Abstract
The intestine interacts with a diverse community of antigens and bacteria. To keep its homeostasis, the gut has evolved with a complex defense system, including intestinal microbiota, epithelial layer and lamina propria. Various factors (e.g., nutrients) affect the intestinal defensive system and progression of intestinal diseases. This review highlights the current understanding about the role of amino acids (AAs) in protecting the intestine from harm. Amino acids (e.g., arginine, glutamine and tryptophan) are essential for the function of intestinal microbiota, epithelial cells, tight junction, goblet cells, Paneth cells and immune cells (e.g., macrophages, B cells and T cells). Through the modulation of the intestinal defensive system, AAs maintain the integrity and function of the intestinal mucosa and inhibit the progression of various intestinal diseases (e.g., intestinal infection and intestinal colitis). Thus, adequate intake of functional AAs is crucial for intestinal and whole-body health in humans and other animals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
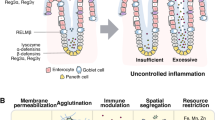
Interactions with pathogens and toxins are a fact of life for almost all animals, and this is more pronounced in the intestine than any other organs. The small intestine is responsible for nutrient digestion and absorption (Wu 2018). In addition, the gut is the home to a diverse community of indigenous microorganisms. Thus, both the small intestine and the large intestine are constantly exposed to various antigens from food and water, as well as a large number of bacteria that coexist in the intestinal lumen. The gastrointestinal tract has evolved with a sophisticated barrier defense system to protect against this exposure and to distinguish “self” from “foreign”. This defense system includes indigenous commensal microorganisms, epithelial layer, and the lamina propria (Fig. 8.1). Intestinal microbiota is associated with the intestinal defensive system through its regulation on intestinal or systemic innate and adaptive immunities (Honda and Littman 2016; Thaiss et al. 2016), as well as direct effects on pathogens via colonization resistance or competition for nutrients (Endt et al. 2010; Seekatz and Young 2014). The epithelial layer includes absorptive enterocytes, hormone-secreting enteroendocrine cells, mucus-secreting goblet cells, antimicrobial-secreting Paneth cells, intraepithelial lymphocytes (IELs), microfold cells, and dendritic cells. The lamina propria harbors various immune cells, such as dendritic cells, neutrophils, macrophages, B lymphocytes (B cells), T lymphocytes (T cells), and fibroblasts. Based on published studies (Johansson and Hansson 2016; Mukherjee and Hooper 2015; Pabst et al. 2016), the intestinal epithelium produces and releases secretory IgA, antimicrobial proteins and mucins in a cell-specific manner.
The mucosal barrier defense system in the intestine. This defense system includes indigenous commensal microorganisms, epithelial layer, and the lamina propria. The epithelial layer consists of absorptive enterocytes, hormone-secreting enteroendocrine cells, mucin-secreting goblet cells, antimicrobial-secreting Paneth cells, intraepithelial lymphocytes (IELs), microfold cells, and dendritic cells. The lamina propria harbors various immune cells [e.g., dendritic cells (DC), neutrophils, macrophages, B cells, and T cells], fibroblasts, and blood vessels. ILC3 = group 3 innate lymphoid cell
Recent years have witnessed growing interest in the biochemistry and physiology of amino acids (AAs) in mammals, such as arginine, glutamine, glycine, and tryptophan (Fan et al. 2019; Le Floc’h et al. 2018; Hou and Wu 2017; Wu 2013). Notably, dietary contents of AAs are crucial for intestinal physiology, especially the intestinal defensive immune (Li et al. 2007; Ren et al. 2016a, b). The review highlights our current understanding of the influences of dietary AAs on intestinal defensive system in humans and animal models, including intestinal microbiota, cells in the epithelial layer and immune cells in the lamina propria.
8.2 Amino Acids and Intestinal Microbiota
Intestinal microbiota is present in virtually any metazoans, ranging from invertebrates to vertebrates. It affects numerous physiological functions of the gut (Lee and Hase 2014; Ren et al. 2016b; Subramanian et al. 2014; Thaiss et al. 2016) and is linked to the pathogenesis of various diseases (Anhe et al. 2014; Lee and Hase 2014; Louis et al. 2014; Qin et al. 2014; Thaiss et al. 2016) through the microbiome and its metabolic products (Lee and Hase 2014; Ren et al. 2016d). Intestinal microbiota has critical roles in intestinal immune response through its regulation of intestinal or systemic innate and adaptive immunities (Honda and Littman 2016; Thaiss et al. 2016), as well as by direct effects on pathogens via colonization resistance (Endt et al. 2010; Seekatz and Young 2014). For example, infection by Clostridium difficile, which is the leading health care-associated illness, usually follows the disruption of the indigenous gut microbiota after antibiotic treatment, leading to the loss of colonization resistance against the pathogen (Britton and Young 2014; Seekatz and Young 2014; Theriot et al. 2014). A successful treatment strategy for C. difficile infection is fecal microbiota transplantation from healthy individuals, which can recover the gut microbiome after transplantation (Fuentes et al. 2014; Seekatz et al. 2014).
Dietary AAs regulate the diversity, composition and metabolism of intestinal microbiota (Dai et al. 2011, 2015). For example, arginine decreases the net utilization of lysine, threonine, isoleucine, leucine, glycine and alanine by jejunal or ileal mixed bacteria (Dai et al. 2012). Arginine supplementation shifts the population of microbes in the jejunum and ileum of mice to favor the growth of Bacteroidetes by decreasing the number of Firmicutes, but increasing the abundance of Bacteroidetes (Ren et al. 2014a). Arginine also enhances the abundance of Lactobacillus in the jejunum and the abundance of Streptococcus in the ileum (Ren et al. 2014a). Thus, feeding Lactobacillus reuteri DSM 17938 to newborn mice increased the concentration of beneficial AAs and their metabolites in the large intestine, while regulating gut microbiota and immune responses (Liu et al. 2019b). In addition, dietary supplementation with glutamine to mice decreases the abundance of Firmicutes in their jejunum and ileum, while increasing the abundance of Streptococcus and Bifidobacterium in their jejunum (Ren et al. 2014b). Furthermore, adding proline to the diet of Huanjiang mini-pigs decreases the amounts of Klebsiella pneumoniae, Peptostreptococcus productus, Pseudomonas, and Veillonella spp. in distal colonic contents (Ji et al. 2018). Likewise, dietary supplementation with gamma-aminobutyric acid (GABA) regulates the community richness and diversity of the ileal microbiota, as well as the abundances of the dominant microbial populations in weaned piglets (Chen et al. 2019b). Interestingly, dietary lysine restriction decreases the bacterial diversity and increases the abundance of Actinobacteria, Saccharibacteria, and Synergistetes in the intestine at the phylum level, as well as the abundances of Moraxellaceae, Halomonadaceae, Shewanellaceae, Corynebacteriaceae, Bacillaceae, Comamonadaceae, Microbacteriaceae, Caulobacteraceae, and Synergistaceae in the intestine at the family level (Yin et al. 2017).
The exact mechanisms whereby AAs modulate intestinal microbiota need further investigation. It is possible that AA supplementation or restriction alters the intestinal microenvironment, and then influences the composition and function of the intestinal microbiota. Notably, beneficial effects of AAs on gut health are associated with similar changes in the intestinal microbiota, but some AAs exert specific effects. Also, the influences of AAs on the intestinal microbiota depend on their supplemental dosages. For example, dietary supplementation with 0.5 and 1% aspartate to mice reduces the ratio of Firmicutes to Bacteroidetes in the ileum and feces, but dietary supplementation with 2% aspartate increases this ratio in the feces (Bin et al. 2017).
Results of our recent studies indicate that arginine or glutamine supplementation promotes the activation of intestinal innate immunity, including expression of factors (e.g., toll-like receptors) and activation of signaling pathways [e.g., mitogen-activated protein kinase (MAPK)] associated with intestinal innate immunity (Ren et al. 2014a, b). Thus, dietary supplementation with arginine or glutamine enhances the ability of the host to clear infections by pathogens (e.g., porcine circovirus type 2 and Pasteurella multocida) (Chen et al. 2014; Ren et al. 2012, 2013a, b, c, d), especially intestinal pathogens (e.g. enterotoxigenic Escherichia coli) (Liu et al. 2017a). However, whether arginine or glutamine promotes the clearance of these pathogens in the host through the intestinal microbiota remains to be explored.
Intestinal microbiota also affects the host AA metabolism and, therefore, the defensive responses. For example, the intestinal microbiota (Clostridium sporogenes) uses aromatic AAs (tryptophan, phenylalanine and tyrosine) as substrates to produce metabolites (e.g., indolepropionic acid), which in turn affect intestinal permeability and systemic immunity (Dodd et al. 2017). The enrichment of the intestinal microbiota that synthesizes the branched-chain amino acids (BCAA), such as Prevotella copri and Bacteroides vulgatus, and that have a low capacity to take up BCAAs, are associated with high concentrations of BCAA in serum (Pedersen et al. 2016). Indeed, the levels of AAs in the ileum differ markedly between conventionally reared and germ-free mice, indicating that the gut microbiota greatly affects the metabolism of AAs in the ileum (Mardinoglu et al. 2015). Those AAs include arginine, asparagine, histidine, isoleucine, leucine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine and glutamine (Mardinoglu et al. 2015). It is unknown whether this alteration in AA metabolism is associated with the abnormities of intestinal immunity in germ-free mice, such as Paneth cell dysfunction (Zhang et al. 2015). During enterotoxigenic Escherichia coli infection, hosts (i.e., piglets and mice) experience remarkable alterations in the intestinal microbiota, especially increases in the abundance of Lactococcus lactis subsp. (Ren et al. 2016d). Lactococcus lactis subsp. regulates the host immune responses against enterotoxigenic Escherichia coli infection through producing GABA, which promotes intestinal expression of IL-17 to activate the mechanistic target of rapamycin complex 1 (mTORC1)-ribosomal protein S6 kinase 1 (S6K1) signaling (Fig. 8.2) (Ren et al. 2016d). Besides glycine and the L-isoform of AAs, the mouse intestine contains high levels of free D-AAs derived from the microbiota (Kepert et al. 2017; Sasabe et al. 2016). Interestingly, the intestinal microbiota stimulates the release of D-amino acid oxidase (DAO) from intestinal epithelial cells (including goblet cells) into the intestinal lumen, resulting in the oxidative deamination of intestinal D-AAs to yield a potent antimicrobial product, H2O2, thereby protecting the mucosal surface in the small intestine from the cholera pathogen (Sasabe et al. 2016). DAO has also been shown to modify the composition of the microbiota and production of intestinal sIgA (Sasabe et al. 2016). This illustrates the importance of D-AAs in nutrition and metabolism. Collectively, there is significant crosstalk between host AAs and the intestinal microbiota, and this interplay regulates the intestinal defensive responses and the progression of intestinal infection.
γ-Aminobutyrate (GABA) mediates intestinal interleukin-17 expression during infection by enterotoxigenic Escherichia coli (ETEC). During ETEC infection, the pathogen induces dysbiosis in the gut microbiota, increasing Lactococcus lactis subsp. The Lactococcus lactis subsp. produces GABA from glutamate through the action of glutamate decarboxylase (GAD). GABA is sensed by Th17 cells through GABA receptors (GABAR), leading to the activation of the mTOR pathway. The mTOR signaling promotes IL-17 expression during infection through the mTOR-S6K1-EGR2-GFI1 pathway. GABA transporter 2 is negatively associated with Th17 response during intestinal infection by terminating the GABA signaling through the translocation of GABA from the extracellular to the intracellular space
8.3 Amino Acids and Intestinal Epithelial Cells
Besides the absorption of nutrients (including AAs, glucose, fatty acids, and electrolytes), intestinal epithelial cells (generated from intestinal epithelial stem cells) represent an effective barrier ling the gastrointestinal mucosal surface, and regulate the functions of intestinal immune cells as well as intestinal homeostatic and inflammatory responses (Nowarski et al. 2017). For example, the villous epithelial cells that express the neonatal Fc receptor (FcRn) play a role in binding intestinal antigens (McDole et al. 2012; Schulz and Pabst 2013). FcRn on villus epithelial cells aids in the secretion of IgG across the intestinal epithelium into the lumen, and also contributes to the uptake of intestinal antigens from the lumen through the IgG-dependent process (Yoshida et al. 2004, 2006). Also, the expression of Ifnlr1 [the receptor for interferon (IFN)-λ] on intestinal epithelial cells in the small intestine and colon is critical for enteric IFN-λ antiviral activity in mice (Baldridge et al. 2017). Importantly, Ifnlr1 expression in intestinal epithelial cells affects the efficacy of IFN-λ in resolving persistent murine norovirus infection, and is necessary for the sterilizing innate immune effects of IFN-λ (Baldridge et al. 2017). Although p40 (a Lactobacillus rhamnosus GG-derived protein) treatment directly on B cells shows little effect on IgA production, p40 promotes the expression of a proliferation-inducing ligand (APRIL) in intestinal epithelial cells, resulting in an increase in fecal IgA levels, as well as IgA+B220+, IgA+CD19+, and IgA+ plasma cells in the lamina propria of mice (Wang et al. 2017). Collectively, intestinal epithelial cells are closely associated with intestinal immunity responses.
It is well known that AAs, such as glutamate, cysteine, glutamine and glycine, promote protein synthesis in intestinal epithelial cells and their growth via various cellular signaling, such as the mTOR signaling (He et al. 2016; Honda and Littman 2016; Wang et al. 2014a, 2016; Ye et al. 2016), as well as nutrient metabolism, glutathione synthesis, and ATP production (Li et al. 2020). For example, arginine enhances DNA synthesis, cell-cycle progression, and mitochondrial bioenergetics in intestinal epithelial cells through mechanisms involving activation of the phosphatidylinositol 3′-kinase (PI3K)-protein kinase B (Akt pathway) (Tan et al. 2015). Given the importance of AAs in these physiological processes, we surmise that AAs may affect intestinal defensive responses by regulating the expression and secretion of immune regulators in intestinal epithelial cells. For example, BCAA stimulate the expression of β-defensin from porcine intestinal epithelial cells possibly through activation of the sirtuin-1(Sirt1)/extracellular regulated protein kinases (ERK) signaling pathway (Ren et al. 2016a). In addition, tryptophan inhibits the secretion of interleukin (IL)-8 in intestinal epithelial cells after tumor necrosis factor (TNF)-α challenge through the calcium-sensing receptor (Mine and Zhang 2015). Glycine attenuates the production of reactive oxygen species (ROS) in intestinal epithelial cells via promoting the synthesis of glutathione and expression of glycine transporter 1, while reducing the activation of the MAPK signaling pathway (Wang et al. 2014a). Amino acids also regulate the function of intestinal epithelial cells and the intestinal immunity. For example, AA starvation in intestinal epithelial cells induces autophagy responses in intestinal epithelial cells, resulting in lower levels of ROS and IL-1beta as well as a reduction in the abundance of IL-17A-producing CD4+ T cells (Ravindran et al. 2016). Collectively, epithelial cells are involved in intestinal immune responses, such as antigen recognition, IgA production, and the killing of pathogens. Some AAs (e.g., arginine, BCAA and glycine) regulate protein synthesis in intestinal epithelial cells, their proliferation and migration, as well as the generation and secretion of immune regulators by the cells.
8.4 Amino Acids and Intercellular Junction
Between intestinal epithelial cells, there are intercellular junctions that include an apical tight junction (TJ), subjacent adheren junction (AJ), and desmosomes, controlling the movement of fluids and solutes in the paracellular space and the establishment of cell polarity (Luissint et al. 2016; Tsukita et al. 2001). Tight junctions reside include claudins, TJ-associated MARVEL domain-containing proteins (TAMPs, including occludin, MARVELD2, and MARVELD3), and members of the cortical thymocyte marker in the Xenopus family, such as junctional adhesion molecules (Luissint et al. 2016; Raleigh et al. 2010). The AJ is an ancient junctional complex that initiates and maintains epithelial cell-cell contacts, while the desmosomes provides mechanical strength to the epithelium. The key transmembrane protein in the epithelial AJ is E-cadherin, while the desmosomes include desmoglein and desmocollin proteins (Green and Simpson 2007; Ivanov and Naydenov 2013). The maintenance of the intestinal epithelial barrier is dependent on the crosstalk among TJs, AJs, and desmosomes (Luissint et al. 2016). A functional intestinal epithelium allows for selective absorption of nutrients, while restricting the passage of pathogens and food-borne antigens. However, various intestinal pathogens have been reported to target the intestinal epithelial barrier to induce disassembly and barrier defects. For example, the enterotoxin produced by Clostridium perfringens has been reported to bind claudins 3, 4, 6, 7, 8, 9, and 14, resulting in their internalization from the TJ and therefore compromising mucosal barrier function (Fernandez Miyakawa et al. 2005; Saitoh et al. 2015; Veshnyakova et al. 2010).
Dietary AAs are important regulators of intercellular function, especially the expression and abundance of TJs. This notion is supported results from both in vitro and in vivo experiments. For example, tryptophan enhances the abundances of occludin, claudin-4, zonula occludens (ZO)-1 and 2 in intestinal porcine epithelial cells (Wang et al. 2015a). Similarly, glutamine decreases the TJ permeability, but increases the monolayer transepithelial electrical resistance (TEER), the abundances of transmembrane proteins (including occludin, claudin-4, ZO-1, ZO-2, and ZO-3) through activation of the calcium/calmodulin-dependent kinase 2 (CaMKK2)-AMP-activated protein kinase (AMPK) signaling (Jiao et al. 2015; Wang et al. 2016). Subsequent investigations with piglets also demonstrate the positive influence of physiological levels of AAs on the expression of TJ proteins. Specifically, dietary supplementation with glutamine to weanling piglets augments the abundances of occludin, claudin-1, ZO-2, and ZO-3 proteins in the jejunum (Wang et al. 2015b). Besides glutamine, dietary supplementation with putrescine or proline to neonatal piglets between day 1 of age and weaning at 14 day of age increases the abundances of ZO-1, occludin, and claudin-3 proteins in the jejunum (Wang et al. 2015c). Similarly, studies with post-weaning pigs have shown that dietary supplementation with 1% glutamine (Wu et al. 1996), 1% proline (Wu et al. 2011), or 1-2% glycine (Wang et al. 2014b) ameliorated intestinal atrophy and improved their growth performance, whereas dietary supplementation with 0.2% putrescine dihydrochloride improved intestinal integrity and decreased the incidence of diarrhea (Liu et al. 2019a). Note that glutamine, glycine and proline are highly abundant in animal-source proteins such as meat & bone meal, poultry by-products, and chicken visceral digest (Wu and Li 2020), whereas the content of glycine and proline is relatively low in all plant-source proteins (Hou et al. 2019).
Animals are frequently exposed to stressful conditions in their life times. Importantly, AAs are beneficial for maintaining the adequate expression of intestinal TJ proteins in subjects with various intestinal diseases, such as intestinal inflammation that is associated with the defect of TJ functions. In the dextran sulfate sodium (DSS)-induced colitic mouse model, which is similar to human ulcerative colitis, dietary supplementation with arginine or glutamine increases the abundance of the claudin-1 protein in the colon (Ren et al. 2014c). Likewise, glutamine administration increases the abundance of the ZO-1 protein in the small-intestinal mucosa of DSS-treated mice (Pai et al. 2014). Similarly, in rats with methotrexate-induced mucositis, glutamine or arginine supplementation enhances the jejunal abundances of claudin-1, occludin and ZO-1 proteins through ERK and NF-κB pathways (Beutheu et al. 2014). In addition to intestinal inflammation, AAs are also essential for the homeostasis of TJ proteins in other situations. For example, although a western-style high-fat diet lowers the levels of occludin and ZO-1 proteins in the upper part of the mouse small intestine, oral administration of arginine restores the abundances of occludin and ZO-1 proteins (Sellmann et al. 2017a). Liang et al. (2018) reported that dietary supplementation with 0.2% tryptophan to weanling pigs increased the abundances of ZO-1 and occludin proteins in the colon. Furthermore, dietary supplementation with 0.2% and 0.4% tryptophan to weanling pigs augmented the abundances of the jejunal ZO-1, ZO-3 and claudin proteins in a dose-dependent manner, whereas dietary supplementation with 0.4% tryptophan also enhanced the abundance of the jejunal occludin protein (Liang et al. 2019). Thus, much evidence shows that intercellular junctions, including TJ, AJ and desmosomes, play a critical role in the hemostasis of the intestinal epithelium. Dietary AAs are essential for the expression of the TJ proteins, especially in various intestinal diseases that are characterized by defects in the intestinal mucosal barrier (Table 8.1). However, it remains unknown how AAs affect the location of intestinal TJ proteins or the homeostasis of the intestinal AJ and desmosomes. This remains to be an active area of research in AA physiology and nutrition.
8.5 Amino Acids and Goblet Cells
In addition to enterocytes, the second subtype of cells in the intestinal epithelium is the mucus-producing goblet cells. Goblet cells are specialized secretory cells lining intestinal mucosal epithelia. The differentiation of goblet cells from intestinal epithelial stem cells is tightly regulated by the sterile α motif pointed domain epithelial specific transcription factor (Spdef), which responds to the downstream of both Notch and Wnt signaling. Spdef-null mice show a reduction in mature, differentiated goblet cells in the intestine, whereas overexpression of Spdef in the intestine displays an expansion of Muc2-expressing goblet cells at the expense of other intestinal cell types (Gregorieff et al. 2009; Noah et al. 2010). Goblet cells have critical roles in maintaining intestinal homeostasis through secreting a variety of factors, such as mucins and trefoil factors (Johansson and Hansson 2016; McCauley and Guasch 2015). The secretion of these factors from goblet cells depends on various stimuli, such as microbial factors, growth factors and inflammatory cytokines (Deplancke and Gaskins 2001; McCauley and Guasch 2015; Wlodarska et al. 2014), as well as the availability of threonine (a major AA in mucins; Wu 2018). These factors entrap external insults such as pathogens, toxins, and allergens, and prevent their translocation into the blood and other extra-intestinal tissues (Johansson and Hansson 2016). Besides the secretory function, goblet cells have recently been implicated as antigen-presenting cells because goblet cells in the small intestine present intestinal luminal antigens to the underlying dendritic cells so that dendritic cells can sense intestinal insults without a break in intestinal barrier integrity (Knoop et al. 2015; McDole et al. 2012).
Increasing evidence has shown that dietary AAs actively maintain the number of intestinal goblet cells and the expression of mucins in the intestine. For example, in healthy mice, dietary supplementation with 1.0% glutamine for 2 weeks promotes the expression of mucin-4 in the jejunum (Ren et al. 2014b). Similar observation has also been reported in various models of intestinal diseases. For example, in rats with DSS-induced colitis, dietary supplementation with a mixture of AAs (containing L-threonine, L-serine, L-proline, and L-cysteine) attenuates reductions in the number of Muc2-containing goblet cells in the intestinal epithelium of the ulcerated area and mucin production in the colon, while restoring the mucin AA composition and mucosal content (Faure et al. 2006). Likewise, in rats with experimental diversion colitis, glutamine supplementation increases the number of goblet cells in the colonic lamina propria (Pacheco et al. 2012). Also, in enterotoxigenic Escherichia coli (ETEC) infected mice, glutamine promotes the expression of mucin-2 in the jejunum (Xu et al. 2017), providing another line of evidence for a crucial role of the functional AA in gut integrity and function (Rhoads and Wu 2009).
Under certain experimental conditions, some AAs have little effect on or even reduce the number of intestinal goblet cells. For example, glutamine supplementation to weaning mice did not affect the number of goblet cells, or the expression of markers for goblet cells (Chen et al. 2018a). In male 50-day-old Wistar rats, dietary supplementation with 2.0% glutamine for 10 days was reported to decrease the numbers of goblet cells in the villi and crypt of the jejunum or ileum (Martins et al. 2016). Similarly, glutamine supplementation reduced the number of goblet cells in the villi and crypt of jejunum or ileum in rats with Walker-256 tumor (Martins et al. 2016). However, the provision of glutamine from the basal diet was not known in all these studies. In weaned piglets, tryptophan supplementation had little effect on the numbers of goblet cells in the duodenum, jejunum and ileum (Tossou et al. 2016). Similarly, threonine supplementation did not influence the numbers of goblet cells in the jejunum and colon or the amounts of mucins in the scrapings of the jejunum and colon in weaning piglets (Trevisi et al. 2015). The possible reasons for these discrepancies include animal models, intakes of the AAs from the basal diets, supplemental dosages of the AAs, and the methods used for the analysis of goblet cells. Thus, the influences of AA in intestinal goblet cells need further investigation. It is interesting to determine whether specific AAs (e.g., glutamine, arginine and glycine) regulates the differentiation of intestinal goblet cells from intestinal epithelial stem cells.
8.6 Amino Acids and Paneth Cells
With the lineage of secretory cells from intestinal epithelial stem cells, Paneth cells produce antimicrobial peptides, which are rich in proline (Hou et al. 2017). Various cellular signaling pathways affect the differentiation of Paneth cells, such as Notch, PKC λ/ι and mTORC1 (Heuberger et al. 2014; Nakanishi et al. 2016; Zhou et al. 2015). Unlike the enterocytes, Paneth cells reside at the base of the small intestinal crypts of Lieberkühn, where epithelial stem cells are also present.
Paneth cells secrete a wide variety of peptides and proteins, such as lysozyme, α-defensin peptides and secretory phospholipase A2 isotype II (Clevers and Bevins 2013; Porter et al. 2002; Salzman and Bevins 2013). Most of these peptides and proteins have antimicrobial effects, which target microorganisms, including the resident microbiota of the small intestine and the intruding pathogens that can potentially penetrate the mucus layer to invade the crypt or other parts of the intestinal epithelium (Ayabe et al. 2000; Bevins and Salzman 2011). Thus, Paneth cells help protect the gut from pathogenic microbes and shape the composition of the intestinal resident microbiota (Brandl et al. 2007; Salzman et al. 2010; Veshnyakova et al. 2010).
Paneth cells also secrete ligands that provide trophic support for the adjacent epithelial stem cells (Sato et al. 2011). These peptides and proteins are stored in the large secretory granules of Paneth cells and secreted into the crypt lumen via mechanisms mediated by KCa3.1 calcium-activated potassium channels in response to a variety of stimuli, including bacterial products (Ayabe et al. 2000, 2002).
Dietary AAs have been reported to regulate the production of antimicrobial peptides by Paneth cells. For example, arginine supplementation upregulates the expression of cryptdins 1, 4, and 5, cryptdin-related sequence 1c (Crs1c), and RNase angiogenin 4 (Ang4) in the jejunum and ileum (Ren et al. 2014a). Similarly, glutamine supplementation increases the mRNA levels for cryptdins 1, 4, and 5 in the jejunum, cryptdins 4 in the ileum, and Reg3g in the colon (Ren et al. 2014b). In ETEC-infected mice, arginine or glutamine supplementation also promotes the expression of the Crs1c and Reg3g genes (Liu et al. 2017a). Although these results indicate the beneficial function of arginine or glutamine in Paneth cells, the underlying mechanisms are largely unknown. It remains to be determined whether other functional AAs regulate the differentiation of Paneth cells or the expression of antimicrobial peptides in the cells. Collectively, arginine or glutamine can modulate the synthesis of antimicrobial peptides in Paneth cells. However, the roles of other AAs in the secretion of intestinal antimicrobial peptides and the differentiation of Paneth cells remain to be explored.
8.7 Amino Acids and Intestinal Immune Cells
There are various types of immune cells in the intestine, including IELs, microfold cells, dendritic cells, macrophages, B cells, and T cells (Fig. 8.1). The intestine has now been characterized as the largest lymphoid organ in humans and other mammals. The intricate intestinal immune system consists of the outer epithelial layer and the inner lamina propria. The components of the outer section include IELs, the dendritic cell extensions and microfold cells. Intraepithelial lymphocytes are an important line of the first defense that maintains the integrity of intestinal epithelial cells, and dendritic cells help to determine the type of immune response as needed by presenting luminal antigens. In pigs, IELs respond well to T-cell mitogens after weaning but not during the preweaning period (Wu 1996). Microfold cells also mediate the transcytosis of antigens across the epithelium. The inner section of the intestinal defense locates below the IELs, and includes dendritic cells, neutrophils, macrophages, immunoglobulin (Ig) A-producing B cells, natural killer (NK) cells, NK T-cells, conventional T-cells, and T-regulatory cells.
The numbers of macrophages, T cells, and B cells in the intestinal mucosa are greater in weanling mammals (e.g., pigs) than in preweaning ones (Wu 1995). These immune cells initiate inflammation and injury in the gut. Available evidence shows that AAs are important regulators of the activation and function of intestinal immune cells. For example, glutamine promotes the secretion of IgA and increases the abundance of IgA-producing B cells in the intestine (Ren et al. 2016b; Wu et al. 2016). Glutamine also highly shapes the polarization of macrophages through mechanisms, including glutamine-UDP-N-acetylglucosamine pathway, glutamine-derivedα-ketoglutarate via glutaminolysis, and glutamine-dependent anerplerosis or the GABA shunt (Ren et al. 2019a; Xia et al. 2019). Dietary deficiency of AAs significantly reduces the number of F4/80+CD11b+ macrophages and the number of IL-10+F4/80+CD11b+ macrophages in the mouse small intestine (Ochi et al. 2016). The influence of dietary AAs on small-intestinal macrophages may depend on mTOR signaling because an inhibition of this signaling by rapamycin also reduces the number of IL-10+F4/80+CD11b+ macrophages in the mouse small intestine (Ochi et al. 2016).
Considering the importance of AAs in T cell fate decision (Ren et al. 2016c, 2017a, b), it is not surprising that AAs regulate intestinal T cell response. For example, during ETEC infection, intestinal GABA promotes the expression of IL-17 in the jejunum of both mice and piglets (Ren et al. 2016d, 2019b). In addition to conventional T-cells, AAs also modulate the intestinal un-conventional T-cell response. For example, in the DSS-treated mice, glutamine administration increases the proportion of small-intestinal IEL γδ-T cells but decreases the expression of genes responsible for immunomodulation in IEL γδ-T cells, such as Ifn-γ, Tnf-α and Il-17 (Pai et al. 2014). Similarly, glutamine decreases the percentage of IEL γδ-T cells, and regulates the mRNA expression of Bcl-xl, Il-7 receptor and Reg3g in IEL γδ-T cells in mice with ischemia/reperfusion injury (Pai et al. 2015). Furthermore, dietary supplementation with L-tryptophan (0.1 g/kg body weight per day) to mice with DSS-induced inflammation reduced the abundances of macrophages and neutrophils in the colon and improved colonic immune responses partly through attenuating the activation of toll-like receptor 4 (TLR4)-STAT3 signaling and nucleus p-65 (Wang et al. 2020). Thus, dietary AAs play an important role in the activation and function of intestinal immune cells (e.g., IgA-producing B cells, macrophages and T cells, Table 8.2). However, the influences of AAs on the number and function of other types of intestinal immune cells, such as M cell, dendritic cells and neutrophils, need further investigation. Besides the mTOR signaling, whether AAs affects the fate of intestinal immune cells through other cellular signaling molecules (such as nitric oxide, kynurenine, glycine, glutamate and hydroxyproline) remain to be determined (Hou and Wu 2018; Li and Wu 2018; Wang et al. 2013, 2020; Wu et al. 2019b).
8.8 Conclusion
The intestine interacts with a diverse community of antigens and bacteria, and has evolved with a complex defense system, including the indigenous intestinal microbiota, epithelial layer and lamina propria. Dietary intakes of AAs profoundly affect this defense system that involves not only luminal microbes but also intestinal epithelial cells, TJs, globet cells, Paneth cells and immune cells (e.g., macrophages, B cells and T cells). It is imperative to explore the roles of AAs on the function of other components of the intestinal defense system, such as tuft cells, enteroendocrine cells and intestinal innate lymphoid cells. Through modulation of the intestine immune and anti-inflammatory systems, AAs can control the progression of various intestinal diseases, such as intestinal infection and intestinal colitis. However, we eagerly await further investigations of the new roles of AAs in intestinal physiology and pathology, and more evidence about the benefits of manipulating AA metabolism for mitigating intestinal diseases. In practice, adequate intakes of dietary AAs, particularly functional AAs (Wu 2010), are crucial for maintaining the integrity and function of the intestine and the whole-body in humans and other animals.
Abbreviations
- AAs:
-
amino acids
- AJ:
-
adheren junction
- Akt:
-
protein kinase B
- AMPK:
-
AMP-activated protein kinase
- Ang4:
-
RNase angiogenin 4
- APRIL:
-
a proliferation-inducing ligand
- BCAAs:
-
branched-chain amino acids
- CaMKK2:
-
calcium/calmodulin-dependent kinase 2
- Crs1c:
-
cryptdin-related sequence 1c
- DAO:
-
D-amino acid oxidase
- DSS:
-
dextran sulfate sodium
- ERK:
-
extracellular regulated protein kinases
- ETEC:
-
enterotoxigenic Escherichia coli
- FcRn:
-
neonatal Fc receptor
- GABA:
-
gamma-aminobutyric acid
- IELs:
-
intraepithelial lymphocytes
- IFN:
-
interferon
- IL:
-
interleukin
- IR:
-
ischemia/reperfusion
- MAPK:
-
mitogen-activated protein kinase
- mTORC1:
-
mechanistic target of rapamycin complex 1
- NK:
-
natural killer
- PI3K:
-
phosphatidylinositol 3′ -kinase
- ROS:
-
reactive oxygen species
- S6K1:
-
ribosomal protein S6 kinase 1
- Sirt1:
-
sirtuin-1
- TEER:
-
transepithelial electrical resistance
- TJ:
-
tight junction
- TNF:
-
tumor necrosis factor
- ZO:
-
zonula occludens
References
Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y et al (2014) A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64:872–883
Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ (2000) Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1:113–118
Ayabe T, Wulff H, Darmoul D, Cahalan MD, Chandy KG, Ouellette AJ (2002) Modulation of mouse Paneth cell alpha-defensin secretion by mIKCa1, a Ca2+−activated, intermediate conductance potassium channel. J Biol Chem 277:3793–3800
Baldridge MT, Lee S, Brown JJ, McAllister N, Urbanek K, Dermody TS, Nice TJ, Virgin HW (2017) Expression of Ifnlr1 on intestinal epithelial cells is critical to the antiviral effects of interferon lambda against norovirus and reovirus. J Virol 91:e02079-16
Beutheu S, Ghouzali I, Galas L, Dechelotte P, Coeffier M (2013) Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin Nutr 32:863–869
Beutheu S, Ouelaa W, Guerin C, Belmonte L, Aziz M, Tennoune N, Bole-Feysot C, Galas L, Dechelotte P et al (2014) Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin Nutr 33:694–701
Bevins CL, Salzman NH (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9:356–368
Bin P, Liu S, Chen S, Zeng Z, Huang R, Yin Y, Liu G (2017) The effect of aspartate supplementation on the microbial composition and innate immunity on mice. Amino Acids 49:2045–2051
Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG (2007) MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 204:1891–1900
Britton RA, Young VB (2014) Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 146:1547–1553
Chen S, Liu S, Zhang F, Ren W, Li N, Yin J, Duan J, Peng Y, Liu G et al (2014) Effects of dietary L-glutamine supplementation on specific and general defense responses in mice immunized with inactivated Pasteurella multocida vaccine. Amino Acids 46:2365–2375
Chen S, Liu Y, Wang X, Wang H, Li S, Shi H, Zhu H, Zhang J, Pi D et al (2016) Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun 22:577–587
Chen S, Xia Y, Zhu G, Yan J, Tan C, Deng B, Deng J, Yin Y, Ren W (2018a) Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice. Food Nutr Res 62:1439
Chen Y, Zhang H, Cheng Y, Li Y, Wen C, Zhou Y (2018b) Dietary L-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br J Nutr 119:1254–1262
Chen Y, Zhang H, Cheng Y, Li Y, Wen C, Zhou Y (2018c) Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br J Nutr 119:1254–1262
Chen J, Zhang D, Tan Q, Liu M, Hu P (2019a) Arginine affects growth and integrity of grass carp enterocytes by regulating TOR signaling pathway and tight junction proteins. Fish Physiol Biochem 45:539–549
Chen S, Tan B, Xia Y, Liao S, Wang M, Yin J, Wang J, Xiao H, Qi M et al (2019b) Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct 10:366–378
Clevers HC, Bevins CL (2013) Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75:289–311
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2012) Regulatory role for L-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Wu ZL, Hang SQ, Zhu WY, Wu G (2015) Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol Hum Reprod 21:389–409
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73:1131S–1141S
Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP et al (2017) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551:648–652
Dong XY, Azzam MM, Zou XT (2016) Effects of dietary L-isoleucine on laying performance and immunomodulation of laying hens. Poult Sci 95:2297–2305
Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ et al (2010) The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 6:e1001097
Fan XX, Li S, Wu ZL, Dai ZL, Li J, Wang XL, Wu G (2019) Glycine supplementation to breast-fed piglets attenuates postweaning jejunal epithelial apoptosis: a functional role of CHOP signaling. Amino Acids 51:463–473
Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuille D, Obled C, Corthesy-Theulaz I (2006) Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr 136:1558–1564
Feng L, Li W, Liu Y, Jiang WD, Kuang SY, Jiang J, Tang L, Wu P, Tang WN et al (2015a) Dietary phenylalanine-improved intestinal barrier health in young grass carp (Ctenopharyngodon idella) is associated with increased immune status and regulated gene expression of cytokines, tight junction proteins, antioxidant enzymes and related signalling molecules. Fish Shellfish Immunol 45:495–509
Feng L, Luo JB, Jiang WD, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Zhang YA et al (2015b) Changes in barrier health status of the gill for grass carp (Ctenopharyngodon idella) during valine deficiency: regulation of tight junction protein transcript, antioxidant status and apoptosis-related gene expression. Fish Shellfish Immunol 45:239–249
Feng L, Gan L, Jiang WD, Wu P, Liu Y, Jiang J, Tang L, Kuang SY, Tang WN et al (2017) Gill structural integrity changes in fish deficient or excessive in dietary isoleucine: towards the modulation of tight junction protein, inflammation, apoptosis and antioxidant defense via NF-kappaB, TOR and Nrf2 signaling pathways. Fish Shellfish Immunol 63:127–138
Fernandez Miyakawa ME, Pistone Creydt V, Uzal FA, McClane BA, Ibarra C (2005) Clostridium perfringens enterotoxin damages the human intestine in vitro. Infect Immun 73:8407–8410
Fuentes S, van Nood E, Tims S, Heikamp-de Jong I, ter Braak CJ, Keller JJ, Zoetendal EG, de Vos WM (2014) Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J 8:1621–1633
Green KJ, Simpson CL (2007) Desmosomes: new perspectives on a classic. J Invest Dermatol 127:2499–2515
Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H (2009) The ETS-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137:1333–1345
Gu M, Bai N, Xu B, Xu X, Jia Q, Zhang Z (2017) Protective effect of glutamine and arginine against soybean meal-induced enteritis in the juvenile turbot (Scophthalmus maximus). Fish Shellfish Immunol 70:95–105
He L, Li H, Huang N, Tian J, Liu Z, Zhou X, Yao K, Li T, Yin Y (2016) Effects of alpha-Ketoglutarate on glutamine metabolism in piglet enterocytes in vivo and in vitro. J Agric Food Chem 64:2668–2673
Heuberger J, Kosel F, Qi J, Grossmann KS, Rajewsky K, Birchmeier W (2014) Shp2/MAPK signaling controls goblet/paneth cell fate decisions in the intestine. Proc Natl Acad Sci U S A 111:3472–3477
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535:75–84
Hou YQ, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Hou YQ, Wu G (2018) L-glutamate nutrition and metabolism in swine. Amino Acids 50:1497–1510
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Hou YQ, Wu ZL, Dai ZL, Wang GH, Wu G (2017) Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8:24
Islam J, Sato S, Watanabe K, Watanabe T, Ardiansyah, Hirahara K, Aoyama Y, Tomita S, Aso H et al (2017) Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J Nutr Biochem 42:43–50
Ivanov AI, Naydenov NG (2013) Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol 303:27–99
Ji Y, Guo Q, Yin Y, Blachier F, Kong X (2018) Dietary proline supplementation alters colonic luminal microbiota and bacterial metabolite composition between days 45 and 70 of pregnancy in Huanjiang mini-pigs. J Anim Sci Biotechnol 9:18
Jiang WD, Deng YP, Liu Y, Qu B, Jiang J, Kuang SY, Tang L, Tang WN, Wu P et al (2015) Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 444:134–142
Jiang WD, Feng L, Qu B, Wu P, Kuang SY, Jiang J, Tang L, Tang WN, Zhang YA et al (2016) Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-kappaB signaling in fish. Fish Shellfish Immunol 56:111–122
Jiang J, Yin L, Li JY, Li Q, Shi D, Feng L, Liu Y, Jiang WD, Wu P et al (2017a) Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol 70:473–484
Jiang WD, Deng YP, Zhou XQ, Liu Y, Jiang J, Kuang SY, Tang L, Tang WN, Wu P et al (2017b) Towards the modulation of oxidative damage, apoptosis and tight junction protein in response to dietary leucine deficiency: a likely cause of ROS-induced gill structural integrity impairment. Fish Shellfish Immunol 70:609–620
Jiao N, Wu Z, Ji Y, Wang B, Dai Z, Wu G (2015) L-glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr 145:2258–2264
Johansson ME, Hansson GC (2016) Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 16:639–649
Kepert I, Fonseca J, Muller C, Milger K, Hochwind K, Kostric M, Fedoseeva M, Ohnmacht C, Dehmel S et al (2017) D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol 139:1525–1535
Kim CJ, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y (2009) L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta 1790:1161–1169
Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD (2015) Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol 8:198–210
Le Floc’h N, Wessels A, Corrent E, Wu G, Bosi P (2018) The relevance of functional amino acids to support the health of growing pigs. Anim Feed Sci Technol 245:104–116
Lee WJ, Hase K (2014) Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol 10:416–424
Li P, Wu G (2018) Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li N, Lewis P, Samuelson D, Liboni K, Neu J (2004) Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol 287:G726–G733
Li P, Yin YL, Li DF, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li W, Sun K, Ji Y, Wu Z, Wang W, Dai Z, Wu G (2016) Glycine regulates expression and distribution of claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J Nutr 146:964–969
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542.
Li XL, Zheng SX, Wu G (2020) Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 52:671–691
Liang HW, Dai ZL, Ma XS, Liu N, Ji Y, Chen JQ, Zhang YC, Yang Y, Li J et al (2018) Dietary L-tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front Microbiol 9:1736
Liang HW, Dai ZL, Kou J, Sun KJ, Chen JQ, Yang Y, Wu G, Wu ZL (2019) Dietary l-tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: implication of tryptophan-metabolizing microbiota. Int J Mol Sci 20:20
Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, Yin Y, Yi G, Shi J et al (2008) Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr 100:552–560
Liu G, Ren W, Fang J, Hu CA, Guan G, Al-Dhabi NA, Yin J, Duraipandiyan V, Chen S et al (2017a) L-glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids 49:1945–1954
Liu W, Mi S, Ruan Z, Li J, Shu X, Yao K, Jiang M, Deng Z (2017b) Dietary tryptophan enhanced the expression of tight junction protein ZO-1 in intestine. J Food Sci 82:562–567
Liu SQ, Wang LY, Liu GH, Tang DZ, Fan XX, Zhao JP, Jiao HC, Wang XJ, Sun SH et al (2018) Leucine alters immunoglobulin a secretion and inflammatory cytokine expression induced by lipopolysaccharide via the nuclear factor-kappaB pathway in intestine of chicken embryos. Animal 12:1903–1911
Liu BM, Jiang XR, Cai L, Zhao XM, Dai ZL, Wu G, Li XL (2019a) Putrescine mitigates intestinal atrophy through suppressing inflammatory response in weanling piglets. J Anim Sci Biotechnol 10:69
Liu YY et al. (2019b) Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am J Physiol Gastrointest Liver Physiol 317:G824–G838
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12:661–672
Luissint AC, Parkos CA, Nusrat A (2016) Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151:616–632
Luo JB, Feng L, Jiang WD, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Zhang YA et al (2014) The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol 40:197–207
Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, Backhed F, Nielsen J (2015) The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 11:834
Martins HA, Sehaber CC, Hermes-Uliana C, Mariani FA, Guarnier FA, Vicentini GE, Bossolani GD, Jussani LA, Lima MM et al (2016) Supplementation with L-glutamine prevents tumor growth and cancer-induced cachexia as well as restores cell proliferation of intestinal mucosa of Walker-256 tumor-bearing rats. Amino Acids 48:2773–2784
McCauley HA, Guasch G (2015) Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 21:492–503
McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483:345–349
Mine Y, Zhang H (2015) Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of L-amino acids in intestinal epithelial cells. J Agric Food Chem 63:9987–9995
Mukherjee S, Hooper LV (2015) Antimicrobial defense of the intestine. Immunity 42:28–39
Mullin JM, Skrovanek SM, Valenzano MC (2009) Modification of tight junction structure and permeability by nutritional means. Ann N Y Acad Sci 1165:99–112
Nakanishi Y, Reina-Campos M, Nakanishi N, Llado V, Elmen L, Peterson S, Campos A, De SK, Leitges M et al (2016) Control of Paneth cell fate, intestinal inflammation, and tumorigenesis by PKClambda/iota. Cell Rep 16:3297–3310
Noah TK, Kazanjian A, Whitsett J, Shroyer NF (2010) SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res 316:452–465
Nowarski R, Jackson R, Flavell RA (2017) The stromal intervention: regulation of immunity and inflammation at the epithelial-Mesenchymal barrier. Cell 168:362–375
Ochi T, Feng Y, Kitamoto S, Nagao-Kitamoto H, Kuffa P, Atarashi K, Honda K, Teitelbaum DH, Kamada N (2016) Diet-dependent, microbiota-independent regulation of IL-10-producing lamina propria macrophages in the small intestine. Sci Rep 6:27634
Pabst O, Cerovic V, Hornef M (2016) Secretory IgA in the coordination of establishment and maintenance of the microbiota. Trends Immunol 37:287–296
Pacheco RG, Esposito CC, Muller LC, Castelo-Branco MT, Quintella LP, Chagas VL, de Souza HS, Schanaider A (2012) Use of butyrate or glutamine in enema solution reduces inflammation and fibrosis in experimental diversion colitis. World J Gastroenterol 18:4278–4287
Pai MH, Liu JJ, Yeh SL, Chen WJ, Yeh CL (2014) Glutamine modulates acute dextran sulphate sodium-induced changes in small-intestinal intraepithelial gammadelta-T-lymphocyte expression in mice. Br J Nutr 111:1032–1039
Pai MH, Shih YM, Shih JM, Yeh CL (2015) Glutamine modulates changes in intestinal intraepithelial gammadeltaT-lymphocyte expressions in mice with ischemia/reperfusion injury. Shock 44:77–82
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E et al (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535:376–381
Porter EM, Bevins CL, Ghosh D, Ganz T (2002) The multifaceted Paneth cell. Cell Mol Life Sci 59:156–170
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J et al (2014) Alterations of the human gut microbiome in liver cirrhosis. Nature 513:59–64
Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR (2010) Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21:1200–1213
Ramalingam A, Wang X, Gabello M, Valenzano MC, Soler AP, Ko A, Morin PJ, Mullin JM (2010) Dietary methionine restriction improves colon tight junction barrier function and alters claudin expression pattern. Am J Physiol Cell Physiol 299:C1028–C1035
Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, Machiah DK, Lawson B, Hakimpour P et al (2016) The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 531:523–527
Ren W, Yin Y, Liu G, Yu X, Li Y, Yang G, Li T, Wu G (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Ren W, Li Y, Yu X, Luo W, Liu G, Shao H, Yin Y (2013a) Glutamine modifies immune responses of mice infected with porcine circovirus type 2. Br J Nutr 110:1053–1060
Ren W, Liu S, Chen S, Zhang F, Li N, Yin J, Peng Y, Wu L, Liu G, Yin Y, Wu G (2013b) Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 45:947–955
Ren W, Luo W, Wu M, Liu G, Yu X, Fang J, Li T, Yin Y, Wu G (2013c) Dietary L-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids 45:479–488
Ren W, Zou L, Li N, Wang Y, Liu G, Peng Y, Ding J, Cai L, Yin Y, Wu G (2013d) Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. Br J Nutr 109:867–872
Ren W, Chen S, Yin J, Duan J, Li T, Liu G, Feng Z, Tan B, Yin Y, Wu G (2014a) Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 144:988–995
Ren W, Duan J, Yin J, Liu G, Cao Z, Xiong X, Chen S, Li T, Yin Y, Hou Y, Wu G (2014b) Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 46:2403–2413
Ren W, Yin J, Wu M, Liu G, Yang G, Xion Y, Su D, Wu L, Li T et al (2014c) Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 9:e88335
Ren M, Zhang S, Liu X, Li S, Mao X, Zeng X, Qiao S (2016a) Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous beta-defensin expression through the Sirt1/ERK/90RSK pathway. J Agric Food Chem 64:3371–3379
Ren W, Wang K, Yin J, Chen S, Liu G, Tan B, Wu G, Bazer FW, Peng Y, Yin Y (2016b) Glutamine-induced secretion of intestinal secretory immunoglobulin a: a mechanistic perspective. Front Immunol 7:503
Ren W, Yin J, Duan J, Liu G, Tan B, Yang G, Wu G, Bazer FW, Peng Y, Yin Y (2016c) mTORC1 signaling and IL-17 expression: defining pathways and possible therapeutic targets. Eur J Immunol 46:291–299
Ren W, Yin J, Xiao H, Chen S, Liu G, Tan B, Li N, Peng Y, Li T et al (2016d) Intestinal microbiota-derived GABA mediates interleukin-17 expression during enterotoxigenic escherichia coli infection. Front Immunol 7:685
Ren W, Liu G, Chen S, Yin J, Wang J, Tan B, Wu G, Bazer FW, Peng Y, Li T, Reiter RJ, Yin Y (2017a) Melatonin signaling in T cells: functions and applications. J Pineal Res 62:e12394
Ren W, Liu G, Yin J, Tan B, Wu G, Bazer FW, Peng Y, Yin Y (2017b) Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis 8:e2757
Ren W, Xia Y, Chen S, Wu G, Bazer FW, Zhou B, Tan B, Zhu G, Deng J, Yin Y (2019a) Glutamine metabolism in macrophages: a novel target for obesity/type 2 diabetes. Adv Nutr 10:321–330
Ren W, Liao Y, Ding X, Jiang Y, Yan J, Xia Y, Tan B, Lin Z, Duan J et al (2019b) Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol 12:531–544
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y (2015) Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 347:775–778
Salzman NH, Bevins CL (2013) Dysbiosis – a consequence of Paneth cell dysfunction. Semin Immunol 25:334–341
Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M et al (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11:76–83
Sasabe J, Miyoshi Y, Rakoff-Nahoum S, Zhang T, Mita M, Davis BM, Hamase K, Waldor MK (2016) Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat Microbiol 1:16125
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418
Schulz O, Pabst O (2013) Antigen sampling in the small intestine. Trends Immunol 34:155–161
Seekatz AM, Young VB (2014) Clostridium difficile and the microbiota. J Clin Invest 124:4182–4189
Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, Young VB (2014) Recovery of the gut microbiome following fecal microbiota transplantation. MBio 5:e00893–e00814
Sellmann C, Degen C, Jin CJ, Nier A, Engstler AJ, Hasan Alkhatib D, De Bandt JP, Bergheim I (2017a) Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 49:1215–1225
Sellmann C, Jin CJ, Engstler AJ, De Bandt JP, Bergheim I (2017b) Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur J Nutr 56:2519–2527
Song Z, Tong G, Xiao K, Jiao le F, Ke Y, Hu C (2016) L-cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-kappaB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun 22:152–161
Stoffels B, Turler A, Schmidt J, Nazir A, Tsukamoto T, Moore BA, Schnurr C, Kalff JC, Bauer AJ (2011) Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil 23:76–87
Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF et al (2014) Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510:417–421
Tan B, Xiao H, Xiong X, Wang J, Li G, Yin Y, Huang B, Hou Y, Wu G (2015) L-arginine improves DNA synthesis in LPS-challenged enterocytes. Front Biosci (Landmark Ed) 20:989–1003
Thaiss CA, Zmora N, Levy M, Elinav E (2016) The microbiome and innate immunity. Nature 535:65–74
Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Young VB (2014) Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:3114
Tossou MC, Liu H, Bai M, Chen S, Cai Y, Duraipandiyan V, Liu H, Adebowale TO, Al-Dhabi NA et al (2016) Effect of high dietary tryptophan on intestinal morphology and tight junction protein of weaned pig. Biomed Res Int 2016:1–6
Trevisi P, Corrent E, Mazzoni M, Messori S, Priori D, Gherpelli Y, Simongiovanni A, Bosi P (2015) Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli K88ac. J Anim Physiol Anim Nutr (Berl) 99:511–520
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Veshnyakova A, Protze J, Rossa J, Blasig IE, Krause G, Piontek J (2010) On the interaction of Clostridium perfringens enterotoxin with claudins. Toxins (Basel) 2:1336–1356
Wang WW, Wu ZL, Dai ZL, Yang Y, Wang JJ, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wang W, Wu Z, Lin G, Hu S, Wang B, Dai Z, Wu G (2014a) Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr 144:1540–1548
Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu G (2014b) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W, Wang B, He B, Zhang Q, Dai Z, Wu Z (2015a) L-tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr 145:1156–1162
Wang H, Zhang C, Wu G, Sun Y, Wang B, He B, Dai Z, Wu Z (2015b) Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J Nutr 145:25–31
Wang J, Li GR, Tan BE, Xiong X, Kong XF, Xiao DF, Xu LW, Wu MM, Huang B et al (2015c) Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets. J Anim Sci 93:1679–1688
Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G (2016) L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr 146:501–508
Wang Y, Liu L, Moore DJ, Shen X, Peek RM, Acra SA, Li H, Ren X, Polk DB, Yan F (2017) An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol 10:373–384
Wang B, Sun SQ, Liu MY, Chen H, Liu N, Wu G et al. (2020) Dietary L-tryptophan supplementation regulates colonic serotonin homeostasis and inhibits gut inflammation in mice with dextran sodium sulfate-induced colitis. J Nutr 150:1966–1976
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN et al (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156:1045–1059
Wu G (1995) Urea synthesis in enterocytes of developing pigs. Biochem J 312:717–723
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G (2010) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (1996) Effects of concanavalin A and phorbol myristate acetate on glutamine metabolism and proliferation of porcine intraepithelial lymphocytes. Comp Biochem Physiol A 114:363–368
Wu G et al. (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton, FL
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton, FL
Wu M, Xiao H, Liu G, Chen S, Tan B, Ren W, Bazer FW, Wu G, Yin Y (2016) Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol Nutr Food Res 60:1637–1648
Wu CH, Ko JL, Liao JM, Huang SS, Lin MY, Lee LH, Chang LY (2019a) Ou CC D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther Adv Med Oncol 11:1758835918821021
Wu ZL, Hou YQ, Dai ZL, Hu CA, Wu G (2019b) Metabolism, nutrition and redox signaling of hydroxyproline. Antioxid Redox Signal 30:674–682
Xia Y, Chen S, Zeng S, Zhao Y, Zhu C, Deng B, Zhu G, Yin Y, Wang W, Hardeland R, Ren W (2019) Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res 66:e12547
Xu T, Stewart KM, Wang X, Liu K, Xie M, Ryu JK, Li K, Ma T, Wang H et al (2017) Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548:228–233
Ye JL, Gao CQ, Li XG, Jin CL, Wang D, Shu G, Wang WC, Kong XF, Yao K, Yan HC, Wang XQ (2016) EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 7:38681–38692
Yi D, Li BC, Hou YQ, Wang L, Zhao D, Chen HB, Wu T, Zhou Y, Ding BY, Wu G (2018) Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino Acids 50:1089–1100
Yin J, Han H, Li Y, Liu Z, Zhao Y, Fang R, Huang X, Zheng J, Ren W et al (2017) Lysine restriction affects feed intake and amino acid metabolism via gut microbiome in piglets. Cell Physiol Biochem 44:1749–1761
Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS (2004) Human neonatal fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20:769–783
Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE et al (2006) Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 116:2142–2151
Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, Li W, Wei H, Liu Z (2015) Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol 16:918–926
Zhang B, Lv Z, Li H, Guo S, Liu D, Guo Y (2017) Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Br J Nutr 118:321–332
Zhang H, Chen Y, Li Y, Zhang T, Ying Z, Su W, Zhang L, Wang T (2019) L-threonine improves intestinal mucin synthesis and immune function of intrauterine growth-retarded weanling piglets. Nutrition 59:182–187
Zhou Y, Rychahou P, Wang Q, Weiss HL, Evers BM (2015) TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis 6:e1631
Zhou X, Zhang Y, He L, Wan D, Liu G, Wu X, Yin Y (2017) Serine prevents LPS-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and AMPK activation. J Funct Foods 39:225–232
Zhou X, Zhang Y, Wu X, Wan D, Yin Y (2018) Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol Biochem 48:993–1002
Acknowledgements
Work in our laboratories was supported by National Natural Science Foundation of China grants (31872365 and 31790411), the Innovation Team Project at Universities of Guangdong Province (2017KCXTD002), and Texas A&M AgriLife Research (H-8200). We thank Mr. Yaoyao Xia for assistance in preparing Fig. 8.1.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ren, W., Bin, P., Yin, Y., Wu, G. (2020). Impacts of Amino Acids on the Intestinal Defensive System. In: Wu, G. (eds) Amino Acids in Nutrition and Health. Advances in Experimental Medicine and Biology, vol 1265. Springer, Cham. https://doi.org/10.1007/978-3-030-45328-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-45328-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45327-5
Online ISBN: 978-3-030-45328-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)