Abstract
Grusonia bradtiana “viejito” (old man cactus) is an endemic species from Cuatro Ciénegas Basin and nearby areas. Grusonia includes 17 clonal species distributed along North American deserts which grow in dense cushion or shrubs. Grusonia bradtiana reproduces sexually by flowering and fruiting, forming seeds with new genetic combinations (new genets). The species clones by fragmentation of stems of different sizes that root independently, producing genetically identical offspring (ramets). Clonal species develop complex reproductive interactions as pollination output depends on pollen transfer between genetically different genets or identical ramets. The hypothesis is that clonality negatively affects sexual reproduction as floral traits are adaptations to promote cross-pollination (among genets) and have evolved to reduce negative effects of inbreeding. We studied the reproductive biology of Grusonia bradtiana and assessed the effect of clonality upon its reproductive success with controlled pollination. We also determined the frequency and taxonomic identity of floral visitors, to assess the pollination syndrome. Flowering occurs once a year during spring. Flowers are diurnal with a life span of 8 h; they are yellow, with radial symmetry, yellow-white lobulated stigma, and produce nectar. Flowers have thigmonastic stamens with red filaments supporting anthers that contain high amount of viable pollen. The flower is perfect, there is no separation of sexual functions in time (dichogamy), but there is in space (herkogamy), attributes that allow selfing and may reduce sexual interference, respectively. The fruit is dry, possibly a trait unique to Grusonia. Pollinators are solitary bees (Diadasia and Melissodes), a melittophily pollination syndrome. The species require the pollinator services to set fruit and seeds, and it suffers inbreeding depression if self-pollinated, so pollination among ramets decreases seed set, representing a cost of clonality. The selfing rate is high when plants are big or if clonality is frequent, as pollinators tend to visit nearby flowers increasing geitonogamy. The species has a mixed mating system with an outcrossing tendency, where delayed selfing (autogamy and geitonogamy) is a mechanism that ensure reproduction when outcrossing fails.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Reproduction in Plants
Several species that inhabit harsh environments display facultative reproduction whereby new offspring are recruited either by sexual and or by clonal means (Harper 1977; Abrahamson 1980). In clonal species, sexual reproduction promotes the production of genetically distinct seeds that are usually capable of long distance dispersal, which potentially will establish genetically new individuals (i.e., genets; Harper 1977; Harder and Barrett 1995), while clonality produces offspring that are an exact genetic copy of the parent plant (i.e., ramets; Harper 1977), usually with limited dispersal (García-Morales et al. 2018), which can be susceptible to diseases or changes in environmental conditions (Harper 1977).
Among angiosperms, a reproductive or sexual system is constituted by the breeding, mating, and pollination systems (Barrett 2013); the first refers to the arrangement in space and time of reproductive structures, while the mating system refers to how and with whom plants mate (Holsinger 2000). Reproductive success of most flowering plants depends on pollination mediated by animals or by abiotic vectors, including bees, mammals, birds, water, and wind. In species pollinated by animals, the frequency of selfing (i.e., autogamy—self-pollination of an hermaphrodite flower and geitonogamy—pollination among flowers of the same plant, de Jong et al. 1993), outcrossing, and final sexual reproductive output are usually dependent on flower phenology (Goulson 2000; Ishii and Sakai 2002), floral displays (Harder and Barrett 1995), floral rewards (Golubov et al. 1999), and resource limitation (Piña et al. 2007) that affect pollinator foraging behavior and in this way determine the amount and destiny of transported pollen (e.g., Snow et al. 1996; Eckert 2000). Hence, pollinator mediated selection of reproductive traits is often considered the main factor that drives evolution of sexual systems (Barrett 2013). Furthermore, diversification in lineages and life history traits in some angiosperm’s families as Agavaceae, Orchidaceae, and Cactaceae are attributed to their reproductive versatility (Mandujano et al. 2010; Barrett 2013).
Plants tend to produce much more flowers than fruits (Stephenson 1981), but some species in Cactaceae have high fruit set - frequency of a hermaphrodite or pistillate flowers to become fruit (ca. 50–98%; Mandujano et al. 1996, 2010) in comparison with other angiosperms (ca. >0–35%; Sutherland 1987), which has been related to the constancy and behavior of pollinators (Mandujano et al. 1996; Pimienta-Barrios and Del Castillo 2002), but may also be due to a large store and availability of resources in these species. For example, small to medium sized solitary bees are usually the main pollinators of several species of Opuntioideae subfamily (Cactaceae) (Grant and Grant 1979; Grant et al. 1979; Ordway 1987; Del Castillo and González-Espinosa 1988; McFarland et al. 1989; Mandujano et al. 1996), and of these bees, the genus Diadasia is one of the most important because of its behavior, size, and abundance (Mandujano et al. 2010). These solitary bees can move from 50 m up to 200 m from their nesting areas (Schlising 1972; Neff et al. 1982; Piña et al. 2007) which is assumed as the distance of pollen transfer (Piña et al. 2007).
The flowers of Opuntioideae species are diurnal and floral life span is from one to 3 days; they open early in the morning and close in the afternoon (Trujillo-Argueta and González-Espinosa 1991; Mandujano et al. 1996; Pimienta-Barrios and Del Castillo 2002). Floral presence is usually restricted to spring, before the rainy season (Grant and Grant 1979; Grant et al. 1979; McFarland et al. 1989; Mandujano et al. 1996). Most Opuntioideae species are hermaphrodite with perfect functional flowers, and dichogamy is absent (Reyes-Agüero et al. 2006). Even when pollen is released before the stigma becomes receptive, several species seem to be self-compatible with a mixed mating system where fruiting relays on pollinators (Mandujano et al. 2010).
All Opuntioideae species produce clonal offspring through some mechanism: agamospermy (seeds), stems (joints—cylindrical stems or cladodes—flattened stems), roots, or plantlets (Mandujano et al. 1996; Palleiro et al. 2006; Carrillo-Ángeles et al. 2011), and clonality is a common strategy to establish crops, for example, several prickly pear species with economic importance are cultivated by planting artificially selected cladodes (Reyes-Agüero et al. 2006); and in the wild, there are extreme cases in which the species sustain the population by clonality as sexual reproduction no longer exist (Grant and Grant 1980), but is more common that clonal species have populations with intermingled individuals of sexual and clonal origin that generate complex clonal architectures at variable scales (Carrillo-Ángeles et al. 2011; García-Morales et al. 2018).
Clonality affects population size, effective population size, density, clonal architecture, and sexual reproduction (Eckert 2000; Charpentier 2002; Carrillo-Ángeles et al. 2011; García-Morales et al. 2018). Furthermore, it is proposed that geitonogamy will increase and reduce plant fitness (Handel 1985; Eckert 2000; Charpentier 2002). Sexual reproduction is affected by clonality as transfer of pollen occurs at different scales: within a flower (intra-flower self-pollination), between flowers of the same plant (geitonogamy), between flowers of spatially independent plants that share the same genotype— ramets (inter-ramet geitonogamy) or among flowers of genetically different plants (cross pollen, outcrossing between genets) (Handel 1985; Mandujano et al. 1996; Eckert 2000). It is expected that any combination of self-pollination will negatively affect the progeny in comparison with outcrossing, that is, it will cause inbreeding depression (Charlesworth and Charlesworth 1987).
In this chapter we describe the reproductive biology and the floral visitors of the clonal cactus Grusonia bradtiana (Opuntioideae, Cactaceae), to determine the effect of clonality upon its sexual reproductive success. We determine the implications that both floral biology and breeding systems can have in the life cycle of this endemic cactus, which in turn is very useful to establish management strategies for this species.
Grusonia bradtiana
Grusonia is a genus that comprises 17 species which are distributed along North American deserts, and Mexico is the center of diversity with 13 species (Guzmán et al. 2003). The boundaries of this genus are not yet clear, but seed studies, and morphological, cytological, and molecular analyses suggest that they are a separate group (Anderson 2001; Pinkava 2002; Guzmán et al. 2003). Bárcenas et al. (2011) confirm that Grusonia bradtiana is not within the Opuntia clade, but it belongs to a polyphyletic unresolved group with other genera of tribe Cylindropuntieae (Cylindropuntia -chollas’ group, Grusonia, Corynopuntia, and Pereskiopsis). All species of Grusonia form dense cushion plants or shrubs and as other species that inhabit unpredictable environments, they exhibit sexual and clonal reproduction.
Grusonia bradtiana (J. M. Coult.) Britton & Rose [= Opuntia bradtiana (J. M. Coult.) Brandegee] (Guzmán et al. 2003), locally known as “viejito,” and in English as “old man cactus,” are short-branched cacti that can form dense shrubs (Fig. 5.1). This cactus reproduces sexually by flowering and fruiting, each fruit contains around 20 seeds (Rosas Barrera et al. 2020, this volume), and a sexual offspring has a new genetic combination (genet, Harper 1977). The species clones by fragmentation of stems (Rosas Barrera et al. 2020, this volume), this generate newborns of different sizes that root independently, and clonal offspring (ramet) has identical genetic composition as parent plant (Harper 1977). Stems are light green, of about 1 m height, from 4 to 7 cm in diameter, with 8–10 short, longitudinal, and tuberculated ribs. Areoles are 3–5 mm in diameter with white wool when young. Leaves, which soon decay, are succulent and green. Yellowish brown spines are found when young, turning white with age. Flowers are yellow, typical of Opuntioideae, 3–4 cm long (Bravo-Hollis 1978) (Fig. 5.2a,b).
Flower of Grusonia bradtiana. Segments of perianth are bright yellow (i.e., petals). It has numerous stamens with red filaments and yellow anthers containing abundant pollen, the yellow lobulated stigma is located at flower’s center (a). Photograph by María C. Mandujano. Longitudinal section of a flower fixed with FAA (10 : 50 : 5 : 35 formalin, 95% ethanol,acetic acid, distilled water) (b), Letters indicate A: stigma, B: anthers and C: ovary chamber with numerous ovules. Photograph by Lucía Plasencia-López
Grusonia bradtiana and Grusonia moelleri are endemic species of CCB and contiguous areas of Mapimi in the Chihuahuan desert; locally abundant in calcareous soils (Bravo-Hollis 1978; Pinkava 1984; Mandujano and Golubov 2000; Guzmán et al. 2003; Martínez-Ávalos et al. 2020, this volume). The study site in CCB, 8 km south from La Becerra at San Marcos y Pinos (26° 45′ 47.8″ N and at 102° 9′ 10.3″ W), has a mean annual precipitation of 200 mm and a temperature that ranges from 0 °C in winter to 44 °C in summer (Marsh 1984; Montiel González et al. 2018).
Grusonia bradtiana establishes in slopes between the alkaline floor of the basin characterized by pastures and gypsum dunes, and oak-pine forests in higher elevations with arid shrubs dominated by Larrea tridentata, Prosopis glandulosa var. torreyana, Acacia greggii, Fouquieria splendens, Suaeda mexicana, and S. suffruticosa (Pinkava 1984; Mandujano and Golubov 2000; Flores-Vázquez et al. 2020, this volume), and other succulents, like Opuntia rufida, Dasylirion sp., Echinocereus engelmannii, Agave lechuguilla, Epithelantha micromeris, Mammillaria pottsii, Euphorbia antisyphilitica, Cylindropuntia imbricata, C. leptocaulis, and Jatropha dioica (Pinkava 1984; Flores-Vázquez et al. 2020, this volume; Martínez-Ávalos et al. 2020, this volume).
Floral Behavior and Flower Production of Grusonia bradtiana in CCB
The flowering period of G. bradtiana was determined in six visits to the study site (March-April 2000, October 2000, June and October 2001, April 2017, and October 2017) and from data of herbaria specimens (MEXU and ENCB-IPN). During June 2001, we measured the diameter of the perianth (corolla) in one flower from each of 40 sampled plants, at six different times from 8:30 h, before the flowers become active, to 20:30 h, when flowers were already closed.
Grusonia bradtiana flowers from May to June, before the main rainy season, and mature fruits appeared in September and October (after the rainy season, Fig. 5.3). This flowering phenology follows the typical pattern of other Opuntioideae and most cacti, showing seasonality and displaying a flowering peak in a specific time of the year (Grant et al. 1979; Mandujano et al. 2010); although in some cactus genera flowering may extend throughout the year (e.g., Ferocactus histrix; Del Castillo 1988; F. robustus, Piña 2000).
Flowers of G. bradtiana were produced on the tip of the stem segments, each producing 1–3 flowers which showed a diurnal flowering cycle: anthesis started at 10:00–10:30 h (1.6 cm ± 0.1 S.E., corolla-perianth opening) and flowers closed at 18:00–18:30 h (1.1 cm ± 0.1 corolla opening), with maximum corolla aperture occurring between 12:30 and 14:30 h (Fig. 5.4). The flowers were active ca. 8 h for a single day. Similar reproductive patterns have been described in related Opuntioideae, such as Cylindropuntia imbricata, Opuntia basilaris, O. lindheimeri, O. rastrera, O. robusta, and O. spinosissima (Bravo-Hollis 1978; Grant et al. 1979; McFarland et al. 1989; Mandujano et al. 1996; Negrón-Ortiz 1998).
Flowers of G. bradtiana produce yellow and abundant pollen displaying thigmonastic stamens (i.e., the stamens always move inwards and toward the center (style) of the flower when are touched, Braam 2005; Cota-Sánchez et al. 2013) that could promote cross-pollination (Ren and Tang 2012) or self-pollination (Mandujano et al. 1996; Nagy et al. 1999), a phenomenon also reported in related Opuntioideae, including O. rastrera, O. brunneogemmia, O. viridirubra, and O. polyacantha (Mandujano et al. 1996; Schlindwein and Wittman 1997; Cota-Sánchez et al. 2013), but absent in O. cochenillifera and Brasiliopuntia brasiliensis (Cota-Sánchez et al. 2013). The stamens movement in G. bradtiana is triggered by activity of floral visitors or mechanical stimuli, in other species the movement is provoked by abiotic environmental stimuli like temperature or light as well as visitors; the stamens movement regulates the rate of pollen dispensation in species of Loasaceae, and thigmonastic stamens optimize pollen transfer (Henning and Weigend 2013).
A linear regression between the number of branches per plant against reproductive structures (fruits, flowers, and buds) showed that flower production in G. bradtiana increased with the plant size (N = 40; r2 = 0.66; P < 0.001). Small plants can have one to 20 stems, up to 4000 stems the largest. Plants start producing flowers when they have around 50 stems, but they can clone at any size (Rosas Barrera et al. 2020, this volume).
Fruits are formed rapidly after pollination in July, but they are ripe (i.e., dry and with mature seeds) by the end of September (Fig. 5.3). Fruits of G. bradtiana are dry or semidry and indehiscent, which contrast with many other Opuntioideae species with fleshy and sweet cactus pears (Reyes-Agüero et al. 2006). It is possible that other Grusonia species have dry fruits but most species descriptions lack of details of reproductive traits.
Breeding System and Pollen Viability in Grusonia bradtiana in CCB
The breeding system and the outcrossing index (OCI) were determined following Cruden (1977). The outcrossing index consists on an estimation of pollen:ovule (P:O) ratios and three characteristics of floral morphology and floral behavior. OCI is the sum of the assigned values of (1) the diameter of the flower (assigned to one of three classes; corolla up to 1 mm wide = 0, 1–6 mm wide = 2, more than 6 mm wide = 3), (2) temporal separation between sexual functions (dichogamy), where homogamy and protogyny received a value of 0 and protandry a value of 1, and (3) spatial relationship of stigma and anthers (herkogamy), if there was contact between stigma and anthers the value was 0, and if they were spatially separated and contact seemed unlikely the value was 1 (Cruden 1977). The spatial or temporal separation between anther dehiscence and stigma receptivity (herkogamy or dichogamy, respectively) was evaluated in one flower per plant in a sample of 40 plants in the spring 2001. The following measurements were made at maximum corolla aperture (i.e., from 12:30 h to 14:30 h): stamen and style length (cm), and corolla, as well as pericarpel size (cm) (Table 5.1, Fig. 5.2b). Each flower was observed every 2 h from 8:30 to 20:30 h to visually evaluate both stigma receptivity (humidity and stickiness of the stigma’s surface) and anther dehiscence (Mandujano et al. 1996). Finally, herkogamy was analyzed through a t-test of paired values of stamen and style lengths.
We did find herkogamy (spatial separation between sexual organs) but no dichogamy (temporal separation between sexual functions) in G. bradtiana, although some flowers present inverse herkogamy, that is, longer stamens than stigma (t = 4.81; df = 37; P < 0.0001; Barrett et al. 2000). Herkogamy is uncommon within Cactaceae but has been reported in Hylocereus undatus, Nopalea spp. and all species of Ariocarpus (Pimienta-Barrios and Del Castillo 2002; Martínez-Peralta et al. 2014). Herkogamy is usually interpreted as a floral adaptation that reduces pollen-stigma interference and as a mechanism to avoid self-pollination (Barrett et al. 2000) and to export pollen in self-incompatible species (Martínez-Peralta et al. 2014).
The stigma of some flowers was receptive 30 min after floral opening, and 60% of the flowers showed an apparently receptive stigma at 10:30 h. Anthers started releasing pollen at the same time (45%, 10:30 h) and after 2 h, all flowers had both receptive stigma and dehiscent anthers, thus showing absence of dichogamy.
Most cacti flowers are perfect (i.e., bisexual), even though some species show functional dioecism (i.e., flower that produce mainly pollen (male) and flowers that produce mainly seeds (female); Anderson 2001). The ancestral condition of the Opuntioideae and probably for all the Cactaceae is the presence of hermaphroditic flowers with mixed mating systems (Pimienta-Barrios and Del Castillo 2002).
Grusonia bradtiana flowers are homogamous, male and female organs mature at the same time inside the flower; like results were found in several Opuntioideae (Bravo-Hollis 1978; Grant et al. 1979; Grant and Grant 1979; Mandujano et al. 1996; Negrón-Ortíz 1998) and other cacti like Ferocactus robustus (Piña 2000). Homogamous flowers allow self-fertilization (Wyatt 1983), and particularly for G. bradtiana, as will be shown below, some individuals have autonomous pollination (Nagy et al. 1999).
Pollen-ovule ratio was estimated from a random sample of one flower from 32 different plants in spring of 2001 and 2002. The flower was longitudinal-sectioned, and the number of ovules counted from one half. The number of anthers per flower was counted from ¼ flower. We quantified the number of pollen grains from these 32 flowers by taking a closed anther from each flower. Each anther was placed in a 1.5 ml microtube and 1 ml of alcohol was added. Samples were homogenized by stirring and an aliquot of 10 μl of the solution was placed in a Neubauer chamber for quantification under a stereoscopic microscope. The relation of the number of pollen grains in 10 μl and in 1000 μl was calculated, and the number of anthers per flower was multiplied by the resulting value. The final P:O was the overall average of all sampled flowers (Kearns and Inouye 1993). Anthers produced large numbers of pollen grains (mean = 287, 023 ± SE = 48,760) per ovule (mean = 156 ± SE =12) in each flower giving a P/O = 2490 ± 601.
The final value of OCI for G. bradtiana is 4, with the following characteristics: flower diameter above 6 mm, spatial separation between sexes, as well as absence of protandry and protogyny. The P/O and the OCI indicated that the breeding system of G. bradtiana is facultative xenogamous. Species of Cactaceae tend to display high P/O ratios which is associated with pollen waste during transfer and with the amount of pollen that is consumed by vectors (Cruden 1977), such as bats in columnar cacti and solitary bees (use to provision their offspring) in Opuntioideae and Cactoideae subfamilies (Fleming et al. 1994; Mandujano et al. 2010; Camacho-Velázquez et al. 2016).
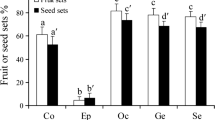
Pollen viability was estimated from a random sample of 15 plants from which one anther of a single flower was collected and placed in 1.5 ml microtubes. The percentage of viable pollen grains from the sampled pollen was obtained as follows: a quarter anther was sectioned and placed in a Petri dish with one drop of water, plus one of lactophenol-aniline blue stain (Kearns and Inouye 1993). After a minute, the pollen samples were observed in a stereoscopic microscope, viable (stained dark blue) and sterile pollen grains (stained light blue) were counted. In general, all sampled anthers showed a large amount of viable pollen grains (85% ± SE 13%). High pollen viability (stainability) correlates with successful set of fruit and seeds in controlled pollination (Dafni and Firmage 2001). Fruit set in control flowers was close to 100% and number of seeds was the highest (Fig. 5.5).
Mean (± s.d.) fruit set (a) and number of seeds per fruit (b) from manual pollination treatments on Grusonia bradtiana in Cuatro Ciénegas Basin, Coahuila, Mexico. 40 different plants were used in a randomized block design (N = 40 flowers per treatment). Pollination treatments were A = forced self-pollination, C = control, E = supplement of pollen, G = automatic self-pollination, T = geitonogamy, and X = outcrossing. Different letters above bars indicate groups that had a significant difference between them and groups with the same letter had not detectable difference (P < 0.005 see Table 5.2)
Mating System and Pollen Limitation in Grusonia bradtiana in CCB
Mating system of G. bradtiana was determined in a manual pollination experiment using a random sample of 40 plants (blocks), during reproductive season of spring 2001. Seven flowers in each plant were randomly assigned to one of the following treatments (Table 5.2 for explanation): apomixis (P), automatic autogamy- autonomous pollination (G), forced autogamy (A), geitonogamy (T), pollination of flowers with pollen of another flower, but from the same ramet, within genet, outcrossing (X) (between genets), supplemental cross pollen (E), and an untreated control (C, Table 5.2). Treatments T, G, and A involved self-pollination at different spatial scales.
Once a flower was treated, it was labeled with its corresponding treatment and attached with a string to the stem to avoid fruit removal by herbivores. Ripe fruits were collected 4 months later, from which we determined fruit set and count of seeds per fruit. Generalized linear models were used to compare seed set (with Poisson distribution) and fruit set percentages (binomial distribution) using the statistical software GLIM (Generalized Linear Models; Crawley 1993). Differences among treatments were calculated with contrast X2 evaluating each model without effect of each treatment (Crawley 1993). Inbreeding depression (δ) was estimated following Charlesworth and Charlesworth (1987) as δ = 1 − ws/wo, the inverse of the ratio of value of selfed fruit or seed (progeny, ws) to value for outcrossed progeny (wo); values towards 1 indicate high inbreeding depression and towards 0 that inbreeding depression is low.
We found significant differences in fruit and seed production among treatments (Fig. 5.5 a,b; Χ2 = 92.58; df = 5; P < 0.001; Χ2 = 1736; df = 5; P < 0.0001, respectively). Fruit and seed production were higher in cross-pollination treatments (X, C, and E) than in self-pollination treatments (A, G, and T, Fig. 5.5a) and fruit set in apomixis (P) was nil. The geitonogamy treatment (T), that is, pollination among flowers of the same ramet showed a significant higher seed production than the other self-pollination treatments, but lower than the cross-pollination treatments (Fig. 5.5a). Fruits that were produced via self-pollination produced less seeds than the fruits of outcrossing-pollination (Fig. 5.5b). Moreover, they were significantly different between contrasts involving the self-pollination group and outcrossing group treatments (Fig. 5.5b, Χ2 = 150; df = 1; P < 0.0001). Manual crossing experiments allowed to conclude that the species is self-compatible, as both fruits and seeds are produced by forced self-pollination (A) and by automatic autogamy (G) treatments, and do not need pollinators to develop some seeds, but more seed and fruit are always produced by outcrossing, involving pollinators. This is a pollination insurance strategy, where autonomous pollination allows the species to produce progeny under unpredictable conditions, being scarce of resources, partners, or pollinators (Nagy et al. 1999). However, there is a marked reduction on fruit set and seed loss by selfing compared with outcrossing as the species has high inbreeding depression as the estimated values are close to one (δ fruit set = 0.74, δ seed set = 0.85; Charlesworth and Charlesworth 1987). However, manual pollinations indicated that G. bradtiana is not limited by pollen and pollen transfer mediated by pollinators is efficient as control treatment showed the highest fruit and seed set. Even though G. bradtiana is self-compatible, as it produces some fruits and seeds by autogamy, self-pollination is clearly less successful than outcrossing (3 and 18 times less fruit and seeds, respectively). The production of significantly more seeds in the control treatment than in supplemental pollen treatment indicates no pollen limitation. Accordingly, Larson and Barrett (2000) reported that pollen limitation for 224 species they analyzed is less intense in self-compatible, autogamous, and nectariferous species than in self-incompatible, non-autogamous, and nectarless species.
Floral Visitors and Nectar Availability Grusonia bradtiana in CCB
Bee species that visited the flowers of G. bradtiana in the study area in CCB were collected, registering date and hour of collection during 2001. The specimens were identified according to Michener et al. (1994) and kept at the Museum of Zoology “Alfonso L. Herrera” at the Facultad de Ciencias (School of Sciences), Universidad Nacional Autónoma de México, Mexico City.
To determine the frequency and type of flower visitors of G. bradtiana we took a random sample of flowers within four areas with patches of reproductive plants. From each area we randomly selected ten flowers, in each of which we recorded the number of visits and the species of floral visitors. These observations were made during a single day from 10:30 h to 18:30 h in two-hour intervals lasting 20 min each, until we completed six and a half hours of observations. We calculated the proportions of visits by means of a contingency table (χ2) and adjusted residuals to determine if significant differences existed among visitors and hours of visits (Everitt 1977).
In addition, we approximated pollinator efficiency in seed production within the population using the same 40 followed flowers. At the end of the day we covered each flower with a fine mesh, and 4 months later we collected the fruits to determine seed production with respect to the number of visits. Data were analyzed using a non-parametric regression (Zar 1996) with JMP (version 3.2.1, SAS Institute 1995).
Flowers of G. bradtiana were visited as soon as they began to open (around 10:00 h, see Figs. 5.4 and 5.6). The flowers are visited by species of Hymenoptera, Coleoptera, and Orthoptera and especially by solitary bees from the genera Diadasia and Melissodes (Table 5.3). Diadasia sp. and Melissodes spp. (two species) were the most frequent visitors (552 visits), followed by Ashmeadiella sp. (92 visits) and Perdita sp. (25 visits). Diadasia, Melissodes, and Dianthidium showed typical pollinator behavior, landing on stigma before searching the base of the flower for nectar, causing their body to touch the anthers, nectar, and pollen assuring the uptake and deposition of pollen grains in the stigma of another flower, and due to their size, they necessarily made contact with the anthers and stigma of the flowers, visiting the flowers in a radius of approximately 10 m. Ashmeadiella and Perdita bees did not show clear contact with the stigmatic surface, but probably stimulate self-pollination by the thigmonastic sensitivity of the stamens (Table 5.3).
In Opuntia species, as well as in Echinocereus viridiflorus and Ferocactus histrix, a similar behavior has been observed and other large pollinators such as Lithurge, Megachile, Xylocopa, and Bombus have been reported to be effective pollinators for Opuntia species (Grant and Grant 1979; Grant et al. 1979; Leuck and Miller 1982; Simpson and Neff 1983; Del Castillo and González-Espinosa 1988; McFarland et al. 1989; Del Castillo 1994; Mandujano et al. 1996; Mandujano et al. 2010).
Beetles (Coleoptera) were also found within the flowers throughout the floral life span, but mostly at 16:00 h (Table 5.3). Sap beetles, family Nitidulidae, were abundant but do not promote self-pollination; visitors from this group stayed in the lower part of the perianth and it seems that they do not produce stamen movements. Sap beetles and grasshoppers were classified as nectar and/or pollen thieves and florivores (Table 5.3). Sap beetle behavior as nectar thieves has also been described for O. basilaris, O. lindheimeri, O. robusta, and F. histrix (Grant and Grant 1979; Grant et al. 1979; Rowley 1980; Del Castillo and González-Espinosa 1988; Del Castillo 1994) and can be considered parasites of Opuntia flowers (as it has been found for O. compressa and O. imbricata; McFarland et al. 1989). Grasshoppers were florivores because during the evening they eat flower anthers, a phenomenon also observed in Echinocactus platyacanthus and Ariocarpus trigonus (Mandujano et al. 2010; Cárdenas-Ramos and Mandujano 2018).
In G. bradtiana there were differences among visitor frequencies (Χ2 = 39.8, df = 8, P < 0.001), and visits are more frequent during the morning (195 visits at 10:30 h), when nectar production was the higher between 12:30 and 14:30 h (78 visits) and increases again after 16:30 h (Fig. 5.6a, b; 161 visits). High frequency of visits during the morning is clearly linked to nectar production. Medium and small-sized visitors of the genera Perdita and Ashmeadiella stimulate thigmonastic movements all day, but preferentially in the evening which could probably favor self-pollination in G. bradtiana, as has been seen in O. littoralis and O. rastrera (Grant and Grant 1979; Mandujano et al. 1996). In contrast with what happens in G. bradtiana, small bees like Ashmeadiella and Dialictus are considered nectar thieves in O. robusta (Del Castillo and González-Espinosa 1988).
Nectar is one of the most important rewards for pollinators (Nicolson 2007), so we quantified accumulated nectar in 70 randomly selected flowers. Nectar was measured using 5 μl capillary tubes, which were inserted into the nectar chamber. To assess the nectar’s consumption of the flower visitors, half of the flowers were covered with a fine mesh to keep visitors away from flowers and the other half (n = 35 flowers) remained uncovered. To avoid non-independence among samples, each flower was only used once. The quantity of nectar for each flower was quantified from seven flowers of each group from 10:30 to 20:30 h in two-hour intervals. Floral visitors consume nectar soon after flower opening. However, we found available nectar during all the floral cycle in uncovered flowers, suggesting that the rate of nectar production exceeds consumption. In covered flowers we found a decrease in nectar production at 12:30 h (Fig. 5.4b) followed by an increase between 16:30 and 18:30 h, which could be explained either by nectar evaporation or nectar reabsorption. Grusonia bradtiana is pollinated by bees and there is an important effect of frequency of visits, the more visits a flower experiments the more seeds it sets (Spearman Rho = 0.3528, P < 0.05). Bees are searching for rewards such as pollen and nectar, which are produced in abundance by all flowers.
General Discussion, Conclusions, and Perspectives
We found that the endemic cactus of the CCB and nearby desert areas G. bradtiana has a complex sexual system combining selfing and outcrossing. It is also able to self-fertilize without pollinators, but the production of seeds decreases considerably, so the species greatly benefits from animal pollination mediated by solitary bees. In addition, control pollination proves that sexual reproduction of the species is highly successful and selfing (autonomous pollination, forced selfing, and geitonogamy) decreases sexual output. Therefore, clonality may limit the production of fruits and seeds as is the case of other clonal species (Liao and Harder 2014) if pollen transfer occurs among genetic similar or identical partners. Our results also indicate that G. bradtiana has a mixed mating system with a tendency towards cross-fertilization, that is, a facultative xenogamous system (P/O = 2490.3,) according to P:O ratio indicated by Cruden (1977). Pollination experiments reinforced the premise that outcrossing is the dominant form of crossing in G. bradtiana, where it is favored by both the fact that pollinators are moving pollen among genets and adaptations to select outcross pollen.
Most cacti, including G. bradtiana, have several adaptations favoring outcrossing, meaning that most of the seeds are produced by pollen that comes from different individuals for fertilization, although self-pollination occurs in some groups (Ross 1981; Clark-Tapia and Molina-Freaner 2004; Mandujano et al. 2010; Camacho-Velázquez et al. 2016). Nevertheless, relatively few species have been studied, considering the large number of species in the family (less than 2% of 2000 species, Mandujano et al. 2010; Camacho-Velázquez et al. 2016) and results show a variety of breeding systems within the cactus family. For example, the genus Frailea and some species of Melocactus are cleistogamous, flowers do not open and self-fertilize (Anderson 2001) while, as we discussed above, dichogamy occurs in Hylocereus spp. cultivars (Mandujano et al. 2010; Camacho-Velázquez et al. 2016). Herkogamy has been reported for Ariocarpus genus (Martínez-Peralta et al. 2014). Trioecy where individuals in the populations have staminate-male, pistillate-female, and hermaphrodite flowers occurs in Pachycereus pringlei (Fleming et al. 1994; Cervantes 2001); functional dioecy, species that show incomplete morphological differentiation as appeared to be hermaphrodite flowers, yet they have complete functional differentiation between male and female flowers, is seen in Mammillaria dioica and Echinocereus coccineus (Bravo-Hollis and Sánchez-Mejorada 1991; Hoffman 1992); gynodioecy, where hermaphrodite and female plants coexist within a population, has been documented in M. blossfeldiana var. shurliana (Rebman 2001; Camacho-Velázquez et al. 2016) and dioecy in Opuntia stenopetala and Pereskia zinniflora (Anderson 2001; Reyes-Agüero et al. 2006).
Grusonia bradtiana is pollinated by bees, mainly by large bees of the genera Diadasia, Melissodes, and Dianthidium, and there is an important effect of frequency of visits, as more visited flowers produce more seeds. Bees visit the flowers by searching for rewards such as pollen and nectar that are produced in abundance by all Grusonia bradtiana flowers.
Bee pollination is very common within the Cactaceae (46% of the genera) and is considered a primitive feature of the family (Anderson 2001; Pimienta-Barrios and Del Castillo 2002; Wallace and Gibson 2002). Bee pollination is typical for the Pereskioideae and Opuntioideae subfamilies, but it is also important for the Cactoideae subfamily (41% of the species; Mandujano et al. 2010; Camacho-Velázquez et al. 2016). Floral visitors among cactus species correlate with life forms and floral features, which may promote specialized pollination by bats, birds, or insects (Rowley 1980; Anderson 2001; Pimienta-Barrios and Del Castillo 2002). In some genera, like Rebutia, pollination is by butterflies, and pollination by birds occurs in many genera whose flowers are zygomorphic (e.g., Cochemiea, Schlumbergera, and Cleistocactus, Rowley 1980; Anderson 2001); butterflies and birds may pollinate despite those functional groups being nectar consumers (Nicolson 2007), while bat pollination is most common in columnar life forms such as Pachycereus, Pilosocereus, Stenocereus, and Neobuxbaumia (Fleming et al. 1994; Nassar et al. 1997; Valiente-Banuet et al. 1997; Casas et al. 1999; Anderson 2001; McIntosh 2005).
Overall, clonality in Grusonia bradtiana imposes a cost as the species displays high inbreeding depression. Despite this, fruits and seeds are produced in all possible ways of selfing, but outcrossing results in a twofold advantage. Moreover, if pollination or resources are scarce, G. bradtiana still has reproductive opportunities, as some fruits and seeds will form, so in unpredictable environments, selfing provides a reproductive insurance for this species. Grusonia bradtiana is a very successful species, its longevity, diversity of reproductive strategies, and its high reproductive success partially explain why this cactus is a dominant species of bajadas at Cuatro Ciénegas Basin.
References
Abrahamson WG (1980) Demography and vegetative reproduction. In: Solbrig OT (ed) Demography and evolution in plant populations. University of California Press, Berkeley, CA, USA, pp 89–106
Anderson EF (2001) The Cactus family. Timber Press, Portland, OR, USA
Bárcenas RT, Yesson C, Hawkins JA (2011) Molecular systematics of the Cactaceae. Cladistics 27:470–489
Barrett SCH (2013) The evolution of plant reproductive systems: how often are transitions irreversible? Proc Royal Soc B 280:1–9
Barrett SCH, Jesson LK, Baker AM (2000) The evolution and function of stylar polymorphisms in flowering plants. Ann Bot 85:253–265
Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165:373–389
Bravo-Hollis H (1978) Las Cactáceas de México Volume 1. Universidad Nacional Autónoma de México, México, DF, Mexico
Bravo-Hollis H, Sánchez-Mejorada H (1991) Las Cactáceas de México Volume 2. Universidad Nacional Autónoma de México, México, DF, Mexico
Camacho-Velázquez A, Ríos-Carrasco S, Vázquez-Santana S (2016) Biología reproductiva de la subfamilia Cactoideae (Cactaceae). Cact Suc Mex 61(4):100–127
Cárdenas-Ramos D, Mandujano MC (2018) Florivory effects on pollinator preference and the reproductive output of a threatened living rock cactus, Ariocarpus retusus (Cactaceae). Haseltonia 25:1–7
Carrillo-Ángeles IG, Mandujano MC, Golubov J (2011) Influences of the genetic neighborhood on ramet reproductive success in a clonal desert cactus. Population Ecology 53:449–458
Casas A, Valiente-Banuet A, Rojas-Martínez A, Dávila P (1999) Reproductive biology and the process of domestication of the columnar cactus Stenocereus stellatus in Central Mexico. Am J Bot 86:534–542
Cervantes SM (2001) Variación geográfica en el sistema reproductivo de Pachycereus pringlei. Bachelor diss., Facultad de Ciencias UNAM, México, DF, Mexico
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Charpentier A (2002) Consequences of clonal growth for plant mating. Evol Ecol 15:521–530
Clark-Tapia R, Molina-Freaner F (2004) Reproductive ecology of the rare clonal cactus Stenocereus eruca in the Sonoran Desert. Plant Syst Evol 247:155–164
Cota-Sánchez H, Almeida OJG, Falconer DJ, Choi HJ, Bevan HL (2013) Intriguing thigmonastic (sensitive) stamens in the plains prickly pear Opuntia polyacantha (Cactaceae). Flora 208:381–389
Crawley MJ (1993) GLIM for ecologists. Blackwell Scientific Publication, Oxford, UK
Cruden RW (1977) Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31:32–46
Dafni A, Firmage D (2001) Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Syst Evol 222:113–132
Del Castillo RF (1988) Fenología y remoción de semillas en Ferocactus histrix. Cact Suc Mex 33:5–14
Del Castillo RF (1994) Polinización y otros aspectos de la biología floral de Ferocactus histrix. Cact Suc Mex 39:36–42
Del Castillo RF, González-Espinosa M (1988) Una interpretación evolutiva del polimorfismo sexual de Opuntia robusta (Cactaceae). Agrociencia 71:185–196
Eckert CG (2000) Contribution of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology 81:532–542
Everitt BS (1977) The analysis of contingency tables. Chapman and Hall, New York, NY, USA
Fleming TH, Mauricem S, Buchmann SL et al (1994) Reproductive biology and relative fitness in a trioecious cactus, Pachycereus pringlei (Cactaceae). Am J Bot 81:858–867
Flores-Vázquez JC, Rosa Barrera MD, Golubov J, Sánchez-Gallén I, Mandujano MC (2020) Ecological importance of Bajadas in the Chihuahuan Desert. In: Mandujano MC, Pisanty I, Eguiarte LE (eds) Plant diversity and Ecology in the Chihuahuan desert. Springer International, Cham, Switzerland
García-Morales E, Carrillo-Angeles IG, Golubov J et al (2018) Influence of fruit dispersal on genotypic diversity and migration rates of a clonal cactus from the Chihuahuan Desert. Ecol Evol 8:12559–12575
Golubov J, Mandujano MC, Franco M et al (1999) Demography of the invasive woody perennial Prosopis glandulosa (honey mesquite). J Ecol 87:955–962
Goulson D (2000) Why do pollinators visit proportionally fewer flowers in large patches? Oikos 91:485–492
Grant V, Grant KA (1979) Pollination of Opuntia basilaris and O. littoralis. Plant Syst Evol 132:321–325
Grant V, Grant KA (1980) Clonal microspecies of hybrid origin in the Opuntia lindheimeri group. Bot Gaz 141:101–106
Grant V, Grant KA, Hurd PD Jr (1979) Pollination of Opuntia lindheimeri and related species. Plant Syst Evol 132:313–320
Guzmán U, Arias S, Dávila P (2003) Catálogo de cactáceas mexicanas. Universidad Nacional Autónoma de México- Comisión Nacional para el conocimiento y uso de la biodiversidad, México, DF, Mexico
Handel SN (1985) The intrusion of clonal growth patterns on plant breeding systems. Am Nat 125:367–383
Harder LD, Barrett SCH (1995) Mating cost of large floral displays in hermaphrodite plants. Nature 373:512–515
Harper JL (1977) Population biology of plants. Academic Press, London, UK, 892 pp
Henning T, Weigend M (2013) Beautiful, complicated—and intelligent? Novel aspects of the thigmonastic stamen movement in Loasaceae. Plant Signal Behav 8(6):E24605. https://doi.org/10.4161/PSB.24605
Hoffman MT (1992) Functional dioecy in Echinocereus coccineus (Cactaceae): breeding systems, sex ratios, and geographic range of floral dimorphism. Am J Bot 79:1382–1388
Holsinger KE (2000) Reproductive systems and evolution in vascular plants. PNAS 97:7037–7042
Ishii HS, Sakai S (2002) Temporal variation in flower display size and individual floral sex allocation in racemes of Narthecium asiaticus (Liliaceae). Am J Bot 89:441–446
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Boulder, CO, USA
Larson BMH, Barrett CH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520
Leuck EE, Miller JM (1982) Pollination biology and chemotaxonomy of the Echinocereus viridiflorus complex (Cactaceae). Am J Bot 69:1669–1672
Liao W, Harder LD (2014) Consequences of multiple inflorescences and clonality for pollinator behavior and plant mating. Am Nat 184:580–592
Mandujano MC, Golubov J (2000) Opuntia bradtiana en la zona calcárea del Bolsón de Mapimí México. Cact Suc Mex 45:66–68
Mandujano MC, Montaña C, Eguiarte LE (1996) Reproductive ecology and inbreeding depression in Opuntia rastrera (Cactaceae) in Chihuahuan Desert: why are sexually derived recruitments so rare? Am J Bot 83:63–70
Mandujano MC, Carrillo-Angeles IG, Martínez-Peralta C et al (2010) Reproductive biology of Cactaceae. In: Ramawat KG (ed) Desert plants-biology and biotechnology. Springer, Berlin, Germany, pp 197–230
Marsh PC (1984) Biota of Cuatro Ciénegas, Coahuila, México: Preface. JANAS 19:1–2
Martínez-Peralta C, Márquez-Guzmán J, Mandujano MC (2014) How common is self-incompatibility across species of the herkogamous genus Ariocarpus? Am J Bot 101(3):1–9
Martínez-Ávalos JG, Martínez-Gallegos R, Guerra-Pérez A, Torres Castillo JA, et al. (2020). Diversity and distribution of cacti species in the Cuatro Ciénegas basin. In: Mandujano MC, Pisanty I, Eguiarte L (eds.) Plant diversity and Ecology in the Chihuahuan desert. Springer International, Cham, Switzerland
McFarland JD, Kevan PG, Lane MA (1989) Pollination biology of Opuntia imbricata (Cactaceae) in southern Colorado. Can J Bot 67:24–28
McIntosh ME (2005) Pollination of two species of Ferocactus: interactions between cactus-specialist bees and their host plants. Funct Ecol 19:727–734
Michener CD, McGinely RJ, Danforth BN (1994) The bee genera of north and Central America (Hymenoptera: Apoidea). Smithsonian Institution Press, Washington, DC, USA and London, UK
Montiel González C, Bautista F, Delgado C, García-Oliva F (2018) The climate of Cuatro Ciénegas Basin: drivers and temporal patterns. In: Souza V et al (eds) Cuatro Ciénegas Ecology, Natural History and Microbiology, Cuatro Ciénegas Basin: an endangered Hyperdiverse oasis. Springer, Cham, Switzerland
Nagy ES, Strong L, Galloway LF (1999) Contribution of delayed autogamous selfing to reproductive success in mountain Laurel, Kalmia latifolia (Ericaceae). Am Midl Nat 142:39–46
Nassar JM, Ramirez N, Linares O (1997) Comparative pollination biology of Venezuelan columnar cacti and the role of nectar-feeding bats in their sexual reproduction. Am J Bot 4:918–927
Neff JL, Simpson BB, Dorr LJ (1982) The nesting biology of Diadasia afflicta Cress. (Hymenoptera: Anthophoridae). J Kansas Entomol Soc 53:499–518
Negrón-Ortiz V (1998) Reproductive biology of a rare cactus, Opuntia spinosissima (Cactaceae), in the Florida keys: why is seed set very low? Sex Plant Reprod 11:208–212
Nicolson SW (2007) Nectar consumers. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, Netherlands, pp 289–342
Ordway E (1987) The life history of Diadasia rinconis Cockerell (Hymenoptera:Anthophoridae). J Kansas Entomol Soc 60:15–24
Palleiro N, Mandujano MC, Golubov J (2006) Aborted fruits of Opuntia microdasys (Cactaceae): insurance against reproductive failure. Am J Bot 93(4):505–511
Pimienta-Barrios E, Del Castillo RF (2002) Reproductive biology. In: Nobel PS (ed) Cacti: biology and uses. University of California Press, Berkeley, CA, USA, pp 75–90
Piña RH (2000) Ecología reproductiva de Ferocactus robustus en el Valle de Zapotitlán Salinas, Puebla. MSc diss., ENCB Instituto Politécnico Nacional, México, DF, Mexico
Piña H, Montaña C, Mandujano MC (2007) Fruit abortion in the Chihuahuan-desert endemic cactus Opuntia microdasys. Plant Ecol 193:305–313
Pinkava D (1984) Vegetation and floral of the bolson of Cuatro Cienegas region, Coahuila, México: IV. Summary, endemism and corrected catalogue. JANAS 19:23–47
Pinkava D (2002) On the evolution of continental North American opuntias. Succulent Plant Research 6. David Hunt, Sherborne, UK
Rebman JP (2001) Las suculentas del Islote Toro, Baja California, México. Cact Suc Mex 46:52–55
Ren MX, Tang JY (2012) Up and down: stamen movements in Rutagraveolens (Rutaceae) enhance both outcrossing and delayed selfing. Annals of Botany 110:1017–1025. https://doi.org/10.1093/aob/mcs181
Reyes-Agüero JA, Aguirre JR, Valiente-Banuet A (2006) Reproductive biology of Opuntia: a review. J Arid Environ 64:549–585
Rosas Barrera MD, Golubov J, Pisanty I, Mandujano MC (2020) Effect of reproductive modes on the population dynamics of an endemic cactus from Cuatro Ciénegas In: Mandujano MC, Pisanty I, Eguiarte LE (eds) Plant diversity and Ecology in the Chihuahuan Desert. Springer International, Cham, Switzerland
Ross R (1981) Chromosome count, cytology and reproduction in the Cactaceae. Am J Bot 68:463–470
Rowley GD (1980) Pollination syndromes and cactus taxonomy. Cact Succ J (Great Britain) 42:95–98
SAS Institute (1995) JMP statistics and graphics guide. SAS Institute, Cary, NC, USA
Schlindwein C, Wittman D (1997) Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst Evol 204:179–193
Schlising RA (1972) Foraging and nest provisioning behavior of the oligolectic bee, Diadasia bituberculata (Hymenoptera: Anthophoridae). Pan-Pac Entomol 48:175–188
Simpson BB, Neff JL (1983) Pollination ecology in the arid southwest. Aliso 11:417–440
Snow AA, Spira TP, Simpson R, Klips RA (1996) The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH (eds) Floral biology studies on floral evolution in animal – pollinated plants. Chapman & Hall, Toronto, Canada, pp 191–216
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Ann Rev Ecol Syst 12:253–279
Sutherland S (1987) Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana. Evolution 41(4):750–759
Trujillo-Argueta S, González-Espinosa M (1991) Hibridización, aislamiento reproductivo y formas de reproducción en Opuntia spp. Agrociencia 1:39–58
Valiente-Banuet A, Rojas-Martínez A, Casas A et al (1997) Pollination biology of two winter-blooming giant columnar cacti in the Tehuacan Valley, Central Mexico. J Arid Environ 37:331–341
Wallace RS, Gibson AC (2002) Evolution and systematics. In: Nobel PS (ed) Cacti: biology and uses, Berkeley, CA, pp 1–21
Wyatt R (1983) Pollinator-plant interactions and the evolution of breeding systems. In: Real L (ed) Pollination biology. Academic Press, New York, pp 51–95
Zar JH (1996) Biostatistical analysis. Prentice-Hall Inc, New Jersey
Acknowledgments
Jordan Golubov, Luis E. Eguiarte, and Francisco Molina-Freaner for comments on previous versions of the chapter. Financial support of project IN205500 PAPIIT-DGPA UNAM to MC Mandujano and PRONATURA provided free stay at La Becerra, Cuatro Ciénegas, during our field trips. Susana Moncada and personnel from the Area de protección de Flora y Fauna Cuatrocienegas provided logistic support. We appreciate the help during field work of Manuel Rosas, Gisela Aguilar Morales and in memoriam of our dearest friend Dolores Rosas Barrera (2018). The first author thanks the help at different stages of the work to M.Sc. Daniel Cervantes.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Plasencia-López, L., Rojas-Aréchiga, M., Mandujano, M.C. (2020). Reproductive Biology of Grusonia bradtiana (Cactaceae): A Dominant Species and Endemic Clonal Cactus from Cuatro Ciénegas Basin and Contiguous Areas in the Chihuahuan Desert. In: Mandujano, M., Pisanty, I., Eguiarte, L. (eds) Plant Diversity and Ecology in the Chihuahuan Desert. Cuatro Ciénegas Basin: An Endangered Hyperdiverse Oasis. Springer, Cham. https://doi.org/10.1007/978-3-030-44963-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-44963-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44962-9
Online ISBN: 978-3-030-44963-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)