Abstract
Issues: In the area of regenerative medicine paradigm, theranostic nanomedicine is emerging as a promising prototype. It has the advantage of both imaging and therapeutic functions in the platform of personalized medicine. Recently scientists have developed smart and hybrid forms of biological molecules, as carriers for various applications in diagnostics and therapeutics.
Major Advances: We reviewed the advances on nanotheranostic materials. Interactions among the particles in carrier payload have critical roles in the efficacy of a drug in clinical translation. Cancer and other degenerative diseases have remained indomitable problems for scientists and doctors due to their diverse nature and etiology. Nanotheranostic agents are now emerging as prudent tools in the detection of diseases, especially cancer. In recent years, the utilization of inorganic particles like iron oxide, gold, and carbon dots has gained massive attention in imaging technologies like MRI, PET, and SPECT. However, cumulative information on nanotheranostic agents, such as nature of materials, cell interaction, toxicity, mode of action at cellular levels, and effects on microenvironmental milieu, are required. Finally, we discuss the progress of different types of theranostic agents, their modes of biodistribution, pharmacokinetic properties, and chemocellular interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
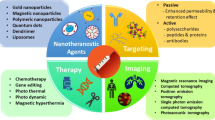
Nanotheranostic, an advanced branch of nanomedicine, is an amalgam of both diagnosis and therapeutics with fewer side effects, even for stubborn pathological conditions (Kojima et al. 2015). The strategy of nanotheranostic involves simple biological probing with easy monitoring of targeted drug delivery with more stability under physiological conditions (Sahoo et al. 2014). Nanotechnology-based theranostics approach is based on manipulating nanoparticles (NPs) (1–200 nm) by exploiting their unique properties, such surface area, optical and magnetic properties, low melting point, and mechanical strength (Horikoshi and Serpone 2013). A theranostic agent consists of a delivery platform that is conjugated either covalently or noncovalently to a therapeutic drug, nucleic acid (miRNA, siRNA), therapeutic proteins, or any other chemotherapeutic agent and signal emitters (with unique radioactive, optical, or magnetic properties) (Baum and Kulkarni 2012). Gold, silver, and magnetic NPs, along with nanoshells and nanocages, are important nanotherapeutical agents, that can be conjugated to either drugs, ligands, or antibodies to enhance the delivery and therapy as well as diagnostic imaging of the stages of diseases (by MRI, CT scan, CAT scan ultrasound, etc.) (Mody et al. 2010). The central principle and multimodal application of different nanotheranostic agents is shown in Fig. 10.1. It is clear from the model that the theranostic principle can be effectively utilized for a variety of activities in the area of biomedical, bioengineering, and biotechnological field. Therapeutic effectiveness of various drugs along with diagnostic agents has emerged as a future disease management system. Centrally, therapeutic drug and carriers are surface conjugated in a nanoform for various diagnostic purposes in the area of biomedical and biotechnological applications by bioengineering designs. Such an artificially designed drug for reliable diagnostic as well as therapeutic purpose enhances the efficacy of drug, especially in the area of cancer treatment. Cooperative joint delivery of drug and diagnostic agents could pave way to new insights for management of various degenerative diseases (Tinwala and Waikar 2019). This intelligent and smart revolutionary synthetic chemistry along with engineering tools is emerging as a powerful platform for modern diseases like cancer, vascular disease, and diabetic. Intracellular biostability, biocompatibility, low toxicity, and point of target are major advantages of this theranostic technology. Therefore, it is gaining importance in modern medicine. Accordingly, many varieties of nanocarriers for efficient conjugation have been developed in the recent past. For example, protein-based NPs and synthesized nano-scale DNA three-dimensional (3D) lattice structures (DNA origami) have been used as theranostic agents where various chemotherapeutic drugs compartmentalized into the hollow spaces inside the lattice can enhance the efficacy of controlled drug delivery and cancer biomarkers (Pinheiro et al. 2011). These nanotherapeutics can also be used for targeted delivery approach with real monitoring of both drug release and biodistribution of the NPs with facilitation of biomedical applications, such as efficient drug delivery across the blood-brain barrier (Tomitaka et al. 2019), multimodal and combinatorial therapies, and co-delivery of antisense oligonucleotides (siRNAs) (Muthu et al. 2014). The NPs can deliver a variety of targeting agents such as peptides, aptamers, monoclonal antibodies, nucleic acids, chemotherapeutics, etc. to malignant cells due to their surface modifications, enhanced permeability, and retention effect (Baetke et al. 2015). Different and promising types of nanotherapeutic agents with properties and target molecule are mentioned (Table 10.1).
10.2 Nomenclature of Nanotheranostic Material
Nomenclature of the nanotheranostic agents is principally based on the arrangement and organization of different drugs, theranostic agents, and target molecules. All three principal molecules are designed with the help of a linker molecule. There are several types of theranostic agents. Linear types have end positions attached to target agents. In polymeric and solid-phase types, diagnostic and theranostic agents are immobilized with linker-assisted target agents. In dendrimer type theranostic material, the endpoint of each branch is conjugated with detecting theranostic and drug molecule. In lipid-based types like liposome- and noisome-based types, micelle compounds are synchronized with diagnostic and theranostic materials. Finally, gold or any metal and carbon-based theranostic agents are prepared by simple chemical reactions. Different base materials are used to synthesize each of these types. Magnetic nanotheranostic is one important and widely used nanotherapeutical, due to its multipurpose functions such as hyperthermal killing of cancer cells, efficient delivery of gene and conventional chemotherapies, as well as multimodal imaging (PET, MRI, and optical imaging) (Singh and Sahoo 2014). Theranostic, coupled with MRI, have various advantages like free ion scanning mode, a high degree of tissue penetration with noninvasive detection mode. Due to their small size, they not only offer a better tissue penetration and faster drug delivery but also can provide a better platform for a diverse array of modifications with chemotherapeutic and target moieties (Xie et al. 2011). They are now used as contrast agents in MRI for diagnosis of cancer cells in soft tissue due to low cytotoxicity (Yoo et al. 2013). It has been shown that the superparamagnetic IONPs (SPIONs) loaded with amphiphilic poly(styrene)-b-poly(acrylic acid)-Dox and folic acid can be used as potential theranostic agents, as demonstrated in SkBr3 (human breast cancer cell) and HCT116 (human colon cancer) cell lines for controlled release, targeting, and anticancer activity (Patra et al. 2014).
Gold- and silver-based nanotheranostic materials are gaining importance in recent years due to their wide application ability in cancer biology. Gold and silver NPs are promising nanotheranostic agents due to their easy synthesis, bioconjugation, and surface modification (Boisselier and Astruc 2009). Use of gold nanoparticles in the diagnostic arena has attained considerable interest in biomedical, agricultural, and environmental including forensic investigation due to availability of highly advanced optical method, namely, surface-enhanced Raman spectroscopy (SERS). Due to laser excitation on gold-labeled drug surface in the SERS analysis, it has a high degree of application in MRI technology in tumor identification. These are used for multimodal imaging as well as to combat different types of cancer by exploiting its efficacy on cancer cell local effect. Cancer cell drug target is focused on the various intracellular cancer cell microenvironments like redox potential, ROS status, and cellular pH. The schematic of gold-based plasmonic SERS system is explained in Fig. 10.2.
Gold as plasmonic materials has been widely used for developing SERS active sensors for intracellular targets. Due to its high specificity, enhanced cellular uptake, and negligible cytotoxicity, gold nanobeacons (AuNBs) are used for specific mRNA silencing (Baptista 2014). Gold nanoclusters (AuNCs) conjugated with chitosan biopolymer are used for imaging due to its properties like low photobleaching, negligible cytotoxicity, and enhanced Stokes-shifted emission (Sahoo et al. 2014). Graphene-based nanotheranostic compounds are recently gaining much attention as inert and nonevasive compounds. Due to their large surface area, colloidal stability, easy surface modification/functionalization), as well as superior electrical and mechanical properties, graphene oxide-based nanomaterials provide significant attention in image-guided molecular ablation of cancerous cells (Draz et al. 2014). With a combination of AuNP, graphene-based nanotherapeutic agents are used in phototherapy; GO-Au-IONP assemblies have been shown to have enhanced superparamagnetism, optical absorbance, and photothermal therapeutic potential (Shi et al. 2013).
Due to distinct properties such as chemical and thermal stability, large surface area, and pore volume, silica NPs (SiNPs) function as an important theranostic agent which can be used for controlled, sequential, and multifunctional delivery drugs to numerous cancer cell types (Draz et al. 2014). Silica nanorattles conjugated with LHRH-PE40 luteinizing hormone-releasing hormone-Pseudomonas aeruginosa exotoxin 40 and docyanine green fusion protein can be used as a nanotheranostic agent in combination with docetaxel (antimitotic chemotherapeutic) for treatment of cancers (Gao et al. 2013). These days, lipid- and polymer-based nanotheranostic agents, mainly liposomes, dendrimers, and polymeric micelles, are widely used as nanocarriers due to their easiness for surface modification, targeting ability, thermal stability, and compatibility with various diagnostic applications (Gu et al. 2007). They have a wide range of capability, such as cellular compatibilities, biodegradability, rapid cellular uptake, and lack of toxicity (Schroeder et al. 2010). Protein-based nanotheranostic agents are made up of proteins which can be used as carriers for both therapeutic and diagnostic agents (Ng et al. 2011). These nanocages can be modified both internally such as loading with conjugable molecules (drugs/aptamers/contrast agents) and externally by ligand conjugation (Lim et al. 2013, Kaur and others 2018). Other classes of protein-based nanotheranostic agents such as nanoradiopeptides and fluorescent peptide nanoprobes (Luo et al. 2012) also hold immense promise as major therapeutic agents for cancer treatment. Targeted binding of ions and small molecule proteins with artificially synthesized RNA or DNA oligonucleotides also called aptamers through a process called systematic evolution of ligands by exponential enrichment (SELEX) method (Tuerk and Gold 1990) have effectively been used in the cell-based theranostic procedure (Kaur 2018). Due to the wide angle of advantage, SELEX has been widely used against cancer cells, tumor-associated proteins, and parasites or virus-infected cells (Rong and others 2016).
10.3 Biodistribution
Biodistribution of the nanotheranostic agents depends upon various factors such as size and shapes. For example, hybrid gadolinium oxide NPs (Gado-6Si-NP) were selectively taken up by circulatory blood pool and finally cleared by renal excretion without accumulation in the liver. The effectiveness of this particular theranostic agent is due to its small size of 3–4 nm diameter (Kryza et al. 2011). The strategy of application of theranostic agents in cancer biology and detection method is depicted in Fig. 10.3. The fate of cancer cell growth or death can be monitored through surface modification of theranostic agents using smart, radiowave-emitting or fluorescent tags that are cancer cell-specific and capturing these signals either by amplifier-assisted receiver or appropriate fluorescence detection instrument. In vivo barriers like cell membrane and blood–brain barriers are major checkpoints for NP movements in the body, by restricting the NP functions like their movement and physical changes and inducing a negative host response (Belting et al. 2005). Blood, its components, anatomical restriction, and different barriers like blood–brain barrier are important components through which the NPs have to pass. Effective traffic control and distribution in the body are key strategical factors for the optical design of NPs. Smart design is an important factor for the biodistribution of nanotheranostic agents through the biological barriers and enabling them to reach their intended destination (Ferrari 2005).
The physiochemical properties, which include morphology, hydrodynamic size, charge, and other surface properties, are important factors for the biodistribution of nanotheranostics (Dobrovolskaia et al. 2008). One of the important physicochemical properties is the hydrodynamic size. Hydrodynamic size helps in governing the NP concentration in the blood vessel by affecting the mechanism of NP clearance and dictates the permeability of NPs out of the vasculature (Chavanpatil et al. 2006). It also affects the NP clearance from the circulation and also determines the passage of it through the blood–brain barrier (Koo et al. 2006). Another aspect of the physicochemical nature and action of a theranostic agent is governed by the shape (Gratton et al. 2007). It was found that the anisotropically shaped NPs can avoid bioelimination better than spherical NPs (Liu et al. 2007). Some scientists also have shown that high aspect ratio shaped MNPs have also been evaluated in vivo and found to have similarly enhanced blood circulation times over the spherical counterparts (Park et al. 2009). Surface properties also play important roles in the target action of theranostic agent because major agents are targeted toward the cell receptors. Surface properties such as NP charge and hydrophobicity can affect biodistribution by minimizing or enhancing the interactions of NPs with the adaptive immune system, plasma proteins, extracellular matrices, and nontargeted cells (Davis 2002). These two factors mainly contribute to the short circulation time, which may be due to the adsorption of plasma proteins recognized by the reticuloendothelial system [RES] (Chouly et al. 1996). Target designed specificity is yet another important aspect to be considered during designing of the theranostic agent. For both diagnostic imaging and drug-based therapies for selected tissues, the specificity of NPs is an important factor for successful nanotheranostics (Leuschner et al. 2006). NPs have been engineered to have an affinity for target tissues either through passive, active, or magnetic targeting approaches. Passive targeting uses the predetermined physicochemical properties of a given NP to specifically migrate to a given tissue region by the phenomenon known as enhanced permeation and retention (EPR) (Maeda et al. 2000). Toxicological preevaluation of NP is an important aspect for end application (Madhyastha et al. 2019). In general, the toxicity of the compound to be conjugated and NP should be considered before development of an appropriate therapeutic agent. It mainly involves considering how the assembled NP will interact with the body and how the independent components will affect the body during biodegradation and liver processing (Lewinski et al. 2008). This leads to a new branch that is nanotoxicology, which mainly deals with an understanding of the body’s response to the NPs’ chemistry (Vega-Villa et al. 2008).
10.4 Pharmacokinetics
Nanotheranostic agents undergo typical pharmacokinetics pathway in which they are clustered within lysosome upon their intracellular internalization via endocytosis. They are degraded into corresponding metal ions by an array of hydrolyzing enzymes at low pH (Gupta et al. 2007). The size, charge, surface chemistry, and route of delivery influence the circulation time and biodistribution pattern inside the body. The spleen is the organ in which the large particles are usually sequestered and small particles are rapidly removed through extravasations and renal clearance upon intravenous injection. (Gupta and Gupta 2005). The final distribution of the particles is observed more in the liver (80–90%), followed by spleen (5–8%), and less in bone marrow (1–2%) which mainly depends upon the surface chemistry and the mechanism of internalization (Unfried et al. 2007). NPs may interact with the extracellular matrix components and the plasma cell membranes of macrophages, endothelial cells, skin epithelium, and respiratory or gastrointestinal tracts during their metabolism (Oberdorster et al. 2005). Upon inhalation, they are accumulated in the brain, liver, spleen, and lungs demonstrating their ability to cross blood–brain barrier (Kwon et al. 2008). Macrophage plays an important role in nanotheranostics clearance. Nanotheranostic agents are challenged by macrophages of the RES upon their administration in vivo (Duguet et al. 2006). Many mechanisms of the internal organization such as phagocytosis (mediated by mannose, complement, Fcγ, and scavenger receptors), endocytosis (clathrin- and caveolin-mediated, fluid-phase), and diffusion are involved for processing of nanotheranostics in macrophage (Dobrovolskaia and McNeil 2007; Unfried et al. 2007). NPs can also get opsonized by plasma proteins (e.g., albumin, apolipoprotein, immunoglobulins, complement, fibrinogen), which promote their recognition and clearance by cells of RES (Park et al. 2008). Iron oxide Nano Particle (IONP) binds to the plasma fibronectin, and vitronectin changed from receptor-mediated to fluid-phase endocytosis (Moore et al. 1997). The polyethylene glycol (PEG), which acts as amphiphilic polymeric surfactants, significantly reduces MNP interactions with plasma proteins, minimizing their internalization and clearance by macrophages (Zhang et al. 2002). PEG along with antitumor drug paclitaxel is being investigated in metastatic breast cancer xenograft mouse model and is found to be effective in reducing the tumor size over the period of administration (Lee et al. 2018), since cancer cell has several receptors for PEG and also free-circulating nature of PEG in bloodstream resulting in remarkable antitumor efficacy.
10.5 Surface Functionalization
Figure 10.4 depicts the various types of nanotheranostic agents by smart designing approach. Linear-type polymer and drugs are conjugated in an exponential manner. Target capture agents are capped at each corner of the polymer edge. Other types are of solid-phase, dendrimer, liposome, and noisome types. In metal variety, gold or silver metals are centrally placed. Surface functionalization, which increases the surface activity and biocompatibility, is an important biological phenomenon needed for all the nanotheranostics reagents (Medha et al. 2018). Biodegradable polymers are safe and green materials that have been widely researched by many investigators. Several formulations such as dextrans, chitosan, polyethylene glycol, polysorbate, and polyaniline are used for increasing the surface functionalization of nanotheranostics agents. Dextran is an important constituent for various formulations such as Ferridex, Resovist, Combidex, and AMI-288/ferumoxytol (McCarthy et al. 2007). Polyethylene glycol due to its hydrophilicity and low antigenicity prevents plasma opsonization and uptake by macrophages and thus increasing the theranostics circulation in vivo (Gupta and Curtis 2004). Its excellent film-forming, emulsifying, and adhesive properties can be utilized in targeted drug delivery, tissue engineering, and biosensor technology (Gupta et al. 2007). Lastly, chitosan provides a natural, biocompatible, cationic, and hydrophilic polymer coating, suitable for affinity purification of proteins and magnetic bioseparation (Sasaki et al. 2008). Inorganic metals are prosperous materials with proper surface modifications. Different inorganic metals such as gold and silver are used for the surface functionalization due to their high stability and low reactivity (Eustis and el-Sayed 2006), but the dissimilarity of the two metallic surfaces causes a pitfall for use of the metal as coating agent (Lu et al. 2007). Inorganic oxides like metals too play a governing role in surface chemistry during synthesis and design of theranostic agents. Inorganic oxides such as silica gel can be used as a coating agent due to their negative charge and stability in aqueous solution. Silica-coated magnetic nanoparticles have longer circulation times, and their hydrophilic negatively charged surface provides ideal anchorage for covalent binding to ligands, presenting an excellent platform for drug delivery (Tartaj et al. 2001). In the area of cancer biology, use of dimercaptosuccinic acid is found to be a suitable agent as a surface modifier. Among all cancer etiology, colorectal cancer is considered as one of the major cancer types with huge mortality worldwide. Cancer of the large intestine (colon, rectum, and anus) is a major cause of morbidity. Nanosized maghemite material precoated with dimercaptosuccinic acid and functionalized anticarcinoembryonic antigen (anti-CEA) is a potent tool in the identification of colon cancer by MRI techniques (Campos da Paz et al. 2012). This system also can be used as a theranostic agent for tumor cells and circulating cancer cells. Surface charges during the synthesis of theranostic agent also play a very important role (Wilhelm et al. 2003). Negatively charged sulfur containing chelating agent prevents the theranostic materials from aggregation and interacts strongly with the positively charged regions of the plasma membrane due to its negative charge.
10.6 Application of Nanotheranostics
Nanotheranostics mainly aims at simultaneous diagnosis and therapy with a wide range of functions in magnetic hyperthermia, drug/gene delivery, tissues engineering, and diagnostic imaging or biosensor platform. For example, the use of magnetic hyperthermia techniques comes under cancer therapy and relies on the localized heating of tumors above 43°c for 30 min (Pankhurst et al. 2003). The magnetic nanoparticle due to its magnetization property can heat the cancer cell, and the selectivity toward tumors was considerably improved through the use of silane coating and through functionalization approaches (Jordan et al. 1999). Tumor growth can be arrested by using magnetic cationic liposomes with a combination approach employing TNF-α gene and stress-inducible gad 153 promoters (Ito et al. 2001). Magnetic resonance imaging is an important diagnostic technique for living tissues in which magnetic NPs are used as contrast agents to identify lymph node metastases and solid tumor (Hogemann et al. 2000). Macrophage-specific MNP labeling protocols are used to image inflammatory pathologies, including atherosclerosis, multiple sclerosis, and rheumatoid arthritis (Berry et al. 2004). MNP-labeled stem cells and neuroprotective glia cells can be guided by in vivo cell tracking of CNS regeneration, while glioma cells are visualized by FITC-conjugated MNPs (Dunning et al. 2004). Theranostic techniques are used to diagnose the rate of bioseparation in vivo (Jian and Rosenberg 2005). Healthy kidney function is evaluated by the rate of glomerular filtration rate. Insulin clearance is the most updated and standard marker for evaluating the glomerular filtrate rate. Alternately, radiolabeled chelating agents like ethylenediaminetetraacetic acid, 51Cr-EDTA; diethylenetriamine pentaacetic acid, 99mTc-DTPA; and radio-iothalamate, 125I-iothalame, are also used in medical pathology. All of these compounds are sensitive to kidney cells and are not cost-effective. Recently iohexol, a nonradiolabeled, nonionic, low osmolar iodinated agent with very high x-ray contrast properties has shown efficacy as theranostic molecule (Berg et al. 2011).
Drug delivery system is a major concern nowadays for appropriate treatment of different chronic diseases, including various cancers. Current chemotherapy drugs attack both normal as well as cancer cells in the tissue, thus becoming a cause of concern with life-threatening side effects. Targeted drug delivery is a novel approach to overcome this lacuna. This can be done by surface functionalization of different NPs. MNP-conjugated drugs have been applied experimentally for cancer therapy (Duguet et al. 2006). Neurological diseases can be treated with these NP-based drugs and gene delivery system, which can cross the blood–brain barriers (Kreuter 2001). However, for efficient drug delivery, the NPs’ surface chemistry, hydrophilicity, and the size are major factors to be considered for the rapid clearance by RES (Torchilin and Trubetskoy 1995). Dual-modality imaging system is comparatively safe, simple, and noninvasive diagnostic and imaging system. But lower image quality is the caveat in this system. Recently magnetic transfection and surface modification of target drug are being used in regenerative medicine. Magnetic transfection is an important application in which NPs are employed as effective transfection system for delivering DNA into the cells. Mainly the MNPs are used for gene introduction to permissive and nonpermissive cells under external magnetic field (Scherer et al. 2002). Effective delivery of antisense oligonucleotide is done through the magnetic field in endothelial cells. (Krotz et al. 2003).
Another hallmark application of theranostic technology is in tissue engineering. During the artificial tissue grafting and developmental studies, varieties of theranostic agents are being used. It is one important application of nanotherapeutics where new tissues are regenerated by using stem cell replacement therapy for cell labeling, sorting, monitoring, and engraftment to the diseased tissue (Bulte et al. 2001). The tissue surfaces are joined under high temperature by using MNPs typically accompanied by protein denaturation followed by repolymerization of adjacent protein chains (Gupta et al. 2007). Keratinocyte sheet-like 3-D constructs have been developed by harvesting MNPs, where self-assembled magnetic nanowire arrays are used (Ito et al. 2005). Another important metal which has been used in theranostic biology is silver. Nanosilver is a major source of antibacterial material because of wide spectrum activity, especially in the wound-healing scenario. Silver, having highest physicochemical properties, very high mechanical strength, and good electrical conductivity, is reported as good sensing as well as imaging agent and is a source of interest for medical and biotechnological research. Silver decahedral NP (Ag10NP) conjugated with fluorophore aptamers (Sgc8-FITC) was found to be the most suitable sensing agent in FRET system (Li et al. 2015). Here target molecule is membrane protein tyrosine kinases-7 (PKT-7) in CCRF-CEM T-cell line. Advantage of using this theranostic agent is to enhance the imaging quality of T cell by disturbing the FERT effect in real-time system.
10.7 Toxicity of Nanotheranostics
Although the theranostic agents display huge advantages, they also exhibit different types of drawbacks like cell stress, cell senescence, and untimely cell death. One of the major concerns of cell stress is oxidative stress. Oxidative stress in the cancer cell is required to cure the cancer; however, the same action in a normal cell can be precancerous. Therefore, fine-tuning between cancer and normal cell is subject for research (Madhyastha et al. 2019). Theranostic agents such as magnetic nanoparticles (MNPs) induce redox cycling and catalytic chemistry, which causes the evolution of reactive oxygen species (ROS), leading to oxidative stress (Borm et al. 2006). ROS induces MMP activity in the nervous system leading to increased blood–brain barrier permeability and neuronal damage (Liu et al. 2007). Application of the nanotheranostics is safe, but an imbalance in the homeostasis gives toxic implications to many organs. The individual ions either iron, gold, or graphene may produce excessive free radicals (Madhyastha et al. 2019) in the brain and could be associated with multiple neurodegenerative disorders, including multiple sclerosis and Alzheimer’s and Parkinson’s diseases (Doraiswamy and Finefrock 2004). Toxicity of the nanotheranostics showed a large effect from cytotoxicity in vitro to transient and acute toxicity to unremarkable changes in vivo (Ma et al. 2008). The most toxic effect showed by MNP is accumulation in tissues, but with unremarkable histological changes in vital organs, concluding safety of the respective formulations. Ultrasound (US) theranostic-based biomedical technology is used frequently in the clinics as HIFU and SDT method. In both technologies, knowledge of material chemistry plays a key factor. In this technology core-to-shell design by solid to gas (perfluorocarbon) phase, interaction is an important factor to be considered. However, biosafety consideration is yet to evolve in this field. Platinum-conjugated drugs like, cisplatin, carboplatin, and oxaliplatin are used extensively in cancer biology. However, these drugs cause extensive damage to the liver and kidney. In recent years, development of Pt-based nanodrugs has seen progress. A major limitation of this nanodrug is toxicity in off-target organ and delay in rapid clearance by the cells of the reticuloendothelial system and mononuclear phagocyte system. Use of graphene in cancer theranostic treatment is still under development but rapidly growing because of its promising results
10.8 Conclusion
Considering the above importance of NPs in both diagnosis and therapeutics, it can be concluded that this is an advanced branch of nanotechnology with inimitable characteristics such as highly specific, targeted, blood–brain barrier crossing, drug delivery, imaging platform, unique transfection, labeling, bioseparation, as well as analytical and tissue engineering approaches. Yet, some challenges exist, such as nanotoxicity, environmental hazards, and target mismatching, which need to be explored in the nearest future. Theranostic NP technology has drawn considerable interest in the cancer field, but application potential is hampered due to its toxicity property. Optical and smart design strategy to control the size and shape is another challenge in the application. Size of a particle plays an important role in the potential activity in vivo. Acceptable and adaptable size of a nanotheranostic drug for cancer cure is of 20 nm, but this size is not suitable or ideal for diagnosis due to poor imaging quality. Importantly posttreatment clearance from the cell is a major area for future research as the nanoresiduals may be a potential threat to cell architecture. More insight and research thoughts are also required on the concept of NP recycling from the cell as a novel challenge in biomedical research.
Abbreviations
- CAT:
-
Computed axial tomography
- CT:
-
Computed tomography
- FITC:
-
Fluorescein isothiocyanate
- FRET:
-
Fluorescence resonance energy transfer
- HIFU:
-
High-intensity focused ultrasound
- IMPT:
-
Intensity modulated proton therapy
- IMXT:
-
Intensity modulated x-ray therapy
- LHRH-PE40:
-
Luteinizing hormone-releasing hormone-Pseudomonas aeruginosa exotoxin 40
- miRNA:
-
Micro-ribonucleic acid
- MNP:
-
Magnetic nanoparticles
- MRI:
-
Magnetic resonance Imaging
- NP:
-
Nanoparticles
- PET:
-
Positron emission tomography
- SDT:
-
Sonodynamic therapy
- SELEX:
-
Systematic evolution of ligands by exponential enrichment (SELEX) method
- SiRNA:
-
Small interfering ribonucleic acid
- SPECT:
-
Single-photon emission computed tomography
References
Baetke SC, Lammers T, Kiessling F (2015) Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol 88:02–07
Baptista PV (2014) Gold nanobeacons: a potential nanotheranostics platform. Nanomedicines 9:2247–2250
Baum RP, Kulkarni HR (2012) Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy – the Bad Berka experience. Theranostics 2:437–447
Belting M, Sandgren S, Wittrup A (2005) Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev 57:505–527
Berg UB, Back G, Celsi SE, Haling I, Hamberg R, Kamer T (2011) Comparison of plasma clearance of iohexol and urinary clearance of insulin for measurement of GFR in children. Am J Kidney Res 57:55–61
Berry CC, Charles S, Wells S, Dalby MJ, Curtis AS (2004) The influence of transferrin stabilized magnetic nanoparticles on human dermal fibroblasts in culture. Int J Pharm 269(1):211–225
Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38:1759–1782
Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, Krutmann J, Warheit D, Oberdorster E (2006) The potential risks of nanomaterials: a review carried out for ECETOC Part. Fibre Toxicol 14(3):11
Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK (2001) Magneto dendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19(12):1141–1147
Campos da Paz M, Santos MF, Santos Camila MB, De Silva SW, De Souza LB, Lima CD, Silva RC, Lucci CM, Morais RB, Lacava GM (2012) Anti-CEA loaded maghemite nanoparticles as theragnostic device for colorectal cancer. Int J Nanomedicine 7:5271–5282
Chavanpatil MD, Khdair A, Panyam J (2006) Nanoparticles for cellular drug delivery: mechanisms and factors influencing delivery. J Nanosci Nanotechnol 6:2651–2663
Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P (1996) Development of super paramagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul 13:245–255
Davis ME (2002) Non-viral gene delivery systems. Curr Opin Biotechnol 13:128–131
Dobrovolskaia MA, McNeil SE (2007) Immunological properties of engineered nanomaterials. Nat Nanotechnol 2:469–478
Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE (2008) Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm 5:487–495
Doraiswamy PM, Finefrock AE (2004) Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol 3(7):431–434
Draz MS, Fang BA, Zhang P, Hu Z, Gu S, Weng KC, Gray JW, Chen FF (2014) Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics 4:872–892
Duguet E, Vasseur S, Mornet S, Devoisselle JM (2006) Magnetic nanoparticles and their applications in medicine. Nanomed 1(2):157–168
Dunning MD, Lakatos A, Loizou L, Kettunen M, Constant CF, Brindle KM (2004) Superparamagnetic iron oxide-labeled Schwann cells and olfactory unsheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci 24(44):9799–9810
Eustis S, el-Sayed MA (2006) Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev 35(3):209–217
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171
Gao F, Li L, Fu C, Nie L, Chen D, Tang F (2013) LHRH-PE40 fusion protein tethered silica nanorattles for imaging-guided tumor-specific drug delivery and bimodal therapy. Adv Mater Deerfield Beach Fla 25:5508–5513
Gratton SEA, Pohhaus PD, Lee J, Guo I, Cho MJ, De Simone JM (2007) Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT (TM) nanoparticles. J Control Release 12:10–18
Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S et al (2007) Targeted nanoparticles for cancer therapy. Nano Today 2:14–21
Gupta AK, Curtis AS (2004) Surface modified super paramagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture. J Mater Sci Mater Med 15(4):493–496
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–3402
Gupta AK, Naregalkar RR, Vaidya VD, Gupta M (2007) Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine (Lond) 2(1):23–39
Hogemann D, Josephson L, Weissleder R, Basilion JP (2000) Improvement of MRI probes to allow efficient detection of gene expression. Bioconjug Chem 11(6):941–946
Horikoshi S, Serpone N (2013) Introduction to nanoparticles. Microw Nanoparticle Synth Fundam Appl 43:1–24
Ito A, Shinkai M, Honda H, Kobayashi T et al (2001) Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther 8(9):649–654
Ito A, Shinkai M, Honda H, Kobayashi T (2005) Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 100(1):1–11
Jian Liu K, Rosenberg GA (2005) Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med 39(1):71–80
Jordan A, Scholz R, Wust P, Schirra H, Schiestel T, Schmidt H et al (1999) Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J Magn Magn Mater 194(1–3):185–196
Kaur H (2018) Recent developments in cell-SELEX technology for aptamer selection. Biochim Biophys Acta Gen Subj 1862:2323–2329
Kojima R, Aubel D, Fussenegger M (2015) Novel theranostic agents for next generation personalized medicine: small molecules, nanoparticles, and engineered mammalian cells. Curr Opin Chem Biol 28:29–38
Koo YEL, Reddy GR, Bhojani MR, Schneider MA, Philbert A, Rehemtulla BD, Ross R, Kopelman R (2006) Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev 58:1556–1577
Kreuter J (2001) Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 47(1):65–81
Krotz F, deWit C, Sohn HY, Zahler S, Gloe T, Pohl U, Plank C (2003) Magnetofection—a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol Ther 7(5 Pt 1):700–710
Kryza D, Taleb J, Janier M, Marmuse L, Milladi I, Bonazza P, Perriat P, Roux S, Tilement O, Billotey C (2011) Blood distribution study of nanometric hybrid gadolinium oxide particles as a multimodal SPECT/MR/optical imaging and theragnostic agent. Bioconjug Chem 22(6):1154–1152
Kwon JT, Hwang SK, Jin H, Kim DS, Minai-Tehrani A, Yoon HJ (2008) Body distribution of inhaled fluorescent magnetic nanoparticles in the mice. J Occup Health 50(1):1–6
Lee JE, Kim MG, Jang YL, Lee MS, Kim MW, Yin Y, Lee JH, Lim SY, Park JW, Kim J, Lee DS, Kim SH, Jeong JH (2018) Self-assembled PEGylated albumin nanoparticles (SPAN) as a platform for cancer chemotherapy and imaging. Drug Deliv 25(1):1570–1578
Leuschner C, Kumar C, Hansel W, Soboyejo W, Zhou JK, Hormes J (2006) LHRH conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat 99:163–176
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49
Li H, Hu H, Xu D (2015) Silver decahedral nanoparticles-enhanced fluorescence resonance energy transfer sensor for specific cell imaging. Anl Chem 87(7):3826–3833
Lim S, Peng T, Sana B (2013) Protein cages as theranostic agent carriers. In: Long M (ed) The World Congress on Medical Physics and Biomedical Engineering, May 26–31 2012 Beijing China. Springer, Berlin/Heidelberg, pp 321–324
Liu Z, Cai WB, He LN, Nakayama N, Chen K, Sun XM, Chen XY, Dai HJ (2007) In vivo biodistribution and highly efficient tumor targeting of carbon nanotubes in mice. Nat Nanotechnol 2:47–52
Lu AH, Salabas EL, Schuth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl 46(8):1222–1244
Luo H, Shi J, Jin H, Fan D, Lu L, Wang F, Zhang Z (2012) An 125I-labeled octavalent peptide fluorescent nanoprobe for tumor-homing imaging in vivo. Biomaterials 33:4843–4850
Ma HL, Qi XR, Ding WX, Maitani Y, Tsuneji N (2008) Magnetic targeting after femoral artery administration and biocompatibility assessment of superparamagnetic iron oxide nanoparticles. J Biomed Mater Res A 84(3):598–606
Madhyastha H, Madhyastha R, Nakajima Y, Daima HK, Navya PN, Maruyama M (2019) An opinion on nanomedicine and toxico-cellular cross talk: considerations and caveats. Mater Today 10:100–105
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–284
McCarthy JR, Kelly KA, Sun EY, Weissleder R (2007) Targeted delivery of multifunctional magnetic nanoparticles. Nanomed (Lond) 2(2):153–167
Medha MU, Sanjana S, Devendra J, Harishkumar M, Radha M, Masugi M, Navya PN, Daima HL (2018) Insight into the composition and surface corona reliant biological behavior of quercetin engineered nanoparticles. Colloids Surf A 5(48):1–9
Mody VV, Siwale R, Singh A, Mody HR (2010) Introduction to metallic nanoparticles. J Pharm Bioallied Sci 2:282–289
Moore A, Weissleder R, Bogdanov A (1997) Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging 7(6):1140–1145
Muthu MS, Leong DT, Mei L, Feng SS (2014) Nanotheranostics – application and further development of nanomedicine strategies for advanced theranostics. Theranostics 4:660–677
Nam JM, Thaxton CS, Mirkin CA (2003) Nanoparticle-based Bio-Bar Codes for the Ultrasensitive Detection of Proteins. Science. (301):1884–1886
Ng KK, Lovell JF, Zheng G (2011) Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res 44:1105–1113
Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K et al (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2:8
Orecchioni M, Cabbiza R, Bianco A, Delogu LG (2015) Graphene as cancer theranostic tool: progress and future challenges. Theranostics 28(7):710–723
Pankhurst QA, Connolly J, Jones SK, Dobson J et al (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36(13):R167–R181
Park J, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, Bhatia SN, Sailor MJ et al (2008) Magnetic iron oxide nano-worms for tumor targeting and imaging. Adv Mater 20:1630–1635
Park JH, Maltzahn GV, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ (2009) Systematic surface engineering of magnetic nano-worms for in vivo tumor targeting. Small 5:694–700
Patra HK, Ul Khaliq N, Romu T, Wiechec E, Borga M, Turner APF, Tiwari A (2014) MRI-visual order-disorder micellar nanostructures for smart cancer theranostics. Adv Healthc Mater 3:526–535
Pinheiro AV, Han D, Shih WM, Yan H (2011) Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol 6:763–772
Rong Y, Chen YH, Zhou XF, Yin CQ, Wang BC, Peng CW, Liu SP, Wang FB (2016) Identification of an aptamer through whole cell-SELEX for targeting high metastatic liver cancers. Oncotarget 7:8282–8294
Sahoo AK, Banerjee S, Ghosh SS, Chattopadhyay A (2014) Simultaneous RGB emitting Au nanoclusters in chitosan nanoparticles for anticancer gene theranostics. ACS Appl Mater Interfaces 6:712–724
Sasaki T, Iwasaki N, Kohno K, Kishimoto M, Majima T, Nishimura S, Minami A (2008) Magnetic nanoparticles for improving cell invasion in tissue engineering. J Biomed Mater Res A 86(4):969–978
Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gänsbacher B (2002) Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 9(2):102–109
Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG (2010) Lipid-based nanotherapeutics for siRNA delivery. J Intern Med 267:9–21
Shi X, Gong H, Li Y, Wang C, Cheng L, Liu Z (2013) Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 34:4786–4793
Singh A, Sahoo SK (2014) Magnetic nanoparticles: a novel platform for cancer theranostics. Drug Discov Today 19:474–481
Tartaj P, Gonzalez-Carreno T, Serna CJ (2001) Single-step nanoengineering of silica coated maghemite hollow spheres with tunable magnetic properties. Adv Mater 13:1620–1624
Tinwala H, Waikar S (2019) Production surface modification and biomedical applications of nanodiamonds: a sparkling tool for theranostics. Mater Sci Eng C Mater Biol Appl 97:913–931
Tomitaka A, Ota S, Nishimoto K, Arami H, Takemura Y, Nair M (2019) Dyanamic magnetic charaterisation and magnetic partcle imaging enhancement of magnetic gold-core shell nanoparticles. Nanoscale 11(13):6489-6496
Torchilin VP, Trubetskoy VS (1995) Which polymers can make nanoparticulate drug carriers long-circulating. Adv Drug Del Rev 16(2–3):141–155
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment ligands to bacteriophage t4 DAN polymerase. Science 249:505–510
Unfried K, Albrecht C, Klotz LO, Mikecz AV, Beck SG, Schins RPF (2007) Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology 1(1):52–71
Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM (2008) Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev 60:929–938
Wilhelm C, Billotey C, Roger J, Pons JN, Bacri JC, Gazeau F (2003) Intracellular uptake of anionic super paramagnetic nanoparticles as a function of their surface coating. Biomaterials 24(6):1001–1011
Xie J, Liu G, Eden HS, Ai H, Chen X (2011) Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res 44:883–892
Yoo H, Moon SK, Hwang T, Kim YS, Kim JH, Choi SW, Kim JH (2013) Multifunctional magnetic nanoparticles modified with polyethyleneimine and folic acid for biomedical theranostics. Langmuir ACS J. Surf Colloids 29:5962–5967
Zhang Y, Kohler N, Zhang M (2002) Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 23(7):1553–1561
Acknowledgments
The authors would like to sincerely acknowledge the university authority for permission to publish the work and several of our collaborators for proofreading.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sahoo, P.R., Madhyastha, H., Madhyastha, R., Maruyama, M., Nakajima, Y. (2020). Recent Progress in Nanotheranostic Medicine. In: Yata, V., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanopharmaceuticals: Principles and Applications Vol. 3. Environmental Chemistry for a Sustainable World, vol 48. Springer, Cham. https://doi.org/10.1007/978-3-030-47120-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-47120-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47119-4
Online ISBN: 978-3-030-47120-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)