Hammer et al. 2019). (7) Early Spathian return to normal marine conditions, significant radiation and rapid recovery (so-called Paris Biota of Brayard et al. 2017). After this radiation, the total number of Spathian gondolellid apparatuses equalled those of the Smithian (Orchard 2007). (8) Late Spathian low stand corresponds to a gradual decline in diversity. Reduction and disappearance of proteromorph stocks with reinitialisation of segminiplanate gondolellids. (9) Uppermost Spathian, large positive carbon isotope excursion, starting in Upper Spathian and peaking with Early Anisian major transgression. Conodont size variation, especially during Late Smithian (after Leu et al. 2018).
Detailed measurements of size variation in conodonts such as Neospathodus show that the clade suffered significant size reduction during the SSB crisis, followed by gradual and steady size increase during the early Spathian.
De Wever et al. (2007) observed that radiolarian biodiversity increased constantly during the Paleozoic and decreased only slightly towards the end of the Permian. Distinctive for the PT event is not the diminishing number of taxa, but the importance of the Triassic diversification that followed. As under anoxic environments radiolarian diversity minima relate to positive δ13C excursions, the end-Permian diversity drops and extinction seems linked to a decrease in δ13C values. About half of the genera that disappeared at the PTB reappeared from the Spathian onwards. The Lazarus effect invoked by Wignall and Benton (1999) seems more probably to be brought by proteromorphosis (sensu Guex 2001).
The second cooling period of the Early Triassic was recognised by Sun et al. (2012) and Romano et al. (2013) in the early Spathian and was followed by climate stabilisation for the remainder of the Spathian (see also Galfetti et al. 2007; Hochuli et al. 2010; Stefani et al. 2010; Hermann et al. 2011).
Middle-Late Spathian Boundary
The second positive shift during the Early Triassic appeared in the middle and late Spathian up to the earliest early Anisian. This global cooling event is recognised worldwide (Galfetti et al. 2007; Hochuli et al. 2010; Stefani et al. 2010; Hermann et al. 2011; Sun et al. 2012; and Romano et al. 2013).
Uppermost Spathian
The uppermost Spathian is considered by Sun et al. (2012) as a third cooling event.
Anisian
The relatively cool climate of the early Middle Triassic Anisian (Aegean-Bithynian) encompasses a period of relative warming that may be related to the Anisian aridisation events. These two noticeable negative shifts should be assigned to the Aegean warming and the mid-Pelsonian event (Korngreen and Bialik 2015), separated by the Bithynian-Early Pelsonian humid event (Stefani et al. 2010).
5.2.6 Stratification of Ocean Water
In the Lower Triassic δ13O isotope curve of Horacek et al. (2009), the Kamura shallow-marine carbonates in Japan (Panthalassa realm) confirm the Tethys curve. These global carbon isotope curves document the profound changes in the global carbon cycle that influenced ecosystems. For these authors, an alleged delay in biological and ecological recovery from the PT extinction event is the result of the stratification of the Tethys and episodical mixing of the stratified ocean waters. These are the most likely processes to produce the observed carbon isotope signatures and seriously hamper a substantial biotic recovery .
5.2.7 Environmental Gaps
A number of gaps affect the Early Triassic.
The Chert Gap
The Early Triassic Chert Gap occurred following the 30 Ma long worldwide Permian Chert Event (Murchey and Jones 1992; Beauchamp and Baud 2002). As a result, radiolarian oozes are absent in Early Triassic oceans. Radiolarian biodiversity increased constantly during the Paleozoic and decreased only slightly towards the end of the Permian. Under anoxic environments radiolarian diversity minima relate to positive δ13C excursions. The end-Permian diversity drops and extinction links to a decrease in δ13C values. About half of the genera that vanished with the PTB reappeared during Spathian times. Invoked as Lazarus effect (Wignall and Benton 1999), it was rather an example of proteromorphosis (sensu Guex 2001) [after De Wever et al. 2007]. A reduced chert gap is apparently present in the Mino Terrane of Japan, where deepwater chert and claystone yield the conodont genus Hindeodus, preserved as natural assemblages on bedding planes in claystone (Agematsu et al. 2014). These Hindeodus assemblages maintain the original composition and structure of an almost complete apparatus: element pairs of P1, P2, M, two digyrate and four bipennate of the S array, and the single alate S0. The conodont biostratigraphy indicates that the lithological boundary between chert and claystone units in the study section corresponds exactly to the Permian-Triassic boundary. The Early Triassic chert gap seems to have caused anoxia, ocean warming and ocean acidification. One basic effect of the Traps volcanism coupled with the release of substantial amounts of hydrate methane was to slow or stop normal thermohaline circulation changing ocean bottom water from oxygenated to anoxic. Radiolarians would have been seriously affected by oceanic acidification because as ocean water became more acidic, less silica was available. Until cold, oxygenated and more alkaline conditions returned, chert production and deposition would have ceased .
The Reef Gap
In the Late Permian, reefs were thriving. In a geologic blink of the eye, the Permian reefs disappeared and were not replaced immediately. The organisms that formed them went extinct, among them rugose corals, fusulinids, many echinoderms, bryozoans and brachiopods. For about 5 Ma years the only few carbonate build-ups were composed of calcareous algae with no larger organisms.
Reef ecosystems are vulnerable to even very small changes in temperature. Oxygen content of the water, its salinity, slight alkalinity (normal ocean pH is 8.1), clarity and nutrient supply are generally quite constant. Each of these conditions can be affected by protracted volcanism and hydrate methane release. The waters would have risen and become dysoxic, and the relative acidity (pH) changed by acid rain. The oceanic nutrient supply would have been enhanced by episodic volcanic ash falls and continuous weathering of continental rock by acid rain, but the upwelling of nutrients would have been much diminished in a stagnant ocean. Two major groups of corals, the rugose corals and the tabulate corals, disappeared forever. In part, this may reflect the need of corals to build calcium carbonate skeletons, which would have been difficult to fulfil as ocean waters became more acidic. In addition, many corals contain photosynthetic algal symbionts that can be ejected during periods of reefal stress. While such ejection may help a coral deal with stress for a short term, in the long term it deprives the coral of a major food source. Photo-synthesisers also contributed oxygen that corals may have been deprived of in increasingly dysoxic waters . The reef gap was apparently filled by microbial build-ups (Friesenbichler et al. 2018; Heindel et al. 2018).
The Coal Gap
Extensive coal deposits extend over Gondwanian. Their absence for the 5 Ma years of the Early Triassic is known as the coal gap (Veevers 1994; Retallack et al. 1996). Peat and coal ceased completely during the Early Triassic as the result of the disappearance of peat-forming plants in the end-Permian extinction. Relatively few plants are adapted to the harsh conditions of waterlogged, acidic, dysaerobic environments in which peat is formed. The end-Permian extinctions decimated these plants and it took millions of years for other plants to become tolerant of such conditions (Retallack et al. 1996).
One considerable impact on terrestrial vegetation is acid rain. Protracted episodes of severe acidity triggered by pulses of hydrate methane release from seafloor slumps or pulses of volcanic eruption over tens to hundreds of thousands of years, coupled with increased precipitation, would have been quite sufficient to cause the extinction of many species of vegetation, including those whose peaty and humified remains became coal. More generally, mycorrhizal fungi, the root symbionts that provide most terrestrial plants with soil nutrients, may have been particularly vulnerable to this altered environment. Increased warmth, lower levels of atmospheric oxygen (hypoxia) and elevated levels of carbon dioxide may also have added to the demise of plants.
The extinction of many peat-forming plants at the end of the Permian, and their slow replacement during the Middle Triassic, has thus been cited as the reason for the coal gap (Retallack et al. 1996). The areas previously covered with forests were replaced by red beds indicating arid conditions. Early Triassic red beds followed the end of Permian throughout much of the world, such as the Moenkopi Formation in North America and the Buntsandstein in Europe .
Changes in Carbon Isotopes at and After the Permian-Triassic Boundary
Following an initial move to negative values at the Permian-Triassic boundary, carbon isotopes swung wildly for roughly 5 million years indicating enormous changes in oceanic chemistry. Aside from confirming that the Early Triassic was a period of continual major instability, the findings provide no insight into the possible causes of the disturbances. As the discoverers of the carbon fluctuations note, these could represent either repeated environmental disturbances or their consequences, as ecosystems were decimated and rebuilt (Payne et al. 2004). In other words, carbon isotope changes could be telling us either of causes (for example, methane outbursts) or of consequences (for example, severe disturbances of the biosphere ) [after Dorritie 2004, 2005, 2006, 2007].
5.2.8 Methane
Recently Rothman et al. (2014) noted that for millions of years prior to the Permian extinction, the world had been in one of its most biologically productive time frames. By 260 million years ago, the level of atmospheric oxygen soared allowing animals to thrive. Yet at the same time, the level of carbon dioxide was still higher than we find today and consequently plants thrived as well. All terrestrial leaf litter and organic waste and all ocean plankton that died carried enormous quantities of energy-rich compounds to the deepest ocean bottoms, and due to temperatures, which were globally very high, there was little ocean circulation. Thus, little wind, few Gulf Stream-like currents and little motion carrying cold, oxygen-rich surface water down to the deep sea took place. Thus, enormous amounts of energy-rich dead bodies from the oxygen-rich world fell onto oxygen-deficient sea bottoms. Simultaneously they became surrounded by the organic compound acetate that prevented the microbes from consuming the dead bodies that filled the ocean bottom. This resulted in deep-sea microbes that exhale methane instead of carbon dioxide, called “methanogens”, to capture two acetate-processing genes from a totally different kind of microbe. That process of gene capture is an epigenetic event called “capture” by Rothman’s MIT team. The results were deadly to all organisms that needed oxygen, and all organisms that died at sustained temperatures at or above about 35 °C, which are mostly multicellular (Ward 2018; p.144).
5.3 Part Two
5.3.1 Phylogenetic Reconstructions
Hirsch (1994a, b) proposed that under lethal stress conditions beginning in the Dienerian, neogondolellin segminiplanate morphs (platforms) were replaced by segminate (cavital) proteromorphs (Neospathodus). After the Dienerian-Smithian boundary reinitialisations of neogondolellin homeomorphs appeared as successive peramorphic trends (Fig. 5.4).
Alternation of morphologies. Darwinian anagenesis undergoes extinction under high external stress; atavistic reversal permits rediversification (after Guex 2001)
Budurov et al. (1988) introduced a parallel Kashmirella lineage , while Kozur et al. (1998), paying attention to the rapid change from neospathodids to gondolellids and vice versa, established the genera Sweetospathodus and Triassospathodus without significant justification. Considering the taxonomy of Permian and Triassic gondolellids in detail, Kozur (1989) considered that Neogondolella Bender and Stoppel had evolved from segminate Neospathodus Mosher through the transitional forms of Chiosella Kozur from the end of the Olenekian stage to the beginning of the Anisian stage and that Neogondolella was the basic group of the Middle to the beginning of the Late Triassic. Klets and Kopylova (2007) illustrated the replacement of Clarkina by Neospathodus, the latter generating up to four lineages of platform-bearing “gondolellids”. These authors also wrote that endemic “Neogondolella” buurensis, “N.” composita, “N.” jakutensis, “N.” taimyrensis and “N.” sibirica, having characteristics of Neogondolella, are widely distributed in the Early Olenekian of the northern latitudes.
Orchard’s (2007) temporal distribution of Lower Triassic conodonts differentiated families and subfamilies, such as the subfamily Neogondolellinae that, having survived the PTB, added a number of Griesbachian species to the lineage before radiating upwards into the Spathian. Next appeared Borinella (Dienerian-Spathian) and the non-platform Sweetospathodus (Dienerian). The latter radiated into the Smithian subfamily Mullerinae that comprises the genera Discretella, Conservatella, Guangxidella and Wapitiodus. New is the appearance of subfamily Scythogondolellinae (Dienerian-Smithian). Spathian “Neogondolella” morphs comprised species such as “regalis”, shevyrevi, jubata and derivatives like the genus Columbitella. Orchard (2007) also proposed Borinella as the origin for the genera Gladigondolella and Cratognathus. For this author, the Dienerian apparition of the segminate genus Neospathodus (Dienerian-Spathian) radiated into the non-platform subfamilies Cornudininae (Smithian-Spathian) and Novispathodinae (Spathian) and the genera Eurygnathodus (Smithian), Icriospathodus (Spathian), Platyvillosus (Spathian), Triassospathodus (Spathian) and Kashmirella (Spathian).
Family Ellisoniidae encompasses the genera Ellisonia, Hadrodontina, Merillina, Parachirognathus, Foliella, Sweetocristatus and Pachycladina.
Except Orchards’ (Orchard 2007) mention of Scythogondolella mosheri as a paedomorphic descendant of Scythogondolella n. sp. in the Canadian Arctic, all phylogenies are exclusively peramorphic for him.
5.3.2 Conodont Radiation
Three peramorphic trends survived the PTB: Anchignathodontidae that became extinct by the Late Griesbachian δ13C minimum; Ellisoniidae that remained dormant until the dawn of the Smithian, when they radiated “until before” the end of the Spathian; and Neogondolellinae that in the course of the Triassic secured the survival of their lineage with atavistic reversals (proteromorphosis).
-
(A)
Family Anchignathodontidae Clark (After Brosse et al. 2015)
The genus Hindeodus (Rexroad and Furnish 1964), type species H. typicalis (Sweet), is widely variable and several species evolved. Conodonts belonging to this genus rapidly differentiated into more than ten species during latest Permian to earliest Triassic time (Kozur 1996, 2004; Angiolini et al. 1998; Orchard and Krystyn 1998; Nicoll et al. 2002; Perri and Farabegoli 2003; Orchard 2007; Chen et al. 2009; Metcalfe 2012). H. typicalis has an apparatus that according to Sweet (in Clark et al. 1981) consists basically of all the elements of Ellisonia teicherti (Sweet 1970). Recent studies on clusters of Hindeodus have identified an octomembrate 15-element apparatus of P1, P2, S0, S1, S2, S3, S4 and M elements (Agematsu et al. 2014). For Sweet (1992), the short-living Isarcicella isarcica as well as Hindeodus parvus are morphologically different elements of a single species.
-
(B)
Family Ellisoniidae Mueller
The genus Ellisonia is confined to shallow environments in the Griesbachian and Dienerian but during the Smithian, the family underwent the outburst of the genera Furnishius, Hadrodontina and Pachycladina, each with several species. Their differentiated distributions define several subprovinces from North America to the Alpine and Levantine Tethys. This Smithian conodont outburst may correspond to a peak of apatite, the mineral of the conodont elements. Smithian Ellisoniidae and Mullerinae both mark the phenomenon with large size and robust elements .
-
(C)
Family Gondolellidae Lindstroem
It is widely agreed that classification within the family is based on the composition of the multi-element apparatus. This is critical for establishing its identity as a member within a subfamily, as in the case of Clarkina and Neospathodus within the subfamily Neogondolellinae.
We have to consider two evolutionary morphotypes: anagenetic and atavistic (retrograding).
Subfamily Neogondollelinae Hirsch
The succession of Late Permian anagenetic “gondolellid” morphs comprises the genera Mesogondolella, Jinogondolella and Clarkina. These form lineages are separated from each other by retrograded morphs. Meso- and jinogondolellin forms have a different apparatuses and they belong in different subfamilies. The first neogondolellin genus is Clarkina . Orchard (2007; p. 16) observed its Early Triassic explosive radiation consisting of the sudden appearance of numerous new species and genera. Most remarkable for the newly emerged conodonts was the morphological diversity of at least 12 multielement apparatuses within Lower Triassic gondolellids alone, compared with perhaps one in the Late Permian. The following taxa occur according to the observed events.
Since the Late Permian-Early Triassic Boundary, the Early Triassic part of the radiation of genus Clarkina (Kozur 1989) (Neogondolella in Orchard 2007, 2008) counts a dozen Griesbachian species, most of them Permian survivors (Orchard 2008). The evolution of this segminiplanate genus in the Meishanensis zone consists of five species ranging upward from the preceding Iranica zone and six new species that appeared, all of which—Clarkina meishanensis Zhang et al., C. orchardi Mei, C. kazi (Orchard), C. nassichuki (Orchard), C. taylorae (Orchard) and C. tulongensis Tian—ranged up into the Induan of Tibet and Spiti (Orchard 2007; Orchard et al. 1994; Orchard and Krystyn 1998) followed by Triassic ones. These Triassic Clarkina krystyni and C. discreta are believed to have radiated into the Dienerian as Clarkina griesbachensis at the starting point of the Dienerian, followed by C. mongeri and the Smithian C. composita that radiated into C. altera, C. jakutensis and C. sibirica. For Orchard (2008), also the appearance of the genus Borinella is presented as the result of Darwinian anagenesis .
The major innovation that took place in the latest Griesbachian Strigatuszone was that of the genus Neospathodus (Mosher 1968), as noted by Orchard (2008). The remarkable survival of the neogondolellin apparatus was enabled by the succession of the large number of retromorphs, known as Neospathodus or other generic names.
The type species of the genus Neospathodus (Mosher 1968) is N. cristagalli that has a multi-element apparatus like that of Neogondolella (Orchard 2005). The appearance of Neospathodus as the result of environmental stress at the end of the Griesbachian (Hirsch 1994a, b) appears as a typical example of retrograde evolution (Guex 2016; Kiliç et al. 2016). The latter authors proposed C. krystyni as possible origin.
Several genera have been detached from the genus Neospathodus based on slightly different morphologies of the P1 as well as the apparatus, when available. Among these, Kozur et al. (1998) separated Triassospathodus from Spathognathodus homeri (Bender 1970) (alias Neospathodus homeri, Bender 1970). This separation from Neospathodus was based on the down-turning of the posterior lower margin of the P1 element, while for Orchard (2005), the elements S2–S4 and S0 significantly differ and the S2 element is particularly distinctive. According to Shigeta et al. (2014), Triassospathodus homeri evolved from T. symmetricus in the Early Spathian. T. homeri extends from Chios and N. Dobrogea to Oman, Salt Range, Kashmir, China and Japan. For Budurov et al. (1988), the Neospathodus lineage comprises Neospathodus cristagalli (Huckriede), N. dieneri Sweet, N. pakistanensis Sweet, N. waageni Sweet, N. conservativus (Mueller), N. zarnikovi Buryi, N. triangularis (Bender) and N. homeri (Bender), N. discretus (Mueller), N. conservativus (Mueller), N. zarnikovi Buryi and N. bransoni (Mueller).
Seen as unrelated Neospathodushomeomorphs by Orchard (1995), Budurov et al. (1988) saw the lineage of Kashmirella Budurov, Sudar and Gupta, 1988, that ranges from latest Griesbachian to Early Aegean as prevailing during the Early Triassic next to that of the genus Neospathodus.
The Kashmirella lineage includes the Latest Griesbachian-Dienerian K. kummeli (Sweet 1970), the Smithian type species K. albertii (Budurov et al. (1988)), the Smithian K. novaehollandiae (McTavish 1973), the Spathian K. spathi (Sweet 1970), the Spathian K. zaksi (Buryi 1979) and the Spathian K. timorensis (junior synonym is Chiosella, Kozur 1989), often seen as the senior synonym of K. gondolelloides (Bender) from which the genus Paragondolella evolved. Characteristic in the type species Kashmirella alberti is a thin blade in the upper part and a lateral thickening in the lower part, similar to a platform; its broad basal cavity resembles that of Metapolygnathus Hayashi and its derivates, and may also be bifurcated. The genus Kashmirella extends to the Tethys (including Romania, Oman, Himalaya, South China, Timor), North America (Nevada, SE Alaska, W. Canada), the Arctic (Svalbard) and Australia. Budurov et al. (1988) saw the lineage of Kashmirella as prevailing during the Early Triassic next to that of the genus Neospathodus. Thus, after the several atavistic retrogradations that were initiated in the latest Griesbachian by the segminate Neospathodus (cristagalli, dieneri) and Kashmirella (kummeli), the apparatus of subfamily Neogondolellinae was preserved in the lineage as Paragondolella (Latest Spathian-Julian) as well as far beyond in Norigondolella (Norian), but not in Epigondolellinae that lacks the bifid S3.
The genus Eurygnathodus Staesche, 1974, type species Eurygnathodus costatus for Platyvillosus costatus Staesche 1964: The morphologically very innovative taxon has an ellipsoid platform with an ornamentation of transversal ridges, and its sub-rounded anterior basal groove is narrow. Eurygnathodus costatus has a very large geographical distribution: North Italy (Staesche 1964); Croatia (Aljinović et al. 2006); Western Serbia (Budurov and Pantić 1973); Kashmir and Spiti, India (Krystyn et al. 2007); South Primorye, Northeastern Asia (Igo 2009); Southwest Japan (Koike 1988); Northeastern Vietnam (Maekawa and Komatsu 2014); Northwest and Western Malaysia (Koike 1982); Nevada, USA (Sweet et al. 1971); and British Columbia, Canada (Beyers and Orchard 1991). In South China, it was reported from western Hubei Province (Wang and Cao 1981; Zhao et al. 2013), Chaohu of Anhui Province (Zhao et al. 2008), Sichuan Province (Tian et al. 1983), Guizhou Province (Wang et al. 2005; Chen et al. 2015), Guangxi Province (Yang et al. 1986), Lowest Middle Smithian Flemingites rursiradiatus in Vietnam (Shigeta et al. 2014), South Tirol (Staesche 1964), Spiti, Kashmir, South China, Malaysia, SW Japan, BC Canada (milleri zone), S. Primorye (dieneri-pakistanensis, waageni-novaehollandiae zones (Upper Dienerian-Smithian)) (Zhang 1990).
The genus Borinella (Budurov and Sudar 1994), type species “Neogondolella” buurensis Dagys, comprises a variety of morphotypes. The Dienerian form described as B. chowadensis has essentially the same apparatus as Neogondolella (Orchard 2007). However, no apparatus was described for Borinella nepalensis, B. buurensis nor B. megacuspa. A number of morphologically related genera are presently combined under this genus. Borinella (Budurov and Sudar 1994) replaces Kozurella (Budurov and Sudar 1993) (Pseudogondolella sensu Kozur 1988, p. 244; Chengyuania, Kozur 1994, p. 529–530), the type species of which is “Gondolella” nepalensis (Kozur and Mostler 1976). Budurov and Sudar (1994) also assigned “G.” nepalensis to the genus Kashmirella (type species, K. albertii). For Orchard (2008), Borinella would be the valid name for Kashmirella and Kozurella, including the species K. albertii, K. nepalensis and N. buurensis. However, separation of these taxa should be based on their apparatuses, which are unknown in detail. Limited material of Borinella buurensis resembles elements of Wapitiodus. For Orchard (2008), an early Borinella megacuspa possibly developed in the Latest Griesbachian, followed in the Dienerian by B. nepalensis and B. chowadensis, from which the Smithian lineage of Borinella sweeti and B. buurensis derived. For Orchard (2005), Borinella largely disappeared at the end of the Smithian , but its characteristic anterior blade denticulation reappeared in the Spathian of Oman next to the dominant genus “Neospathodus ” and with early Tethyan forms of the genus Gladigondolella. If in these collections, no apparatus of the type species of Gladigondolella occurs, the P1 element of G. ex gr. carinata Bender, and perhaps of Borinella n. sp. do, can be thought as the first stage in the development of Gladigondolella. If Borinella was the root stock for Gladigondolella, then it would represent a migration of the former into lower latitude environments, as Kozur et al. (1998) postulated to have occurred among conodonts after the PTB extinction (in Orchard 2007; p. 100). The earliest occurrence of Borinella cf. nepalensis was in the uppermost Dienerian of the South Primorye Abrek Bay (Shigeta et al. 2009) and in Spiti (Krystyn et al. 2007), while its range extended to the Early Smithian in pelagic environment (Orchard 2007).
The genus Smithodus Budurov, Buryi and Sudar, 1988, type species Smithodus longiusculus (Buryi 1979), originally attributed to Neospathodus, is characterised by a sturdy blade at the end, a strong wide concave basal cavity below the main cusp. The species is found within the waageni zone of northeastern Vietnam (Shigeta et al. 2014).
The genus Platyvillosus Clark, Sincavage and Stone 1964, type species Platyvillosus asperatus Clark, Sincavage and Stone 1964, has a platform with a large concave basal cavity with the basal pit located in the centre. A basal furrow can be clearly observed from the basal pit to the anterior end. Protrusions on the upper surface are either low nodes or high denticles, which are either randomly arranged or in a definite pattern.
The genus Scythogondolella (Kozur 1989), after which the subfamily Scythogondolellinae (Orchard 2007) was proposed, is a Smithian genus having derived from the genus Clarkina discreta. However, for Orchard (2007) the type species Scythogondolella milleri developed from Sc. mosheri that would appear to descend as a paedomorph from Scythogondolella? n. sp. B in the Canadian Arctic. Klets and Kopylova (2007) proposed its derivation from Neospathodus . The genus extends from Siberia to Arctic Canada and Nevada.
From the genus Paragondolella (Mosher 1968), type species Paragondolella excelsa (Mosher 1968), the Spathian-Aegean Paragondolella regale derived from Kashmirella timorensis, as the first species of the Paragondolella lineage that ranges up to the Julian. Budurov (in Muttoni et al. 2000; p. 233), classified the species N. ex gr. regalis Mosher as Paragondolella regale (Mosher). Deriving from Kashmirella timorensis, it was the first species of the anagenetic Paragondolella lineage. Supporting this attribution is the mention in Golding (2018) of the suggestion by Henderson (2006) and Henderson and Mei (2007) that the S0 element morphology found in the species regale should refer the species to Clarkina or Neoclarkina instead of Neogondolella. For Chen (2015), the segminate derivation of Paragondolella, i.e. the genus Triassospathodus as proposed by Mosher (1968), has been challenged by an apparatus comparison of Paragondolella and Triassospathodus. The S3 of the former has an accessory anterior process branching from its anterior end, which is missing in the latter (Orchard 2005). Kozur (1989) suggested a derivation of Paragondolella from the Early Triassic genus Pseudogondolella, now viewed as a synonym of the genus Borinella (Orchard 2007). But Borinella went extinct in the late Smithian (Orchard 2007) and thus cannot be the direct forebear of Paragondolella. Chen (op. cit) favours Nicora’s (1977) link of Paragondolella bulgarica (Budurov and Stefanov (1975)) with Neogondolella ex gr. regale, which are both close morphologically and overlap during the early Bithynian. The Paragondolella lineage leads thus from P. regale to P. bulgarica, P. hanbulogi, P. praezsaboi, P. bystrickyi, P. excelsa, P. fueloepi and P. inclinata (Budurov, in Muttoni et al. 2000).
Genera Incertae Sedis in Gondolellids
Rediversifications into independent peramorphic lineages took place for a number of Dienerian to Spathian morphotypes that do not harbour some of the neogondolellin features. These morphotypes were mostly assigned to the genus Neogondolella ; however, their multi-elements may often be unavailable or differ from the neogondolellin apparatus. Several of these taxa did not evolve through Darwinian anagenesis but through atavistic reversal instead. Such rediversification from proteromorphs, as it repeated several times each rediversification, possibly issued from a distinct proteromorph, resulted in neogondolelli-morph, only similar in appearance yet not part of a same anagenetic lineage. Rediversifications into independent peramorphic lineages may be differentiated on the basis of platform shape and blade characteristics, as in Orchard (2007).
The genus Siberigondolella Kiliç and Hirsch comprises the Boreal lineage described as Neogondolella by Dagys (1984) and Klets and Yadrenkin (2001) that includes Griesbachian-Dienerian S. griesbachensis Orchard, S. mongeri Orchard, the Smithian S. composita Dagys, S. altera Klets, S. sibirica Dagys and S. jakutensis Dagys (Kiliç and Hirsch 2019).
Under the generic name of Neogondolella are the Spathian forms N. jubata Sweet, N. dolpanae Balini, Gavrilova and Nicora, N. shevyrevi Kozur and Mostler, N. taimyrensis Dagys, N. elongata Sweet, N. paragondolellaeformis Dagys, N. amica Klets, N. captica Klets and a large number of unnamed taxa (C, D, E, F, G, H and K), all derived by Orchard (2007) from Smithian Borinella sweeti. Among these, N. elongata Sweet, common in the USA (Orchard and Tozer 1997), was referred to the genus Columbitella (Orchard 2007), based on modifications to its apparatus (Orchard 2005). Having characteristics from pointed posterior platform and terminal cusp to reduced anterior platforms or a rounded posterior platform and brim surrounding the cusp, they produced successor species with a stronger cusp.
The Smithian genus Paullella (Orchard 2008) was established for the species “Gladigondolella” meeki (Paull (1983)) of the Meekoceras beds, Thaynes Formation, Idaho. The assemblage of Paullella meeki, Neospathodus posterolongatus, N. pakistanensis and Guangxidella bransoni appears as an outburst within a level equivalent to the lower part of the waageni zone (Golding 2014).
The Spathian genus Icriospathodus (Krahl et al. (1983)) was established for Neospathodus collinsoni (Solien 1979) (= Spathoicriodus, Budurov et al. 1987). Type locality: Thaynes Formation, near Salt Lake City, Utah, USA.
-
(D)
Family Gladigondolellidae Ishida and Hirsch
After genus Gladigondolella (Mueller 1956), type species Gladigondolella tethydis (Huckriede), the probably monogeneric taxon has an octomembrate apparatus in which all elements fundamentally differ from their equivalents in Gondolellidae. For Kiliç et al. (2013), the two P2 elements are sexual dimorphs. For Koike (1999), followed by Orchard (2005), one of these is considered as the P1 element of form-genus “Cratognathus”. The basinal open marine habitat of the family was, as indicated by high fluorapatite δ18Ophos values of ~21–21.5‰ in Gladigondolella, compared to 20‰ in coeval eurythermal Paragondolella (Rigo and Trotter 2014), deep cold oceanic. The family originated in the cool environment that first reappeared in the Late Spathian. For Orchard (2005), the denticulation in Gladigondolella and Cratognathodus resembles that of Guangxidella robusta, except for the significantly different M and S3elements .
Wapitiodus might well be the Smithian reinitialisation of the Permian genus Vjalovognathus Kozur (1977), type species V. shindyensis Kozur and Mostler (1976), revised in Nicoll and Metcalfe (1998, p. 435–436). Vjalovognathus was restricted to the northern margin of eastern Gondwanaland, suggested to have been tolerant to cool temperate conditions, a characteristic shared by Gladigondolellidae (Rigo and Trotter 2014). Orchard (2005) named the genus Wapitiodus after Lake Wapiti, Sulphur Mountain Formation, British Columbia, Canada, and saw it as a diversification issued from Kashmirella (Sweetognathodus) kummeli, based on S3 elements with an accessory anterolateral process branching from the cusp. The genus is included in subfamily Mullerinae (Orchard (2005)) that comprises the genera Conservatella and Discretella that Orchard (2005) established after the original descriptions of Ctenognathodus conservativus and Ctenognathodus discretus by Mueller (1956). Neoprioniodus bransoni (Mueller (1956)) belongs in genus Guangxidella (Zhang and Yang (1991)). However, the genera Conservatella, Discretella and Guangxidella, instead of related to Kashmirella, are rather the Smithian reinitialisation of Permian taxa like Vjalovognathus. Guangxidella is the origin of Cratognathodus and Gladigondolella.
5.3.3 Griesbachian Zonation
Brosse et al. (2016) have put in evidence six unitarian associations of up to 16 species in UAZ 4. The first Griesbachian level UAZ 3 counts Clarkina planata and C. taylorae; level UAZ 4 is marked by the coming in of the genus Isarcicella and level UAZ 5 is marked by Isarcicella isarcica, while UAZ 6 still yields C. taylorae (Figs. 5.5 and 5.6). The Griesbachian is thus marked by the Clarkina taylorae zone and the subdivisions of the C. planata subzone (UAZ 3–4) and Isarcicella isarcica subzone (UAZ 5), the latter being the most reliable marker. The Griesbachian is also marked by several Hindeodus specimens, depending on factors of depth and temperature, as discussed later. It is noteworthy that Zhao et al. (2007) in Chaohu, Anhui Province, China, have established the Hindeodus typicalis zone for the Griesbachian, instead of Hindeodus parvus, not found in Chaohu! Hindeodus typicalis (Sweet), found in the Elika Formation (Iran) by Hirsch and Suessli (1973), was recently illustrated in Maaleki-Moghadam et al. (2019; p. 377, fig. 13). These authors identified Hindeodus typicalis (Sweet), H. praeparvus Kozur and H. cf. parvus. Their complete H. cf. parvus specimen differs from H. praeparvus by a narrower and acute-angled main denticle, not high enough to be a typical H. parvus. While this sample indicates a stratigraphic position close to the Permian-Triassic boundary, it again wakes up doubts about the many alleged species of the highly variable genus Hindeodus .
The six unitarian association zones (UAZ 1–6) (after Brosse et al. 2016)
The poor reproducibility of the UA2, UA3, UA4 and UA6: (a) the grey shades indicating the UAs that are merged into UAZs, (b) the lateral traceability of UAZ 1–6, (c) a sequence of eight UAs subsequently grouped into six UAZs of higher lateral reproducibility (Brosse et al. 2016)
5.3.4 Conodont Morphogenesis
The evolution of the genera in the subfamily Neogondolellinae consists of a number of reiterating trends: (1) displacement of the basal cavity from its posterior position towards the middle, accompanied by the modification of its shape from loop-like to amygdaloid; (2) reduction of the platform, that in most Early and Middle Triassic genera of the family borders the entire unit of adult specimens, by the formation of a free blade; and (3) splitting of the monolobate basal groove into a bilobate, forked platform. The evolutionary trends are paced by recurring retrogradations as proteromorphic neospathid morphs followed by accelerated rates of rediversified peramorphic speciations, such as the Smithian radiations of Scythogondolella milleri, Borinella sibiriensis, B. nepalensis and Spathian Columbitella jubata.
Evolutionary “simplifications ” have been interpreted in terms of heterochrony such as paedomorphism, progenesis and neoteny (Gould 1977). Paedomorphosis is when the descendant species is underdeveloped relatively to the ancestor, smaller in size and simpler in shape, resembling juvenile ancestors; peramorphosis is when the descendant species transcends its ancestor in terms of size and shape; and a neotenous descendant is of the same size as the adult ancestor but is underdeveloped (simpler) in terms of shape (Lieberman 2011; p. 35).
In their cladogram of gondolellid taxa , Henderson and Mei (2007) consider the evolution of bifid S3 elements as transitional between Mesogondolella and Jinogondolella: the changes in ontogenetic developmental timing, including lack of a platform or its reduction to a narrow rib in juvenile specimens within the development of Clarkina (Neoclarkina). Newly, the sudden loss of platform in Neospathodus is attributed to proteromorphosis (Guex 2001) and the platform development in the succeeding peramorphic series to reinitialisation (Guex 2006). This is seen in Borinella and Scythogondolella, evolving from Neospathodus, consisting of peramorphic processes in which ontogeny was restored and additional stages are added in which the platform is developed in intermediate and adult forms.
The Early Triassic is characteristic not only for a high frequency of retrogradations that are followed by reinitialisation to peramorphic lineages, but also for the frequent appearance of totally new morphologies with previously unseen ornamentations, often of limited duration.
The Middle Triassic lineage of Paragondolella derived from Kashmirella and prevails in the more open marine scene.
A so far unidentified event during the Early Anisian of the central part of the Northern Tethys has precipitated the appearance of the forms Kamuellerella-Ketinella-Gedikella. These small-size ramiform units, found in the Turkish Istanbul zone (Gedik 1975; Kiliç et al. 2018), may suggest some extraordinary local warming event.
During the Early Carnian Pluvial interval the main evolutionary trends are (a) reduction of the platform with development of a free blade and (b) the trend of splitting the basal groove (Budurov 1977).
5.3.5 Palaeogeography (After Brosse et al. 2016)
The structurally distinctive oceanic patterns of the gradually closing Palaeotethys (N. and S. China) and opening Mesotethys (Pakistan, India) are essentially tropical. The Mesotethys opens into pan-latitudinal Panthalassa that also caps the Earth with an immense Boreal (Arctic) realm (Fig. 5.7).
Early Triassic paleogeography (Brosse et al. 2016). T Tethys, B Boreal, W Werfen, P Pacific
Tethys
The Early Triassic tropical Tethyan realms encompass the following zones: Griesbachian typicalis, isarcica and carinata; Dienerian kummeli, dieneri and cristagalli; Smithian pakistanensis, waageni, conservativus and milleri; and Spathian jubata, triangularis, homeri and timorensis.
The Northern Palaeotethyan area of Mangyshlak (West Kazakhstan) is characterised by the Homeri zone that, next to Neospathodus homeri, also yields N. abruptus, N. symmetricus and “Neogondolella” dolnapae . For Balini et al. (2000) this species has affinities to “N.” jubata (Sweet 1970).
Boreal
The Canadian Arctic, British Columbia and Western USA during the Smithian comprise next to Neospathodus waageni, Borinella buurensis, Scythogondolella milleri, S. mosheri, Guangxidella bransoni, Conservatella spp., Discretella spp., Paullella meeki, Wapitiodus robustus and N. posterolongatus, some of them providing a rather Boreal identity.
In Spitsbergen, Nakrem et al. (2008) list next to Clarkina meishanensis C. taylorae, C. orchardi, C. hauschkei, C. carinata, Neospathodus dieneri, Ns. pakistanensis, Ns. cristagalli, Ns. waageni, Scythogondolella milleri, Sc. mosheri, Sc. sweeti, Borinella buurensis, Bo. nepalensis, Eurygnathodus, Columbitella paragondolellaeformis, Neospathodus svalbardensis and Merillina peculiaris. The Boreal belt of Siberia (Klets and Kopylova 2004; Fig. 2, Table 1) includes the cosmopolitan Neospathodus with Scythogondolella and the endemic species Borinella buurensis (Dagys), “Neogondolella” composita Dagys and N. jakutensis Dagys (Dagys 1984; Kuzmin and Klets 1990; Klets and Yadrenkin 2001; Klets and Kopylova 2004). For Klets and Kopylova (2007), Neospathodus replaced Clarkina and generated the above-mentioned segminiplanate “gondolellids”, next to “N.” taimyrensis and “N.” sibirica that are widely distributed in the Smithian of the northern latitudes.
“Werfen”-Like
Ellisoniids seem rather restricted to shallower shelf-conditions (Carr et al. 1984) with Furnishius triserratus in the Western USA and Western South Pacific (including Japan), East Asia and Eastern Europe. Similarly, the Alpine-Mediterranean Werfen province yields the Ellisoniids Pachycladina and Hadrodontina (Staesche 1964; Hirsch 1975; Aljinovic et al. 2006) that also extend to the “Cimmerian” Elikah Formation of the Alborz Mountains (BadriKolalo et al. 2015).
In the Far East (Vietnam), Shigeta et al. (2014) report the Lower Smithian Neospathodus waageni zone extending from the Owenites koeneni ammonoid zone to the Lower Middle Smithian Flemingites rursiradiatus and Urdiceras tulongensis ammonoids. The zone yields Conservatella conservativa, Discretella discreta, D. robusta, Guangxidella bransoni, Neospathodus spitiensis and N. waageni. In its lower part appear Eurygnathodus costatus, Neospathodus cristagalli, N. dieneri, N. posterolongatus and Kashmirella novaehollandiae. In the middle part appear Smithodus longiusculus and Hadrodontina.
The Upper Smithian Neospathodus pingdingshanensis zone represents the Xenoceltites variocostatus beds (= Anasibirites novelise zone to Tirolites cassianus zone) is coeval to the Werfen Formation of the Southern Alps and represents the Smithian-Spathian transition, yielding Neospathodus pingdingshanensis, N. waageni, N. triangularis, Icriospathodus collinsoni, I. crassatus and I. zaksi. The Lower Spathian Triassospathodus symmetricus zone is marked by ammonoids of the Tirolites zone and yields Neospathodus triangularis, Triassospathodus symmetricus and T. homeri. Triassospathodus homeri evolved from T. symmetricus in the Early Spathian , extending from Chios and N. Dobrogea to Oman, Salt Range, Kashmir, China and Japan.
Pacific Terranes
The Abrek-Lazurnaya Bay area of Primorye, Russian Far East, yields the Griesbachian Clarkina carinata; Dienerian Neospathodus cf. cristagalli, N. dieneri, Borinella cf. nepalensis and Eurygnathodus costatus; Dienerian-Smithian N. pakistanensis; and Smithian Ellisonia cf. peculiaris, Neospathodus ex. gr. waageni, N. novaehollandiae, N. aff. posterolongatus, N. concavus, N. spitiensis and Foliella gardenae (Igo 2009).
In Japan, Koike (1988, 2004, 2016), Nakazawa et al. (1994) and Agematsu et al. (2014) described Ellisoniidae: Ellisonia triassica, Hadrodontina aequabilis, H. agordina, H. anceps, Cornudina breviramulis, Pachycladina obliqua, P. eromera, Furnishius triserratus and Staeschegnathus perrii; Anchignathodontidae: Hindeodus typicalis and H. parvus; Gondolellidae: Neospathodus dieneri, N. conservativus, N. waageni, N.cf. robustus, N. homeri, Icriospathodus collinsoni, Platyvillosus costatus, P. hamadai, Kashmirella timorensis and Paragondolella regale; Gladigondolellidae: form element Cratognathodus kochi, followed higher up by Gladigondolella tethydis. Nakazawa et al. (1994) see two different faunas in Japan, one resembling the Primorye fauna, while the other is closely related to the Tethyan fauna .
5.4 Conclusions
Early Triassic conodont faunas developed under the impact of succeeding ecological crises of their marine ecosystems. Early Triassic environmental changes (e.g. temperature) and opening of new niches were accompanied by the appearance of new genera.
From the three surviving families—Anchignathodontidae, Ellisoniidae and Gondolellidae—only the late Griesbachian, possibly new Spathognathid genus Isarcicella became an important short-lived event.
Among Gondolellidae, the genus Clarkina of the latter’s subfamily Neogondolellinae had ten species surviving the Permian-Griesbachian boundary, producing the two additional short-living Griesbachian species C. krystyni and C. discreta. From the latter, the latest Griesbachian genera Neospathodus and Kashmirella emerged by retrogradation becoming the dominant Dienerian taxa that gave origin to the reinitialisation of all short- or long-term lineages with a “Neogondolella-like” appearance. Thus Neospathodus and Kashmirella were at the origin of the wealth of genera that radiated until the end of the Spathian. Among these are the segminiplanate Dienerian-Smithian genera Borinella and Eurygnathodus, the Smithian subfamily Scythogondolellinae and genus Paullella (not illustrated). Among uncertain segminate Spathognathodontid genera are Smithian Guangxidella, Conservatella, Discretella and Wapitiodus and the Spathian Gladigondolellid form-genus Cratognathodus. The uncertain subfamily Cornudininae and genus Icriospathodus remain unrepresented in our scheme.
Boreal reinitiated segminiplanate genera encompass the lineages of the Late Griesbachian-Smithian genus Siberigondolella Kiliç and Hirsch and of the Spathian genus Columbitella Orchard.
The third surviving Paleozoic family Ellisoniidae is well represented in the Smithian-Spathian Thaynes/Werfenian environments by the genera Furnishius, Pachycladina and Hadrodontina, their huge size and massive build that during the Smithian may concur with an apatite peak.
A great wealth of new forms appeared and went extinct during the time span of less than 5 million years between the Permian-Triassic boundary mass extinction and the Spathian-Anisian environmental bottleneck. Of the lineages that appeared during the Early Triassic, none persisted beyond the Spathian, with the exception of genera Paragondolella and Gladigondolella. The former survived by repeated retrogradations until the Rhaetian final conodont extinction and the latter, Gladigondolella, which ascended in the Late Spathian with the reappearance of cooler pelagic conditions, was mostly found in equatorial environments until the end of the Julian.
Environmental instabilities are a key to morphogenesis in biology and palaeontology .
During the Early Triassic, each event was marked by short-lived entirely new morphologies, while the long-lasting “Gondolellid” segminiplanate retrograded into segminate proteromorphs. These occur as two parallel 4 Ma long lineages, each characterised by its own evolution of successive morphs, interpreted by some as genera. In this chapter, we analyse this succession in an attempt to define their taxonomy (Fig. 5.8).
Main Gondolellid segminiplanate, segminate and uncertain segminate lineages. The latest Griesbachian Gondolellid genus Clarkina, of subfamily Neogondolellinae, initialised the genera Neospathodus and Kashmirella by retrogradation. Becoming dominant Dienerian-Spathian taxa, Neospathodus gave origin to the reinitialisation of all lineages of “Neogondolella”-like appearance that radiated until the end of the Spathian. Among these are the segminiplanate Dienerian-Smithian genera Borinella and Eurygnathodus and the Smithian genus Paullella and subfamily Scythogondolellinae. Spathian Kashmirella was at the origin of the genus Paragondolella. Among uncertain segminate genera, Smithian Guangxidella, Conservatella, Discretella and Wapitiodus and Spathian Cratognathodus apparently initiated the family Gladigondolellidae. Boreal-reinitiated segminiplanate genera encompass the lineages of the Dienerian-Smithian genus Siberigondolella Kiliç and Hirsch and of the Spathian genus Columbitella Orchard
The wealth of new morphs that appeared during the Early Triassic evokes the Cambrian explosion that Stephen Gould compared with the many models of the first automobiles (Gould 1996). In the animal world, a similar comparison can be made with Darwin’s finches, well known for their phenotypic variability and evolution in response to changing environmental conditions. Recently, McNew et al. (2017) documented epigenetic variations within each of the two species of Darwin’s finches. This suggests that evidence of phenomena observed within extinct taxa in the fossil state may be compared with phenomena for which living organisms can provide the evidence in the laboratory.
Conodonts, as a biotic group, next to ammonoids, pollen and spores, crustaceans and vertebrates, provide proxy clues for environment and age assessments of the rocks in which they occur.
References
Agematsu S, Sano H, Sashida K (2014) Natural assemblages of Hindeodus conodonts from the Permian-Triassic boundary sequence, Japan. Palaeontology 57(6):1277–1289
Aljinović D, Kolar-Jurkovsek T, Jurkovsek B (2006) The lower Triassic marine succession in Gorse Kotari Region (External Dinarides, Croatia): Lithofacies and conodont dating. Rivista italiana di Paleontologia e Stratigrafia 112:35–53
Angiolini L, Nicora A, Bucher H, Vachard D, Pillevuit A, Platel JP, Roger J, Baud A, Broutin J, Hashmi HA, Marcoux J (1998) Evidence of a Guadalupian age for the Khuff Formation of southeastern Oman: preliminary report. Rivista italiana di Paleontologia e Stratigrafia 104:329–340
BadriKolalo N, Bahaeddin H, Seyed Hamid V, Seyed AA (2015) Biostratigraphic correlation of Elikah formation in Zal section (Northwestern Iran) with Ruteh and type sections in Alborz Mountains based on conodonts. Iranian J Earth Sci 7:78–88
Balini M, Gavrilova VA, Nicora A (2000) Biostratigraphical revision of the classic Lower Triassic Dolnapa section (Mangyshlak, west Kazakhstan): Zentralblatt für Geologie und Paläontologie 1998, v. 1, p. 1441–1462
Barash MS (2006) Development of marine biota in the Paleozoic in response to abiotic factors. Oceanology 6:848–858. ISSN 0001-4370 (Original Russian Text, in Okeanologiya 46: 899–910)
Baud A, Magaritz M, Holser WT (1989) Permian-Triassic of the Tethys: carbon isotope studies. Geol Rundschau 78(2):649–677
Beauchamp B, Baud A (2002) Growth and demise of Permian biogenic chert along northwest Pangaea: evidence for end-Permian collapse of thermohaline circulation. Palaeogeogr Palaeoclimatol Palaeoecol 184:37–63
Bender H (1970) Zur gliederung der Mediterranen Trias II. Die Conodontenchronologie der Mediterranen Trias. Annales Geologiques des Pays Helleniques 19:465–540
Beyers JM, Orchard MJ (1991) Upper Permian and Triassic conodont faunas from the type areas of the Caches Creek complex, south-central British Columbia, Canada. Geol Surv Can Bull 417:269–297
Brayard A, Krumenacker LJ, Botting JP, Jenks JF, Bylund KG, Fara E, Vennin E, Olivier N, Goudemand N, Saucède T, Charbonnier S, Romano C, Doguzhaeva L, Thuy B, Hautmann M, Stephen DA, Thomazo C, Escarguel G (2017) Unexpected Early Triassic marine ecosystem and the rise of the Modern evolutionary fauna. Sci Adv 3(2):e1602159. https://doi.org/10.1126/sciadv.1602159
Brosse M, Bucher H, Bagherpour B, Baud A, Frisk ÅM, Guodun K, Goudemand N (2015) Conodonts from the Early Triassic microbialite of Guangxi (South China): implications for the definition of the base of the Triassic System. Palaeontology 58(3):563–584
Brosse M, Bucher H, Goudemand N (2016) Quantitative biochronology of the Permian-Triassic boundary in South China based on conodont unitary associations. Earth Sci Rev 155:153–171
Budurov KJ (1977) Revision of the late Triassic platform conodonts. Geologica Balcanica 7(3):31–48
Budurov KJ, Pantić S (1973) Conodonten aus den Campiler Schichten von Brassica (Westserbien). Bulgarian Acad Sci Bull Inst Seri Paleontol 22:49–64
Budurov KJ, Stefanov S (1972) Platform-conodonten und ihre Zonen in der Mittleren Trias Bulgariens. Mitt Ges Geol Bergbaustud 21:829–852
Budurov KJ, Stefanov S (1975) Middle Triassic conodonts from drillings near the town of Knezha. Palaeontol Stratigr Lithol 3:11–18. Sofia
Budurov KJ, Sudar M (1993) Kozurella gen. n. (Conodonta) from the Olenekian (Early Triassic). Geologica Balcanica 23:24
Budurov KJ, Sudar M (1994) Borinella Budurov and Sudar, nomen novum for the Triassic conodont genus Kozurella Budurov and Sudar, 1993. Geologica Balcanica 24(3):30
Budurov KJ, Sudar M, Gupta VJ (1987) Spathoicriodus, a new Early Triassic conodont genera. Bull Indian Geol Assoc 20:175–176
Budurov KJ, Buryi GI, Sudar MN (1988) Smithodus n. gen. (Conodonta) from the Smithian stage of the Lower Triassic. Mitteilungen der Gesellschaft der Geologie- und Bergbaustudenten, Osterreich 34/35:295–299
Burgess SD, Muirhead JD, Bowring SA (2017) Initial pulse of Siberian Traps sills as the trigger of the end-Permian mass extinction. Nat Commun 8:164
Buryi G (1979) Lower Triassic conodonts of southern Primorye. Institut Geologii i Geofiziki, Sibirskoye Otdeleniye, Akademiya Nauk SSSR, Moskva [In Russian]
Carpenter SJ, Lohmann KC (1995) δ18O and δ13C values of modern brachiopod shells. Geochim Cosmochim Acta 59:3749–3764
Carr TR, Paull RK, Clark DL (1984) Conodont paleoecology and biofacies analysis of the Lower Triassic Thaynes Formation in the Cordilleran Miogeocline. Geol Soc Am Spec Pap 196:283–294
Chen Y (2015) A multi-method study on Triassic conodonts. Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften an der Karl-Franzens-Universität Graz Institut für Erdwissenschaften, 219 pp
Chen J, Beatty TW, Henderson C, Rowe C (2009) Conodont biostratigraphy across the Permian–Triassic boundary at the Dawen section, Great Bank of Guizhou, Guizhou Province, South China: implications for the Late Permian extinction and correlation with Meishan. J Asian Earth Sci 36:442–458
Chen B, Joachimski MM, Shen SZ, Lambert LL, Lai XL, Wang XD, Chen J, Yuan DX (2013a) Permian ice volume and palaeoclimate history: oxygen isotope proxies revisited. Gondwana Res 24:77–89
Chen Y, Twitchett RJ, Jiang H, Richoz S, Lai X, Yan C, Sun Y, Liu X, Wang L (2013b) Size variation of conodonts during the Smithian–Spathian (Early Triassic) global warming event. Geology 41(8):823–826
Chen Y, Jiang H, Lai X, Yan C, Richoz S, Liu X, Wang L (2015) Early Triassic conodonts of Jiarong, Nanpanjiang Basin, southern Guizhou Province, South China. J Asian Earth Sci 105:104–121
Clark DL, Sincavage JP, Stone DD (1964) New conodont from the lower Triassic of Nevada. J Paleontol 38:375–377
Clark DL, Sweet WC, Bergstrom SM, Klapper G, Austin RL, Rhodes FHT, Muller KJ, Ziegler W, Lindstrom M, Miller JF, Harris AG (1981) Conodonta. Part W, Miscellanea, Supplement 2 of R. A. Robison, ed. Treatise on invertebrate paleontology. Geological Society of America, Boulder, Colo., and University of Kansas, Lawrence
Clutson M, Brown D, Tanner L (2018) Distal processes and effects of multiple late Triassic terrestrial bolide impacts: insights from the Norian Manicouagan Event, Northeastern Quebec, Canada. In: Tanner L (ed) The late Triassic world. Topics in geobiology, vol 46. Springer, Cham
Cohen KM, Harper DAT, Gibbard PL, Fan JX (2018) The ICS international chronostratigraphic chart. Updated from Cohen KM, Finney SC, Gibbard PL, Fan JX (2013) Episodes 36:199–204, 2013, (c) International Commission on Stratigraphy, August 2018
Dagys AA (1984) Early Triassic conodonts of northern Middle Siberia (in Russian). Trans Inst Geol Geophys Acad Sci USSR Siberian Branch 554:1–69
De Wever P, O’Dogherty L, Gorican S (2007) The plankton turnover at the Permo-Triassic boundary, emphasis on radiolarians. Eclogae Geol Helv 99:S49–S62
Dorritie D (2004, 2005, 2006, 2007) Killer in Our Midst. www.killerinourmidst.com
Friesenbichler E, Richoz S, Baud A, Krystyn L, Sahakyan L, Vardanyan S, Peckmann J, Reitner J, Heindel K (2018) Sponge-microbial build-ups from the lowermost Triassic Chanakhchi section in southern Armenia: microfacies and stable carbon isotopes. Palaeogeogr Palaeoclimatol Palaeoecol 490:653–672
Galfetti T, Hochuli P, Brayard A, Vigran J (2007) Smithian-Spathian boundary event: evidence for global climatic change in the wake of the end-Permian biotic crisis. Geology 35(4):291–294
Garbelli C, Angiolini L, Brand U, Shen S-Z, Jadoul F, Posenato R, Azmy K, Cao C-Q (2015) Neotethys seawater chemistry and temperature at the dawn of the end Permian mass extinction. Gondwana Res 35:272–285
Gedik I (1975) Die Conodonten der Trias auf der Kocaeli-Halbinsel (Turkei). Palaeontogr Abt A 150:99–160
Golding M (2014) Biostratigraphy and sedimentology of Triassic hydrocarbon-bearing rocks in Northeastern British Columbia. PhD Thesis, The University of British Columbia, Vancouver, 388 pp
Golding M (2018) Reconstruction of the multielement apparatus of Neogondolella ex gr. regalis Mosher, 1970 (Conodonta) from the Anisian (Middle Triassic) in British Columbia, Canada. J Micropalaeontol 37:2124
Gould JS (1996) Full house: the spread of excellence from Plato to Darwin. Harmony, New York
Gould JS (1977) Ontogeny and Phylogeny. Belknap, Harvard Univ. Press, Cambridge, Mass. 490p
Gradstein FM, Ogg JG, Smith AG (2005) A geological time scale. Cambridge University Press, Cambridge, 589 pp
Guex J (2001) Environmental stress and atavism in ammonoid evolution. Eclogae Geol Helv 94:321–328
Guex J (2006) Reinitialization of evolutionary clocks during sublethal environmental stress in some invertebrates. Earth Planet Sci Lett 242:240–253
Guex J (2016) Retrograde evolution during major extinction crises. Springer briefs in evolutionary biology. Springer, Cham 77 pp
Haas J, Demény A, Hips K, Zajzon N, Weiszburg T, Sudar M, Palfy J (2007) Biotic and environmental changes in the Permian–Triassic boundary interval recorded on a western Tethyan ramp in the Bükk Mountains, Hungary. Global Planet Change 55:136–154
Hammer Ø, Jones M, Schneebeli-Hermann E, Hansen B, Bucher H (2019) Are Early Triassic extinction events associated with mercury anomalies? A reassessment of the Smithian/Spathian boundary extinction. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2019.04.016
Harland WB, Smith AG, Wilcock B (1964) Phanerozoic time-scale. Geological Society of London, 466 pp
Heindel K, Foster WJ, Richoz S, Birgel D, Roden VJ, Baud A, Brandner R, Krystyn L, Mohtat T, Kosun E, Twitchett RJ, Reitner J, Peckmann J (2018) The formation of microbial-metazoan bioherms and biostromes following the latest Permian mass extinction. Gondwana Res 61:187–202
Henderson CM (2006) Multielement definition of Clarkina Kozur. Permophiles 48:23–24
Henderson C, Mei S (2007) Geographical clines in Permian and lower Triassic gondolellids and its role in taxonomy. Palaeoworld 16:190–201
Hermann E, Hochuli PA, Mehay S, Bucher H, Bruehwiler T, Hautmann M, Ware D, Roohi G, Rehman K, Yaseen A (2011) Organic matter and palaeoenvironmental signals during the Early Triassic biotic recovery: the Salt Range and Surghar Range records. Sediment Geol 234:19–41
Heydari E, Hassanzadeh J, Wade WJ (2000) Geochemistry of central Tethyan Upper Permian and Lower Triassic strata, Abadeh region, Iran. Sediment Geol 137:85–99
Hirsch F (1975) Lower Triassic conodonts from Israel. Bull Geol Surv Isr 66:39–48
Hirsch F (1994a) Triassic conodont multielements versus eustatic cycles. Lausanne, Mémoires de Géologie 22:35–52
Hirsch F (1994b) Triassic conodonts as ecological and eustatic sensors. In: Embry AF (ed) Pangea: global environments and resources. Memoir, vol 17. Canadian Society of Petroleum Geologists, Calgary, pp 949–959
Hirsch F, Suessli P (1973) Lower Triassic conodonts from the Lower Elikah formation, Central Alborz, North Iran. Eclogae Geol Helv 66(3):525–531
Hochuli PA, Vigran JO, Hermann E, Bucher H (2010) Multiple climatic changes around the Permian–Triassic boundary event revealed by an expanded palynological record from mid-Norway. Geol Soc Am Bull 122:884–896
Holser WT, Schoenlaub HP, Attrep M, Boeckelmann K, Klein P, Magaritz M, Orth CJ, Fenninger A, Jenny C, Kralik M, Mauritsch H, Pak E, Schramm JM, Stattegger K, Schmoller R (1989) A unique geochemical record at the Permian/Triassic boundary. Nature 337:39–44
Horacek M, Koike T, Richoz S (2009) Lower Triassic δ13C isotope curve from shallow-marine carbonates in Japan, Panthalassa realm: confirmation of the Tethys δ13C curve. J Asian Earth Sci 36:481–490
Igo H (2009) Conodonts. In: Shigeta Y, Zakharov Y, Maeda H, Popov AM (eds) The Lower Triassic system in the Abrek Bay area, South Primoriye, Russia. National Museum of Nature and Science Monographs 38, Tokyo, pp 44–40
Joachimski MM, Lai X, Shen S, Jiang H, Luo G, Chen B, Chen J, Sun Y (2012) Climate warming in the latest Permian and the Permian-Triassic mass extinction. Geology 40:195–198
Kiliç AM, Hirsch F (2019) Siberigondolella n. gen., a Boreal Early Triassic Lanceolate Conodont. Turk J Zool 43(5):536–539
Kiliç AM, Plasencia P, Ishida K, Hirsch F, Yumun ZU (2013) Reflections on the apparatus of Triassic Gondolellacea (Conodonta) and the question of sexually dimorphs. In Albanesi GL, Ortega G (eds) Conodonts from the Andes: Proceedings of the 3rd international conodont symposium Asociacion Paleontologica Argentina, publicacion especial no. 13, pp 63–67
Kiliç AM, Plasencia P, Ishida K, Guex J, Hirsch F (2016) Proteromorphosis of Neospathodus (conodonta) during the Permian-Triassic crisis. Rev Micropaleontol 59:33–39
Kiliç AM, Plasencia P, Onder F (2018) Debate on skeletal elements of the Triassic conodont Cornudina Hirschmann. Acta Geologica Polonica 68(2):147–159
Kiparisova LD, Popov YD. (1956) Subdivision of the lower series of the Triassic system into stages. Doklady AN SSSR 109:842–845 (in Russian)
Kiparisova LD, Popov YD (1964) The project of the subdivision of the Lower Triassic into stages. In: XXII International Geological Congress, Reports of Soviet Geologists, pp 91–99 (in Russian)
Klets TV, Kopylova AV (2004) New findings of Triassic Conodontophoridae in northeastern Asia. News of paleontology and stratigraphy. Supplement to Geologiya i Geofizika (Russia)
Klets T, Kopylova S (2007) The problem of Triassic Gondolellid conodont systematics Conodontophorida, Conodonta). In: Lucas SG, Spielmann JA (eds) The Global Triassic. New Mexico Museum of Natural History and Science Bulletin 41, Albuquerque, NM, pp 131–133
Klets TV, Yadrenkin AV (2001) Lower Triassic conodonts from Kotelny island (taxonomic composition, correlation): news of paleontology and stratigraphy. Suppl J “Geologiya I Geofizika” 42(4):14–21. (in Russian)
Knell SJ (2012) The great fossil enigma, the search of the conodont animal. Indiana University Press Science, Bloomington, IN, 440 pp
Koike T (1982) Triassic conodont biostratigraphy in Kedah, West Malaysia. Geol Palaeontol Southeast Asia 23:9–51
Koike T (1988) Lower Triassic Conodont Platyvillosus from the Taho Limestone in Japan. Sci Rep Yokohama Natl Univ II 35:61–79
Koike T (1999) Apparatus of a Triassic conodont species Cratognathodus multihamata (Huckriede). Paleontol Res 3:234–248
Koike T (2004) Early Triassic Neospathodus (Conodonta) apparatuses from the Taho Formation, southwest Japan. Paleontol Res 8:129–140
Koike T (2016) Multielement conodont apparatuses of the Ellisoniidae from Japan. Paleontol Res 20:161–175
Korngreen D, Bialik OM (2015) The characteristics of carbonate system recovery during a relatively dry event in a mixed carbonate/siliciclastic environment in the Pelsonian (Middle Triassic) proximal marginal marine basins: a case study from the tropical Tethyan northwest Gondwana margins. Palaeogeogr Palaeoclim Palaeoecol 440:793–812
Korngreen D, Zilberman T (2017) The role of land-marine teleconnections in the tropical proximal Permian-Triassic Marine Zone, Levant Basin, Israel: insights from stable isotope pairing. Global Planet Change 154:44–60
Korte C, Kozur HW, Veizer J (2005) δ13C and δ18O values of Triassic brachiopods and carbonate rocks as proxies for coeval seawater and palaeotemperature. Palaeogeogr Palaeoclimatol Palaeoecol 226:287–306
Kozur H (1977) Beitrage zur Stratigraphie des Perms, Teil I: Probleme der Abgrenzung und Gliederung des Perms. Freiberger Forschungsheft C319:79–121
Kozur H (1988) Division of the gondolellid platform conodonts. In: Abstract. Proceedings of the fifth European conodont symposium, contributions 1. Courier Forchungsinstitut Senckenberg, vol 102, pp. 244–245
Kozur H (1989) The taxonomy of the gondolellid conodonts in the Permian and Triassic. Courier Forschungsinstitut Senckenberg 117:409–469
Kozur H (1994) Chengyuania, a new name for Pseudogondolella Kozur 1988 (Conodonta) [non Pseudogondolella, Yang, 1984 (hybodont fish teeth)]. Palaontologische Zeitschrift 68:529–530
Kozur H (1996) The conodonts Hindeodus, Isarcicella and Sweetohindeodus in the uppermost Permian and lowermost Triassic. Geol Croat 49(1):81–115
Kozur H (2004) Pelagic uppermost Permian and the Permian–Triassic boundary conodonts of Iran: part I: taxonomy. Hall Jb Geowiss 18:39–68
Kozur H (2006) Remarks to the base of Olenekian. Albertiana 34:66–70
Kozur H, Mostler H (1976) Neue Conodonten aus dem Jungpaläozoikum und der Trias. Geologische und Paläontologische Mitteilungen. Innsbruck 6:1–33
Kozur H, Mostler H, Krainer K (1998) Sweetospathodus n. gen. and Triassospathodus n. gen., two important lower Triassic conodont genera. Geol Croat 51:1–5
Krahl J, Kauffmann G, Kozur H, Richter D, Foerster O, Heinritzi R (1983) Neue Daten zur Biostratigraphie und zur tektonischen Lagerung der Phyllit-Gruppe und der Trypali-Gruppe auf der Insel Kreta (Greichenland). Geologische Rundschau 72:1147–1166
Krause AJ, Mills BJW, Zhang S, Planavsky NJ, Lenton TM, Poulton SW (2018) Stepwise oxygenation of the Paleozoic atmosphere. Nat Commun 9:4081. https://doi.org/10.1038/s41467-018-06383-y
Krystyn L, Bhargava ON, Richoz S (2007) A candidate GSSP for the Olenekian Stage: Mud at Pin Valley; district Rahul and Spiti, Himachal Pradesh (Western Himalaya), India. Albertiana 35:5–29
Kuzmin SP, Klets TV (1990) On biostratigraphy of Lower Triassic deposits of the Shevli River basin. In: Abstracts of the fourth FE RMSS, Khabarovsk, Russia, pp 146–147
Leu M, Bucher H, Goudemand N (2018) Clade-dependent size response of conodonts to environmental changes during the late Smithian extinction. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2018.11.003. 49 pp
Lieberman DE (2011) The evolution of the human head. The Belknap Press of Harvard University Press, Cambridge, MA. ISBN: 978-0674046368, ISBN-10. xii, 756 pp
Lucas S (2018) The GSSP method of chronostratigraphy: a critical review. Front Earth Sci. https://doi.org/10.3389/feart.2018.00191
Maaleki-Moghadam M, Rafiei B, Richoz S, Woods AD, Krystyn L (2019) Anachronistic facies and carbon isotopes during the end-Permian biocrisis: evidence from the mid-Tethys (Kisejin, Iran). Palaeogeogr Palaeoclimatol Palaeoecol 516:364–383
Maekawa T, Komatsu T (2014) Conodont succession. In Shigeta Y, Komatsu T, Maekawa T, Tran HD (eds) Olenekian (Early Triassic) stratigraphy and fossil assemblages in Northeastern Vietnam. Nat Mus Nat Sci Monogr 45:51–54. Tokyo, Japan
McNew SM, Beck D, Sadler-Riggleman I, Knutie S, Koop J, Clayton D, Skinner M (2017) Epigenetic variation between urban and rural populations of Darwin’s finches. BMC Evol Biol 17:183. https://doi.org/10.1186/s12862-017-1025-9
McTavish RA (1973) Triassic conodont faunas from Western Australia. Neus Jahrbuch fur Geologie und Palaontologie Abhandlungen 143:275–303
Metcalfe I (2012) Changsinghian (Late Permian) conodonts from Son La, northwest Vietnam and their stratigraphic and tectonic implications. J Asian Earth Sci 50:141–149
Mosher LC (1968) Evolution of Triassic platform conodonts. J Paleontol 42(4):895–946
Mueller KJ (1956) Triassic conodonts from Nevada. J Paleontol 44:818–830
Murchey BL, Jones LD (1992) A mid-Permian chert event: widespread deposition of biogenic siliceous sediments in coastal, island arc and oceanic basins. Palaeogeogr Palaeoclimatol Palaeoecol 96(1):161–174
Muttoni G, Gaetani M, Budurov K, Zagorchev I, Trifonova E, Ivanova D, Petrounova L, Lowrie W (2000) Middle Triassic paleomagnetic data from northern Bulgaria: constraints on Tethyan magnetostratigraphy and paleogeography. Palaeogeogr Palaeoclimatol Palaeoecol 160:223–237
Nakazawa K, Ishibashi T, Kimura T, Koike T, Shimizu D, Yao A (1994) Triassic biostratigraphy of Japan based on various taxa. Lausanne, Mémoires de Géologie 22:83–101
Nakrem HA, Orchard MJ, Weitschat W, Hounslow M, Beatty TW, Mørk A (2008) Triassic conodonts from Svalbard and their Boreal correlations. Polar Res 27:523–539
Nicoll RS, Metcalfe I (1998) Early and Middle Permian Conodonts from the Canning and Southern Carnarvon Basins, Western Australia: their implications for regional biogeography and palaeoclimatology. Proc Roy Soc Victoria 110:419–461
Nicoll RS, Metcalfe I, Wang C (2002) New species of the conodont genus Hindeodus and the conodont biostratigraphy of the Permian–Triassic boundary interval. J Asia Earth Sci 20:609–631
Nicora A (1977) Lower Anisian platform-conodonts from the Tethys and Nevada; taxonomic and stratigraphic revision. Palaeontographica 157:88–107
Orchard MJ (1995) Taxonomy and correlation of Lower Triassic (Spathian) segminate conodonts from Oman and revision of some species of Neospathodus. J Paleontol 69:110–122
Orchard MJ (2005) Multielement conodont apparatuses of Triassic Gondolelloidea. Spec Pap Palaeontol 73:73–101
Orchard MJ (2007) Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeogr Palaeoclimatol Palaeoecol 252:93–117
Orchard MJ (2008) Lower Triassic conodonts from the Canadian Arctic, their intercalibration with ammonoid-based stages and a comparison with other North American Olenekian faunas. Polar Res 27:393–412
Orchard MJ, Krystyn L (1998) Conodonts of the lowermost Triassic of Spiti, and new zonation based on Neogondolella succession. Rivista Italiana di Paleontologia e Stratigrafia 104:341–368
Orchard MJ, Tozer ET (1997) Triassic conodont biochronology, its calibration with the ammonoid standard, and a biostratigraphic summary for the Western Canada Sedimentary Basin. Bull Can Petrol Geol 45(4):675–692
Orchard MJ, Nassichuk WW, Lin R (1994) Conodonts from the Lower Griesbachian Otoceras latilobatum bed of Selong, Tibet and the position of the Permian-Triassic boundary. Memoir Can Soc Petrol Geol 17:823–843
Paull RK (1983) Definition and stratigraphic significance of the Lower Triassic (Smithian) conodont Gladigondolella meeki n. sp. in western United States. J Paleontol 57:188–192
Payne JL, Clapham ME (2012) End-Permian mass extinction in the oceans: an ancient analog for the twenty-first century? Annu Rev Earth Planet Sci 40:89–111
Payne J, Van de Schootbrugge B (2007) Life in Triassic oceans: links between plankton and benthic recovery and radiation, Chapter 9. In: Falkowski PG, Knol AH (eds) Evolution of primary producers in the sea. Academic Press, London, pp 165–189
Payne JL, Lehrmann DJ, Wei J, Orchard MJ, Schrag DP, Knoll AH (2004) Large perturbations of the carbon cycle during recovery from the end-Permian extinction. Science 305:506–509
Péron S, Bourquin S, Fluteau F, Guillocheau F (2005) Paleoenvironment reconstructions and climate simulations of the Early Triassic: impact of the water and sediment supply on the preservation of fluvial system. Geodinamica Acta 18(6):431–446
Perri MC, Farabegoli E (2003) Conodonts across the Permian boundary in the Southern Alps. Cour Forsch Inst Senckenberg 245:281–313
Petsios E, Thompson J, Pietsch C, Bottjer D (2017) Biotic impacts of temperature before, during, and after the end-Permian extinction: a multi-metric and multi-scale approach to modeling extinction and recovery dynamics. Palaeogeogr Palaeoclimatol Palaeoecol 513:86–99
Rampino MR, Eshet Y (2017) The fungal and acritarch events as time markers for the latest Permian mass extinction: an update. Geosci Front 9(1):147–154
Retallack GJ (2013) Permian and Triassic greenhouse crises. Gondwana Res 24:90–103
Retallack GJ, Veevers JJ, Morante R (1996) Global coal gap between Permian-Triassic extinction and Middle Triassic recovery of peat-forming plants. Geol Soc Am Bull 108:195–207
Rexroad CB, Furnish WM (1964) Conodonts from the Pella Formation (Mississippian), south-central Iowa. J Paleontol 38(4):667–676
Richoz S, Krystyn L, Baud A, Brandner R, Horacek M, Mohtat-Aghai P (2010) Permian-Triassic boundary interval in the Middle East (Iran and N. Oman): progressive environmental change from detailed carbonate carbon isotope marine curve and sedimentary evolution. J Asian Earth Sci 39:236–253
Rigo M, Trotter J (2014) Stenothermal habits of Gladigondolella and Norigondolella: constraints from δ18O of conodont apatite. In: Abstract in 2014 GSA annual meeting, Vancouver, BC
Romano C, Goudemand N, Vennemann TW, Ware D, Schneebeli-Herman E, Hochuli PA, Brühwiler T, Brinkmann W, Bucher H (2013) Climatic and biotic upheavals following the end-Permian mass extinction. Nat Geosci 6:57–60
Rothman DH, Fournier GP, French KP, Alm EJ, Boyle EA, Cao C, Summons RE (2014) Methanogenic burst in the end-Permian carbon cycle. PNAS 111(15):5462–5467
Schaal E (2014) Permian-Triassic Global Change: the Strontium cycle and body size evolution in marine clades. Dissertation, Dept. Geol. & Environs. Sciences, Stanford University, 266 pp
Schneebeli-Hermann E, Kuerschner WM, Hochuli PA, Ware D, Weissert H, Bernasconi SM, Roohi G, Rehman K, Goudemand N, Bucher H (2013) Evidence for atmospheric carbon injection during the end-Permian extinction. Geology 41(5):579–582
Sellwood B, Valdes P (2006) Mesozoic climates: general circulation models and the rock record. Sediment Geol 190:269–287
Shevyrev AA (2005) Triassic biochronology: state of the art and main problems. Stratigr Geol Correlation 14:629–641
Shigeta Y, Zakharov Y, Maeda H, Popov A (2009) The Lower Triassic system in the Abrek Bay Area, South Primorye, Russia. Natl Mus Nat Sci Monogr 38:24–27
Shigeta Y, Komatsu T, Maekawa T, Tran HD (2014) Olenekian (Early Triassic) stratigraphy and fossil assemblages in Northeastern Vietnam. Natl Mus Nat Sci Monogr 45, 190–271
Silva-Tamayo JC, Lau KV, Jost AB, Payne JL, Wignall PB, Newton RJ, Eisenhauer A, Depaolo DJ, Brown S, Maher K, Lehrmann DJ, Altiner D, Yu M, Richoz S, Paytan A (2018) Global perturbation of the marine calcium cycle during the Permian-Triassic transition. GSA Bull 130(7-8):1323–1338
Solien MA (1979) Conodont biostratigraphy of the Lower Triassic Thaynes Formation, Utah. J Paleontol 53:276–306
Staesche U (1964) Conodonten aus dem Skyth von Suedtirol. Neues Jahrbuch für Geologie und Paläontologie 119:247–306
Stefani M, Furin S, Gianolla P (2010) The changing climate framework and depositional dynamics of Triassic carbonate platforms from the Dolomites. Palaeogeogr Palaeoclimatol Palaeoecol 290:43–57
Sun T, Joachimski MM, Wignall PB, Yan C, Chen Y, Jiang H, Wang L, Lai X (2012) Lethally hot temperatures during the Early Triassic greenhouse. Science 388:366–370
Sweet WC (1970) Permian and Triassic conodonts from a section at Guryul Ravine, Vihi District, Kashmir. Univ Kansas Palaeontol Contrib 49:1–10
Sweet WC (1992) A conodont-based high-resolution biostratigraphy for the PermoTriassic boundary interval. In: Sweet WC, Zunyi Y, Dickins JM, Hongfu Y (eds) Permo-Triassic events in the Eastern Tethys. Cambridge University Press, Cambridge, pp 120–133
Sweet WC, Mosher LC, Clark DL (1971) Conodont biostratigraphy of the Triassic. Geol Soc Am 127:441–465
Thomazo C, Vennin A, Brayard I, Bour I, Mathieu O, Elmeknassi S, Olivier N, Esquarguel G, Bylund KG, Jenks J, Stephen DA, Fara E (2016) A diagenetic control on the Early Triassic Smithian–Spathian carbon isotopic excursions recorded in the marine settings of the Thaynes Group (Utah, USA). Geobiology 14(3):220–236
Tian C, Dai J, Tian S (1983) Triassic conodonts. In: Chengdu Institute of Geology and Mineral Resources (ed) Paleontological atlas of Southwest China. Microfossils. Geological Publishing House, Beijing, pp 345–398
Tozer ET (1967) A standard for Triassic time. Geol Surv Can Bull 156:332
Tozer ET (1994) Canadian Triassic ammonoid faunas. Geol Surv Can Bull 467:1–663
Trotter JA, Williams IS, Nicora A, Mazza M, Rigo M (2015) Long-term cycles of Triassic climate change: a new δ18O record from conodont apatite. Earth Planet Sci Lett 415:165–174
Veevers JJ (1994) Pangaea: evolution of a supercontinent and its consequences for Earth’s paleoclimate and sedimentary environments. In: Klein GD (ed) Pangaea: paleoclimate, tectonics and sedimentation during accretion, zenith, and breakup of a supercontinent. Geological Society of America Special Paper 288, Boulder, CO, pp 12–24
Von Mojsisovics E, Waagen W, Diener C (1895) Entwurf einer Gliederung der pelagischen Sedimente des Trias-Systems. Sitzungsber Akad Wiss Wien 104:1271–1302
Wang ZG, Cao YY (1981) Early Triassic conodonts from Lichuan, Western Hubei. Acta Palaeontologica Sinica 20(4):363–373. (in Chinese with English abstract)
Wang YB, Tong JN, Wang JS, Zhou XG (2005) Calcimicrobialite after end-Permian mass extinction in South China and its palaeo-environmental significance. Chin Sci Bull 50(7):665–671
Ward PD (2018) Lamarck’s revenge: heritable epigenetic consequences of stress and environmental change, from deep time to near future. The Stuart Agency. 240 pp
Wignall P, Benton M (1999) Discussion on Lazarus taxa and fossil abundance at times of biotic crisis—reply. J Geol Soc 156(3):453–456
Yang SR, Wang X, Hao WC (1986) Early and Middle Triassic conodonts sequence in Western Guangxi. Acta Scientiarum Naturalium Universitatis Pekinensis 4:90–106
Zakharov Y (1974) Importance of palaeobiogeographical data for the solution of the problem on the Lower Triassic Division. In: Zapfe H (ed) The stratigraphy of the Alpine-Mediterranean Triassic, vol 2. Oesterr. Akad. Wiss. Schiftenreihe Erdwissensch. Komm., pp 237–243
Zakharov Y, Biakov D, Horacek M (2014) Global correlation of basal Triassic layers in the light of the first carbon isotope data on the Permian–Triassic boundary in Northeast Asia. Russ J Pac Geol 8(1):1–17
Zapfe H (1983) Das Forschungsprojekt “Triassic of the Tethys Realm” (IGCP Projekt 4) Abschlussbericht. Oesterreichische Akad. Wissenschaften, Schriftenreihe der Erdwissenschaftliche Kommissionen 5:7–16
Zhang SX (1990) On the lower Triassic conodont sequence of western Guangxi. J Grad Sch China Univ Geosci 4(2):1–15
Zhang S, Yang Z (1991) On multielement taxonomy of the Early Triassic conodonts. Stratigr Paleontol China 1:17–47
Zhao L, Orchard MJ, Tong J, Zhang S, Sun Z (2007) Lower Triassic conodont sequence in Chaohu, Anhui Province, China and its global correlation. Palaeogeogr Palaeoclimatol Palaeoecol 252:24–38
Zhao L, Tong J, Sun Z, Orchard M (2008) A detailed Lower Triassic conodont biostratigraphy and its implications for the GSSP candidate of the Induan-Olenekian boundary in Chaohu, Anhui Province. Prog Nat Sci 18:79–90
Zhao L, Chen Z, Algeo T, Chen J, Chen Y, Tong J, Gao S, Zhou L, Hu Z, Liu Y (2013) Rare-earth element patterns in conodont albid crowns: evidence for massive inputs of volcanic ash during the latest Permian bio-crisis? Global Planet Change 105:135–151
Acknowledgements
We are indebted to Prof. H. Bucher (University of Zurich, Switzerland), Dr. E.S. Carter (University of Portland, USA), for reading the first draft of the manuscript, and to our reviewers. They all permitted us to improve the text.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kiliç, A.M., Guex, J., Hirsch, F. (2020). Proteromorphosis in Early Triassic Conodonts. In: Guex, J., S. Torday, J., Miller Jr., W. (eds) Morphogenesis, Environmental Stress and Reverse Evolution. Springer, Cham. https://doi.org/10.1007/978-3-030-47279-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-47279-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47278-8
Online ISBN: 978-3-030-47279-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)