Abstract
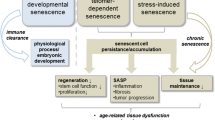
Cellular senescence is one of the fundamental mechanisms of aging. Senescent cells accumulate at etiological sites of age-related diseases and can secrete factors that cause dysfunction at local and systemic levels. The discovery of senolytics, drugs that specifically target senescent cells, has opened an innovative pathway for treating age-related diseases. Successes in pre-clinical models have led to first-in-human trials. If effective, senolytics could have a profound impact on alleviating age-related disorders and diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Aging is the leading risk factor for most serious chronic diseases and disabilities including dementias, cancers, cardiac disease, vascular diseases, atherosclerosis, osteoporosis, arthritis, diabetes, metabolic syndrome, renal failure, blindness, and frailty (Miller 2002; Kirkland 2013). Although age-related chronic conditions are the major drivers of morbidity, mortality, and health costs, most have been difficult to control. The number of chronic disease conditions per individual increases with aging, leading to multi-morbidity (St Sauver et al. 2015), thus circumventing the public health impact of preventing any single age-related disease (Miller 2002; Fried et al. 2009). In addition to chronic diseases, aging predisposes to geriatric syndromes and reduced resilience. Geriatric syndromes include frailty, sarcopenia, immobility, falling, depression, mild cognitive impairment, incontinence, and weight loss, among other problems (Inouye et al. 2007). Decreased physiological resilience entails failure to respond to or recover from stresses, such as pneumonia, stroke, heart attacks, dehydration, chemotherapy, surgery, fractures, or vaccinations (Kanapuru and Ershler 2009; Bandeen-Roche et al. 2009; Qu et al. 2009; Walston et al. 2009; Leng et al. 2007; Walston et al. 2006; Fried et al. 2001; Walston et al. 2002; Bandeen-Roche et al. 2006; Rockwood et al. 2006; Kirkland et al. 2016; Hadley et al. 2017).
An ideal strategy for addressing age-related chronic diseases, geriatric syndromes, and decreased resilience may be a “root cause” approach: targeting the fundamental aging mechanisms that represent shared upstream contributors or drivers of all of these conditions. Such a strategy could delay, prevent, or alleviate these conditions as a group, instead of adhering to the traditional one-disease-at-a-time approach. By one estimate, a 2% delay in the progression of aging processes would lead to an increase of 10 million healthy, as opposed to disabled, elderly people in the US by 2060 compared to doing nothing, delaying cancer, or delaying heart disease, with a savings in US health costs of $7.1 trillion over 50 years (Goldman et al. 2013).
Aging has long been recognized as the leading risk factor for most chronic diseases, geriatric syndromes, and decreased resilience, yet the fundamental aging processes that predispose to these conditions have only recently become viewed as potentially modifiable (Kirkland 2013; Tchkonia et al. 2013; Kirkland 2016). Supporting the view that interventions targeting basic aging processes could be feasible are the findings that: (1) Maximum lifespan is extended and age-related diseases are delayed across species by a number of single gene mutations (Bartke 2011; Pilling et al. 2017), suggesting pathways affected by these mutations could be therapeutic targets; (2) Humans who live beyond age 100, a partly heritable trait, frequently have delayed onset of age-related diseases and disabilities (Lipton et al. 2010), leading to compression of morbidity and enhanced healthspan; (3) Caloric restriction, which increases maximum lifespan, is associated with delayed onset of multiple chronic diseases in animal models (Anderson and Weindruch 2012); (4) Rapamycin increases lifespan and appears to delay cancers, age-related cognitive decline, and frailty in mouse models (Harrison et al. 2009; Bitto et al. 2016); (5) Factors produced by stem cells or in blood from young individuals may alleviate dysfunction in older individuals (Conboy et al. 2005; Lavasani et al. 2012; Villeda et al. 2014); and (6) Senescent cell accumulation is associated with chronic inflammation, fibrosis, and stem and progenitor cell dysfunction that in turn promote many age-related chronic diseases and geriatric syndromes (Tchkonia et al. 2013; Zhu et al. 2014). Senolytic drugs, which selectively eliminate senescent cells, delay age-related disorders, and enhance both health and life span in mice (Tchkonia 2017; Kirkland et al. 2017; Zhu et al. 2015; Roos et al. 2016; Schafer et al. 2017; Ogrodnik et al. 2017; Farr et al. 2017), as do a number of other drugs and lifestyle interventions. While senolytics and other agents that target fundamental aging processes were discovered only recently and in some cases are not yet published, the aging field is at the point of beginning to translate these interventions from lower mammals to humans
Timeline depicting the discovery of senolytics. The discovery of senolytics began with the discovery of senescent cells in 1961 by Hayflick and Moorehead (1961). Developing senolytics began before and independently from making or studying INK-ATTAC mice
2 Cellular Senescence
Hayflick and Moorehead (1961) (Fig. 2.1) discovered senescent cells in 1961. These cells, which appeared after serial subculturing of human embryonic fibroblasts, had loss of replicative capacity but remained viable. This prompted work to test the hypothesis that aging leads to accumulation in vivo of pre-senescent cells, which have limited remaining replicative potential, and senescent cells, which are viable but cannot replicate. In 1979, Schneider et al. found this to be true in human skin fibroblasts (Schneider 1979). In 1990, we observed this to be the case in primary fat cell progenitors (preadipocytes) in adipose tissue cloned from rats across the age spectrum, one of the cell types employed to discover senolytics (Kirkland et al. 1990).
A key article by Sharpless et al. in 2004 showed in Ames dwarf mice with pituitary hormone deficiencies as well as in calorically restricted mice, models in which both healthspan and lifespan are increased, that senescent cell accumulation is delayed (Krishnamurthy et al. 2004). This article was critical in prompting us to begin testing the hypothesis that targeting senescent cells may alleviate multiple age-related disorders. We had noted that the preadipocytes with restricted replicative potential that accumulate with aging in adipose tissue and fail to differentiate into fat cells also have increased expression of the inflammatory cytokine, TNFα (Kirkland et al. 2002). Before their key publication in 2008 (Coppé et al. 2008), Campisi et al. presented their finding that senescent cells can secrete a range of inflammatory and pro-apoptotic factors, the senescence-associated secretory phenotype (SASP) at meetings. This suggested that senescent cells have the potential to damage tissues in vivo, further prompting us to test if the increased burden of senescent cells with aging could be a cause of local and systemic dysfunction. Thus, in 2004/5 we began to test the hypothesis that selectively targeting senescent cells is a promising strategy for restoring function in old age and for delaying or preventing age-related disease onset. We began exploratory efforts to develop senolytic agents, a strategy we published several years later (Kirkland and Tchkonia 2014).
3 Senescent Cell Burden/Accumulation
Senescent cells are resistant to apoptosis (Munoz-Espin and Serrano 2014). Senescent cells appear with aging in a number of tissues and develop at sites of pathogenesis of several chronic diseases. Accumulation of senescent cells can cause extensive local and systemic dysfunction due to their pro-inflammatory SASP (Coppé et al. 2008, 2010; Kuilman and Peeper 2009). In pre-clinical experiments, we showed transplanting small numbers of senescent cells around the knee joints of young mice induces an osteoarthritis-like phenotype, while transplanting non-senescent cells did not (Xu et al. 2018). Additionally, transplanting 1 million radiation- or chemotherapy-induced senescent autologous ear fibroblasts or syngeneic preadipocytes intraperitoneally into lean, adult mice, so that only 1/10,000 of all cells in the transplanted mice were senescent cells, induced impaired physical function and shortened lifespan due to early onset of all of the same age-related diseases that cause death in older naturally-aged mice (Xu et al. 2018). Transplanting non-senescent cells into control mice did not do this, nor did transplanting 500 thousand as opposed to 1 million senescent cells. However, transplanting 500 thousand senescent cells into diet-induced obese (DIO) adult mice, which have more senescent cells than lean mice, or into old mice, was sufficient to cause the accelerated aging-like state. This suggests that there is a threshold of senescent cells, pre-existing plus transplanted, above which the accelerated aging-like state occurs. These observations further suggest the potential value of interventions that target senescent cells.
4 Development of Senolytics
The first senolytics were ultimately identified using a mechanism-based approach informed by their modes of action and targets, rather than a random approach such as screening libraries of compounds. As stated above, our efforts to find senolytics began in 2004/5 (Fig. 2.1), with initial attempts to create fusion proteins comprising a senescent cell surface binding domain coupled to a toxin, high throughput compound library screens for agents that eliminate senescent but not non-senescent cells, and other approaches. These traditional approaches did not achieve our goal. We therefore turned to a hypothesis-driven drug discovery approach. Our hypotheses were: (1) senescent cells resist apoptotic stimuli, implying the existence of pro-survival—anti-apoptotic defenses against their own SASP and harsh metabolic internal state and (2) in some respects, senescent cells are like cancer cells that do not divide (Zhu et al. 2015). Our hypothesis driven, mechanism-based approach led to discovery of the first senolytics, the combination of Dasatinib plus Quercetin, within a month after starting this work in May, 2013 [published in March, 2015 (Zhu et al. 2015)] and more senolytics subsequently (Zhu et al. 2016, 2017; Fuhrmann-Stroissnigg 2017).
We asked how senescent cells expressing a SASP can survive, despite their own highly pro-apoptotic and metabolically-distinct, potentially damaging milieu. Building upon bioinformatics data derived from proteomic and transcriptomic profiles of senescent versus non-senescent cells, we searched for senescent cell anti-apoptotic pathways (SCAPs). We identified several such potential SCAPs (ephrins/dependence receptors; PI3Kδ/Akt/metabolic; Bcl-2, Bcl-xl, Bcl-w; p53/FOXO4a/p21/serpine [PAI-1&2]; HIF-1α) (Zhu et al. 2015; Baar et al. 2017) (Fig. 2.2) and then another, the HSP-90 pathway (Fuhrmann-Stroissnigg 2017). We tested whether these SCAPs are essential for senescent cell survival by targeting key proteins within these SCAP pathways using RNA interference in senescent versus non-senescent human primary preadipocytes and human umbilical vein endothelial cells (HUVECs). Of the 39 small interfering RNA’s (siRNA’s) targeting possible SCAPs, 17 caused death of senescent but not non-senescent cells. We noted the patterns of SCAP pathways that prevent self-induced death of senescent human preadipocytes differed considerably from those required for survival of senescent endothelial cells. Senescent preadipocytes rely on pathways related to tyrosine kinases involved in the apoptosis that can be caused by dependence receptors (receptors that, if present on a cell but that are unoccupied by a ligand, induce cell death by apoptosis, such as the ephrin receptors) as well as p53- and p21-related pro-survival mechanisms and metabolically-related apoptosis involving PI3-kinase, AKT, and, again, p53. Senescent preadipocytes did not depend on BCL-2 family pro-survival proteins. Conversely, senescent endothelial cells depended for survival on BCL-2 family members, particularly BCL-xL, compared to non-senescent endothelial cells, as well as components of the PI3 kinase and HIF-1α pathways.
To discover senolytic agents, senescent and non-senescent cells were treated with 46 drugs and natural products previously reported to target the key SCAP proteins that we had identified through our RNA interference studies. Based on our starting hypotheses, agents selected for testing included those reputed to have an effect against cancers. By using this mechanism-based approach, Dasatinib (D) and Quercetin (Q) were among the first senolytics we selected for more intensive investigation based on their ability to target multiple nodes in the pro-survival SCAP networks we had discovered. We also selected these compounds because D has been approved by the FDA for use in humans since 2006 and Q is a natural product with a favorable safety profile, potentially enabling progression to clinical trials. Furthermore, these agents were selected because both have short elimination half-lives, <11 h, in humans, facilitating a “hit-and-run” intermittent treatment approach. As predicted, we found D and Q are senolytic. From the RNA interference studies, D was predicted to be senolytic for senescent human cultured preadipocytes but not human endothelial cells, which turned out to be true. Also as predicted, Q was senolytic for endothelial cells but not preadipocytes. The combination of D+Q, which together targets at least 8 SCAP pathway nodes (Fig. 2.2), was senolytic for both senescent preadipocytes and endothelial cells. Thus, different types of senescent cells employ distinct SCAPs to defend themselves against their own SASP and pro-apoptotic milieu. In some types of senescent cells, more than one SCAP pathway is engaged and these pathways can be redundant. Therefore, for each type of senescent cell, targeting only one SCAP pathway may not be sufficient to induce apoptosis. Combinations of senolytics that target different SCAPs or individual drugs that are active against multiple targets are required to overcome this redundancy. This novel concept goes against the traditional drug development paradigm of “one drug-one target-one disease” (Fig. 2.3).
Single target pathway versus multiple targets. The first generation of senolytics were discovered based on their mechanisms of action and targets. Senolytics, such as D+Q or Fisetin, go against the “old-fashioned” model of one-drug, one target, and one disease. Ideally, senolytics should act on senescent cells specifically versus causing apoptosis in multiple cell types (“panolytics”)
Of note, work to discover senolytics began before development of transgenic mice from which highly p16Ink4a-expressing cells can be removed using a drug that acts on a cell-killing construct, ATTAC that was originally devised by P. Scherer et al. (Trujillo et al. 2005; Baker et al. 2011). Similarly, in p16-3MR mice, a drug-inducible killing construct is expressed in highly p16Ink4a-expressing cells (Demaria et al. 2014). In these engineered mice, the drug-inducible killing construct is expressed in an inactive form by placing the transgene under the control of the p16Ink4a promoter, either as an inserted construct or, in the case of p16-3MR mice, within a minigene. Some, but not all senescent cells express p16Ink4a and not every cell with high p16Ink4a expression is senescent, for example non-senescent activated macrophages (Hall et al. 2016; 2017). Unlike in the transgenic mice, senolytics do not act through targeting cells that have high expression of p16Ink4a. Rather, senolytics selectively eliminate those senescent cells that cause tissue damage by releasing the pro-apoptotic, proinflammatory factors and proteases that are part of the SASP. Senolytics can also kill cancer cells, in particular those types of cancer cells that release pro-apoptotic factors, such as certain lymphoid malignancies. Indeed, one of the hypotheses used to develop the strategy for discovering senescent cells is that senolytics should do so (Zhu et al. 2015). Thus, senolytics act in a manner distinct from the removal of highly p16Ink4a-expressing cells from the engineered animal models. It is therefore not a “given” that eliminating highly p16Ink4a-expressing cells from the transgenic animal models will faithfully mimic the effects of removing senescent cells using senolytic agents. Unlike the transgenic animal approaches, senolytics are effective in wild-type mice without an inserted transgene, the discovery of senolytics did not depend on or involve use of the transgenic mice, and efforts to discover senolytics began before and independently from development of the transgenic mice.
5 Target of Senolytics: Senescent Cells
Completely new drug development paradigms are needed to take senolytics into clinical application, as may be the case for other types of interventions that target “root cause” fundamental aging processes (Kirkland 2013, 2016; Newman et al. 2016; Huffman et al. 2016; Justice et al. 2016; Burd et al. 2016). Perhaps the closest analogy regarding potential strategies for translating senolytics into clinical application is that of antibiotics. Antibiotics or antibiotic combinations are developed to target bacteria or other pathogens, not necessarily single molecular targets. In developing antibiotics, often a range of infections, for example respiratory and urinary tract infections, skin infections, and septicemia, are tested using candidate agents, rather than testing their effectiveness against only a single disease. Additionally, a range of pathogens is generally tested for susceptibility to the antibiotic. Effects of antibiotics alone or in combination are tested. The same may be the case for effectively developing and translating senolytics into clinical application. The key drug targets are senescent cells and the networks that sustain them, not a single molecule, not a single biochemical pathway, nor a single receptor. Multiple senescent cell types, senescence-associated diseases, and combinations of senolytics may need to be considered to successfully translate senolytics into clinical application, unlike the traditional one-drug/one-target/one-disease approach used for developing drugs that target a receptor, an enzyme, or a biochemical pathway.
Combination of senolytic agents with SASP inhibitors, such as rapamycin, metformin, or ruxolitinib, might possibly interfere with effectiveness of the senolytics, since senolytics act by allowing pro-apoptotic, proinflammatory SASP factors to kill the senescent cells from which these factors are released by transiently disabling the SCAPs that protect the senescent cells. This theoretical problem may turn out to be avoided by holding administration of SASP inhibitors for periods of time before and after senolytics are administered in the course of intermittent senolytic drug treatment regimens.
Since our report of D+Q, over a dozen more senolytics have been published using essentially the same hypothesis-driven approach [reviewed in (Tchkonia 2017; Kirkland et al. 2017)]. One such senolytic, Fisetin, is a naturally occurring flavonoid that selectively induces apoptosis in senescent but not proliferating human umbilical vein endothelial cells (HUVECs) (Zhu et al. 2017; Yousefzadeh et al. 2018). Similarly to quercetin, Fisetin selectively reduced viability and numbers of senescent HUVECs. This flavonoid is present in many fruits and vegetables such as apples, persimmon, grapes, onions, and cucumbers, with high concentrations found in strawberries (160 μg/g) (Khan et al. 2013). Its hydrophobic properties allow Fisetin to penetrate cell membranes and accumulate within cells to exert antioxidant effects (Ishige et al. 2001). It is widely available as a nutritional supplement and has a low side effect profile, which makes it an attractive option for clinical trials.
We first reported that targeting the BCL-2 pathway is a senolytic strategy in March, 2015 (Zhu et al. 2015). Knocking down BCL-xL mRNA by RNA interference killed senescent human endothelial cells, but not senescent human preadipocytes or non-senescent cells. Ten months later, we reported that Navitoclax, which targets BCL-2 family members including BCL-xL and BCL-w, induces apoptosis in senescent human endothelial cells, but not senescent human preadipocytes or non-senescent cells (Zhu et al. 2015). Within 15 days of that report, another group had found Navitoclax is senolytic and enhances bone marrow recovery following radiation in mice (Chang et al. 2016). However, Navitoclax only eliminates a subset of senescent cell types (e.g., endothelial cells) and not others (e.g., senescent preadipocytes) and can cause off-target side-effects through eliminating non-senescent cells, such as neutrophils and megakaryocytes or platelets. Also, unlike Dasatinib, which has been approved by the US Food and Drug Administration (FDA) for clinical use since 2006, Navitoclax is still not approved by the FDA for general clinical use. We subsequently found the more specific BCL-xL inhibitors, A1331852 and A1155463, are senolytic, at least for endothelial cells (Zhu et al. 2017), but again they have not been approved for general clinical use by the FDA.
6 Mouse Models of Aging and Disease
After the initial finding that the combination of D+Q is senolytic for both senescent preadipocytes and endothelial cells, we tested this first generation senolytic drug combination in naturally-aged animals. In early 2015, we published our findings that a single course of senolytics enhanced cardiac ejection fraction and improved vascular reactivity in 24 month old mice, the equivalent to 75–80 years of age in humans (Zhu et al. 2015). This was the first demonstration that targeting senescent cells enhances healthspan parameters in naturally-aged mice. Later that year, we confirmed and extended that finding by treating naturally-aged mice with a SASP inhibitor, Ruxolitinib. Metabolic healthspan parameters, including preservation of subcutaneous fat and insulin sensitivity, were enhanced by targeting senescent cells in these naturally-aged mice (Xu et al. 2015). Furthermore, total daily activity, rearing activity, ambulation, ability to remain suspended by hanging on to a wire, grip strength, and coordination were improved by targeting senescent cells in naturally-aged mice (Xu et al. 2015). Subsequently, we and others found that senolytics alleviate an impressive range of not only age-related phenotypes, but also chronic diseases in pre-clinical animal models, including high fat diet-induced vascular hyporeactivity, vascular calcification, damaged cardiac muscle, age-related cardiac hypertrophy and fibrosis, metabolic dysfunction and diabetes, chronic kidney disease, liver steatosis and fibrosis, pulmonary fibrosis, hyperoxia-induced airway disease, age-related bone loss, and obesity-related neuropsychiatric dysfunction, among others (Roos et al. 2016; Schafer et al. 2017; Ogrodnik et al. 2017; Farr et al. 2017; Xu et al. 2018; Moncsek et al. 2017; Ogrodnik et al. 2019; Parikh et al. 2018; Lewis-McDougall et al. 2019; Palmer et al. 2019; Lewis-McDougall et al. 2019; Anderson et al. 2019; Kim et al. 2019; Musi et al. 2018; Zhang et al. 2019). Senolytics can reduce chronic low-grade inflammation, protein aggregation, calcification, and fibrosis, the pathological processes active at sites of etiology in many of the major chronic diseases (Tchkonia 2017; Kirkland et al. 2017; Tchkonia and Kirkland 2018).
In a key study first demonstrating that targeting senescence is a potentially disease-modifying treatment for Alzheimer’s disease and other protein aggregation-related neurodegenerative diseases, D+Q was shown to decrease brain senescence markers, SASP factors, neurofibrillary tangles, and neuro-inflammation and to partially reverse a measure of brain hypo-perfusion, decrease brain atrophy, and enhance cognition in several different Tau+ Alzheimer’s disease mouse models (Musi et al. 2018; Zhang et al. 2019). D+Q was next shown to be effective in β-amyloid-expressing mice, another Alzheimer’s disease mouse model (Zhang et al. 2019). D+Q was effective even if administered to 23 month old Tau+ mice with clinically-evident dementia (Musi et al. 2018). Thus, it may be feasible to alleviate features of Tau+ dementia even after the dementia has become clinically manifest, a scenario that is much more translatable into initial clinical application than would be trials of preventing development of dementia, since the latter would involve discerning which subjects are most likely develop dementia before it becomes clinically evident, a difficult task and one that would entail treating many subjects unnecessarily (since many subjects with risk factors do not develop dementia or do so after a prolonged lag). Therefore, clinical trials of senolytics for clinically manifest Alzheimer’s and related dementias are about to start.
Based on our findings of pro-survival SCAP networks, we and others identified the flavonoid Fisetin as senolytic in both HUVEC and IMR 90 cells. The senolytic efficacy of Fisetin was tested in both murine models (progeroid and chronological aged mice) and human tissues (Yousefzadeh et al. 2018). Intermittent treatment with Fisetin reduced abundance of some types of senescent cells in multiple tissues. Also, late intervention with Fisetin restored tissue homeostasis, alleviated age-related pathology, and extended median and maximum lifespan. These characteristics suggested the feasibility of translating Fisetin into human clinical studies, which are now underway.
The first article about senolytics demonstrated that targeting senescent cells improves function in naturally-aged animals (Zhu et al. 2015). Beneficial effects on function in naturally-aged mice were subsequently confirmed in studies using the original and later senolytics as well as SASP inhibitors (Xu et al. 2018; Yousefzadeh et al. 2018; Xu et al. 2015).
7 Testing If a Drug Acts as a Senolytic Using a Modified Set of Koch’s Postulates
Although there is evidence that senolytics may alleviate multiple conditions as considered above, proving that drugs actually alleviate a given age-related phenotype, disorder, or disease because of senolytic as opposed to off-target effects is not trivial and has not been established beyond doubt for many such conditions so far. To prove conclusively that a candidate agent alleviates a condition because of senolytic effects, we propose a set of 8 criteria based on Koch’s postulates of the type used to prove causation in the case of infectious agents. These are considered below.
To establish causality using this modified set of Koch’ postulates, it would be first necessary to show that senescent cells occur in tandem with the condition in question: (1) Are senescent cells present in animals or humans with the condition? (2) Do individuals without senescent cells have the condition? Next, a way to test if cellular senescence is sufficient to cause a condition is to (3) show that the condition can be reproduced by inducing local accumulation of senescent cells. This can be achieved by transplanting senescent cells, focal irradiation, or tissue-specific genetic approaches to cause local senescent cell accumulation. For example, it was first demonstrated that senescent cells are sufficient to cause osteoarthritis by transplanting small numbers of syngeneic senescent mouse cells around the knee joints of younger mice (Xu et al. 2016). After a couple of months, this resulted in decreased mobility, knee joint pain, and radiographic changes characteristic of age-related osteoarthritis, while transplanting equal numbers of non-senescent cells did not cause this. In another example (also considered above), transplanting small numbers of senescent cells into middle-aged mice caused development of a frailty-like state after a few weeks, with decreased physical function and endurance, as well as accelerated onset of age-related diseases as a group (Xu et al. 2018). Causality can be tested further by (4) determining if removing these transplanted or induced senescent cells prevents or alleviates the condition. In the case of frailty or accelerated onset of age-related diseases caused by transplanting senescent cells, D+Q reduced senescent cell burden and indeed delayed or prevented these conditions. (5) It is then important to test if targeting naturally-occurring senescent cells alleviates the condition in question. This was achieved in the cases of frailty and age-related disease onset by treating naturally-aged, as opposed to younger transplanted mice with D+Q. (6) Administering the potentially senolytic drugs being investigated should have few or no effects related to the condition being tested in individuals without senescent cells, for example in young mice. (7) Senolytics should alleviate the condition even if given intermittently, at intervals longer than the drugs’ half lives, since senescent cells can take 2–6 weeks to re-accumulate, at least in cell culture. In the case of D+Q, the elimination half life is 11 h, but the drugs are as effective if administered monthly as continuously, at least in the case of age-related osteoporosis (Farr et al. 2017). (8) Finally, if an agent is truly senolytic, it should alleviate multiple age-related conditions.
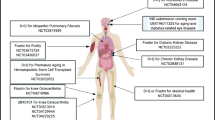
8 Clinical Trials
Senolytics were first developed using cells cultured from human subjects in order to facilitate the path to translation into human application. Senolytics were next shown to be effective in clearing senescent cells from mice and also for alleviating a range of age- and senescence-related disorders in mice, including cardiac and vascular dysfunction, insulin resistance, cognitive dysfunction, age-related osteoporosis, and frailty, among many others (see below). Furthermore, treatment with senolytics alleviated the accelerated aging-like state induced by transplanting senescent versus non-senescent cells into young mice (Xu et al. 2018). Recently, senolytics were shown to decrease senescent cell abundance in adipose tissue of humans with diabetes and obesity (Hickson et al. 2019) and to alleviate physical dysfunction in patients with idiopathic pulmonary fibrosis, a cellular senescence-driven disease [see below (Justice et al. 2019)].
Obesity with diabetes leads to accumulation of senescent cells in adipose tissue of humans (Minamino et al. 2009). To test if senolytics can clear senescent cells from human tissues, adipose tissue biopsied from obese, diabetic human subjects undergoing surgery was treated with D+Q or vehicle for 48 h (Xu et al. 2018). Within a few hours, the senescent cells in the freshly-isolated tissue began to undergo cell death through apoptosis. This was associated with reduced release of SASP factors, and increased expression of transcription factors that promote adipose tissue insulin sensitivity and of metabolically beneficial factors, including adiponectin and adipsin. Fisetin was also shown to reduce senescent cells from freshly-isolated human fat (Yousefzadeh et al. 2018).
8.1 First-in-Human Trial of Senolytics: D+Q for Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a senescence-associated, progressive, fatal disease of the elderly for which treatments, other than lung transplantation, are at best modestly useful. Senescent cells accumulate in subjects with IPF and are a source of inflammatory and fibrotic mediators in this disease. In the Bleomycin inhalation mouse model of IPF, D+Q was more effective than another senolytic agent, Navitoclax, in clearing senescent cells and D+Q alleviated pulmonary dysfunction, attenuated weight loss, and improved exercise endurance in these mice (Schafer et al. 2017). A pilot, open-label clinical trial of 9 doses of oral D+Q over 3 weeks led to improved 6-min. walk distance, walking speed, ability to get up from a chair, and short physical performance battery by 5 days after the final dose in 14 subjects with IPF (Justice et al. 2019). These results led to initiation of a Phase IIb randomized, placebo-controlled, double-blind trial that is currently underway.
Approaches were devised to gain approvals of new tests for senescent cell burden, a rapidly developing field, and of other tests of fundamental aging mechanisms to be incorporated into the study without stopping the primary study each time an amendment was submitted. Subject recruitment strategies had to be developed, including adjusting and simplifying the study burden for the elderly subjects in the trial.
8.2 Systemic Sclerosis
A reanalysis of archived skin biopsies from a trial of Dasatinib administered continuously for 6 weeks to 3 subjects with systemic sclerosis with evidence of skin senescent cells before treatment was conducted to test if senescent cells were removed (Martyanov et al. 2019). Of 65 SASP factors assayed, 55 had decreased after the course of Dasatinib, as were gene signature profiles linked to senescence.
8.3 Chronic Kidney Disease and Diabetes
A Phase 1, open-label, clinical trial of D+Q for subjects with diabetic kidney disease (DKD) is underway at Mayo (ClinicalTrials.gov Identifier: NCT02848131). Interim results were encouraging, showing that a 3 day oral course of D+Q in 9 subjects with DKD caused reduced adipose tissue senescent cell burden by 11 days after the last dose. Furthermore, a composite score of 10 circulating SASP factors was significantly decreased 11 days after completing the 3 day D+Q intervention. This trial is continuing (goal = 30) to test effects of senolytics on adipose tissue and skin senescent cell abundance, blood and urine SASP factors, metabolic and renal function, inflammation, quality of life, and safety (drug toxicity) and tolerability. No serious drug side effects have emerged so far and evidence continues to show clearance of senescent cells. Each subject will be followed for 4 months after the single course of D+Q. The goal is to provide data for a larger Phase IIb randomized, placebo-controlled, double-blind trial of senolytics for DKD.
8.4 Alleviation of Frailty, Inflammation, and Related Measures in Older Women
This Phase IIb double-blind, placebo-controlled clinical trial of a different senolytic drug, Fisetin, to reduce senescent cell burden and alleviate frailty and inflammation in older women (AFFIRM) is underway at Mayo (ClinicalTrials.gov Identifier: NCT03430037). Our groups recently reported an association between frailty in elderly women and senescent cell burden in adipose tissue biopsies (Justice et al. 2017). In preclinical studies, we found Fisetin causes apoptosis of senescent human endothelial cells (Zhu et al. 2017), among other senescent cell types. Fisetin alleviated frailty in progeroid and naturally-aged mice and extended median and maximum lifespan (Yousefzadeh et al. 2018). The latter finding has prompted testing of continuous versus intermittent Fisetin administration in the NIA-Interventions Testing Program (ITP). We arranged for isolation of Fisetin from plants in which it is present, conducted FDA-required testing for contaminants, completed stability and toxicity testing, prepared placebo capsules, conducted dose-escalation studies in Rhesus monkeys, and gained an FDA-IND for testing effectiveness of Fisetin in alleviating physical dysfunction in elderly women with the Fried frailty phenotype and slow gait speed, as well as ancillary testing of senescent cell burden, metabolic function, inflammation, and bone turnover. Along with the clinical trials mentioned here, there are other trials launching in the near future, including 3 studying senolytics for Alzheimer’s disease.
9 Translational Geroscience Network
In conducting the pre-clinical laboratory studies, completing the FDA and institutional approval processes, arranging for clinical trials to be conducted at multiple sites, enrolling subjects, coordinating specimen and data processing and storage, and harmonizing standard operating procedures (SOPs), many issues became apparent that a coordinated research network could resolve.
The Translational Geroscience Network (TGN), made up of 8 academic medical centers (Mayo, Harvard, Connecticut, Hopkins, Michigan, Wake Forest, UTHSCSA, and Minnesota), was established to develop, implement, and test SOPs for translational early phase trials of agents that target fundamental aging processes. The overall goal is to facilitate and speed translation by optimizing resource utilization, while avoiding duplication and counter-productive competition. This initial network will engage in preclinical translational research, completing the appropriate regulatory steps (e.g., acquiring INDs from the FDA), and assisting in developing several “use case” proof-of-concept Phase I and II trials. By pursuing steps to translate drugs that target fundamental aging mechanisms from bench to bedside concurrently and by doing so in a coordinated way, it is hoped the TGN will accelerate development of effective treatments. With luck, such interventions might be introduced clinically within the next 5–15 years. Additionally, the creation of this network will allow researchers from different sites to select, optimize, and validate ancillary measures of fundamental aging processes to be assayed across all trials. Ultimately, one of the long term goals of the TGN is to develop a biobanking and repository network where researchers can share samples and data, allowing comparisons among interventions targeting fundamental aging processes, including different senolytic drug regimens.
10 Enhancing Healthspan
A method for increasing healthspan may be to remove damaging and potentially cancerous senescent cells and next to treat with trophic or anabolic factors. Removing senescent cells before administering treatments that augment stem and progenitor cell function or anabolic agents may: (1) increase effectiveness of the trophic or anabolic agents by removing the “brake” on stem and progenitor cell function and growth exerted by senescent cells and (2) reduce the risk of cancer that could complicate administering trophic or anabolic agents to elderly individuals harboring pre-cancerous or cancerous cells. Many of the recently discovered senolytic agents have been in use for inducing apoptosis of cancer cells before their senolytic effects were discovered (Tchkonia 2017; Kirkland et al. 2017). For example, Dasatinib is used for treating certain hematological and other cancers (Keating 2017) and Quercetin can delay or alleviate cancers (Chikara et al. 2018). Thus, treating with senolytic drugs first might remove cancerous cells and senescent cells harboring potentially cancerous mutations before trophic and anabolic factors are administered. This approach for enhancing healthspan needs to be validated in pre-clinical experimental animal studies.
Perhaps better senolytics reported after the discovery that D+Q is senolytic will result in even more improvement of physical function, delay and alleviation of age-related diseases, and lifespan extension than D+Q, although it is likely that these improvements will not persist indefinitely. It seems much more likely that age-related dysfunction and death will occur at some point because of other fundamental aging processes, even if repeated administration of senolytics can completely and durably eliminate senescent cells. To achieve a more substantial increase in healthspan than is possible through senolytics alone, combining senolytic treatments with interventions that target other aging processes may be more effective.
11 Conclusions
Aging is a complex process that involves multiple factors that are comprised in a network driving this process. Cellular senescence is a key aging mechanism that is part of this network of fundamental aging mechanisms. Many fundamental aging processes, including genomic instability, telomere dysfunction/shortening, metabolic dysregulation, protein aggregation and misfolding, and nuclear membrane and mitochondrial dysfunction, can initiate or drive cellular senescence, and senescent cells coupled with their SASP factors can causally prime multiple aging processes, including stem cell exhaustion, disrupted intercellular communications, protein aggregation, NAD+ depletion, and chronic inflammation, which eventually lead to aging phenotypes and chronic diseases. If senescent cells are targeted and eliminated by senolytics, many or perhaps all other aging processes might be alleviated, the Unitary Theory of Targeting Fundamental Aging Mechanisms.
Since interventions that increase health- and life-span in mammals now exist, we hypothesize that by targeting fundamental mechanisms of aging, clinical interventions can be envisaged that could delay, prevent, or alleviate age-related conditions as a group, instead of one-disease-at-a-time. If effective in humans, interventions targeting basic aging processes could have a substantially larger impact on healthspan and costs than curing any one chronic disease, a potentially fundamental transformation in health care (Miller 2002; Kirkland 2013, 2016).
Until safety and effectiveness of senolytic drugs have been established in clinical trials, we strongly emphasize that these drugs are at an early stage of development. There could be profound, serious, as yet unforeseen adverse effects of senolytics in humans. At this point, senolytic agents must not be used outside of clinical trials involving intensive monitoring for potential adverse effects. Senolytics are not ready for general prescribing or clinical use.
References
Anderson RM, Weindruch R (2012) The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol 24(2):101–6
Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J et al (2019) Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38(5)
Baar MP, Brandt RM, Putavet DA, Klein JD, Derks KW, Bourgeois BR et al (2017) Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169(1):132–47 e16
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–6
Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P et al (2006) Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 61(3):262–6
Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L (2009) Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res 12(6):403–10
Bartke A (2011) Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci 366(1561):28–34
Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E et al (2016) Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife 5
Burd CE, Gill MS, Niedernhofer LJ, Robbins PD, Austad SN, Barzilai N et al (2016) Barriers to the preclinical development of therapeutics that target aging mechanisms. J Gerontol A Biol Sci Med Sci 71(11):1388–94
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22(1):78–83
Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS (2018) Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett 413:122–34
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–4
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Coppé JP, Patil C, Rodier F, Sun Y, Muñoz DP, Goldstein J et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–68
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR et al (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31(6):722–33
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL et al (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–56
Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R et al (2009) Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 64(10):1049–57
Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Stripay JL, Gregg S et al (2017) Identification of HSP90 inhibitors as senolytics for extending healthspan. Nat Commun (in press)
Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D et al (2013) Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff 32(10):1698–705
Hadley EC, Kuchel GA, Newman AB, Allore HG, Bartley JM, Bergeman CS et al (2017) Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci 72(7):980–90
Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP et al (2016) Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 8(7):1294–315
Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP et al (2017) p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K et al (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253):392–5
Hayflick L, Moorehead P (1961) The serial cultivation of human diploid strains. Exp Cell Res 25:585–621
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK et al (2019) Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47:446–56
Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN (2016) Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J Gerontol A Biol Sci Med Sci 71(11):1395–406
Inouye SK, Studenski S, Tinetti ME, Kuchel GA (2007) Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 55(5):780–91
Ishige K, Schubert D, Sagara Y (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30(4):433–46
Justice J, Miller JD, Newman JC, Hashmi SK, Halter J, Austad SN et al (2016) Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontl A Biol Sci Med Sci 71(11):1415–23
Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB et al (2017) Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine
Kanapuru B, Ershler WB (2009) Inflammation, coagulation, and the pathway to frailty. Am J Med 122(7):605–13
Keating GM (2017) Dasatinib: a review in chronic myeloid leukaemia and Ph+ acute lymphoblastic leukaemia. Drugs 77(1):85–96
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19(2):151–62
Kim SR, Jiang K, Ogrodnik M, Chen X, Zhu XY, Lohmeier H et al (2019) Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res J Lab Clin Med
Kirkland JL (2013) Translating advances from the basic biology of aging into clinical application. Exp Gerontol 48(1):1–5
Kirkland JL (2016) Translating the science of aging into therapeutici interventions. Cold Spring Harb Perspect Med 6(3):a025908
Kirkland JL, Tchkonia T (2014) Clinical strategies and animal models for developing senolytic agents. Exp Gerontol
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine
Kirkland JL, Hollenberg CH, Gillon WS (1990) Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol 258:C206–C10
Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I (2002) Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 37:757–67
Kirkland JL, Stout MB, Sierra F (2016) Resilience in aging mice. J Gerontol A Biol Sci Med Sci 71(11):1407–14
Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD (2017) The clinical potential of senolytic drugs. J Am Geriatr Soc 65(10):2297–301
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L et al (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114(9):1299–307
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9:81–94
Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B et al (2012) Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun 3:608
Leng SX, Xue QL, Tian J, Walston JD, Fried LP (2007) Inflammation and frailty in older women. J Am Geriatr Soc 55(6):864–71
Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ et al (2019) Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. e12931
Lipton RB, Hirsch J, Katz MJ, Wang C, Sanders AE, Verghese J et al (2010) Exceptional parental longevity associated with lower risk of Alzheimer’s disease and memory decline. J Am Geriatr Soc 58(6):1043–9
Martyanov V, Whitfield ML, Varga J (2019) Senescence signature in skin biopsies from systemic sclerosis patients treated with senolytic therapy: potential predictor of clinical response? Arthritis Rheumatol
Miller RA (2002) Extending life: scientific prospects and political obstacles. Milbank Q 80(1):155–74
Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T et al (2009) A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15(9):1082–7
Moncsek A, Al-Suraih MS, Trussoni CE, O’Hara SP, Splinter PL, Zuber C et al (2017) Targeting senescent cholangiocytes and activated fibroblasts with Bcl-xL inhibitors ameliorates fibrosis in Mdr2-/- mice. Hepatology
Munoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–96
Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q et al (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. e12840
Newman JC, Milman S, Hashmi SK, Austad SN, Kirkland JL, Halter JB et al (2016) Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci 71(11):1424–34
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A et al (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8:15691
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E et al (2019) Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab
Palmer AK, Gustafson B, Kirkland JL, Smith U (2019a) Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62(10):1835–41
Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM et al (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. e12950
Parikh P, Britt RD, Jr., Manlove LJ, Wicher SA, Roesler A, Ravix J et al (2018) Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Amer J Respir Cell Mol Biol
Pilling LC, Kuo CL, Sicinski K, Tamosauskaite J, Kuchel GA, Harries LW et al (2017) Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging 9(12):2504–20
Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA et al (2009) Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev 130(3):161–6
Rockwood K, Mitnitski A, Song X, Steen B, Skoog I (2006) Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 54(6):975–9
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. https://doi.org/10.1111/acel.12458. [Epub ahead of print]
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ et al (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8:14532
Schneider EL (1979) Aging and cultured human skin fibroblasts. J Invest Dermatol 73(1):15–8
St Sauver JL, Boyd CM, Grossardt BR, Bobo WV, Finney Rutten LJ, Roger VL et al (2015) Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open 5(2):e006413
Tchkonia T, Kirkland JL (2018) Aging, cell senescence, and chronic disease: emerging therapeutic strategies. J Am Med Assoc 320(13):1319–20
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123(3):966–72
Trujillo ME, Pajvani UB, Scherer PE (2005) Apoptosis through targeted activation of caspase 8 (“ATTAC-mice”): novel mouse models of inducible and reversible tissue ablation. Cell Cycle 4(9):1141–5
Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J et al (2014) Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20(6):659–63
Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH et al (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med 162(20):2333–41
Walston J, Hadley E, Ferrucci L, Guralnick JM, Newman AB, Studenski SA et al (2006) Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on aging research conference on frailty in older adults. J Am Geriatr Soc 54:991–1001
Walston JD, Matteini AM, Nievergelt C, Lange LA, Fallin DM, Barzilai N et al (2009) Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol 44(5):350–5
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA et al (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. https://doi.org/10.7554/elife.12997
Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T et al (2015b) JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci (USA) 112(46):E6301–E10
Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T et al (2016) Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M et al (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S et al (2019) Senolytic therapy alleviates abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci
Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL (2014) Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17(4):324–8
Zhu Y, Tchkonia T, Pirtskhalava T, Gower A, Ding H, Giorgadze N et al (2015a) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–58
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB et al (2015) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. https://doi.org/10.1111/acel.12445. [Epub ahead of print]
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB et al (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15(3):428–35
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H et al (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wissler Gerdes, E.O., Zhu, Y., Tchkonia, T., Kirkland, J.L. (2020). Discovery of Senolytics and the Pathway to Early Phase Clinical Trials. In: Muñoz-Espin, D., Demaria, M. (eds) Senolytics in Disease, Ageing and Longevity. Healthy Ageing and Longevity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-44903-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-44903-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44902-5
Online ISBN: 978-3-030-44903-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)