Abstract
The majority of the heavy metals are considered toxic to the human beings by interfering with the normal functions that are taking place in the human body by disrupting metabolic processes and their exposure may be due to natural or anthropogenic sources. Heavy metals act as endocrine-disrupting chemicals (EDCs) by disrupting the mechanism of action of endogenous substances. Heavy metals such as cadmium and arsenic have a negative impact on some enzymes that are involved in carbohydrates and lipids metabolism and lead to an abnormal level of glucose and lipid, cholesterol, and triglycerides. This is responsible for inducing the pathogenesis associated with diabetes mellitus and insulin resistance. These metals are also responsible to induce reactive oxygen species and suppress antioxidant defense mechanism. The stress-induced by oxidation is highly linked with metabolic syndrome. These conditions lead to a risk of diabetes-associated cardiovascular diseases. While on the other side, some of the heavy metals notably zinc which is considered as an essential nutrient, play its significant role in metabolic disorders by suppressing oxidant effect, reducing obesity and lipogenesis. In this chapter, we have briefly overviewed the role of heavy metals that act as EDCs in metabolic disorder via interfering various transcriptional and metabolic pathways while the other heavy metals which have a beneficial role in the amelioration of metabolic disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
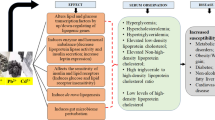
Endocrine-disrupting chemicals (EDCs) can be defined as exogenous substances or mixtures of substances that interact in any way with endogenous hormonal signaling, not only affecting the production, secretion, and transportation of hormones but also has an effect on their cellular metabolism, binding action and elimination [1]. EDCs play a crucial role in obesity, diabetes, and cardiovascular system. In case when EDCs act as obesogens, they show their effect on adipocyte tissues and also on the brain for induction of obesity which causes glucose intolerance, insulin resistance, dyslipidemia and enhanced expression susceptibility towards cardiovascular disorders and type 2 diabetes mellitus. In case when EDCs act as diabetogenic, they have a direct effect on the islet of Langerhans due to which insulin synthesis and release may be increased or decreased, as a result, may be hyperglycemia or hypoglycemia occur. Due to the impairment of insulin signaling and insulin resistance, the metabolic syndrome occurs. Some EDCs also have a direct effect on the heart and cause cardiovascular diseases [1]. EDCs are basically chemical substances which exhibit agonist and antagonist effect on the endocrine system. Many heavy metals such as lead, cadmium, mercury, nickel, and arsenic have endocrine-disrupting activities [2].
Heavy metals are considered as a broad class of metals and metalloids having a relatively high density and are very toxic in nature even at parts per billion levels. Examples include lead, arsenic, mercury, cadmium, zinc, silver, copper, iron, chromium, nickel, palladium, and platinum. Both natural and anthropogenic sources such as mining, automobiles exhaust, and industrial discharge are major sources of releasing these metals into the environment [3]. Heavy metals in the form of oxides and sulfides ore naturally occur in the earth’s crust and rocks. Heavy metals can be halted out in the form of minerals from many different types of ores such as sulfides of cobalt, lead, cadmium, mercury, iron, and arsenic. The leaching of heavy metals may occur due to naturally occurring mechanisms such as weathering of rocks, mining, and volcanic eruptions processes. Leaching of heavy metals into oceans, rivers, and lakes can cause pollution and affects its surrounding environment by acidic rain [4]. Heavy metals can also cause environmental pollution due to metal corrosion, deposition of metals in the atmosphere, leaching of heavy metals, soil erosion of heavy metals and metallic ions, re-suspension of sediments, and evaporation of metals from water reservoirs to soil and groundwater [5]. These heavy metals can accumulate in the human body by different processes and cause harmful effects on them. These heavy metals have the ability to bind with macromolecules and alter their normal cellular functions. It may lead to many adverse effects on the health of humans by affecting their central nervous system, lungs, liver, and kidney [6]. In the proceeding sections, we have briefly summarized the role of most important heaving metals that act as EDCs in metabolic disorders.
Cadmium
Sources and Exposure
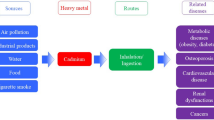
Cadmium is a heavy metal that naturally occurs in ores. It generally acts as a stabilizer in many products such as polyvinyl chloride containing products, many alloys, and color pigments. Phosphate fertilizers are also a major source of cadmium exposure [7]. The activities in the environment such as the burning of fossil fuels, electroplating phenomena, usage and production of pigments and batteries containing alkaline nickel-cadmium and welding have more contribution to acting as a source of cadmium as compared to naturally occurring process in the environment. The naturally occurring processes such as volcanic activity, forest fires, erosion of soil, and weathering of rocks are major sources for releasing cadmium into the environment [8]. The human being may be exposed to cadmium in several ways (Fig. 13.1). Mining and smelting of non-ferrous metals and synthesis of compounds containing cadmium are known as occupational sources of cadmium exposure. Non-occupational sources of cadmium exposure may include smoking, diet, and house dust [9].
Role of Cadmium on Carbohydrates Metabolism
Effect of Cadmium Glycolysis
Glycolysis is a process of conversion of a six-carbon compound into three-carbon compounds. This process occurs in the cytoplasm [10]. Glucose is converted into glucose-6-phosphate (G-6-P) by the mechanism of phosphorylation with the help of an enzyme glucokinase [11]. The G-6-P is converted into fructose-6-phosphate (F-6-P) by an enzyme phosphohexose isomerase. F-6-P is phosphorylated by an enzyme phosphofructokinase to form fructose 1, 6-bisphosphate. Fructose 1, 6-bisphosphate is broken down to glyceraldehyde-3-phosphate (G-3-P) and dihydroxyacetone phosphate (DHAP), which is catalyzed by an enzyme aldolase. Then fructose 1,6-bisphosphate passes by several series of chemical reactions and conversion of phosphoenolpyruvate into pyruvate by utilizing an enzyme pyruvate kinase [10].
It has been demonstrated [12] that the glycolysis process can be limited by cadmium exposure because it has great potential to decrease the level of phosphofructokinase that is involved in the glycolysis process as shown in Fig. 13.1. The studies also show that cadmium exposure alters the chemical composition of muscles and liver [13]. Cadmium is also responsible to increase the activity of some enzymes that are responsible for many catabolic processes such as glutamate dehydrogenase, amino acid oxidase, and xanthine oxidase [14]. Several studies show that cadmium has an adverse effect on metabolic enzymes [15] and antioxidants [16, 17] and also on metallothionein expression [18, 19].
Cadmium has great potential to inhibit the hexokinase and phosphofructokinase by a mechanism in which cadmium has a great affinity towards a pair of free electrons present in the cysteine—SH group. Hexokinase and phosphofructokinase structure show that it has a great number of cysteine residues [20]. Studies revealed that by increasing the cadmium concentration, glycolysis can be inhibited as discussed earlier. The same case is observed for pyruvate kinase enzymes that are involved in glycolysis [21].
Effect of Cadmium Pentose Phosphate Pathway
Pentose phosphate pathway consists of two steps, the first step is the oxidative production of NADPH and the second step is non-oxidative inter-conversion of sugar [22]. Pentose phosphate pathway is a biochemical process that is parallel to glycolysis and it is a vital source for the production of NADPH [23]. In the process of glycolysis, glucose-6-phosphate is produced on which glucose-6-phosphate dehydrogenase converts it into 6-phosphogluconate. In this process, NADPH is produced which is used to maintain the level of glutathione in a reduced form whose function to kill the oxidative metabolites that are dangerous [24]. Cadmium has great potential to decrease the level of glutathione by inhibiting the activity of glucose-6-phosphate dehydrogenase (Fig. 13.2). The decreased activity of glucose-6-phosphate dehydrogenase may cause the induction of diabetes mellitus due to the oxidative stress-induced by oxidation [25]. The studies also show that the production of glucose-6-phosphate is decreased due to inhibition of hexokinase, an enzyme involved in glycolysis, and pentose phosphate pathway suppressed due to reduced level of glucose-6-phosphate [26].
Schematic representation of the mechanism of cadmium and arsenic interfering with enzymes that are involved in carbohydrates metabolism. Adopted from [12]. GP glycogen phosphorylase, GS glycogen synthetase, UDP uridine diphosphate glucose, PGM phosphoglucomutase, HK hexokinase, G6Pase glucose-6-phosphatase, PFK phosphofructokinase, FBase fructose-1,6-bisphosphatase, PK pyruvate kinase, PC pyruvate carboxylase, PEPC phosphoenolpyruvate carboxykinase, G6PDH glucose-6-phosphate dehydrogenase, NADP nicotinamide adenine dinucleotide phosphate, GSH glutathione
Effect of Cadmium Glycogenolysis
In the process of glycogenolysis, glycogen is phosphorylated by glycogen phosphorylase and as a result, glucose-1-phosphate is produced. Glucose-1-phosphate is converted into glucose-6-phosphate and this reaction is catalyzed by an enzyme phosphoglucomutase [27]. The experimental study reveals that the storage ability of glycogen in animals is decreased due to the exposure of heavy metals such as cadmium [28]. The phenomenon of glycogenolysis occurs due to a higher level of glycogen phosphorylase and its activity. The glycogen level may be reduced due to decreased activity of glycogen transferase. The decrease in the production of glucose-6-phosphate which is a very essential substance for glycogen synthesis may occur due to reducing the glucokinase activity [29].
If cadmium is exposed to placenta, then increased glycogen phosphorylase activity is observed [30]. Exposure of cadmium may increase the level of cortisol in plasma which may contribute to the activation of glycogenolysis [31]. Cadmium has the ability to accumulate in the pancreas and increases oxidative stress [32]. Cadmium is divalent which has the ability to interact with thiol group and zinc-binding site, which is generally present in proteins [33]. It has been revealed that chronic exposure of cadmium has a positive effect on the activity of serum amylase [34].
Effect of Cadmium Gluconeogenesis
In gluconeogenesis, pyruvate is formed from amino acids and lactate then transported into mitochondria from the cytosol. In mitochondria, pyruvate is converted into oxaloacetate with the help of an enzyme pyruvate carboxylase. Phosphoenolpyruvate carboxykinase acts on oxaloacetate and converts it into phosphoenolpyruvate. Then phosphoenolpyruvate is passed through a series of reactions and converted into fructose-6-phosphate. Then it is converted into glucose-6-phosphate by an enzyme phosphohexose isomerase that acts on fructose-6-phosphate. Finally, glucose-6-phosphate is converted into glucose after releasing the phosphate group [35].
Only a high dose of cadmium has an effect on enzymes that are involved in gluconeogenesis such as glucose-6-phosphatase, phosphoenolpyruvate carboxykinase, and fructose 1, 6-bisphosphatase [36]. It has been proposed that phosphoenolpyruvate carboxykinase is very important to target to treat diabetes mellitus associated with hyperglycemia [12].
Effect of Cadmium on Lipid Metabolism
The exposure of cadmium has a great potential on acetyl CoA carboxylase and fatty acid synthase that is involved in the synthesis of fatty acids [37]. Cadmium induces the lipid peroxidation of polyunsaturated fatty acids [38]. Chronic exposure to cadmium causes impairment in the storage and metabolism of lipids. Higher mobilization of lipids to mitochondria cause lower lipid content and decrease the level of triglycerides and ATP [39]. Further exposure to cadmium decreases the level of NADPH. Cadmium has a negative impact on digestion, transportation, synthesis of fatty acids, and even on the metabolism of fatty acids [38].
Arsenic
Sources and Exposure
Arsenic is naturally present in the earth’s crust and it is a very toxic element in an inorganic form that is present in air, land, and water. Exposure of arsenic to human beings (Fig. 13.3) is mostly through drinking contaminated water, using this contaminated water in the preparation of food and processing in the industries [40]. Arsenic contents are also found in the smoke of a cigarette. It is proved that by smoking a single cigarette, about 0.25 μg inhalation of arsenic occurs. Arsenic can be exposed to humans by the skin when there is the frequent use of cosmetic products [41].
Effect Arsenic on Carbohydrates Metabolism
Arsenic has the potential to inhibit the activity of hexokinase [42] production of ATP by substituting the phosphate group of ATP with arsenate, this process is known as arsenolysis [43]. The ability of arsenic to replace the phosphate group is due to its structural similarity with the phosphate group [44]. This process may occur during oxidative phosphorylation and as a result, adenosine diphosphate arsenate is produced (Fig. 13.4). Arsenic can react with glucose to form glucose-6-arsenate. Similarly, when arsenic reacts with gluconate it can produce 6-arsenogluconate [45]. Trivalent arsenicals can inhibit many enzymes that participate in the metabolism of carbohydrates such as α-ketoglutarate dehydrogenase, pyruvate dehydrogenase, and succinyl COA synthase. One of these following enzymes, pyruvate dehydrogenase is highly sensitive to arsenic toxicity. This causes a reduction in the production of ATP [12].
Role of arsenic as an endocrine disruptor. Arsenic binds with a disulfide bridge present in insulin and insulin receptors and makes them non-functional. Arsenic impairs the translocation of GLUT by decreasing the phosphorylation of Akt. Arsenic competes with Pi and binds with ADP in order to make ADP-arsenate that is subsequently used in the formation of glucose-6-arsenate. Arsenic causes the elevation of oxidative stress level through which it increases the expression of pro-inflammatory cytokines (TNF-α and IL-6) and decreases the expression of PPAR-γ and these two factors play a key role in the development of insulin resistance. Adopted from [12]. As arsenic, ROS reactive oxygen species, NF-kB nuclear factor kappa B, PPAR-γ Peroxisome proliferator-activated receptor-gamma, TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, PIP2 Phosphatidylinositol 4,5-bisphosphate, PIP3 phosphatidylinositol (3,4,5)-trisphosphate, IRS insulin receptor substrate, PI3k phosphoinositide 3-kinase, GLUT glucose transporter, Pi inorganic phosphorus, ADP adenosine diphosphate, ATP adenosine triphosphate
Arsenic-Induced Diabetes Mellitus
The subjects that are exposed to arsenic show similar symptoms as a patient suffering from type 2 diabetes mellitus [46].This is due to the same pathophysiology of T2DM which is induced by arsenic toxicity (Fig. 13.4). The possible pathway through which arsenic can induce diabetes mellitus has been well determined [47].
Arsenic can induce oxidative stress by suppressing the antioxidant enzyme [48]. Arsenic can induce adverse effects on health by increasing the production of reactive oxygen species [49]. The chief house for the production of reactive oxygen species is mitochondria which may be due to the alteration in electron transfer through respiratory chain that enhances the production of hydrogen peroxide, hydroxyl radicals and superoxide anion [50].
Sulfhydryl group present in glucose transporter at the outer surface of the plasma membrane can form a bond with a polypeptide chain of insulin [51]. This sulfhydryl group has an essential role in the transportation of glucose either insulin-dependent or insulin-independent [52]. Arsenic has a high affinity for enzyme-containing thiols group and inhibits the binding of a substrate with the active site of an enzyme (Fig. 13.4). Trivalent arsenate reacts with molecules containing sulfhydryl group such as glutathione and cysteine [53]. Arsenic can induce alteration in the expression of genes that causes diabetes. Due to arsenic, the expression of mRNA and secretion of insulin is decreased [54]. The most important gene for a transcription factor peroxisome proliferative-activated receptor-γ control expression of a gene for the sensitivity of insulin. Arsenic has the ability to alter the expression of this gene and as a result synthesis of mRNA is inhibited and adipocytes differentiation is reversed [55].
Lead
Sources and Exposure
Lead is a toxic metal, which is widely used and responsible for contamination in the environment and many health-related problems. The common sources of lead are found in the environment and mainly in food and smoking, drinking water, industrial process, and domestic sources [56]. The common route of exposure to lead is inhalation and ingestion. Inhalation of lead particles may be due to the burning of lead-containing materials. The lead may produce during the process of smelting, deterioration of lead-coated paint and by using gasoline loaded with lead. The ingestion of lead may take place due to lead-contaminated soil, water, and food [47]. Another important pathway of lead intake is gastrointestinal absorption and retention but it depends upon the chemical environment of gastrointestinal lumen and iron stored in GIT [57].
Lead-Induced Oxidative Stress
Lead induces oxidative stress by interfering with many biochemical processes (Fig. 13.5). Lead has the ability to mimic or inhibit the calcium action by interacting with proteins. The biological molecules that are bound with lead, have not the ability to perform a number of biochemical processes. Lead has the ability to bind with sulfhydryl group and amide group present in enzymes, as a result, alter the configuration of enzymes and diminish the activity of enzymes. Lead also interferes with the transport of some cations by exhibiting the competition with other metallic cations for binding with the active site of enzymes [5]. This oxidative damage of membrane induced by lead is due to a change in fatty acids composition present in the membrane [59].
Schematic representation of the mechanism of toxic effects of lead. Lead exposure causes anemia because of interference with heme-synthesis. δ-aminolevulinic acid dehydratase (δ-ALAD) enzyme is inhibited resulting in increased δ-aminolevulinic acid (δ-ALA) levels which can cause oxidative stress and may result in the production of genotoxic effects. Adopted from [58]
The lipid peroxidation induces oxidative stress and the production of reactive oxygen species in the lipid membrane. These free radicals abstract the electron from lipids that are present in the membrane and cause oxidative damage to the cell membrane. The free radicals are also responsible for the oxidation of hemoglobin and the destruction of red blood cells. The oxidation of lipids and hemoglobin results due to inhibition of δ-aminolevulinate dehydratase(ALAD), the substrate level of δ-aminolaevulinic acid (ALA) is increased in the blood and urine. The generation of superoxide and hydrogen peroxide results due to the elevated level of ALA. These oxides and peroxides react with oxyhemoglobins and hydroxyl radicals generated [60]. Lead has the ability to form a covalent bond with sulfhydryl group of antioxidant enzymes such as glutathione (GSH) and causes inactivation of this enzyme. The level of GSH is decreased which is not compensated by a γ-glutamyl cycle that is also responsible for the synthesis of GSH from cysteine [61]. Lead has the ability to bind with an enzyme ALAD, glutathione peroxidase (GPX), glutathione reductase, and glutathione-S-transferase, causes inactivation of these enzymes, as a result, depresses the level of GSH [62]. Due to exposure of lead, alteration in gene expression occurs. The mechanism that is involved in alteration of gene expression is binding of lead with DNA associated protein, protamine, by interaction with zinc-binding site [63].
Lead has the ability to interact with enzymes that catalyze the synthesis of vitamin D and involve in the maintenance of the cell membrane. Lead disintegrates the cell membrane and RBCs with a membrane that has no integrity become fragile and results in anemia [64]. Lead also affects glucose-6-phosphate dehydrogenase, the enzyme responsible for catalyzing the initial step in the pentose phosphate pathway. Lead enhances the level of this enzyme in RBCs in human beings [58].
Lead-Induced Inflammatory Responses
Lead exhibits a negative impact on the immune system which is an important key element for the process of inflammation and plays a defensive role in injury within a living organism [65]. Lead exposure does not cause complete deficiencies of immune cells but it has a negative impact on the regulation of the immune system [66]. COX-2, an enzyme that is responsible for catalyzing the formation of prostaglandins H2 from arachidonic acid. The resultant prostaglandins H2 are involved in a unique series of enzymatic and non-enzymatic reactions for the formation of primary prostanoids; PGE2, PGF2α, PGI2, PGD2, and TXA2, and also the generation of reactive oxygen species [67]. Lead has the ability to influence the COX-2 gene by alteration in a nuclear factor of activated T-cells(NFAT) which is a transcription factor. Lead causes mutation in the NFAT binding site which is responsible for the eradication of COX-2 gene transcription [68]. IL-8 which has the ability to exhibit antioxidant response, is also bound with Nrf2. Lead is responsible for activation of IL-8 synthesis but their secretion depends upon Nrf2. The blocking of Nrf2, by small interfering RNA (siRNA), causes the complete blocking of transcription, translation, and secretion of IL-8 produced by lead [69].
Zinc
Zinc is a very important element and has an essential role in many events that occur in the cell. Zinc plays an important role in the functioning of enzymes when acting as a cofactor and also plays an important role in transcription [70]. Zinc is present as an integral constituent in a large number of enzymes and proteins and contributes to a wide range of metabolic processes such as carbohydrates metabolism, lipid metabolism, protein metabolism, and generation and degradation of nucleic acid [71]. Zinc has a unique role in metabolic syndrome by participating in cell events such as the expression of cytokines and suppression of inflammation. Zinc is responsible for activating antioxidant enzymes that reduce the level of reactive oxygen species which in return decreases stress-induced by oxidation. Zinc is also present in supplement whose function to improve blood pressure, level of glucose and cholesterol in the body. This suggests that zinc plays an important role in the regression of metabolic syndrome [72].
Effect of Zinc on Oxidative Stress
Oxidative stress is strongly associated with metabolic syndrome and has a connection with it through dyslipidemia, hypertension, diabetes, and obesity [73]. Zinc has the potential to inhibit the production of reactive oxygen species including hydrogen peroxide, superoxide anion, and hydroxyl radical [74]. Zinc also inhibits the reactive nitrogen species such as peroxynitrite [75]. Zinc also has an antioxidant effect by direct-acting on antioxidant proteins and the production of structurally modified metallothionein. Zinc performs the antioxidant activity by direct binding of zinc ion with thiol groups [76].
Effect of Zinc on Lipid Metabolism
In the living organisms, the lipid is accumulated in adipose tissues which lead towards obesity. Previous studies show that there is a connection between zinc serum level and metabolism of lipids. Zinc intake causes a decrease in the level of total cholesterol, triglycerides, and low-density lipoprotein cholesterol, and also causes an increase in the level of HDL cholesterol [72]. Leptin is a hormone produced by adipocytes which play an important role in the regulation of energy by increased energy expenditure and reduce the need for food uptake [77]. Zinc status is also an essential factor for determining the normal functioning of adipocytes and for the production of leptin for maintenance of negative feedback which is mediated by leptin. Zinc intake in obese patients who are resistant to leptin increases the serum leptin level and helps in the improvement of weight that is associated with metabolism [78].
Zinc has a significant role in adipokines. Zinc helps in the oligomerization of adiponectin which is a high molecular weight molecule by modulation of disulfide bond formation [79]. There is the existence of a positive relationship between adiponectin and serum leptin level in obese patients [80]. Adipokine, Zinc-α-2-glycoprotein is reduced in obesity, high fat diet, and inflammatory stimuli. Zinc-α-2-glycoprotein is responsible for the regulation of lipid metabolism in adipose tissues. Zinc-α-2-glycoprotein decreases the level of fatty acid synthase, acyl-coenzyme A and acetyl-coenzyme A carboxylase 1. It causes an increase in the hormone-sensitive lipase activity as a result of lipolysis occur and decreases in lipogenesis [72].
Conclusion
Heavy metals are substances which have high density and can cause toxicity to a human being even at very small concentration. The major source for exposure to heavy metal is an environment and anthropogenic sources. These metals expose to human beings may be by ingestion and inhalation. Heavy metals have a major contribution in inducing the various metabolic disorders that lead to cardiovascular diseases as endocrine-disrupting chemicals. Some heavy metals such as arsenic, cadmium, and lead show significant harm to health. Cadmium is a major contributor to disrupting carbohydrates and lipid metabolism and leads to various disease conditions. Arsenic is responsible for inducing diabetes mellitus type 2 by various different mechanisms such as alter gene expression, glucose transportation, and glucose metabolism. Lead has significant potential to induce oxidative stress and inflammatory response which causes various metabolic syndromes. Zinc is a necessary element that plays an important role in the prevention of metabolic syndrome by reducing reactive oxygen species and altering lipid metabolism.
References
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150.
Georgescu B, Georgescu C, Dărăban S, Bouaru A, Paşcalău S. Heavy metals acting as endocrine disrupters. Sci Pap Anim Sci Biotechnol. 2011;44(2):89–93.
Yadav M, Gupta R, Sharma RK. Chapter 14 - green and sustainable pathways for wastewater purification. In: Ahuja S, editor. Advanced water purification techniques. Amsterdam: Elsevier; 2019. p. 355–83.
Verma N, Kaur G. Chapter 2 - trends on biosensing systems for heavy metal detection. In: Scognamiglio V, Rea G, Arduini F, Palleschi G, editors. Comprehensive analytical chemistry, vol. 74. Amsterdam: Elsevier; 2016. p. 33–71.
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Experientia Suppl. 2012;101:133–64.
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN. Mechanism and health effects of heavy metal toxicity in humans. In: Karcioglu O, Arslan B, editors. Poisoning in the modern world-new tricks for an old dog? London: Intechopen; 2019.
Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51.
Huang Y, He C, Shen C, Guo J, Mubeen S, Yuan J, et al. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017;8(4):1373–401.
Schwartz GG, Reis IM. Is cadmium a cause of human pancreatic cancer? Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Am Soc Prev Oncol. 2000;9(2):139–45.
Kumari A. Chapter 1 - glycolysis. In: Kumari A, editor. Sweet biochemistry. Cambridge: Academic Press; 2018. p. 1–5.
Veramendi J, Fernie AR, Leisse A, Willmitzer L, Trethewey RN. Potato hexokinase 2 complements transgenic Arabidopsis plants deficient in hexokinase 1 but does not play a key role in tuber carbohydrate metabolism. Plant Mol Biol. 2002;49(5):491–501.
Sabir S, Akash MSH, Fiayyaz F, Saleem U, Mehmood MH, Rehman K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: inserting the association into perspectives. Biomed Pharmacother. 2019;114:108802.
Sastry KV, Subhadra K. Effect of cadmium on some aspects of carbohydrate metabolism in a freshwater catfish Heteropneustes fossilis. Toxicol Lett. 1982;14(1):45–55.
Cicik B, Engin K. The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turk J Vet Animal Sci. 2005;29:113–7.
De la Torre FR, Salibian A, Ferrari L. Biomarkers assessment in juvenile Cyprinus carpio exposed to waterborne cadmium. Environ Pollut. 2000;109(2):277–82.
Romero-Ruiz A, Amezcua O, Rodriguez-Ortega MJ, Munoz JL, Alhama J, Rodriguez-Ariza A, et al. Oxidative stress biomarkers in bivalves transplanted to the Guadalquivir estuary after Aznalcollar spill. Environ Toxicol Chem. 2003;22(1):92–100.
Barata C, Lekumberri I, Vila-Escale M, Prat N, Porte C. Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the tricoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat Toxicol. 2005;74(1):3–19.
Ma W, Wang L, He Y, Yan Y. Tissue-specific cadmium and metallothionein levels in freshwater crab Sinopotamon henanense during acute exposure to waterborne cadmium. Environ Toxicol. 2008;23(3):393–400.
Fang Y, Yang H, Wang T, Liu B, Zhao H, Chen M. Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp Biochem Physiol Toxicol Pharmacol: CBP. 2010;151(3):325–33.
Ramirez-Bajo MJ, de Atauri P, Ortega F, Westerhoff HV, Gelpi JL, Centelles JJ, et al. Effects of cadmium and mercury on the upper part of skeletal muscle glycolysis in mice. PloS One. 2014;9(1):e80018.
Li L, Tian X, Yu X, Dong S. Effects of acute and chronic heavy metal (Cu, Cd, and Zn) exposure on sea cucumbers (Apostichopus japonicus). BioMed Res Int. 2016;2016:13.
Berg JM, Tymoczko JL, Stryer L. Biochemistry. New York: WH Freeman; 2012.
Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography–mass spectrometry. J Chromatogr A. 2007;1147(2):153–64.
Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008;31(6):703–17.
Santra A, Maiti A, Chowdhury A, Mazumder DN. Oxidative stress in liver of mice exposed to arsenic-contaminated water. Indian J Gastroenterol: Official J Indian Soc Gastroenterol. 2000;19(3):112–5.
Viselina TN, Luk’yanova ON. Cadmium-induced changes in the activity of carbohydrate metabolism enzymes in mollusks. Russ J Mar Biol. 2000;26(4):289–91.
Rines AK, Sharabi K, Tavares CDJ, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15:786.
Haque M, Roy S, Islam M, Roy N. Role of arsenic on the regulation of glycogen metabolism in liver of Taki fishes (Channa Punctatus) exposed to cold. Thai J Agric Sci. 2009;42(3):159–66.
Reddy S, Venugopal N. In vivo effects of cadmium chloride on certain aspects of protein metabolism in tissues of a freshwater field crab Barytelphusa guerini. Bull Environ Contam Toxicol. 1991;42(6):847–53.
Hazelhoff Roelfzema W, Hacker HJ, Van Noorden CJ. Effects of cadmium exposure on glycogen phosphorylase activity in rat placenta as demonstrated by histochemical means. Histochemistry. 1989;91(4):305–8.. Epub 1989/01/01. eng
Soengas JL, Agra-Lago MJ, Carballo B, Andres MD, Veira JA. Effect of an acute exposure to sublethal concentrations of cadmium on liver carbohydrate metabolism of Atlantic salmon (Salmo salar). Bull Environ Contam Toxicol. 1996;57(4):625–31.
Lei LJ, Jin TY, Zhou YF. The toxic effects of cadmium on pancreas. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi. Chin J Ind Hyg Occup Dis. 2005;23(1):45–9.
Burzlaff N, Sigel A, Sigel H, Sigel RKO, editors. Cadmium: from toxicity to essentiality, metal ions in life sciences, vol. 11. Dordrecht: Springer; 2013. p. 560. https://doi.org/10.1007/978-94-007-5179-8.
Kalahasthi RB, Hirehal Raghavendra Rao R, Bagalur Krishna Murthy R, Karuna Kumar M. Effect of cadmium exposure on serum amylase activity in cadmium electroplating workers. Environ Bioindic. 2006;1(4):260–7.
Champe PC, Harvey RA. Biochemistry (Lippincott’s illustrated reviews). Philadelphia: Lippincott; 1994.
Rajanna B, Hobson M, Reese J, Sample E, Chapatwala KD. Chronic hepatic and renal toxicity by cadmium in rats. Drug Chem Toxicol. 1984;7(3):229–41.
Lucia M, Andre JM, Gonzalez P, Baudrimont M, Bernadet MD, Gontier K, et al. Effect of dietary cadmium on lipid metabolism and storage of aquatic bird Cairina moschata. Ecotoxicology. 2010;19(1):163–70.
Yang J, Liu D, Jing W, Dahms HU, Wang L. Effects of cadmium on lipid storage and metabolism in the freshwater crab Sinopotamon henanense. PLoS One. 2013;8(10):e77569.
Digel M, Ehehalt R, Stremmel W, Fullekrug J. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol Cell Biochem. 2009;326(1–2):23–8.
Kordinas V, Ioannidis A, Chatzipanagiotou S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes. 2016;7(9):60.
Chung JY, Yu SD, Hong YS. Environmental source of arsenic exposure. J Prev Med Public Health (Yebang Uihakhoe chi). 2014;47(5):253–7.
Zhang HN, Yang L, Ling JY, Czajkowsky DM, Wang JF, Zhang XW, et al. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc Natl Acad Sci U S A. 2015;112(49):15084–9.
Tawfik DS, Viola RE. Arsenate replacing phosphate: alternative life chemistries and ion promiscuity. Biochemistry. 2011;50(7):1128–34.
Dixon HBF. The biochemical action of arsonic acids especially as phosphate analogues. In: Sykes AG, editor. Advances in inorganic chemistry, vol. 44. Cambridge: Academic Press; 1996. p. 191–227.
Xu Y, Ma B, Nussinov R. Structural and functional consequences of phosphate-arsenate substitutions in selected nucleotides: DNA, RNA, and ATP. J Phys Chem B. 2012;116(16):4801–11.
Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, et al. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect. 2000;108(9):847–51.
Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, et al. Telomere Phenotypes in Females with Heterozygous Mutations in the D yskeratosis Congenita 1 (DKC 1) Gene. Hum Mutat. 2013;34(11):1481–5.
Kannan GM, Flora SJ. Chronic arsenic poisoning in the rat: treatment with combined administration of succimers and an antioxidant. Ecotoxicol Environ Saf. 2004;58(1):37–43.
Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255(1-2):67–78.
Naranmandura H, Xu S, Sawata T, Hao WH, Liu H, Bu N, et al. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem Res Toxicol. 2011;24(7):1094–103.
Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol Appl Pharmacol. 2007;222(3):281–8.
Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985;260(5):2646–52.
Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem-Biol Interact. 1994;90(2):139–55.
Diaz-Villasenor A, Burns AL, Hiriart M, Cebrian ME, Ostrosky-Wegman P. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol Appl Pharmacol. 2007;225(2):123–33.
Wauson EM, Langan AS, Vorce RL. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol Sci: Official J Soc Toxicol. 2002;65(2):211–9.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72.
Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125.
Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. 2018;119(1):157–84.
Knowles SO, Donaldson WE. Dietary modification of lead toxicity: effects on fatty acid and eicosanoid metabolism in chicks. Comp Biochem Physiol C, Comp Pharmacol Toxicol. 1990;95(1):99–104.
Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev: J Clin Ther. 2006;11(2):114–27.
Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58.
Ahamed M, Siddiqui MK. Environmental lead toxicity and nutritional factors. Clin Nutr. 2007;26(4):400–8.
Quintanilla-Vega B, Hoover D, Bal W, Silbergeld EK, Waalkes MP, Anderson LD. Lead effects on protamine-DNA binding. Am J Ind Med. 2000;38(3):324–9.
White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225(1):1–27.
Skoczynska A, Poreba R, Sieradzki A, Andrzejak R, Sieradzka U. The impact of lead and cadmium on the immune system. Medycyna Pracy. 2002;53(3):259–64.
Dietert RR, Piepenbrink MS. Lead and immune function. Crit Rev Toxico. 2006;36(4):359–85.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38:97–120.
Wei J, Du K, Cai Q, Ma L, Jiao Z, Tan J, et al. Lead induces COX-2 expression in glial cells in a NFAT-dependent, AP-1/NFkappaB-independent manner. Toxicology. 2014;325:67–73.
Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int J Mol Sci. 2018;19(6):1813.
Cai L, Li XK, Song Y, Cherian MG. Essentiality, toxicology and chelation therapy of zinc and copper. Curr Med Chem. 2005;12(23):2753–63.
Wang S, Liu GC, Wintergerst KA, Cai L. Chapter 14 - Metals in diabetes: zinc homeostasis in the metabolic syndrome and diabetes. In: Mauricio D, editor. Molecular nutrition and diabetes. San Diego: Academic Press; 2016. p. 169–82.
Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 2018;68(1):19–31.
Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20(1):72–7.
Ogawa D, Asanuma M, Miyazaki I, Tachibana H, Wada J, Sogawa N, et al. High glucose increases metallothionein expression in renal proximal tubular epithelial cells. Exp Diabetes Res. 2011;2011:534872.
Hadwan MH, Almashhedy LA, Alsalman AR. Study of the effects of oral zinc supplementation on peroxynitrite levels, arginase activity and NO synthase activity in seminal plasma of Iraqi asthenospermic patients. Reprod Biol Endocrinol. 2014;12:1.
Saarni H, Tamminen-Peter L. Physical stress and strain in catering work on the Baltic car ferries. Bull Inst Marit Trop Med Gdynia. 1987;38(1–2):25–31.
Payahoo L, Ostadrahimi A, Mobasseri M, Khajebishak Y, Asghari Jafarabadi M. Effects of zinc supplementation on serum leptin level and insulin sensitivity in obese people. Trace Elem Electrolytes. 2014;31:27–32.
Baltaci AK, Mogulkoc R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J Endocrinol Metab. 2012;16(Suppl 3):S611–6.
Briggs DB, Giron RM, Schnittker K, Hart MV, Park CK, Hausrath AC, et al. Zinc enhances adiponectin oligomerization to octadecamers but decreases the rate of disulfide bond formation. Biometals: Int J Role Metal Ions Biol Biochem Med. 2012;25(2):469–86.
Mazloomi S, Alizadeh N, Aminzare M, Niroomand S, Mousavi SN. Retracted article: serum zinc and adiponectin levels in patients with polycystic ovary syndrome, adjusted for anthropometric, biochemical, dietary intake, and physical activity measures. Biol Trace Element Res. 2018;181(2):388.
Acknowledgments
This work has been financially supported by the research grant (8365/Punjab/NRPU/R&D/HEC/2017) received from the Higher Education Commission (HEC) of Pakistan.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Irshad, K. et al. (2021). Role of Heavy Metals in Metabolic Disorders. In: Akash, M.S.H., Rehman, K., Hashmi, M.Z. (eds) Endocrine Disrupting Chemicals-induced Metabolic Disorders and Treatment Strategies. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-45923-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-45923-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45922-2
Online ISBN: 978-3-030-45923-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)