Abstract
The clearance of senescent cells has the potential to become a therapeutic strategy that could be used in many pathologies, ranging from pulmonary fibrosis and diabetes to ageing itself. The initial genetic experiments performed in mouse models are now being recapitulated using chemical compounds that preferentially kill senescent cells, known as senolytics. Senolytic drugs hold an immense clinical potential but their lack of specificity could lead to side effects that would limit their use, especially when treating otherwise healthy aged individuals. Thus, it would be convenient to develop more targeted approaches to the elimination of senescent cells. Here, we discuss the ongoing efforts to design targeted senolytics, with special focus on the utilization of the extracellular epitopes displayed by the senescent surfaceome, and we summarize the avenues of research that have shown the most promising results so far: nanotechnology, cellular therapies and antibody-based drug delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Road to Targeted Senolytics
According to the current theories of ageing, therapeutic clearance of senescent cells could prevent comorbidities and prolong lifespan in mammals (Naylor et al. 2013; de Magalhães and Passos 2017; Demaria 2017). This is because the progressive accumulation of senescent cells in tissues is thought to play an active role in ageing and in the reduction of healthspan (Deursen 2014; de Keizer 2016). This hypothesis is supported by the fact that genetic or chemical interventions to remove senescent cells have been recently shown to improve organismal health in several models (Kirkland and Tchkonia 2015; Moreno-Blas et al. 2018).
The hallmark study of Baker and colleagues (Baker et al. 2011), which used an elegant transgenic mouse model to prevent the build-up of senescent cells, was the first to demonstrate this possibility in vivo. As shown in this paper, the apoptosis induced in p16Ink4a-positive cells from muscle, fat and the lens of a BubR1H/H progeroid mice promoted normal functioning of these tissues, delayed the onset of age-related diseases, slowed down the progression of already established age-related disorders and extended animal lifespan. The same group later confirmed that clearance of p16 positive cells from normally aged mice had similar effects (Baker et al. 2016). Other mouse models followed, including one in which the clearance of p19ARF-expressing cells from mice was achieved using a toxin receptor-mediated cell knockout system, which ameliorated age related decline in lung function (Hashimoto et al. 2016). Also, the elimination of senescent cells by a suicide gene-meditated ablation of p16Ink4a-expressing senescent cells in the INK-ATTAC mouse model was shown to reduce overall hepatic steatosis (Ogrodnik et al. 2017). These ground-breaking experiments started a race to translate these observations made in transgenics into a clinically applicable strategy based on chemical compounds.
The first preliminary results were provided by a new class of chemicals, which were named ‘senolytics’ for their selective ability to induce death in senescent cells. For instance, a pharmacological approach with drugs that inhibit Bcl-2 and activate caspase 3/7 to partially eliminate senescent cells in vivo, was shown to decrease age-related phenotypes in wild type C57BL/6 mice and prolonged their lifespan (Zhu et al. 2015, 2016). The senolytic drug ABT263 was found to rejuvenate haematopoietic and muscle stem cells, and also improve healthspan in mice (Chang et al. 2016). Additionally, using a suicide gene as well as senolytic compounds, senescent cell clearance was found to prevent ageing-associated bone loss in mice (Farr et al. 2017). Senolytics have been shown to clear senescent type II pneumocytes and alveolar epithelial cells, suggesting and application in the treatment of idiopathic pulmonary fibrosis (Lehmann et al. 2017; Pan et al. 2017) and have also been proposed to be useful in Alzheimer’s disease (Zhang et al. 2019) and tau-dependent pathologies (Bussian et al. 2018).
Many other senolytic compounds are currently being developed and tested (Fuhrmann-Stroissnigg et al. 2017), including some derived from natural products (Li et al. 2019), but so far they all share the issue of a lack of specificity. The action of these drugs are not exclusively limited to senescent cells and the potential side effects could be significant, although preliminary experiments in mouse models of ageing seem promising (van Deursen 2019; Xu et al. 2018). Moreover, the first in human clinical trial of senolytics using dasatinib plus quercetin have already shown positive results in alleviating physical dysfunction in IPF patients (Justice et al. 2019). In this context, it would now be convenient to develop more efficient strategies to clear senescent cells as the field of senolytics moves towards the first clinical applications (Justice et al. 2019). This is where the concept of targeted senolytics comes to relevance.
2 Recognizing Senescent Cells
The first step in the design of targeted senolytics approaches, and probably the most important, would be to devise the best possible system to identify the cells to be eliminated. Several characteristics that distinguish senescent cells from normal proliferating cells could be used for this purpose. As it was originally observed (Hayflick and Moorehead 1961), the main feature of these cells is being growth inhibited and not able to respond to growth factors or mitogens. Moreover, they express unique characteristic features, including morphological changes, senescence-associated β-galactosidase (SA β-Gal) positivity, increased expression of certain proteins (such as p21, p53, p16, p15, p27 and ARF), presence of senescence-associated heterochromatin foci (SAHF), enlarged and prominent nucleoli and a senescence-associated secretory phenotype (SASP) (Naylor et al. 2013; Hayflick 1965; Narita et al. 2003; Campisi 2011; Rodier and Campisi 2011; Rufini et al. 2013; Munoz-Espin and Serrano 2014; Pérez-Mancera et al. 2014).
To date, several of these markers have been extensively used to detect senescent cells in vitro and in vivo, alone or in combination. The fact that none of them is exclusive to the senescent state (Rodier and Campisi 2011; Munoz-Espin and Serrano 2014; Sikora et al. 2011) highlights the need to search for novel and more reliable substitutes for clinical applications. A universal senescence marker would need to be very robust and exclusive to the senescent state (Lawless et al. 2010; Matjusaitis et al. 2016). Also, a suitable biomarker should be able to distinguish between senescent and non-senescent cells in vitro and in vivo, its expression should be detectable regardless of sample preparation and it should be minimal in non-senescence states. Considering the limitations faced so far, it seems unlikely that such a marker will ever be found, although it would be expected that many that at least fulfil some of those criteria will be eventually identified.
The senescence program harbours transcriptional heterogeneity with a dynamic phenotype, which changes at variable intervals (Sharpless and Sherr 2015; Hernandez-Segura et al. 2017). Thus, the expression of thousands of genes is significantly altered in cellular senescence, and this could be the basis of new screens for markers. However, these changes are largely conserved within individual cell types and are specific to these cells only (Coppe et al. 2010). Of all the remarkable changes in gene expression observed during senescence, very few of them are stably expressed, specific and of great enough magnitude to be considered proper markers (Sharpless and Sherr 2015; Wang et al. 2009).
The fact that none of the markers has so far been proven to be sufficiently specific, having many false positives and negatives as well as cell type- and tissue-dependent expression, would be the main limiting factor when it comes to designing targeted senolytic strategies. Thus, a single strategy to clear all senescent cells from the body without noticeable side effects is a goal that may prove to be too ambitious. It will be more likely that a multi-faceted approach would be the most successful when translated to clinical use. We will first review some of the most used markers before discussing the surfaceome as a source of novel clinical opportunities.
2.1 Staining of Senescent Cells
The most used protocol to identify senescent cells ex vivo is the classic senescence associated β-galactosidase [SA-β-Gal] assay, in which senescent cells are stained blue at a pH of 6.0 (Dimri et al. 1995). Several human cells express high levels of the enzyme β-galactosidase upon becoming senescent, which is detectable cytologically or histochemically in freshly fixed cells or tissues at pH 6.0 (Campisi 2011; Munoz-Espin and Serrano 2014; Dimri et al. 1995). SA-β-Gal was originally observed in senescent fibroblasts and keratinocytes but not in terminally differentiated keratinocytes, quiescent fibroblasts or immortal cells, and an age-dependent increase was described in dermal fibroblasts and epidermal keratinocytes in human skin samples of different ages (Dimri et al. 1995).
Although the functional implications are not clear, the increased levels of lysosomal β-galactosidase activity in senescent cells is thought to be as a result of increased content and mass of the lysosomes (Kurz et al. 2000a). However, it is worthy of note that almost all cells show endogenous lysosomal β-galactosidase activity at pH 4.0 and certain cell types, such as human epithelial cells and mouse fibroblasts, stain with some intensity for SA-β-Gal at pH 6.0 (Itahana et al. 2013). Moreover, when cells are maintained at confluency for long periods, a false positive density-induced SA-β-Gal activity can be sometimes detected, and serum starvation is also known to produce false positives (Itahana et al. 2007; Campisi et al. 2009; Evangelou et al. 2017). Despite this, SA-β-gal is still considered to be one of the best senescence markers available (Kurz et al. 2000b) and it has been used as the basis for the first nanoparticle-based targeted senolytics (see below).
Senescent cells also stain positive for the histochemical Sudan Black-B (SBB), which stains the lysosomal aggregate and age-pigment lipofuscin (Terman and Brunk 2004; Georgakopoulou et al. 2013). SBB stains senescent cells in tissues regardless of sample preparation and can be used on formalin-fixed, paraffin-embedded tissues, giving it an advantage over SA-β-Gal, which requires the use of fresh cells or tissue samples (Munoz-Espin and Serrano 2014; Georgakopoulou et al. 2013).
2.2 The Senescence-Associated Secretory Phenotype
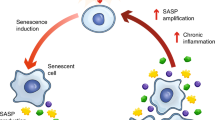
Senescent cells secrete factors collectively known as the senescence associated secretory phenotype (SASP), which includes inflammatory cytokines, chemokines, matrix remodelling proteins, damage-associated molecular pattern proteins (DAMPs), growth factors (such as interleukins 6, 7, and 8), Macrophage Inflammatory Protein 3α (MIP-3α), Growth Regulated Oncogene alpha (GROα), Monocyte Chemoattractant Proteins 1 and 2 (MCP-1 and MCP-2), Insulin-like Growth Factor Binding Protein (IGFBP) and Hepatocyte Growth Factor (HGF), among others (Kuilman and Peeper 2009; Young et al. 2013; Salama et al. 2014; Laberge et al. 2015; Maciel-Barón et al. 2016; Kirkland and Tchkonia 2017; McHugh and Gil 2018). It is thought that these molecules are able to disrupt tissue structure and function, being then responsible in part for chronic age-related diseases and cancer progression (Cahu 2013).
The mechanisms involved in the generation of the SASP are still being investigated. It has been found that the production of SASP is not tied to characteristics of senescence such as enlarged cell morphology and SA-β-Gal expression or even cell cycle arrest (Laberge et al. 2015; Herranz et al. 2015; Wang et al. 2017). Also, ectopic expression of p16INK4a and p21WAF1/Cip1/Sdi1 can induce senescence without production of SASP (Coppe et al. 2011). It has been suggested that the SASP is not an essential feature of senescent cells and can develop independently of p16INK4a status (Coppe et al. 2011). The SASP may thus be a result of severe DNA damage, which can activate secretion independently of p53 (Coppe et al. 2008). The SASP is also regulated by microRNAs, the mTOR, cGAS/STING and JAK/STAT signalling pathways, the cytokine receptor for interleukin–1 [IL-1] and IL-8 chemokine receptor CXCR2, as well as transcription factors such as NF-kB (Coppe et al. 2011). It has also recently been reported that NOTCH1 plays a vital role as both a regulator of the composition SASP and a regulator of juxtacrine signalling within the context of oncogene-induced senescence (OIS) (Hoare and Narita 2017; Ito et al. 2017; Hoare et al. 2016).
The SASP elements could be good markers to identify senescent cells, given the fact that they constitute a protein profile not observed in normal cells. Pro-inflammatory cytokines such as IL-6 and IL-8 seem to be the most conserved aspects of the secretome, and they play key roles in the maintenance of the SASP (Lasry and Ben-Neriah 2015; Kuilman et al. 2008). However, the SASP varies significantly between tissues and different senescence triggers, and the molecular regulation of the SASP program is complex and multifaceted in space and time (Wang et al. 2017; Malaquin et al. 2016). Moreover, it has been shown that mitochondrial dysfunction can lead to a type of cellular senescence, known as mitochondrial dysfunction associated senescence (MiDAS) (Wiley et al. 2016; Gallage and Gil 2016; Herranz and Gil 2016; Wiley and Campisi 2016), which produces a distinct secretory phenotype (Wiley et al. 2016). Also, using models of stress induced premature senescence (SIPS), proteasome inhibition induced premature senescence (PIIPS) and replicative senescence (RS), it was shown that the SASP due to PIIPS was substantially different, with significantly lower amounts of cytokines and chemokines when compared to the SASP due to RS or SIPS (Maciel-Barón et al. 2016). Thus, markers based on the SASP are unlikely to be specific enough for targeted senolytics, although a profile of secreted factors specific to certain type of senescent cells could alternatively be used in certain situations.
2.3 Nuclear Features
Cellular senescence is associated with an altered chromatin assembly, forming easy to visualize heterochromatin regions called senescence associated heterochromatic foci (SAHF) (Narita et al. 2003; Cichowski and Hahn 2008). These are highly condensed regions of chromatin characterised by a build-up of histone H3 that is tri-methylated at lysine 9 (K9M-H3), as well as heterochromatin proteins, such as high-mobility group A (HMGA) proteins, macroH2A and heterochromatin protein 1 (HP1) (Salama et al. 2014; Adams 2007; Kuilman et al. 2010). In proliferating and quiescent cells, the euchromatin markers K9Ac-H3 (histone H3 acetylated on lysine 9) and K4M-H3 (histone H3 methylated on lysine 4) homogeneously stain the DNA. The SAHF usually lack lysine 9 acetylated H3 (K9Ac-H3) and lysine 4 methylated H3 (K4M-H3) but rather are enriched in K9M-H3 (Narita et al. 2003).
p16INK4A, Rb, p53, interleukin-6 and C/EBPβ are thought to be vital for the formation of SAHF, which can be prevented by interfering with the signalling of these pathways (Narita et al. 2003; Cichowski and Hahn 2008; Kuilman et al. 2010). It is likely that SAHF contribute to the collective changes in gene expression observed in senescent cells (Young et al. 2013; Mduff and Turner 2011). The SAHF are mainly found only in human cells, and even taking this in consideration, they are not consistently expressed in all models (Narita et al. 2003; Swanson et al. 2013), which limits their use as markers.
Senescent cells also present a senescent-associated distension of satellites (SADS), a display of constitutive peri/centromeric satellite heterochromatin decondensation, which are not exclusive to either the p53–p21 or the p16 INK4A/Rb pathways. These SADS occur prior to and independently of SAHF formation (Ogrodnik et al. 2017; Swanson et al. 2013).
2.4 Molecular Markers
Although it has been over half a century since it was first described (Hayflick and Moorhead 1961), the molecular pathways involved in senescence are yet to be fully understood. Senescence can be signalled through various routes, many of which activate p53 and/or Rb and the cyclin dependent kinase [CDK] inhibitors p16INK4A, p15INK4B, p21WAF1/Cip1/Sdi1 and p27 (Munoz-Espin and Serrano 2014; Campisi 2005). Upregulation of these and other genes has been used as markers of senescence, although none of them is specific of the senescent phenotype. Nevertheless, p16 is currently the most widely used genetic marker of senescence and many in vivo experiments have equated p16-expresing cells to senescent cells (Baker et al. 2011; Baker et al. 2016; Palmer et al. 2019).
The changes in gene expression patterns of senescent cells also affect cell cycle regulatory genes, extra-cellular matrix remodelling genes, as well as genes involved in cytokine signalling and inflammation (Mduff and Turner 2011). These various senescence-associated gene expression changes are specific to and conserved within individual cell types (Coppe et al. 2010). It has also been observed that mTOR activation is necessary for cellular senescence to take place, as mTOR enables transition from cell cycle arrest and quiescence to senescence (Blagosklonny 2014; Leontieva and Blagosklonny 2017). Rapamycin, an inhibitor of the mTOR pathway, was found to slow down cellular senescence and prevent the accompanying irreversible loss of proliferative capacity (Demidenko et al. 2010; Xu et al. 2014).
It is also interesting to consider that ontologic and gene expression analysis of skin from Caucasian females between the ages of 20 years and 70 years revealed changes in the expression of thousands of genes with age (Kimball et al. 2018). Studies have shown that the expression of cellular damage related genes such as inflammatory or stress response genes increase with age while biosynthetic and metabolic genes expression decrease with age (Kimball et al. 2018; Edwards et al. 2007).
In order to facilitate research on the mechanisms of gene regulation in cellular senescence, a database of senescence associated genes is greatly needed. To this end, Dong and colleagues have established the Human Cellular Senescence Gene Database (HCSGD), using a combination of data from published literature sources, gene expression profiling as well as protein-protein interaction networks (Dong et al. 2017). Profiles of gene expression taken from databases such as HCSGD could provide useful alternatives to single-gene markers, which are the ones mostly used now and heavily rely on the p16/Rb and the p53/p21 pathways.
2.5 The P16/Rb Axis
Rb is an important gatekeeper during cell cycle progression through the G1 phase and its activity is tightly controlled by several post-translational modifications, such as phosphorylation, acetylation and ubiquitination (Campisi 2005; Takahashi et al. 2007). p21Cip1/Waf1/Sdi1 and p16INK4A inhibit the kinases that phosphorylate Rb, leading to the accumulation of its active, hypo-phosphorylated form (Xu et al. 2014; Herbig et al. 2004; di Fagagna and Campisi 2007). The two products of the INK4a/ARF locus, p16Ink4a and p19Arf [p14ARF in humans], are key tumour suppressors which regulate the activities of p53 and Rb and are expressed from partly overlapping nucleotide sequences read in alternative reading frames (Lowe and Sherr 2003). p14ARF increases the growth inhibitory functions of p53 by sequestering its negative regulator, Mdm2. Both p16INK4a and p19ARF could be used as markers, since they have been found to accumulate in many senescent cells and their overexpression also promotes senescence (Lundberg et al. 2000). Mutations affecting INK4a or ARF can compromise senescence on various levels depending on the cell type and species (Narita et al. 2003; Lowe and Sherr 2003; Park and Sin 2014).
As previously mentioned, p16INK4A is considered a leading marker for indicating the presence of senescent cells, as most senescent cells express it (Campisi 2011; Taniguchi et al. 1999; Simboeck and Di Croce 2013). The levels of p16INK4A have even been used as a biomarker of ageing in humans as it is seen to increase with ageing—up to 7 fold in some human tissues and up to 30 fold in mouse tissues (Wang et al. 2009; Krishnamurthy et al. 2004; Collado et al. 2007; Romagosa et al. 2011; Sherr 2012). However, p16 does not seem to be a key regulator of development, as it is not expressed during embryogenesis, even though senescence is known to play an important role during these stages (Guney and Sedivy 2006). Instead, this is usually mediated by p21 in a p53-independent manner (Storer et al. 2013; Munoz-Espin et al. 2013).
Immunostaining of benign tumours show a positive p16INK4A expression and a negative or very low Ki67 index (a marker of proliferation), while malignant tumours often stain positive for Ki67 but negative for p16INK4A (Romagosa et al. 2011; Collado et al. 2005). However, in high grade malignant tumours, where there are alterations in the p16INK4A/Rb pathway, a high p16INK4A as well as a high Ki67 immunostaining has been observed. In human tumours, the overexpression of p16 could either be a result of OIS, as seen in benign or pre-malignant lesions, or a result of an Rb pathway failure, as seen in some malignant lesions. Hence, p16INK4A is often used as a prognostic marker for several cancers in order to grade or distinguish between benign and malignant lesions (Romagosa et al. 2011). Staining for p16 in vivo has been quite challenging and requires robust positive and negative controls.
2.6 The P53/P21 Axis
p53 is a cell cycle regulator and guardian of the genome, and an important tumour-suppressor protein involved in senescence (Munoz-Espin and Serrano 2014). p53 induction after genotoxic stress and DNA damage can lead to either DNA repair, transient cell cycle arrest, senescence or apoptosis, depending on various factors (Sionov and Haupt 1999; Macip et al. 2002; Bieging et al. 2014). The activity of p53 as well as levels of its downstream effector p21 increase during late passage of cells, leading to a slowdown in proliferation (Murray-Zmijewski et al. 2008; Kastan et al. 1991; Lane 1992; Levine 1997). It has been proposed that p53 and p21 are not required for the maintenance of senescence, even though they are important in inducing the senescence-like proliferation arrest (Chang et al. 1999). Linked to this, p16 expression has been proposed to be a second barrier to cell proliferation during senescence to ensure irreversibility of the arrest (Macip et al. 2002; Beausejour et al. 2003).
p21Waf1/Cip1/Sdi1 is a well characterised transcriptional target of p53 and links the p53 and Rb pathways to enhance tumour suppression (Takahashi et al. 2007). p21 is a dual inhibitor of proliferating cell nuclear antigen (PCNA) and cyclin dependent kinases, both crucial in the cell cycle, and is significantly overexpressed during cellular senescence (El-Deiry et al. 1993; Waldman et al. 1995; Brugarolas et al. 1995). In late passage human diploid fibroblasts, an increase in p21Waf1/Cip1/Sdi1 expression was observed, and targeting the deregulation of p21Waf1/Cip1/Sdi1 in these cells increased their lifespan. In p21-null mice, p53 is unable to cause G1 cell cycle arrest upon DNA damage (Shiohara et al. 1994; Abbas and Dutta 2009; Romanov and Rudolph 2016). Expression of p21 can also induce apoptosis and this is thought to be mediated by reactive oxygen species (Masgras et al. 2012). Genomic and phenotypic analyses using p21-inducible, p53-null, malignant as well as near-normal cellular models has shown that a subpopulation of proliferating cells emerged after an initial senescence-like phase, which expressed p21 and exhibited increased genomic instability, aggressiveness and resistance to chemotherapeutic agents (Galanos et al. 2016; Georgakilas et al. 2017).
The Bruton’s tyrosine kinase (BTK) is a dual-specificity non-receptor tyrosine kinase belonging to the Tec family that is vital for B cell maturation (Mohamed et al. 2009) and it has recently been reported to be a part of the p53 senescent pathway, phosphorylating p53 and modulating its responses (Althubiti et al. 2016). BTK was found to be expressed in response to damage and induces phosphorylation of p53 at serine 15 at the N-terminus, increasing its protein levels and activity. In addition, it was found that BTK binds to and phosphorylates MDM2, facilitating its loss of ubiquitination activity and further stabilising p53 and also plays a role in the p73 pathway (Rada et al. 2017, 2018). Inhibition of BTK reduced the expression of p53 and interfered with the upregulation of p53 target genes, leading to an impairment in the induction of p53-mediated senescence. Thus, BTK has been proposed to be useful as a novel marker of p53-induced senescence (Rada et al. 2018).
3 The Senescent Surfaceome
In the search for better markers of senescence, the profile of proteins present on the surface of these cells, known as the senescent surfaceome, has emerged as a potentially interesting alternative (Table 6.1). In general, the surfaceome could be defined as the panel of all plasma membrane proteins of a given cell type that exhibit at least one amino acid residue at the extracellular space. The majority of the human surfaceome consist of all types of transmembrane proteins, single and or polytopic ɑ-helical or β-sheet transmembrane proteins (Bausch-Fluck et al. 2018). Like other senescent markers, those in the surfaceome are expressed varyingly but consistently across different tissues and conditions and are unlikely to be universal. One of the most interesting features of the novel markers of senescence present in the senescent surfaceome are their extracellular epitopes, which could be used for targeted therapies, as discussed below.
The cell surface is a hub that coordinates information transmission to and from the outside environment. The proteins present at the cell surface are crucial factors connecting cellular signaling networks and determining the cell’s interaction and communication with its neighbourhood. Thus, the surfaceome consists of a broad range of receptors, transporters, channels and enzymes, and each of them could be a valuable diagnostic marker of cellular senescence, as well as a potential therapeutic target.
3.1 Current Known Members of the Senescent Surfaceome
CD264 (also known as TRAIL-R4, DcR2, TRUNDD and TNFRSF10D) was one of the first proteins preferentially expressed on the surface of senescent cells identified, and was accordingly proposed as a biomarker of cellular aging (Madsen et al. 2017). CD264 is a decoy receptor for tumour necrosis factor-related apoptosis- inducing ligand (TRAIL) (Falschlehner et al. 2007). Due to the presence of a truncated intercellular death domain, CD264 competes for TRAIL binding and prevents the formation of a death-inducing signalling complex (Pan et al. 1998; Lee et al. 2005). CD264 upregulation was observed in senescent premalignant tumours induced by ras, whereby there was downregulation of CD264 in the malignant ras-induced tumours that had escaped senescence (Collado et al. 2005). CD264 was also further detected in stress-induced senescence of non-malignant smooth muscle cells (Zhu et al. 2011) and, in contrast, showed to be not upregulated in replicative senescence (Collado et al. 2005).
NOTCH1 was one of the first membrane-associated proteins shown to play a role in senescence, specifically as a key determinant of SASP expression (Hoare et al. 2016). NOTCH3, a member of the Notch family receptors, which also reported to have tumour suppressor functions and is overexpressed in senescent fibroblasts (Cui et al. 2013; Althubiti et al. 2014), is another classic member of the senescent surfaceome that has been known for a while.
A mass spectrometry screening of the membrane fraction of senescent cell lysates revealed a new subset of proteins that were preferentially expressed in the membranes of senescent cells over their proliferative counterparts (Althubiti et al. 2014), in what, to the best of our knowledge, was the first systematic screening of the senescent surfaceome. Later, gene expression profiling analysis of G protein-coupled receptor kinase (GRK) 4-induced senescent HEK293 cells showed a further differential expression of a new set of 17 senescence-related genes compared to control cells (Xiao et al. 2017). Also, a more comprehensive screen of membrane-associated proteins, this time focusing on oncogene-induced senescence, was later published as part as the characterization of the involvement of Notch signalling in SASP (Hoare et al. 2016). In this study, 521 proteins were identified on the surface, 32 increasing in senescence and 135 being downregulated instead. Most of these candidates were not found in the first screen described above, but some were present in both (such as NTAL). This confirms that different cell types and methods of induction will lead to completely different surfaceomes, although some elements may still be common in more than one model.
It has to be taken in consideration that the plasma membrane proteome is only a subset of all membrane proteins within the cells. Thus, in any proteomic screen of the senescent surfaceome, it would be challenging to distinguish between intercellular membrane proteins (i.e. endoplasmic reticulum, Golgi), the ones bound to the intracellular part of the plasma membrane (such as BTK (Althubiti et al. 2016)) and the ones present in the plasma membrane that actually cross over the cell surface. Thus, a biochemical validation and characterization of the individual hits of the proteomic screen would always be necessary.
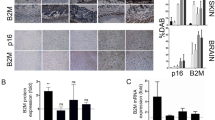
One of the approaches to identifying proteins uniquely expressed on the surface of senescent cells mentioned above was done in our lab and focused on previously described genetic models of senescence (Althubiti et al. 2014). EJp16 and EJp21 are derivatives of a bladder carcinoma cell line (EJ) with a tetracycline regulatable expression system for p16 and p21, respectively, that entered senescence after 3–4 days of induction (Macip et al. 2002; Chang et al. 1999). EJ-based models allow for the establishment of a genetically induced senescent state due to the prolonged expression of core genes from the main pathways responsible for the induction and maintenance of the phenotype, without any additional effects caused by the trigger of senescence itself, as seen in more physiological models. Thus, the proposed panel of surfaceome proteins presented in this study can be linked to the activation of either the p53/p21 or the pRb/p16 pathways and could help determine which markers are activated preferentially in response to each of them. In this regard, 107 proteins were reported to be exclusively present on the plasma membrane in EJp21 senescent cells and 132 in EJp16 were identified.
Ten proteins that had not been previously linked with cellular senescence were validated using immunofluorescence microscopy and Western blots: DEP1, NTAL, EBP50, STX4, VAMP3, ARMCX3, B2M, LANCL1, VPS26A and PLD3. The biological role of the proposed markers is heterogeneous. Some, like DEP1 or EBP50, have been identified as important players in the regulation of cancer progression (Liu et al. 2018; Iuliano et al. 2010). Others, including VPS26A and PLD3, have been found to be involved in Alzheimer’s disease, providing a direct link to an age-associate disorder (Tan et al. 2019; Choi et al. 2018). Some of them, like VPS26A, VAMP3, PLD3 and STX4, may have a role in vesicle trafficking and therefore could potentially contribute to some aspect of the SASP (Veale et al. 2011; Caceres et al. 2016; Bugarcic et al. 2011). All of them showed a tissue- and pathway-specific profile. For instance, in tissue sections collected from a V600EBRAF lung adenoma mouse model, previously showed to have a high percentage of senescent cells (Mercer et al. 2005; Carragher et al. 2010), DEP1, STX4, B2M and NTAL were preferentially detected. Analysis of human naevi also revealed positive staining for the same markers.
An alternative approach to these three screens described, focused only on replicative senescence, using proliferating passages of WI-38 human diploid fibroblasts [population doubling level (PDL) = 23) compared with their senescent counterparts, in which senescence was induced by extensive cell culture (PDL = 59) (Kim et al. 2017). Following the fractionation of membrane-associated proteins, the samples were analysed using mass-spectrometry as well. The results revealed 2 groups of proteins: those abundant in intracellular membranes [i.e. endoplasmic reticulum, mitochondria] and those in the cell surface. There were 118 proteins at the overlap of these two groups and this list was narrowed down to 15. Out of these, the leading candidate marker to be further studied was DPP4 (CD26). DPP4 is responsible for deactivation of the glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) (Zhong et al. 2013). GIP and GLP-1 hormones are beneficial in diabetes as they induce the pancreatic β cells to release insulin after a meal, which results with the suppression of the glucose level in the blood (Wu et al. 2016). However, the role of the elevation of DPP4 in senescent cells remains unclear and further tests will need to be done to confirm its impact on biological ageing.
The expression of DPP4 in the membrane fraction of senescent but not proliferating cells was further validated by Western blot and RT-qPCR. Validation experiments included also different cell models (WI-38, IMR-90, HUVEC, HAEC) subjected to ionizing radiation (IR). The expression of DPP4 was confirmed in IR models, although with more modest effects than in replicative senescence. The expression level of DPP4 was next validated with other triggers of stress-induced senescence, including doxorubicin and OIS in MEFs expressing the oncogene HRASG12V. DPP4 was upregulated in all of them, demonstrating that is a widely expressed marker.
Besides DPP4, SCAMP4 has also been proposed by the same group to be preferentially expressed on the surface of senescent cells (Kim et al. 2018). They confirmed its membrane localization and its abundancy in different senescent models (Lee et al. 2005; Kim et al. 2018). Western blot analysis revealed increased level of SCAMP4 in replicative senescence, together with the upregulation of other family proteins (SCAMP1-3). The SCAMP4 upregulation was also successfully determined in models of SIPS, including doxorubicin-induced and oncogene-induced senescence or HAECs and HUVECs cells subjected to IR. However, SCAMP4 mRNA levels remained unchanged in all the tested models, suggesting a post-translational effect. Testing its half-life in WI-38 fibroblasts after treatment with the translational inhibitor cycloheximide showed a SCAMP4 decline in proliferating cells, without the changes in the protein stability in senescent cells. After proteasome inhibition, SCAMP4 protein was found to accumulate in proliferating but not in senescent cells. In turn, autophagy inhibition revealed SCAMP4 accumulation in both proliferating and senescent cells, suggesting proteasome but not autophagy involvement in the control of protein levels. This was further supported by previous findings on SCAMP4 ubiquitination in Lys4 and Lys185 in lung cancer (Wu et al. 2015).
It is worth mentioning that the level of SASP also increased after proteasome inhibition and that SCAMP4 protein stabilization occurred before the induction of SASP. On the other hand, the secretion of many SASP factors decreased after SCAMP4 silencing (IL6, IL8, GDF-15, IL1B, MIF and CCL2), together with the reduced level of core senescence markers (p53, p21 and SA-β-gal activity). These findings were further confirmed by short-term and stable overexpression of SCAMP4 in proliferating cells, in which there was increased mRNA and protein level of several senescent and SASP markers (p16, IL1A, IL1B, IL6, and IL8). SCAMP4 overexpression resulted also in increased SA-β-gal activity and reduced [3H]-thymidine incorporation.
3.2 Clinical Relevance
The potential clinical implications for the senescence surfaceome have already started to be discussed in the literature. For instance, CD264 has been proposed to be specific for the detection of senescent mesenchymal stem cells (MSC) population. The regenerative capacity and therapeutic applications of MSC are well documented (Barrilleaux et al. 2006; Caplan 2007). However, the main limiting aspect that impedes the standardization and optimization of MSC therapies for clinical use is their cellular heterogeneity, which is considered the main cause of strong variability in treatment outcomes. The immunolabelling of surface antigens can be an effective approach for standardization of MSC composition, for instance before transplantation. The identification of aging cells among the MSC population would facilitate the enrichment of multipotent progenitors by negative selection, slowing cellular aging during MSC expansion by the improvement of culture conditions, and allowing for selective elimination of aging MSC. The proliferation potential of MSC cultures has been shown to be inversely related to the CD264 cellular content. The expression of CD264 was also tested in replicative senescence by serial passage of MSCs, and the number of CD264+ cells increased from 30% at the passage 3–7 to 90% by passage 14.
After these initial studies, more detail on the senescent surfaceome will no doubt appear in the future. As the profiles of extracellular epitopes of different types of senescent cells become clearer, new markers will emerge, which could be used for diagnostic and therapeutic purposes. Apart from that, the surfaceome will also generate information on novel effectors and modulators of senescence, like the first reports have shown. Thus, the proteomic screen of the membrane of senescent cells will not only provide a list of extracellular epitopes that could be used for detection and targeting of the cells, but will likely uncover new important members of the senescent pathways.
4 Targeted Senolytics as a New Anti-senescence Approach
Targeted therapies have been developed for the management of various cancers and they have proven over the years to be more specific and more effective than the traditional approaches. Unlike classic antineoplastic drugs, which are highly cytotoxic, targeted therapies can inhibit specific molecular targets of interests without acting in a non-specific cytotoxic manner. Although not without adverse effects, targeted therapies are often better tolerated and generally more efficient (Walter and Ahmed 2017; Ke 2017). This principle can be applied to other diseases as well (Florence and Lee 2011; Daste et al. 2016; Pauliah et al. 2018). Indeed, targeted therapies tailored to individual patients, with new approaches towards disease assessment and dosing, is likely to be the choice for most treatments in the near future, provided that the technical and financial hurdles are resolved.
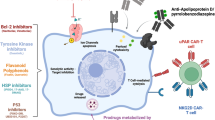
Senescence-related conditions, including ageing, could benefit from targeted drugs as well. In this instance, the goal would be to eliminate senescent cells in a more specific way than the current available senolytics, which could be compared to blunt treatments, such as classic chemotherapy for cancer. Several avenues for these novel targeted senolytics are currently being developed, some of them based on epitopes present on the senescent surfaceome, some against intracellular targets (Fig. 6.1). The three main categories of potential targeted therapies for clearance of senescent cells that have been most investigated are based on nanoparticles, the immune system and monoclonal antibodies.
4.1 Nanoparticles
Unlike cancer, senescence lacks the concept of an oncogene-like abnormal protein that triggers the phenotype. It is, instead, the result of the interaction of different networks of physiological signals that respond to damage. Thus, it is unlikely that drugs that inhibit a single protein to induce apoptosis only in senescent cells will ever be identified. One alternative approach for targeted senolytics could be to design a specific mechanism of toxic drug delivery that would preferentially reach senescent cells. As we will discuss below, this kind of tools have been successfully used in cancer. Nanotechnology has already provided proof of principle examples that this could also be a relevant avenue for senescence.
The first published report of nanoparticles applied to targeting senescent cells used a galactosidase-encapsulated drug (Munoz-Espin et al. 2018). Sugar-coated beads of ~100 nm diameter produced using MCM-41, a silica porous scaffold, had been previously shown to be internalized by endocytosis and digested in the lysosomes. This approach was modified using a 6-mer galacto-oligosaccharide to coat the silica scaffold and containing in their core drugs such as the cytotoxic doxorubicin or the senolytic navitoclax. Due to their high levels of lysosomal β-galactosidase, these nanoparticles only released the drug in senescent cells, which were able to lyse the galactosidase coat, while the cargo was protected in other cells. The same principle was modified to detect senescent cells, using a fluorescent cargo for the nanoparticles instead of drugs.
Importantly, it was shown that this drug delivery system worked in vivo. Toxic or senolytic drugs were specifically released in xenografts of cancer cells after senescence-inducing treatments, which importantly reduced the size of the tumours formed in the mice. Also, a mouse model of bleomyicin-induced pulmonary fibrosis responded to the nanoparticles with a severe decrease of the associated respiratory symptomatology and a reduction of damage in the tissues, consistent with the clearance of the senescent cells known to aggravate this disease (Munoz-Espin and Serrano 2014).
β-galactosidase was also used as a senolytic target by a later study that showed that enzyme-instructed peptide self-assembly (EISA) can lead to the formation of nanofibers and hydrogels specifically in senescent cells (Xu et al. 2019). These nanofibers inhibit the expression of senescence-driving proteins [p53, p21 and p16] and eventually cause death by apoptosis. This is the first example of the use of peptide-based supramolecular nanomaterials directed by the high levels of an enzyme (β-galactosidase in this case) to eliminate senescent cells.
Other delivery systems used porous calcium carbonate nanoparticles (CaCO3) loaded with the mTOR inhibitor rapamycin, which then blocked SASP (Thapa et al. 2017). Similarly to the previous examples, these nanoparticles were coated with a conjugate of lactose to increase specificity based on β-galactosidase enzymatic activity. On top of that, another layer of specificity was added by tagging them to a monoclonal antibody against CD9, which some senescent cells express. These nanoparticles were shown to be senolytic in human fibroblasts.
Another alternative to these senolytic strategies that bypass the need for an antibody would be the use Molecularly Imprinted NanoPolymers (nanoMIPs), which are synthetic antibody-like nanoparticles with high stability, cost effectiveness, ease of preparation and adaptation (Sellergren and Allender 2005; Piletsky et al. 2006; Vasapollo et al. 2011; Canfarotta et al. 2016a, b) to deliver drugs only to cells that express a certain surface marker. NanoMIPs are synthesized from methacrylic or acrylic monomers polymerized by creating a matrix around a template target molecule, which is later removed, leaving behind cavities complementary to its shape and functional groups. The produced nanoMIPs are thereafter able to selectively re-bind to their target molecule (Canfarotta et al. 2016a; Poma A, Turner APF, Piletsky SA. Advances in the manufacture of MIP nanoparticles 2010).
The advantage of this approach is that it relies on high affinity and specificity of the final product toward their targets, and the binding site affinities exhibit homogenous distribution (Canfarotta et al. 2016c). These “plastic antibodies” have recently found application in the detection of different cancers (Sengupta and Sasisekharan 2007; Voigt et al. 2014; Tyagi et al. 2016; Cecchini et al. 2017), in sustained drug release and drug delivery systems using small molecules (Sellergren and Allender 2005; Cunliffe et al. 2005; Puoci et al. 2011; Tieppo et al. 2012; Kempe et al. 2015; Luliński 2017), and also for transport across an in vitro model of the blood-brain barrier (Dadparvar et al. 2011). This suggests that nanoMIPs could be used as a system to deliver drugs to senescent cells, providing an extracellular marker as an epitope for the nanoparticles to bind.
NanoMIPs were created in our lab to specifically recognize one of the previously identified members of the senescent surfaceome, β2-Microglobulin (B2M) (Ekpenyong-Akiba et al. 2019). These MIPs, fluorescently-tagged, were tested in vitro and the selective detection of senescent cells using FACS was confirmed. Furthermore, it was observed that the nanoMIPs preferentially accumulated on the membrane surface of these cells, consistent with their selective detection of the extracellular epitope. The internalization of nanoMIPs was observed after binding to the marker, together with its accumulation in the cytosol forming perinuclear aggregates.
In order to analyse their potential for the detection of senescent cells in vivo, the B2M nanoMIPs were conjugated with a DyLight 800 NHS ester and injected intravenously into mice. A strong fluorescent signal was detectable in old animals, suggesting that the nanoMIPs were selectively binding to senescent cells, specifically in the gastrointestinal tract. These proof-of-principle experiments demonstrate that B2M nanoMIPs accumulate in certain senescent cells in vitro and in vivo and supports the hypothesis that nanoMIPs could be a promising approach for targeted drug delivery into senescent cells. To test this, B2M nanoMIPs were loaded with different drugs, including dasatinib, to minimize the off-target toxicity and impact on other cells. The dasatinib-containing nanoMIPs significantly reduced the senescent cells viability comparing to the proliferating cells and the effect was significantly stronger than the activity of dasatinib as a drug alone. Of note, nanoMIPs had no cytotoxic effects on their own, as the long-term exposure of the B2M targeted nanoparticles did not affect the viability and survival of senescent and control cells. This had been previously shown for other nanoMIPs (Canfarotta et al. 2016b; Hoshino et al. 2010). Also, the administration of B2M targeted nanoMIPs to mice did not significantly affect the health of the animals during 14-days follow-up after treatment despite the route of injection (oral gavage, intraperitoneally and intravenously).
These experiments show that nanoparticles, in different formats, are a stable and inexpensive tool for the detection and targeting of senescent cells. The senolytic nanoparticles tested so far have been shown to be biocompatible, non-toxic to cells in the long-term and non-toxic to animals in the short-term. However, not much is currently known about the bio-distribution and clearance of nanoparticles from the body when applied in vivo. For instance it has been proposed that nanoMIPs are cleared from the animal’s blood by the liver’s mononuclear phagocytes (Hoshino et al. 2010). Moreover, technical and economic limitations may occur when nanoparticle production needs to be scaled for clinical use. Further studies to study these and other parameters related to biosafety will be needed before nanoparticles could be used in humans, but these tools can already provide useful pre-clinical information on potential targets for targeted clearance of senescent cells.
4.2 Antibody-Drug Conjugates
A more readily translatable approach to the same principle would be to use antibodies instead on the nanoparticles for drug delivery. Antibody-drug conjugates (ADCs) are monoclonal antibodies to which cytotoxic drugs are bound through a chemical linker to reduce systemic toxicity and increase the therapeutic benefit for patients (Strohl and Strohl 2012; Casi and Neri 2012; Perez et al. 2014; Gébleux and Casi 2016; Kumar et al. 2017). The concept of ADCs was first introduced over a century ago by Paul Ehrlich, a German physician who proposed the use of a targeting agent to selectively deliver a cytotoxic drug to a tumour (Perez et al. 2014; Ehrlich 1906). From the introduction of the idea by Ehrlich to date, advances have been made in the development of ADCs for cancer therapy (Trail et al. 1993; Sievers and Senter 2013; Sau et al. 2017; Donnell et al. 2017). So far, they have only been used to kill cancer cells, but their therapeutic potential could be exploited for other targets, such as senescent cells.
Several ADCs have entered clinical trials that have been developed to target breast cancer (Trail et al. 1993; Kolodych et al. 2017; Trail et al. 2018), ovarian cancer (Jiang et al. 2016), lung and colon cancers (Trail et al. 1993) and hematologic malignancies (Sievers and Senter 2013), among others (Birrer et al. 2019). The US Food and Drug Administration (FDA) has so far approved three ADCs: Mylotarg® and Adcetris® for the treatment of haematological cancers, and Kadcyla® for the treatment of HER2 positive breast cancer. Of note, Mylotarg® was withdrawn from the market a decade after its approval due to poor overall survival of patients (Perez et al. 2014; Gébleux and Casi 2016; Sau et al. 2017).
An ADC typically consists of three parts namely, an antibody, a linker and a cytotoxic drug or “payload” (Kumar et al. 2017; Thomas et al. 2016). In the design of ADCs, the antibody’s specificity, stability of the linker, potency of the payload, the drug to antibody ratio (DAR) as well as rate of internalization are important determinants of efficacy (Perez et al. 2014; Gébleux and Casi 2016; Sievers and Senter 2013; Kolodych et al. 2017; Trail et al. 2018). ADCs are eventually cleared from circulation either via the renal or hepatobiliary route and studies have been carried out to evaluate the toxicity, higher-than-normal tissue exposure and to improve the localization and clearance of ADCs from the body (Casi and Neri 2012; Sievers and Senter 2013).
An anti-senescent ADC would have to be targeted against an epitope on the surfaceome and based on a monoclonal antibody that was easily internalized after binding. Several drugs could be chosen as payloads, from the most non-specific (we have observed that senescent cells are sensitive to different concentrations of doxorubicin and duocarmycin, for instance) to actual senolytics (like we used in some of the mentioned nanoparticles). The linker should be degraded once the antibody has been internalized, in order to free the payload. Also, the DAR should be high enough to elicit a strong effect. Preliminary data from our laboratory shows that an ADC against B2M with an average DAR of 2 (in line with currently used ADCs (Birrer et al. 2019)), generated using a commercially available monoclonal antibody linked to duocarmycin, can selectively kill senescent cells in culture, as seen with the targeted nanoMIPs. This suggests that ADCs could be used in humans to specifically clear senescent cells. However, further in vitro and in vivo tests would be necessary to understand the action of these treatments and evaluate their safety. More importantly, it has to be taken in consideration that the choice of the right surface marker would determine their specific biological effect.
4.3 Cellular Therapies
Antibodies can also be used to elicit cytotoxic effects independently of drug delivery. New approaches have already been developed for other diseases, such as antibody-dependent cell-mediated cytotoxicity (ADCC), which guides natural killer (NK) cells to selectively destroy a target (Lo Nigro et al. 2019). This has already been tested in senescence using a protein from the surfaceome. In this previously mentioned study (Kim et al. 2017), an anti-DPP4 antibody was added to both proliferating and senescent WI-38 cells. NK cells were isolated from human peripheral blood mononuclear cells and added to the conditioned media of WI-38 previously treated with a specific anti-DPP4 antibody. Results showed up to 40% reduction in cell viability relative to proliferating cells, proving that the antibody attracted the NK cells to the DPP4-expressing senescent targets. One variant of these immune-based therapies that does not require the use of antibodies would be to engineer NK cells to be attracted to SASP molecules such as IL-6 (Qudrat et al. 2017).
5 Conclusions: The Road Ahead
Targeted senolytics represent an attractive mode of therapy that could help speed up the translational applications of senescent cell clearance. The increased effectivity and, more importantly, reduced side effects could allow a widespread use of senolytics in senescence-related pathologies, including ageing. However, this is an area of research still in its infancy and many issues will have to be resolved before the first clinical trials can be designed. Given that the cellular events and molecular pathways that lead to senescence vary, it would be necessary to define clearly the pharmacodynamics of each potential anti-senescence therapeutic candidate not only to minimise off-target effects, ensure sensitivity and specificity, but to also identify potential synergistic effects or even contraindications when they are used in combination with other medications.
Identifying specific targets is key to the selectivity of these targeted therapies, since all studies so far seem to point to the fact that each tissue and model will have a particular set of senescent markers preferentially expressed. Thus, knowing the profile of senescent proteins present in each particular condition will allow the development of the right drug. In this context, the senescent surfaceome has the potential to provide several novel candidates, with the added value of determining extracellular epitopes for nanoparticle- and antibody-based strategies. Many proteins overexpressed on the surface of senescent cells have already been described in several labs, and more are likely to be published in the near future. However, like all known senescent markers, their expression is not universal, showing both tissue and disease specificity.
Since none of the current markers of senescence is exclusively specific to the senescent state, there is still the need for more reliable alternatives. One could be to use a combination of markers to increase specificity. Following the examples described above, a cytotoxic mechanism split in two portions (such as those used in certain mouse models (Baker et al. 2011)) could be delivered into senescent cells by separate ADCs or nanoparticles, thus killing only the cells that express both markers simultaneously. This and other approaches could help solve the issue of lacking a distinct and unique marker of senescence.
In parallel, better and more exhaustive screens would need to be developed. Mass spectrometry is still the best option for an unbiased approached. However, it would be interesting to compare the results of different pathways of induction of senescence (OIS, RS, MiDAS, radiotherapy, etc.) on the same cellular model in the same experiments, in order to provide a more balanced comparison of the surfaceomes. Manual validation of the main targets will still be essential. Therefore, high throughput techniques will need to be implemented to confirm the hits of the proteomic screens, which it has not been done in the published studies so far.
In light of recent findings (Zhang et al. 2019; van Deursen 2019; Xu et al. 2018; Roos et al. 2016), there is no doubt that senolytics are going to become a new class of drugs that will be intensively studied for their wide range of clinical applications. Their potential impact in the healthcare of an ever-growing aged population is immense. Targeted senolytics could be the most useful form of these drugs and efforts should be made to propose options that could be tested in specific diseases as soon as possible. In order to do this, better markers of senescence need to be identified, while the first generation of targeted senolytics are generated against the ones that are currently available. In following years, important advances are likely to be made in these areas and is to be expected that senolytics and targeted senolytics will routinely reach the patients.
References
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9(6):400–414
Adams PD (2007) Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging 84–93
Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A et al (2014a) Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis 5(11):e1528
Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A et al (2014b) Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis 5:e1528
Althubiti M, Rada M, Samuel J, Escorsa JM, Najeeb H, Lee KG et al (2016) BTK modulates p53 activity to enhance apoptotic and senescent responses. Can Res 76(18):5405–5414
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–236
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J et al (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530(7589):184–189
Barrilleaux B, Phinney DG, Prockop DJ, O’Connor KC (2006) Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng 12(11):3007–3019
Bausch-Fluck D, Goldmann U, Muller S, van Oostrum M, Muller M, Schubert OT et al (2018) The in silico human surfaceome. Proc Natl Acad Sci USA 115(46):E10988–E10997
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P et al (2003) Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22(16):4212–4222
Bieging KT, Mello SS, Attardi LD (2014) Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 14:359
Birrer MJ, Moore KN, Betella I, Bates RC (2019) Antibody-Drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst
Blagosklonny MV (2014) Geroconversion: irreversible step to cellular senescence. Cell Cycle (Georgetown, Tex) 13(23):3628–3635
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377(6549):552–557
Bugarcic A, Zhe Y, Kerr MC, Griffin J, Collins BM, Teasdale RD (2011) Vps26A and Vps26B subunits define distinct retromer complexes. Traffic 12(12):1759–1773
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562(7728):578–582
Caceres PS, Mendez M, Haque MZ, Ortiz PA (2016) Vesicle-associated membrane protein 3 (VAMP3) mediates constitutive trafficking of the renal co-transporter NKCC2 in thick ascending limbs: ROLE IN RENAL FUNCTION AND BLOOD PRESSURE. J Biol Chem 291(42):22063–22073
Cahu J (2013) SASP: roadblock for tissue re-organization. Aging 5(9):641–642
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens. Bad Neighb Cell 120(4):513–522
Campisi J (2011) Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 21(1):107–112
Campisi J, Debacq-Chainiaux F, Erusalimsky JD, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4(12):1798–1806
Canfarotta F, Poma A, Guerreiro A, Piletsky S (2016a) Solid-phase synthesis of molecularly imprinted nanoparticles. Nat Prot 11(3):443–455
Canfarotta F, Waters A, Sadler R, McGill P, Guerreiro A, Papkovsky D et al (2016b) Biocompatibility and internalization of molecularly imprinted nanoparticles. Nano Res 9(11):3463–3477
Canfarotta F, Poma A, Guerreiro A, Piletsky S (2016c) Solid-phase synthesis of molecularly imprinted nanoparticles. Nat Prot 11:443
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213(2):341–347
Carragher LA, Snell KR, Giblett SM, Aldridge VS, Patel B, Cook SJ et al (2010) V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol Med 2(11):458–471
Casi G, Neri D (2012) Antibody–drug conjugates: basic concepts, examples and future perspectives 422–428
Cecchini A, Raffa V, Canfarotta F, Signore G, Piletsky S, MacDonald MP et al (2017) In Vivo recognition of human vascular endothelial growth factor by molecularly imprinted polymers. Nano Lett
Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J et al (1999) Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18(34):4808–4818
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22(1):78–83
Choi SA, Kim YH, Park YH, Yang HJ, Jeong PS, Cha JJ et al (2018) Novel crosstalk between Vps26a and Nox4 signaling during neurogenesis. Cell Death Differ
Cichowski K, Hahn WC (2008) Unexpected pieces to the senescence puzzle 958–961
Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M et al (2005) Tumour biology: senescence in premalignant tumours. Nature 436(7051):642
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130(2):223–233
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6(12):2853–2868
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann Rev Pathol 5:99–118
Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J (2011) Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 286(42):36396–36403
Cui H, Kong Y, Xu M, Zhang H (2013) Notch3 functions as a tumor suppressor by controlling cellular senescence. Cancer Res 73(11):3451
Cunliffe D, Kirby A, Alexander C (2005) Molecularly imprinted drug delivery systems 1836–1853
Dadparvar M, Wagner S, Wien S, Kufleitner J, Worek F, von Briesen H et al (2011) HI 6 human serum albumin nanoparticles—development and transport over an in vitro blood–brain barrier model 60–66
Daste A, Chakiba C, Domblides C, Gross-goupil M, Quivy A, Ravaud A et al (2016) Targeted therapy and elderly people: a review 199–215
de Keizer PL (2016) The fountain of youth by targeting senescent cells? Trends Mol Med
de Magalhães JP, Passos JF (2017) Stress, cell senescence and organismal ageing
Demaria M (2017) Senescent cells: New target for an old treatment? Mol Cell Oncol 4(3):e1299666
Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV (2010) Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA 107(21):9660–9664
Deursen JMAV (2014) The role of senescent cells in ageing 509(7501):439–446
di Fagagna FDA, Campisi J (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92(20):9363–9367
Dong Q, Han H, Liu X, Wei L, Zhang W, Zhao Z et al (2017) HCSGD: an integrated database of human cellular senescence genes 227–234
Donnell AF, Zhang Y, Stang EM, Wei DD, Tebben AJ, Perez HL et al (2017) Macrocyclic pyrrolobenzodiazepine dimers as antibody-drug conjugate payloads 5267–5271
Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA (2007) Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genom 8:80
Ehrlich P (1906) The relationship existing between chemical constitution, distribution and pharmacological action. Collect Stud Immun 441–450
Ekpenyong-Akiba AE, Canfarotta F, Abd HB, Poblocka M, Casulleras M, Castilla-Vallmanya L et al (2019) Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horiz. 4(3):757–768
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al (1993) WAF1, a potential mediator of p 53 tumor suppression. Cell 75(4):817–825
Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Munoz-Espin D et al (2017) Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16(1):192–197
Falschlehner C, Emmerich CH, Gerlach B, Walczak H (2007) TRAIL signalling: Decisions between life and death. Int J Biochem Cell Biol 39(7):1462–1475
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL et al (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23(9):1072–1079
Florence AT, Lee VHL (2011) Personalised medicines: More tailored drugs, more tailored delivery 29–33
Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW et al (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8(1):z
Galanos P, Vougas K, Walter D, Polyzos A, Maya-Mendoza A, Haagensen EJ et al (2016) Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat Cell Biol 18:777
Gallage S, Gil J (2016) Mitochondrial dysfunction meets senescence. Trends Biochem Sci 41(3):207–209
Gébleux R, Casi G (2016) Antibody-drug conjugates: current status and future perspectives 48–59
Georgakilas AG, Martin OA, Bonner WM (2017) p 21: a two-faced genome guardian 310–319
Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP et al (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging. 5(1):37–50
Guney I, Sedivy JM (2006) Cellular senescence, epigenetic switches and c-Myc. Cell Cycle (Georgetown, Tex) 5(20):2319–2323
Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K et al (2016) Elimination of p19(ARF)-expressing cells enhances pulmonary function in mice. JCI insight. 1(12):e87732
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37(3):614–636
Hayflick L, Moorehead P (1961) The serial cultivation of human diploid strains. Exp Cell Res 25:585–621
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25(3):585–621
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14(4):501–513
Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M (2017) Unmasking transcriptional heterogeneity in senescent cells. Curr Biol: CB
Herranz N, Gil J (2016) Mitochondria and senescence: new actors for an old play. The EMBO J 35(7):701–702
Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ et al (2015) mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17:1205
Hoare M, Narita M (2017) NOTCH and the 2 SASPs of senescence. Cell Cycle 16(3):239–240
Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA et al (2016) NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18(9):979–992
Hoshino Y, Koide H, Urakami T, Kanazawa H, Kodama T, Oku N et al (2010) Recognition, neutralization and clearance of target peptides in the blood stream of living mice by molecular imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc 132(19):6644–6645
Itahana K, Campisi J, Dimri GP (2007) Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol (Clifton, NJ) 371:21
Itahana K, Itahana Y, Dimri GP (2013) Colorimetric detection of senescence-associated beta galactosidase. Methods Mol Biol 965:143–156
Ito Y, Hoare M, Narita M (2017) Spatial and temporal control of senescence 820–832
Iuliano R, Palmieri D, He H, Iervolino A, Borbone E, Pallante P et al (2010) Role of PTPRJ genotype in papillary thyroid carcinoma risk. Endocr Relat Cancer 17(4):1001–1006
Jiang J, Dong L, Wang L, Wang L, Zhang J, Chen F et al (2016) HER2-targeted antibody drug conjugates for ovarian cancer therapy 274–286
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p 53 protein in the cellular response to DNA damage. Cancer Res 51(23 Part 1):6304–6311
Ke X (2017) Shen L. The progress and future prospect, Molecular targeted therapy of cancer, pp 69–75
Kempe H, Parareda Pujolràs A, Kempe M (2015) Molecularly imprinted polymer nanocarriers for sustained release of erythromycin. Pharm Res 32(2):375–388
Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE et al (2017) Identification of senescent cell surface targetable protein DPP4. Genes Dev 31(15):1529–1534
Kim KM, Noh JH, Bodogai M, Martindale JL, Pandey PR, Yang X et al (2018) SCAMP4 enhances the senescent cell secretome. Genes Dev 32(13–14):909–914
Kimball AB, Alora-Palli MB, Tamura M, Mullins LA, Soh C, Binder RL et al (2018) Age-induced and photoinduced changes in gene expression profiles in facial skin of Caucasian females across 6 decades of age 39.e7
Kirkland JL, Tchkonia T (2015) Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 68:19–25
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28
Kolodych S, Michel C, Delacroix S, Koniev O, Ehkirch A, Eberova J, et al (2017) Development and evaluation of β-galactosidase-sensitive antibody-drug conjugates 376–382
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L et al (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Investig 114(9):1299–1307
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9(2):81–94
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133(6):1019–1031
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24(22):2463–2479
Kumar A, White J, James Christie R, Dimasi N, Gao C (2017) Chapter twelve–antibody-drug conjugates. In: Goodnow RA (ed). Academic Press, pp 441–480
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000a) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113(Pt 20)(Pt 20):3613–3622
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000b) Senescence-associated beta-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113(Pt):3613–3622
Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L et al (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17:1049
Lane DP (1992) p53, guardian of the genome. Nature 358:15
Lasry A, Ben-Neriah Y (2015) Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 36(4):217–228
Lawless C, Wang C, Jurk D, Merz A, Zglinicki Tv, Passos JF (2010) Quantitative assessment of markers for cell senescence, pp 772–778
Lee HW, Lee SH, Lee HW, Ryu YW, Kwon MH, Kim YS (2005) Homomeric and heteromeric interactions of the extracellular domains of death receptors and death decoy receptors. Biochem Biophys Res Commun 330(4):1205–1212
Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN et al (2017) Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 50(2):2016. Print 7 Aug
Leontieva OV, Blagosklonny MV (2017) While reinforcing cell cycle arrest, rapamycin and Torins suppress senescence in UVA-irradiated fibroblasts. Oncotarget 8(65):109848–109856
Levine AJ (1997) p 53, the cellular gatekeeper for growth and division 323–331
Li W, Qin L, Feng R, Hu G, Sun H, He Y et al (2019) Emerging senolytic agents derived from natural products. Mech Ageing Dev 181:1–6
Liu H, Zhao WL, Wang JP, Xin BM, Shao RG (2018) EBP50 suppresses the proliferation of MCF-7 human breast cancer cells via promoting Beclin-1/p62-mediated lysosomal degradation of c-Myc. Acta Pharmacol Sin 39(8):1347–1358
Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC (2019) NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med 7(5):105
Lowe SW, Sherr CJ (2003) Tumor suppression by Ink4a–Arf: progress and puzzles 77–83
Luliński P (2017) Molecularly imprinted polymers based drug delivery devices: a way to application in modern pharmacotherapy. A Rev 1344–1353
Lundberg AS, Hahn WC, Gupta P, Weinberg RA (2000) Genes involved in senescence and immortalization 705–709
Maciel-Barón LA, Morales-Rosales S, Aquino-Cruz A, Triana-Martínez F, Galván-Arzate S, Luna-López A et al (2016) Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age 38(1):26
Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW et al (2002) Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J 21(9):2180–2188
Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, O’Connor KC (2017) Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther 8(1):201
Malaquin N, Martinez A, Rodier F (2016) Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype 39–49
Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W et al (2012) Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem 287(13):9845–9854
Matjusaitis M, Chin G, Sarnoski EA, Stolzing A (2016) Biomarkers to identify and isolate senescent cells, pp 1–12
McHugh D, Gil J (2018) Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 217(1):65
Mduff FKE, Turner SD (2011) Jailbreak: oncogene-induced senescence and its evasion 6–13
Mercer K, Giblett S, Green S, Lloyd D, DaRocha Dias S, Plumb M et al (2005) Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Can Res 65(24):11493–11500
Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A et al (2009) Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 228(1):58–73
Moreno-Blas D, Gorostieta-Salas E, Castro-Obregón S (2018) Connecting chaperone-mediated autophagy dysfunction to cellular senescence 34–41
Munoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–496
Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S et al (2013) Programmed cell senescence during mammalian embryonic development. Cell 155(5):1104–1118
Munoz-Espin D, Rovira M, Galiana I, Gimenez C, Lozano-Torres B, Paez-Ribes M et al (2018) A versatile drug delivery system targeting senescent cells. EMBO Mol Med
Murray-Zmijewski F, Slee EA, Lu X (2008) A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9(9):702–712
Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA et al (2003) Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113(6):703–716
Naylor RM, Deursen JMAV, Baker DJ (2013) Senescent cells: a novel therapeutic target for aging and age-related diseases 93(1):105–116
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A et al (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8:15691
Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM et al (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18(3):e12950
Pan G, Ni J, Yu G-l, Wei Y-F, Dixit VM (1998) TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett 424(1):41–45
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M et al (2017) Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in Mice, pp 353–361
Park HY, Sin DD (2014) Chapter 16—stress-induced premature senescence: another mechanism involved in the process of accelerated aging in chronic obstructive pulmonary disease. In: Rahman I, Bagchi D (eds) Inflammation, advancing age and nutrition. Academic Press, San Diego, pp 193–202
Pauliah M, Zanganeh S, Erfanzadeh M, Ho JQ (2018) Chapter 10—tumor-targeted therapy. In: Mahmoudi M, Laurent S (eds). Elsevier; pp 273–290
Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD (2014) Antibody–drug conjugates: current status and future directions 869–881
Pérez-Mancera PA, Young ARJ, Narita M (2014) Inside and out: the activities of senescence in cancer. Nat Rev Cancer 14(8):547
Piletsky SA, Turner NW, Laitenberger P (2006) Molecularly imprinted polymers in clinical diagnostics–future potential and existing problems. Med Eng Phys 28(10):971–977
Poma A, Turner APF, Piletsky SA. Advances in the manufacture of MIP nanoparticles. 2010. p. 629–37
Puoci F, Cirillo G, Curcio M, Parisi OI, Iemma F, Picci N (2011) Molecularly imprinted polymers in drug delivery: state of art and future perspectives. Expert Opin Drug Deliv 8(10):1379–1393
Qudrat A, Wong J, Truong K (2017) Engineering mammalian cells to seek senescence associated secretory phenotypes. J Cell Sci
Rada M, Althubiti M, Ekpenyong-Akiba AE, Lee KG, Lam KP, Fedorova O et al (2017) BTK blocks the inhibitory effects of MDM2 on p53 activity. Oncotarget 8(63):106639–106647
Rada M, Barlev N, Macip S (2018a) BTK modulates p73 activity to induce apoptosis independently of p53. Cell Death Discov 4:30
Rada M, Barlev N, Macip S (2018b) BTK: a two-faced effector in cancer and tumour suppression. Cell Death Dis 9(11):1064
Rodier F, Campisi J (2011) Four faces of cellular senescence. J cell Biol 192(4):547–556
Romagosa C, Simonetti S, López-Vicente L, Mazo A, Lleonart ME, Castellvi J et al (2011) p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 30(18):2087–2097
Romanov VS, Rudolph KL (2016) p21 shapes cancer evolution. Nat Cell Biol 18:722
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15(5):973–977
Rufini A, Tucci P, Celardo I, Melino G (2013) Senescence and aging: the critical roles of p53. Oncogene 32(43):5129
Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28(2):99–114
Sau S, Alsaab HO, Kashaw SK, Tatiparti K, Iyer AK (2017) Advances in antibody–drug conjugates: a new era of targeted cancer therapy 1547–1556
Sellergren B, Allender CJ (2005) Molecularly imprinted polymers: a bridge to advanced drug delivery. Adv Drug Deliv Rev 57(12):1733–1741
Sengupta S, Sasisekharan R (2007) Exploiting nanotechnology to target cancer. Br J Cancer 96(9):1315–1319
Sharpless NE, Sherr CJ (2015) Forging a signature of in vivo senescence. Nat Rev Cancer 15(7):397–408
Sherr CJ (2012) Ink4-Arf locus in cancer and aging. Wiley Interdiscip Rev Dev Biol 1(5):731–741
Shiohara M, El-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R et al (1994) Absence of WAF1 mutations in a variety of human malignancies. Blood 84(11):3781
Sievers EL, Senter PD (2013) Antibody-drug conjugates in cancer therapy. Annu Rev Med 64:15–29
Sikora E, Arendt T, Bennett M, Narita M (2011) Impact of cellular senescence signature on ageing research, pp 146–152
Simboeck E, Di Croce L (2013) p16INK4a in cellular senescence. Aging 5(8):590
Sionov RV, Haupt Y (1999) The cellular response to p53: the decision between life and death. Oncogene 18(45):6145–6157
Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V et al (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155(5):1119–1130
Strohl WR, Strohl LM (2012) 15—Antibody-drug conjugates. In: Strohl WR, Strohl LM (eds) Therapeutic antibody engineering. Woodhead Publishing Series in Biomedicine, Woodhead Publishing; pp 345–595
Swanson EC, Manning B, Zhang H, Lawrence JB (2013) Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol 203(6):929–942
Takahashi A, Ohtani N, Hara E (2007) Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Div 2:10
Tan M, Li J, Ma F, Zhang X, Zhao Q, Cao X (2019) PLD3 rare variants identified in late-onset alzheimer’s disease affect amyloid-beta levels in cellular model. Front Neurosci 13:116
Taniguchi K, Kohsaka H, Inoue N, Terada Y, Ito H, Hirokawa K et al (1999) Induction of the p16INK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med 5(7):760
Terman A, Brunk UT (2004) Lipofuscin 1400–1404
Thapa RK, Nguyen HT, Jeong JH, Kim JR, Choi HG, Yong CS et al (2017) Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep 7:43299
Thomas A, Teicher BA, Hassan R (2016) Antibody–drug conjugates for cancer therapy e262
Tieppo A, White CJ, Paine AC, Voyles ML, McBride MK, Byrne ME (2012) Sustained in vivo release from imprinted therapeutic contact lenses 391–397
Trail PA, Willner D, Lasch SJ, Henderson AJ, Hofstead S, Casazza AM et al (1993) Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Sci (New York, NY) 261(5118):212–215
Trail PA, Dubowchik GM, Lowinger TB (2018) Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design 126–142
Tyagi N, Arora S, Deshmukh SK, Singh S, Marimuthu S, Singh AP (2016) Exploiting nanotechnology for the development of MicroRNA-Based cancer therapeutics. J Biomed Nanotechnol 12(1):28–42
van Deursen JM (2019) Senolytic therapies for healthy longevity. Science 364(6441):636–637
Vasapollo G, Sole RD, Mergola L, Lazzoi MR, Scardino A, Scorrano S et al (2011) Molecularly imprinted polymers: present and future prospective. Int J Mol Sci 12(9):5908–5945
Veale KJ, Offenhauser C, Lei N, Stanley AC, Stow JL, Murray RZ (2011) VAMP3 regulates podosome organisation in macrophages and together with Stx4/SNAP23 mediates adhesion, cell spreading and persistent migration. Exp Cell Res 317(13):1817–1829
Voigt J, Christensen J, Shastri VP (2014) Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc Natl Acad Sci USA 111(8):2942–2947
Waldman T, Kinzler KW, Vogelstein B (1995) p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Can Res 55(22):5187–5190
Walter HS, Ahmed S (2017) Targeted therapies in cancer. Surgery (Oxford)
Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8(3):311–323
Wang R, Sunchu B, Perez VI (2017) Rapamycin and the inhibition of the secretory phenotype 89–92
Wiley CD, Campisi J (2016) From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23(6):1013–1021
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A et al (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype 303–314
Wu Q, Cheng Z, Zhu J, Xu W, Peng X, Chen C et al (2015) Suberoylanilide hydroxamic acid treatment reveals crosstalks among proteome, ubiquitylome and acetylome in non-small cell lung cancer A549 cell line. Sci Rep 5:9520
Wu T, Rayner CK, Horowitz M (2016) Incretins. In: Herzig S (ed) Metabolic control. Springer International Publishing, Cham, pp 137–171
Xiao P, Huang X, Huang L, Yang J, Li A, Shen K et al (2017) G protein-coupled receptor kinase 4-induced cellular senescence and its senescence-associated gene expression profiling 273–280
Xu Y, Li N, Xiang R, Sun P (2014) Emerging roles of the p 38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence 268–276
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8):1246–1256
Xu T, Cai Y, Zhong X, Zhang L, Zheng D, Gao Z et al (2019) Beta-Galactosidase instructed supramolecular hydrogelation for selective identification and removal of senescent cells. Chem Commun (Camb)
Young ARJ, Narita M, Narita M (2013) Cell senescence as both a dynamic and a static phenotype. In: Galluzzi L, Vitale I, Kepp O, Kroemer G (eds) Cell senescence: methods and protocols. Humana Press, Totowa, NJ, pp 1–13
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S et al (2019) Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22(5):719–728
Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J et al (2013) A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 62(1):149
Zhu C, Zhang L, Zheng Y, Xu J, Song J, Rolfe BE et al (2011) Effects of estrogen on stress-induced premature senescence of vascular smooth muscle cells: a novel mechanism for the “time window theory” of menopausal hormone therapy. Atherosclerosis 215(2):294–300
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14(4):644–658
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB et al (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15(3):428–435
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ekpenyong-Akiba, A.E., Poblocka, M., Macip, S. (2020). Targeted Senolytic Strategies Based on the Senescent Surfaceome. In: Muñoz-Espin, D., Demaria, M. (eds) Senolytics in Disease, Ageing and Longevity. Healthy Ageing and Longevity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-44903-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-44903-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44902-5
Online ISBN: 978-3-030-44903-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)