Abstract
Dynamic preservation strategies are a promising option to improve graft quality and to extend preservation time for either logistic or treatment reasons. In contrast to normothermic oxygenated perfusion, which is based on physiologic conditions, thereby aiming to simulate the human body, hypothermic oxygenated liver perfusion appears un-physiologic and induces a unique, mitochondrial response for its protective effect. Both ex vivo perfusion techniques can be used for viability assessment, which will open the door for an increased liver utilization in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Machine perfusion of organs before transplantation is currently a hot topic, as many organs are declined due to the lack of methods ensuring graft quality, for example, steatotic grafts or livers donated after circulatory death (DCD) [34, 69]. The utilization rate of marginal livers is therefore highly different between centers and countries and is influenced by donation rates, risk strategies, and transplanting surgeon’s experience [9, 34]. Often, however, the decision to reject livers is not based on objective parameters, but rather on gut feeling [69]. In contrast, machine perfusion concepts would potentially offer the advantage to test organ function before transplantation and to optimize metabolic deficiencies. Despite numerous research efforts in this field during the last 20 years, it remains unclear which perfusion procedures and which ex vivo viability tests are most reliable and also practical today.
This chapter provides an overview of current machine liver perfusion techniques and focuses on different achievements through hypothermic perfusion of liver grafts from DCD donors.
Machine Liver Perfusion Concepts
Two perfusion approaches for liver grafts have been recently introduced in clinical practice, which differ fundamentally in terms of their logistic efforts and protective mechanism. Firstly, an upfront machine perfusion, immediately after standard procurement, with the aim to replace conventional cold storage [45]. For this purpose, the organ is placed directly after procurement on a transportable device and undergoes continuous perfusion until implantation in the recipient center [16, 45]. Sophisticated and expensive systems are used for this approach, mostly at normothermic (NMP) or subnormothermic (SMP) temperatures, with a blood-based perfusate (Organox®, Transmedics®, Liver Assist®) [6, 7, 16, 21, 37, 45]. A modification of this technique involves an even earlier start of machine liver perfusion already in the donor, e.g., normothermic regional perfusion (NRP), instead of the routine super rapid cannulation and cold in situ organ flush [24, 66, 67]. A logical extreme would be the combination of NRP and NMP, in order to keep the perfused organ without any intermittent cooling and therefore preventing interruption of normothermic perfusion until implantation. This concept leads to complete abundance of cold ischemia and has been introduced as “ischemia free organ transplantation” (IFOT) in a few human livers [23]. Although such procedure avoids repeated temperature changes during liver preservation, the enormous technical complexity appears as a clear hurdle for a broad clinical introduction. Additionally, the IFOT technique should be compared to other perfusion techniques.
An alternative machine liver perfusion approach is applied endischemically, after initial cold storage and liver transport to the recipient center (repair centers) [36, 57, 71]. Subsequently, organs are perfused for a relatively short period prior to implantation. Such endischemic perfusion techniques have been applied at various temperatures, including normothermic and hypothermic temperatures or by a combination of both conditions, defined as controlled oxygenated rewarming (COR) [41, 64]. Although these techniques are logistically easier and cheaper, because a device transport is not necessary, the initial period of cold ischemia induces severe metabolic depletion before perfusion is started, particularly in high-risk grafts, such as steatotic livers or livers from DCD donors [4, 38, 69].
Besides the timing of machine perfusion, the perfusate composition varies substantially among techniques at all temperatures [7, 56]. While normothermic or subnormothermic perfusions require the presence of red blood cells or artificial oxygen carriers, cold perfusion technologies rely on the presence of dissolved oxygen in the perfusate [7, 58]. Accordingly, hypothermic oxygenated perfusion (HOPE) is performed with high oxygen concentrations (>80 kPa) at low temperatures between 8–12 degrees (Fig. 13.1) [11, 49, 58, 59].
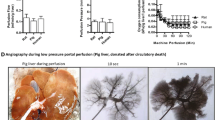
Example of hypothermic oxygenated perfusion (HOPE) of DCD livers prior to transplantation: (a, b): Preparation (a) of human livers and hypothermic oxygenated perfusion (HOPE) (b), performed with UW gluconate (Belzer Machine perfusion solution) at 10 °C with a high oxygen concentration of 80–100 kPa and a perfusion pressure limited to 3 mmHg. (c) Angiograpy confirmed rapid and complete liver imaging through single portal vein perfusion of pig DCD liver example with the HOPE technique. (d) The entire liver including the tip of the extrahepatic bile duct is entirely stained by fluorescein during early HOPE through the portal vein alone. HOPE with fluorescein was performed in discarded human DCD liver (concentration: 0.5 g/5 ml) through the portal vein. Images obtained under dark light confirmed complete perfusion of liver graft by macroscopy and microscopic assessment of portal triad (e)
Of note, as liver architecture always implies sinusoidal fusion of both the portal and the arterial system, perfusion of human or pig livers through the portal system reaches every single liver cell (Fig. 13.1), including the tip of the extrahepatic bile duct and all epithelial cell layers [52]. The benefit of dual perfusion in the cold appears therefore unclear. While a direct clinical comparison of hypothermic single vs. dual perfusion has not been performed yet (HOPE vs. D-HOPE), recent experimental studies showed no difference between single and dual liver perfusion even under subnormothermic conditions in rats [5]. An additional often underestimated but important factor is the much lower perfusion pressure needed during hypothermic liver perfusion to avoid sinusoidal shear stress at low temperatures. Therefore, perfusion flow should be approximately ten-fold reduced during hypothermic liver perfusion compared to normothermic perfusion [49].
Protective Mechanism of Cold Liver Perfusion
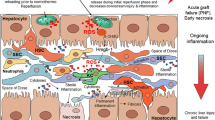
Ischemic cells, regardless of the organ type, experience a rapid loss of nucleotides, and most adenosine triphosphate (ATP)-dependent processes are subsequently on hold [39, 61]. This phenomenon is paralleled by a massive accumulation of nicotine adenine dinucleotide (NADH), citric acid cycle- and purine-metabolites, mainly succinate, hypoxanthine, and xanthine (Fig. 13.2) [14, 40, 60]. Upon normothermic liver reperfusion, accumulated electron donors, such as NADH and succinate, deliver high amounts of electrons to mitochondrial complex I and II, while ADP is not yet available for ATP synthetase, due to previous nucleotide breakdown during ischemia [14, 63]. This results in over-reduction of complex I, either through electron back flow through complex II (reverse electron transfer, RET) or by accumulation of NADH. Both modes lead to a dissociation of reduced flavin mononucleotide (FMNH2) from mitochondrial complex I, with sudden oxidation to FMN and reactive oxygen species (ROS) release [19, 40, 44] (Fig. 13.2). Of note, RET supports the highest rate of ROS generation in mitochondria [15, 29], and complex I has been identified as the main site of ROS production [44, 62]. Any machine perfusion with an oxygenated perfusate after ischemia will therefore induce reperfusion injury to some extent. Such mitochondrial ROS release occurs within the first minutes of reintroduction of oxygen to ischemic tissues and triggers an opening of the mitochondrial membrane pore with further release of mitochondrial DNA together with other DAMPs and multiple cytokines [31, 32, 44, 50]. Accordingly, the release of signaling proteins has been recently confirmed during endischemic normothermic perfusion of several organs, including kidneys, lungs and also livers [4, 22, 25, 26, 30, 51].
Mechanism of liver protection and viability assessment through hypothermic oxygenated perfusion. This chart presents the underlying mechanisms of liver injury during warm and cold ischemia, which subsequently becomes evident at oxygenated reperfusion under normothermic conditions. Initial ROS and FMNH2 release from complex 1 presents the instigators of the entire reperfusion injury cascade with downstream DAMPs and cytokine release with increasing inflammation throughout continuous normothermic reperfusion in vivo after graft implantation or ex vivo on a perfusion device. Endischemic HOPE perfusion has been shown to protect mitochondria from this initial injury and such cold oxygenated perfusion induces a complex 1 repair with subsequent improved function of the respiratory chain, which lead to recharging of ATP at complex V and metabolism of metabolites which accumulate during warm and cold ischemia. When livers become rewarmed at implantation or normothermic perfusion on a device, the injury is significantly less, due to such improvement of mitochondrial function during previous HOPE treatment. Furthermore, the entire metabolism of the liver can be captured by fluorometric analysis of mitochondrial function (NADH) and injury (FMNH2) using the auto-fluorescent properties of such two molecules, representing complex I behavior during reoxygenation in the cold. Importantly, the quantification of FMNH2 and NADH predicts liver function and further outcomes after transplantation and therefore guides surgeons to decide, if a high-risk DCD liver with prolonged warm ischemia is metabolically “good enough” to become utilized or not
In contrast, a newly recognized but decisive option to minimize upfront mitochondrial injury during re-oxygenation is cooling of mitochondria below the Arrhenius breakpoint temperature of 15 °C [1, 2, 17], thereby inducing significant changes in the reactivity of mitochondrial transfer processes, as seen in hibernating animals or plants [18, 33]. Consistently, FMNH2 release and injury of mitochondrial complex I occur less frequently during cold oxygenated reperfusion when compared to normothermic oxygenated reperfusion [4] (Fig. 13.2).
Likewise and surprisingly, mitochondria work more effectively at hypothermic temperatures in uploading cellular ATP, when consuming processes are significantly reduced [4, 8, 51, 70]. A similar central role of attenuating mitochondria-derived oxidative injury has currently also been recognized in other biological fields, such as aging and cancer development [3, 28, 65]. Hypothermic oxygenated perfusion (HOPE) after ischemia protects therefore, first, from significant mitochondrial ROS release and, secondly, provides uploaded cellular energy reserves before implantation [19, 31, 51]. Both effects depend, however, on the number of accumulating metabolites during ischemia, which in principle may also lead to an oxidative injury during HOPE. Of note, the changes in mitochondrial metabolism during HOPE are detectable by perfusate analysis during cold perfusion, which will likewise be available as viability parameters in the future (see paragraph on viability assessment [31, 58]).
The clinical effect of the hypothermic perfusion approach has been demonstrated in recent observational studies in Maastricht III DCD livers [48, 55]. Accordingly, despite extended donor warm ischemia, HOPE-treated DCD liver transplants achieved similar overall graft survival, compared to standard DBD liver transplants. Particularly, graft loss due to non-tumor-related causes occurred in 8% (4/50) of cases. In contrast, one-third of untreated DCD livers (16/50) were lost due to non-tumor-related graft failure, despite significantly shorter functional donor warm ischemia time (p < 0.0001) [55]. Five-year graft survival, censored for tumor death, was 94% for HOPE-treated DCD liver transplants vs. 78% in untreated DCD liver transplants (p = 0.024). Similar results were recently presented by a group from Milan, where Maastricht II and III DCD livers are routinely transplanted with a combination of NRP, cold storage, and endischemic HOPE treatment [10,11,12,13] (Fig. 13.3). These results have been achieved despite the use of extended DCD liver grafts and are strikingly different from recent outcomes after endischemic normothermic perfusion of human livers [68, 69]. The findings by the Italian groups suggest that a simple endischemic perfusion approach is very effective and may open the field for safe utilization of extended DCD liver grafts. Recent clinical studies on hypothermic liver perfusion are summarized in Fig. 13.3. Results of most randomized controlled trials in DBD and DCD livers are awaited.
Overview of clinical studies with hypothermic oxygenated perfusion with implantation. HOPE Hypothermic oxygenated perfusion, ATP Adenosine triphosphate, ROS Reactive oxygen species, 8-OHdG Hydroxydesoxyguanosin, DAMPs Danger-associated molecular pattern, HMGB-1 High-mobility-group-box-protein-1, HSC Hepatic stellate cells, SEC Sinusoidal endothelia cells, KC Kupffer cells, PNF Primary non-function, RET Retrograde electron transport CI Complex I, ∗ same series 50 DCD include the earlier reports of 25 and 8, § continue to recruit
Which Livers Benefit from Cold Machine Perfusion?
Current benchmark analysis suggests that ideal liver transplants, defined as primary low risk DBD transplants, show excellent outcome by conventional cold storage [42]. This has also been confirmed for low-risk DCD liver transplants, defined by the recent UK DCD risk score [54]. Importantly, the former criteria for extended criteria donors, based on donor age > 65 years, hepatitis C core antigen positivity, donor BMI > 30 kg/m2, elevated sodium >165 mmol/l, ICU stay >7 days and hepatic steatosis >40% [20], require an urgent refinement, because such grafts are frequently considered by many transplant programs today [34, 58]. For example, several reports have demonstrated safe utilization of DBD livers with advanced donor age, elevated sodium, prolonged ICU stay, high donor BMI, or elevated liver enzymes [53, 27]. Graft optimization by any sort of machine perfusion is therefore likely to be reserved for marginal DBD and extended DCD livers, with, for example, advanced donor age and expected severely prolonged cold ischemia (more than 12 h for DBD, or more than 6 h for DCD), or increased donor warm ischemia (more than 30 minutes functional warm ischemia), or for livers with significant macrosteatotic livers (more than 30%), in contrast to only microsteatotic livers [11, 31, 46] (Fig. 13.4).
Clinical application of hypothermic oxygenated perfusion in liver transplantation. This chart represents current clinical application of the HOPE technique in liver transplantation. Hypothermic perfusion is used to improve high-risk DCD livers or steatotic grafts. Additionally, this technique is routinely applied to use such high-risk livers for sick recipients to also improve safety and to confirm liver function before transplantation of high MELD recipients, even when ventilated on intensive care unit before transplantation. Finally, the HOPE approach is of great importance to bridge potential prolonged cold ischemia times when recipients are suddenly unfit for transplantation or when logistical issues, including an exchange of recipient, are required. In Switzerland, the HOPE approach is also used to treat DCD liver grafts and confirm viability with the fluorometric analysis, prior to liver transport to other transplant centers
Viability Assessment During Hypothermic Liver Perfusion
Measuring graft function before clinical use has been a dream of many transplant surgeons. Normothermic physiologic liver or kidney perfusion appears logical to determine visible signs of liver or kidney function. Yet, the current set of parameters used for the determination of viability during ex vivo normothermic liver perfusion failed to predict function or irreversible injury [38, 66, 67, 69]. For example, lactate clearance, bile production, and liver enzyme release were identified to be weak predictors. In addition, bile glucose and pH have been suggested to be more informative for post-transplant biliary injury; however, validation of this data set remains awaited [35].
While normothermic perfusion appears advantageous for measuring organ function ex vivo, recent work has shown that the metabolic status of organs can also be easily monitored during hypothermic oxygenated perfusion. Especially mitochondrial injury and function can be assessed by measuring perfusate Flavin, released from complex I (flavin mononucleotide, FMN) [62]. Current data suggest, accordingly, that perfusate analysis during hypothermic oxygenated perfusion is predictive for later graft function (Fig. 13.4) [43]. These results are in clear contrast with the low predictive value of conventional perfusate parameters, including liver transaminases or perfusate lactate levels, which repeatedly failed to recognize impaired liver function after implantation [66, 67]. Instead of solely focusing on the release of cytosolic compounds, future perfusate analysis should target on real-time monitoring of mitochondrial metabolism to enable an accurate prediction of oxidative stress and downstream activation of the hepatic inflammasome upon transplantation [47, 50]. The combination of several key mitochondrial metabolites including FMN, NADH, succinate, and purine metabolites, e.g., inosine monophosphate, xanthine and hypoxanthine, may allow future detailed assessment of mitochondrial function of any solid organ.
Ideal Hypothermic Machine Perfusion Design
An underestimated hurdle for the widespread use of machine perfusion techniques is the complicated design and application. All liver machines suffer from the need for extra man power to connect livers to the device and the need for extra support during perfusion. Even an easy perfusion approach, as, for example, single portal vein perfusion requires repeated calibration of perfusion pressure, temperature, and flow control. Device alarming leads frequently to full perfusion stop, requiring reset and additional calibration with subsequent repeat liver connection. Although transport of livers on machines has been reported, most centers try to avoid continuous perfusion from donor center to transplant center with device transport, due to the additional need of travelling perfusion experts. From our point of view, instead, perfusion at recipient centers has clear advantages and should be performed by small, automatic devices, fully blue tooth connectable to, for example, smart phones or tablets, with full screen information of perfusion pressures, flow, oxygenation, temperature, and mitochondrial metabolism. Calibration should be as easy as possible with automatic perfusion start and stop. All perfusion machines should work with minimal heat or noise effects, and the liver basin should be either designed to cope with all possible liver sizes or the device should be connected to a simple metal liver bench bowl, routinely in use. Disposables should be kept as cheap as possible, e.g., less than approximately 1500 € per perfusion. We may envision that liver perfusion machine design will substantially improve and adapt according to the clinical need in the next years. The hypothermic LifePort Liver Transporter machine by Organ Recover Systems can be seen in Figure 13.5a. The VitaSmart hypothermic oxygenated machine perfusion platform by Bridge to Life can be seen in Figure 13.5b. Such two devices are currently avaible to provide hypothermic oxygenated perfusion only.
Summary
Hypothermic liver perfusion (HOPE) achieves excellent clinical outcome in extended DCD liver transplantations, despite an endischemic application, e.g., perfusion after organ procurement and organ transport. This technique is currently the cheapest and easiest perfusion concept, requiring no transport of perfusion equipment to donor locations, and only short perfusion periods through the portal vein. Recent experimental studies have unravelled the protective mechanism of cold re-oxygenation of ischemic liver tissues and have confirmed a novel and unique mitochondrial response compared to any form of re-oxygenation under normothermic conditions. Based on these results, the assessment of mitochondrial function and injury is possible during the initial first 30 minutes of HOPE and allows recognition of later graft function already before implantation. This will likewise have an effect on the future safe utilization of extended DCD and steatotic liver grafts.
References
Abele D, et al. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya Mya arenaria. J Exp Biol. 2002;11:1636. https://doi.org/10.1016/j.ecolind.2011.04.007.
Abele D. Toxic oxygen: the radical life-giver. Nature. 2002;420:27. https://doi.org/10.1038/420027a.
Blier PU, et al. What modulates animal longevity? Fast and slow aging in bivalves as a model for the study of lifespan. Semin Cell Dev Biol. 2017;70:130. https://doi.org/10.1016/j.semcdb.2017.07.046.
Boteon Y, et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl. 2018;24:1699–715. https://doi.org/10.1002/lt.25315.
Brüggenwirth IMA, et al. A comparative study of single and dual perfusion during end-ischemic subnormothermic liver machine preservation. Transplant Direct. 2018;4:e400. https://doi.org/10.1097/TXD.0000000000000840.
Bruinsma BG, et al. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int J Artif Organs. 2013;36(11):775–80. https://doi.org/10.5301/ijao.5000250.
Bruinsma BG, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2014;14(6):1400–9. https://doi.org/10.1111/ajt.12727.
Burlage L, et al. Opposite acute potassium and sodium shifts during transplantation of hypothermic machine perfused donor livers. Am J Transplant. 2018;19:1061. https://doi.org/10.1111/ajt.15173.
Croome KP, Lee DD, Keaveny AP, Taner CB. Noneligible donors as a strategy to decrease the organ shortage. Am J Transplant. 2017;17(6):1649–55.
De Carlis L, et al. Sequential use of normothermic regional perfusion and hypothermic machine perfusion in donation after cardiac death liver transplantation with extended warm ischemia time. Transplantation. 2016;100:e101. https://doi.org/10.1097/TP.0000000000001419.
De Carlis R, et al. Hypothermic machine perfusion of liver grafts can safely extend cold ischemia for up to 20 hours in cases of necessity. Transplantation. 2017;101:e223–4. https://doi.org/10.1097/TP.0000000000001753.
De Carlis R, et al. Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl. 2017;23:166. https://doi.org/10.1002/lt.24666.
De Carlis R, et al. Donation after cardiac death liver transplantation with normothermic regional perfusion and hypothermic machine perfusion: follow-up of the first Italian series. Portugal: ILTS Conference Lisbon; 2018.
Chouchani ET, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(V):431–5. https://doi.org/10.1038/nature13909.
Chouchani ET, et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabolism. 2016;23:254–63. https://doi.org/10.1016/j.cmet.2015.12.009.
Detelich D, Markmann JF. The dawn of liver perfusion machines. Curr Opin Organ Transplant. 2018;23:151. https://doi.org/10.1097/MOT.0000000000000500.
Dufour S, et al. Top-down control analysis of temperature effect on oxidative phosphorylation. Biochem J. 1996;314:743. https://doi.org/10.1042/bj3140743.
Dugbartey GJ, et al. Renal mitochondrial response to low temperature in non-hibernating and hibernating species. Antioxid Redox Signal. 2017;27:599. https://doi.org/10.1089/ars.2016.6705.
Dutkowski P, Clavien P. Uploading cellular batteries: caring for mitochondria is key. Liver Transpl. 2018;24(4):462–4. https://doi.org/10.1002/lt.25036.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–85.
Gringeri E, et al. Subnormothermic machine perfusion for non-heart-beating donor liver grafts preservation in a swine model: a new strategy to increase the donor pool? Transplant Proc. 2012;4:2026–8. https://doi.org/10.1016/j.transproceed.2012.06.014.
Hashimoto K, et al. Soluble adhesion molecules during ex vivo lung perfusion are associated with posttransplant primary graft dysfunction. Am J Transplant. 2017;17(5):1396–404. https://doi.org/10.1111/ajt.14160.
He X, et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transplant. 2018;18(3):737–44. https://doi.org/10.1111/ajt.14583.
Hessheimer A, et al. Superior outcomes using normothermic regional perfusion in cDCD liver transplantation. Portugal: ILTS Conference Lisbon; 2018.
Hosgood SA, et al. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J Transl Med. 2017;15(1):216. https://doi.org/10.1186/s12967-017-1314-5.
Iskender I, et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion [Abstract]. J Heart Lung Transplant. 2016;35(4):S142–3. https://www.jhltonline.org/article/S1053-2498(16)00441-1/fulltext.
Kaltenbach M, et al. Trends in deceased donor liver enzymes prior to transplant: the impact on graft selection and outcomes. Am J Transplant. 2019;20:213. https://doi.org/10.1111/ajt.15573.
Karki R, Man SM, Kanneganti T-D. Inflammasomes and cancer. Cancer Immunol Res. 2017;5:94. https://doi.org/10.1158/2326-6066.CIR-16-0269.
Kim M, et al. Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic Biol Med. 2018;124:517. https://doi.org/10.1016/j.freeradbiomed.2018.06.040.
Kron P, et al. Short, cool, and well oxygenated – HOPE for kidney transplantation in a rodent model. Ann Surg. 2016;264:815. https://doi.org/10.1097/SLA.0000000000001766.
Kron P, et al. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol. 2018;68(1):82–91. https://doi.org/10.1016/j.jhep.2017.08.028.
Land WG. Emerging role of innate immunity in organ transplantation. Part I: evolution of innate immunity and oxidative allograft injury. Transplant Rev. 2012;26:60–72. https://doi.org/10.1016/j.trre.2011.05.001.
MacDonald JA, Storey KB. cAMP-dependent protein kinase from brown adipose tissue: temperature effects on kinetic properties and enzyme role in hibernating ground squirrels. J Comp Physiol. 1998;168:513. https://doi.org/10.1007/s003600050172.
Marcon F, et al. Utilisation of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002127.
Matton APM, et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2019;103:1405. https://doi.org/10.1097/TP.0000000000002500.
De Meijer V, Fujiyoshi M, Porte R. Ex situ machine perfusion strategies in liver transplantation. J Hepatol. 2018;70:203. https://doi.org/10.1016/j.jhep.2018.09.019.
Mergental H, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–45. https://doi.org/10.1111/ajt.13875.
Mergental H. et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion: the VITTAL (VIability Testing and Transplantation of mArginal Livers) trial outcomes’, AASLD 2018, Abstract. 2018.
Michel SG, et al. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann Transplant. 2015;20:461–8. https://doi.org/10.12659/AOT.893784.
Mills EL, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–470.e13. https://doi.org/10.1016/j.cell.2016.08.064.
Minor T, et al. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13(6):1450–60. https://doi.org/10.1111/ajt.12235.
Muller X, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2017;267(3):419–25. https://doi.org/10.1097/SLA.0000000000002477.
Muller X, et al. Novel real time prediction of liver graft function during hypothermic oxygenated machine perfusion prior to liver transplantation. Ann Surg. 2019;270(5):783–90.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. https://doi.org/10.1042/BJ20081386.
Nasralla D, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50. https://doi.org/10.1038/s41586-018-0047-9.
Patrono D, et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci Rep. 2019;9(1):1. https://doi.org/10.1038/s41598-019-45843-3.
Pinto C, et al. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim Biophys Acta Mol basis Dis. 2017;1864:1270. https://doi.org/10.1016/j.bbadis.2017.07.024.
Van Rijn R, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907–17. https://doi.org/10.1002/bjs.10515.
Schlegel A, et al. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278–86.
Schlegel A, et al. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. Elsevier. 2014;61(6):1267–75.
Schlegel A, et al. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61(6):1267–75. https://doi.org/10.1016/j.jhep.2014.07.023.
Schlegel A, et al. Is single portal vein approach sufficient for hypothermic machine perfusion of DCD liver grafts? J Hepatol. Elsevier. 2016;64:239–41.
Schlegel A, et al. Impact of donor age in donation after cardiac death liver transplantation: Is the cut-off “60” still of relevance? Liver Transplantation. 2017; https://doi.org/10.1002/lt.24865. [Epub ahead of print].
Schlegel A, et al. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018c;68(3):456–64. https://doi.org/10.1016/j.jhep.2017.10.034.
Schlegel AA, et al. Outcomes of liver transplantations from donation after circulatory death (DCD) treated by hypothermic oxygenated perfusion (HOPE) before implantation. J Hepatol. 2019;70(1):50–7. https://doi.org/10.1016/j.jhep.2018.10.005.
Schlegel A, Kalisvaart M, Muiesan P. Machine perfusion in liver transplantation - an essential treatment or just an expensive toy? Minerva Anestesiol. 2018;84(2):236–45. https://doi.org/10.23736/S0375-9393.17.12016-X.
Schlegel A, Muller X, Dutkowski P. Hypothermic liver perfusion. Curr Opin Organ Transplant. 2017;22:563.
Schlegel A, Muller X, Dutkowski P. Hypothermic machine preservation of the liver: state of the art. Current Transpl Rep. 2018;5:93. https://doi.org/10.1007/s40472-018-0183-z.
Selten J, et al. Hypo- and normothermic perfusion of the liver: which way to go? Best Pract Res Clin Gastroenterol. 2017;31:171–9. https://doi.org/10.1016/j.bpg.2017.04.001.
Siebels I, Dröse S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta Bioenerg. 2013;1827(10):1156–64. https://doi.org/10.1016/j.bbabio.2013.06.005.
Stegemann J, Minor T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology. 2009;58(3):331–6. https://doi.org/10.1016/j.cryobiol.2009.03.003.
Stepanova A, et al. Redox-Dependent Loss of Flavin by Mitochondrial Complex I in Brain Ischemia/Reperfusion Injury. Antioxid Redox Signal. 2019;31(9):608–22. https://doi.org/10.1089/ars.2018.7693. Epub 2019 Jul 1.
Takakuwa Y, et al. Properties and kinetics of membrane-bound enzymes when both the enzyme and substrate are components of the same microsomal membrane. Studies on lathosterol 5-desaturase. J Biol Chem. 1994;269(45):27889–93. https://doi.org/10.1074/jbc.270.8.4180.
de Vries Y, et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant. 2019;19:1202. https://doi.org/10.1111/ajt.15228.
Wang CH, et al. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med. 2013;238:450. https://doi.org/10.1177/1535370213493069.
Watson C, et al. Normothermic regional perfusion (NRP) for DCD liver transplantation in the UK: better graft survival with no cholangiopathy. Portugal: ILTS Conference Lisbon; 2018.
Watson CJE, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005. https://doi.org/10.1111/ajt.14687.
Watson CJE, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: Hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation. 2017;101:1084. https://doi.org/10.1097/TP.0000000000001661.
Watson CJE, Jochmans I. From “gut feeling” to objectivity: machine preservation of the liver as a tool to assess organ viability. Curr Transpl Rep. 2018;5:72. https://doi.org/10.1007/s40472-018-0178-9.
Westerkamp A, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825–35. https://doi.org/10.1097/TP.0000000000001081.
Westerkamp AC, et al. End-ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transpl. 2015;21(10):1300–11. https://doi.org/10.1002/lt.24200.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schlegel, A., Mueller, M., Dutkowski, P. (2020). Hypothermic Machine Perfusion in Liver Transplantation Using Grafts From Donation After Circulatory Death Donors. In: Croome, K., Muiesan, P., Taner, C. (eds) Donation after Circulatory Death (DCD) Liver Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-030-46470-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-46470-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46469-1
Online ISBN: 978-3-030-46470-7
eBook Packages: MedicineMedicine (R0)