Abstract
The generation of three-dimensional (3D) organoids can allow researchers to resemble in vitro distinct types of cellular compartments within specific organs, including exocrine glands. The development of salivary gland organoids can entail the use of biomaterial substrates (usually hydrogel- or Matrigel-based) or can be substrate-free to allow cells to produce their own extracellular matrix molecules. Our research strategies focus on the latter and use innovative biofabrication platforms via 3D bioassembly of salivary gland primary cells or oral stem cells using magnetic nanoparticles. These magnetic nanoparticles can tag these cells and spatially arrange these either at the bottom of a culture well (magnetic 3D bioprinting) or at the air-media interface (magnetic 3D levitation) with specific magnetic fields. In this chapter, we discuss how epithelial compartments can be formed within these organoids with different 3D culture platforms and the importance of the presence of a neuronal network to make them achieve a functional status. A neuronal network that responds to parasympathetic and sympathetic neurotransmitters is crucial for testing saliva secretion arising from the epithelia compartment. These neuroepithelial organoids will support the discovery and cytotoxicity screening of novel drugs for dry mouth syndrome or xerostomia. The advantages and limitations of both magnetic bioassembly platforms will be discussed to optimize these salivary gland-like organoids.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
Exocrine glands, such as mammary, prostate, thyroid, pancreas, lacrimal, and salivary glands, are organs frequently targeted by cancer or autoimmune disorders (Stewart and Wild 2018; Lombaert et al. 2017). Functional damage in these glands usually occurs after conventional cancer therapies are delivered, thereby impairing the health of patients. In the salivary gland (SG), following radiotherapy for head and neck cancers, up to 60% of the secretory epithelia can be irreversibly damaged, and saliva secretion is severely compromised in those cases. No pharmacological or medical interventions exist to fully rescue the saliva production in these cancer patients. Immunosuppression-free gland transplants are currently not available for gland replacement. Organoid bioprinting has been emerging as a promising 3D biofabrication strategy toward (1) the development an in vitro and “off-the-shelf” gland transplant from patient’s own cryopreserved cells (Mironov et al. 2009a; Groll et al. 2016); or (2) the discovery of new saliva stimulants (sialogogues) or drugs in in vitro organoid platforms (Adine et al. 2018; Ferreira et al. 2019). To avoid immunological incompatibilities and potential cytotoxicity from scaffold biomaterials in the long-term, our group generated a scaffold-free culture platform to promptly produce 3D functional salivary gland-like organoids using magnetic nanoparticles (Adine et al. 2018; Ferreira et al. 2019). This biofabrication process induces cellular spatial arrangement in 3D and scalable organoid production within 24h. In addition, this process facilitates organoid handling and transfer as well as high-throughput analysis and imaging. A magnetic nanoparticle solution, consisting of gold, iron oxide, and poly-L-lysine, is used in this 3D bioassembly process. These nanoparticles support cell proliferation and metabolism, without increasing deleterious processes such as inflammation and oxidative stress (Tseng et al. 2015). In addition, negligible immune response after transplantation is reported in animal models (Lin et al. 2016).
Thus, in this chapter, we will discuss the development secretory neuroepithelial organoids similar to the native SG using stem cells and two 3D bioassembly platforms in vitro. The overall objective was to generate a scalable SG organoid with innervation and bio-functional properties upon neurostimulation to overcome current limitations in stem cell therapies for dry mouth or xerostomia.
2 Organotypic Three-Dimensional (3D) Cell Culture System
2.1 Overview of 3D Cell Culture Systems
In this last decade, the organotypic three-dimensional (3D) cell culture system has been utilized as a promising strategy for the ex vivo generation of robust 3D organoids or miniature glands for transplantation in patients suffering from xerostomia (Ferreira et al. 2016). Also, 3D cell culture models serve as an auspicious and feasible model for the study of the molecular and cellular mechanisms underlying diseases as well as for drug discovery (Langhans 2018; Hagemann et al. 2017).
The 3D cell culture system is rapidly prevailing over the two-dimensional (2D) culture system and in vivo models, because better mimics physiologic conditions and maintains the cellular and tissue function by establishing appropriate cell-signaling pathways and extracellular matrix interactions (Campbell and Watson 2009). These unique properties enable it to overcome many of the shortcomings associated with both in vivo animal experimentation and two-dimensional (2D) tissue culture. In vivo transgenic animal models, while undoubtedly representing the gold standard for basic molecular studies and diagnostic work, are expensive to produce and are subject to stringent regulation, resulting in studies utilizing low replicate numbers, with consequences for scientific rigor (Campbell and Watson 2009).
2.2 Spheroid 3D Culture Systems
Scaffold-based tissue engineering faces some challenges including (i) immunogenicity, (ii) acute and long-term inflammatory response resulting from the host response to the scaffold and its biodegradation products, (iii) mechanical mismatch with the surrounding tissue, (iv) difficulties in incorporating high numbers of cells uniformly distributed within the scaffold, and (v) limitations in introducing multiple cell types with positional specificity (Jakab et al. 2010). In contrast, in scaffold-free spheroids culture, cellular cross talk proceeds naturally (Jakab et al. 2010).
Table 1 summarizes the most common 3D spheroid culture techniques currently employed. All these techniques have their own advantages and challenges (Lombaert et al. 2017; Ferreira et al. 2016; Achilli et al. 2012; Fennema et al. 2013). A few of 3D bioengineering systems have effectively shown the capability of primary SG epithelial cells to self-assemble and display polarization properties on engineered scaffolds incorporating SG basement membrane molecules such as Matrigel as well as other natural polymers (chitosan) and synthetic polymers (PEG, PLGA). However, some of these matrices (Matrigel) contain xenogeneic materials and thus limit its application in vivo (Ferreira et al. 2016). Other polymers may degrade too fast before epithelial cells self-assemble and tight junctions can form (Sfakis et al. 2018). In contrast, scaffold-free bioengineered platforms can facilitate a rapid cell clustering in 3D as well as cell-cell interactions to allow the generation of a tightly packed epithelium to finally become a functional organ. Scaffold-free or spheroid culture can be efficiently produced through seeding cells in nonadherent surfaces and suspension culture. However, these methods will form nonuniform spheroids, and the size of the spheroids cannot be controlled for proper mass transfer and nutrition (Ferreira et al. 2016; Achilli et al. 2012; Fennema et al. 2013).
In contrast, cell patterning techniques offer uniformly formed spheroids. Cell patterning arranges cells into desired patterns mimicking the real tissue by applied external guiding or manipulation. In general, cell patterning can be classified as passively and actively direct cell patterning. Passive cell patterning requires a cell-adhesive ECM protein-coated onto regions defined by photoresistance or applied by a micro-fabricated stamp, leaving other uncoated regions unfavorable for cell adhesion. The seeded cells attach and occupy ECM-coated regions and hence form a clean-cut pattern (Fukuda et al. 2006; Fu et al. 2011).
Despite promising results, the effectiveness of passive cell patterning is limited by the natural cell adhesion process, which is slow, irregular, and divergent from cell type to cell type. In contrast, active cell patterning employs techniques that apply force to actively direct cells to desired positions (Table 1).
Similar to the in vivo environment, cells in a 3D spheroid are free to assemble without constraint. They establish 3D gradients of nutrients, metabolites, and cell signals as well as barriers to the transport of molecules (Achilli et al. 2012). In addition to gradients, spheroids create an in vivo-like microenvironment by forming more complex cell-to-cell interactions and cell-to-ECM adhesions. They maximize cell-to-cell contact, form numerous adhesions between surface adhesion molecules, and even form direct cell couplings such as gap junctions (Fennema et al. 2013).
Thus, spheroids from adult stem cells bioprinted onto the 3D printed scaffold are a fascinating approach for future clinical trials, since they can form larger, complex, and functional autologous tissues and mini-organs (Fennema et al. 2013).
In summary, there are several advantages of using spheroids as building blocks for organoid development (Achilli et al. 2012; Fennema et al. 2013; Parfenov et al. 2018a; Mironov et al. 2009b). First, spheroids have the highest theoretically possible cell density equivalent to native tissue. Second, spheroids have a compact rounded shape, which is ideally suitable for their handling, manipulation, transfer, processing, and bioprinting. Third, they have a complex internal structure, multicellular composition and lumens and can be pre-vascularized. Finally, when the spheroids are closely placed and touching each other, they inherently begin to fuse and producing complex 3D tissue constructs. This phenomenon was found during fetal development and organogenesis, and it is a fundamental principle of bioprinting and biofabrication technologies (Mironov et al. 2009b).
2.3 Potential Cell Sources for Salivary Gland Tissue Engineering
The ideal cell source for SG tissue engineering should entail cell types such as primary, progenitor, or stem cells isolated from autologous tissue (Lombaert et al. 2017). These cells can be isolated from the patient’s SG prior to radiotherapy (RT), grown and expanded in the laboratory, and transplanted into the gland after RT has been completed. Autologous SG cells would be accepted by the host with no risk of immune rejection. Although studies on SG epithelial transplants from digested tissue have demonstrated potential, most reveal a partial capability of transplanted cells to restore SG function. Isolated primary SG cells rapidly dedifferentiate and undergo structural changes as a result of the loss of extracellular and intercellular communications (Nelson et al. 2013). Unfortunately, harvesting such cells generally causes great patient discomfort and morbidity, and thus such cell lines are not widely available. Furthermore, these cells do not grow efficiently in vitro (Nelson et al. 2013) as they become apoptotic when dissociated into single-cell suspensions and difficult to achieve acinar formation (Szlavik et al. 2008). Differentiation of human submandibular gland (HSG) intercalated duct-like cell lines toward the acinar phenotype is possible on a substrate containing laminin-1 or Matrigel (Nelson et al. 2013). However, the lack of tight junctions (TJs) crucial for the development of polarized epithelia and unidirectional secretion has become a major limitation of this cell line. Moreover, transfection of tight junction proteins did not improve HSG polarity and form weak epithelial resistance (Aframian et al. 2002). Therefore, this cell line is not suitable for tissue engineering of SG epithelia. Currently, no primary cell line fully recapitulates the morphological and functional features of the native salivary acinar cells (Hegde et al. 2014).
Other potential cell sources are embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC). These are considered pluripotent stem cells because they can differentiate into all cell types from all three germinal layers. A few studies have differentiated ESC into SG tissues. Mouse ESC was shown to successfully differentiate into SG-like cells through direct co-culture with human SG cells (Kawakami et al. 2013) or in the presence of mouse fetal rudiments by induction of Sox9 and Foxc1 transcription factors (Tanaka et al. 2018). However, the translation of these ESC mouse studies into human ESC is still far for becoming a reality due to the tumorigenicity of these cells lines and rudiments. Moreover, multiple studies have reported the generation of iPSC from various dental tissues, but very few targeted the SG (Hynes et al. 2015). In the scarce literature, mouse iPSC has been shown to support SG differentiation (Ono et al. 2015) and is capable to partially regenerate damaged SG (Alaa El-Din et al. 2018). iPSC differentiation into SG-like cells still warrants further investigation. Despite the preliminary promising results, both stem cells (ESC, iPSC) have technical and moral obstacles. In addition, these cells are not easy to control, due to their genomic instability and propensity to form tumor (Hynes et al. 2015).
In contrast, adult stem cells become a very attractive cell source because they are widely available and have multi-lineage differentiation capacity. Adult stem cells (somatic stem cells) are undifferentiated cells in the body after a multiplication process during its development when the cells will eventually cease to exist or are damaged. Stem cells are generally considered to be more “primitive” and are the precursors of progenitor cells, which are more lineage-committed, have less capacity to self-renew, and maybe organ-specific. Multiple groups have been evaluating the potential of different stem cell sources for SG repair or regeneration which will be discussed in the following section. Furthermore, there are four clinical trials evaluating the effectiveness of adult stem cells to alleviate xerostomia, listed in Table 2.
2.3.1 Salivary Gland Stem/Progenitor Cells
As stem cells might be organ-specific, it is therefore obvious to explore the potential of stem cells derived from the SG to repair or regenerate the damaged gland. Earlier on, the SG organ was thought to have a slow cellular turnover, and therefore the presence of stem cells was seen unlikely. However, ductal ligation experiments in rat parotid and submandibular glands identified proliferating cells with regenerative capabilities. These cells emerged upon deligation and were labeled stem/progenitor cells, and gland atrophy was reversed with the appearance of new acinar cells (Burford-Mason et al. 1993; Takahashi et al. 2004). These and other studies have shown that SG stem/progenitors cells are present among the differentiated cells, and therefore, it is necessary to have an effective isolation methodology to test their regenerative potential.
Nanduri et al. (Nanduri et al. 2013a) have shown that isolation with the c-Kit marker was enough to restore morphology and functionality of irradiated salivary glands. Salisphere derived c-Kit+ cells expressing CD24 and/or CD49f markers, successfully restored tissue homeostasis in the submandibular gland of irradiated mice. However, the population of c-Kit+ is very limited (<1%) in the human SG and greatly diminishes in aging patients (Pringle et al. 2016).
Furthermore, Jeong et al. (Jeong et al. 2013) develop a method to efficiently isolate SG progenitor without cell surface marker selection but repeated subculturing over five passages. These cells exhibited MSC-like characteristics and successfully differentiated into amylase-secreting cells in vitro. More importantly, these cells were able to ameliorate hyposalivation and rescued SG morphology in irradiated rat SG.
In addition, an interesting study by Mishima et al. (Mishima et al. 2012) has identified and isolated side population (SP) cells from mouse SG via cell sorting. This cell population was able to recover the radiotherapy-induced hypofunction of salivary and lacrimal glands, although cell reconstitution was not observed in vivo.
Table 3 summarizes studies of SG-derived stem/progenitor cells (SGSC) isolation and evaluation of their regenerative capacity, especially for radiotherapy-induced xerostomia. Although studies have shown that origin-specific stem cells are favorable for treatment of the tissue origin, SGSC has its own limitations. The treatment is heavily dependent on residual endogenous acinar cell, and therefore it is not suitable for severe cases. Hence it is worthwhile to explore the use of other sources of adult stem cells.
2.3.2 Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSC)
Several groups have reported the potential of bone marrow-derived cells (BMC) for salivary gland regeneration (Sumita et al. 2011; Lombaert et al. 2006; Lin et al. 2011). These studies used very heterogeneous stem cell populations, which may produce different functional effects when transplanted in vivo. In order to address this, Lim et al. utilized a subfractionation culturing method to obtain highly homogenous MSC cell lines (Lim et al. 2013a). The intraglandular injection of these MSC restored the morphology and function of the submandibular gland after radiation injury. The paracrine effect appears to induce functional restoration through the survival of epithelial secretory cells, mobilization of host progenitor cells, and vascularization.
To further investigate this matter, Tran et al. (Tran et al. 2013) evaluated the paracrine effect of BM-MSC. These researchers extracted and lysed the crude bone marrow cells and its soluble intracellular content (termed BM soup) and then injected into irradiated mice. Injection of BM soup was as effective as the transplantation of BM cells in restoring the function of irradiated salivary glands, highlighting the importance of paracrine effects in salivary gland regeneration. The utilization of cell extract treatment strategies can definitely overcome the issues related to immune rejection after allogeneic transplantation of MSC or other adult stem/progenitor cells.
Table 4 displays the studies evaluating BMC and BM-MSC for SG regeneration.
Multiple research groups have also identified several reprogramming factors that can lead to transdifferentiation to SG-like epithelial cell types and/or SG regeneration. These reprogramming factors include ankyrin repeat domain-containing protein 56 (ANKRD56), high mobility group protein 20B (HMG20B), transcription factor E2a (TCF3), pancreas-specific transcription factor 1a (PTF1α), muscle, intestine, and stomach expression-1 (MIST-1), achaete-scute complex homolog 3 (ASCL3), bone morphogenetic protein 6 (BMP-6), interleukin-6 (IL-6), and/or CCAAT enhancer-binding protein beta (CEBPB) .
2.3.3 Adipose-Derived Stem Cells
Although BM-derived cells hold a relevant potential for SG tissue regeneration, autologous or allogeneic cell harvesting from BM or transplantation may not be possible for a variety of reasons (compromised immunity, abnormal hematopoiesis, hip morbidity, lack of patient acceptance, etc.). Alternatively, researchers explored other sources of mesenchymal stem cells (MSC). Studies have shown that one can have a high yield of MSC from adult adipose tissues, and fat tissue biopsies are easier to perform when compared to bone marrow biopsies and produce less morbidity (Konno et al. 2013).
Therefore, several studies using adipose-derived stem cells are currently registered in clinical trial databases (Table 2). Direct administration of adipose-derived stromal cells into the SG can improve saliva production by stimulating vascularization within salivary gland tissues, since transplanted ASC differentiate into endothelial cells as well as ductal epithelial cells (Kojima et al. 2011). However, the secretory acinar compartment did not display prominent signs of regeneration. Later, Lim et al. (Lim et al. 2013b) administered systemically human adipose-derived MSC (hAd-MSC) to subdue radiotherapy-induced SG damage. This strategy improved salivary gland function and epithelial proliferation, and suppressed cellular apoptosis. Furthermore, like BM-MSC studies, a limited number of adipose-derived MSC transdifferentiate into epithelial secretory cells. These findings may suggest that MSC, regardless of their source, have a paracrine effect rather than a transdifferentiation effect.
A phase 1/2 clinical trial evaluating the safety and efficacy of autologous hAd-MSC in 15 subjects with radiotherapy-induced xerostomia was recently reported (NCT02513238) (Gronhoj et al. 2018). At 4 months, this clinical trial reported 50% improvement in saliva secretion and no major adverse events. Thus, ASC treatment was safe and exhibited promising efficacy in a short-term follow-up on both objective and patient-reported outcomes. It would be interesting to assess if these clinical outcomes can be maintained or improved beyond 4 months.
2.3.4 Dental Pulp Stem Cells
Dental pulp stem cells (DPSC) arise from the neural crest-derived mesenchyme surrounding the oral primitive ectoderm during craniofacial morphogenesis. These cells possess several MSC-like markers and share a common mesenchymal embryonic origin with the salivary gland stroma (Adine et al. 2018). Therefore, DPSC can be part of regeneration strategies for the SG epithelia (severely damaged in radiotherapy-induced xerostomia), the stroma compartment, and the neuronal network (neurons are derived from neural crest mesenchymal lineages).
In 2D cultures, Janebodin et al. (Janebodin and Reyes 2012) co-cultured DPSC with a human submandibular gland cell line (HSG) in Matrigel which increased the number of secretory epithelial cells in vitro. In vivo subcutaneous co-transplantation of HSG and DPSC with hyaluronic acid (HA) substrate led to an increase of SG epithelial tissue, innervation, and vascularization. Yamamura et al. (Yamamura et al. 2013) successfully differentiated DPSC into endothelial cells (DPECs) and demonstrated that radiotherapy-induced SG hypofunction was partially reverted following in vivo transplantation of DPECs.
Recently, our laboratory was able to differentiate human DPSC in 3D culture platform toward SG-like cells that express epithelial acinar and ductal markers as well as neuronal markers in the presence of FGF10 (Adine et al. 2018). This 3D cellular assembly created the first innervated SG epithelial organoid via 3D bioprinting.
3 Incorporating Different Biomaterials for Salivary Gland Bioengineering
A recent advancement in SG regenerative medicine showed that a bioengineered gland made from embryonic epithelium and mesenchyme can be transplanted into an adult mouse to produce a whole functional SG (Ogawa et al. 2013). This bioengineered SG was composed of a variety of progenitor and stem cells, including cell from epithelial, mesenchymal, endothelial, and neuronal origins. More interestingly, the SG reconnected with the existing ductal system and possessed functional activity. The new SG was able to secrete saliva, protect the oral cavity from bacteria, and restore swallowing functions.
Hence, future research may translate these bioengineering strategies to animal models with salivary glands that have more morphological and functional similarities to the human SG. Further studies may also focus on the usage of stem cells or adult salivary progenitors with high expansion capabilities in 3D scaffolds in order to form a bioengineered construct that grows into a functional gland in the adult microenvironment.
Salivary gland tissue engineering requires three essential components: (1) the stem/progenitor cells that retain epithelial progenitor biomarkers typical of the native salivary gland (SG); (2) the extracellular matrix (ECM) proteins that can orchestrate the differentiation of progenitor cells into functional structures; and (3) a biocompatible and biodegradable three-dimensional (3D) scaffold that can hold these components together to recreate the microenvironment found in the native SG (Aframian and Palmon 2008).
Since dynamic cell-ECM interactions are essential in processes such as epithelial ductal formation/branching, a recent strategy has been to engineer scaffolds that structurally and functionally resemble native ECM architecture. Three-dimensional (3D) collagen matrices have been used for homing salisphere stem/progenitor cells which form epithelial ductal structures with mucin-positive acini, indicating their capability to differentiate in response to the ECM environment (Nanduri et al. 2014). Various biomaterials such as collagen type I, Matrigel, and other animal-derived products have been showing promising results in the differentiation and organization of human SG cells (Nanduri et al. 2014; Maria et al. 2011; Pradhan et al. 2009); nevertheless, these biomaterials are not human-compatible. Thus, tissue engineering-based research is gearing toward the creation of xeno-free biomaterials, which can eventually be transplanted into humans.
In 2010, researchers have started to utilize the soft hyaluronic acid (HA) hydrogels, which are human-compatible, as biocompatible substrates for SG tissue engineering (Pradhan et al. 2010). When encapsulated in HA hydrogels, human SG cells can grow into organized spheroid structures that merge and proliferate to form larger acini-like structures with a central lumen and are maintained for long-term in these gels in vitro (Pradhan et al. 2010). These in vitro 3D acini-like structures also secrete α-amylase, express β-adrenergic and muscarinic receptors that activate protein transport, and induce calcium oscillations upon treatment with cholinergic stimulants. Furthermore, these 3D spheroids continue to secrete α-amylase when hydrogels were implanted in vivo in an athymic rat model (Pradhan-Bhatt et al. 2013). However, these latter 3D structures have reversed polarity suggesting that further environmental cues from the ECM and the myoepithelial cells may be needed to reverse inside out acini and correct the polarity. Culture of salivary gland progenitor cells on human perlecan domain IV peptide has been shown to support the formation of 3D acini-like salivary units that express α-amylase (Pradhan et al. 2009). It will be useful to incorporate the perlecan IV domain peptide into biomaterial scaffolds to mediate differentiation, correct polarity, and directional secretion of the 3D salivary gland cell cultures in the future.
Other research groups have used poly(lactic)-co-glycolic acid (PLGA) , an FDA approved constituent in implantable dental and orthopedic devices, as a synthetic material to show that it can support the attachment, proliferation, and survival of salivary gland epithelial cells (Jean-Gilles et al. 2010). The same group further shows that nanofiber PLGA scaffolds can support development and morphogenesis of intact fetal SMG organ cultures and promote natural self-organization of dissociated SMG cells into branched SG-like structures (Sequeira et al. 2012). However, adult SG cells grown on flat polymeric substrates fail to form a complex 3D branching structure and are unable to assemble tight junctions that are needed for unidirectional flow of saliva. To overcome this, recent studies generated lithographically based micropatterning curved “craters” that mimic the physical structure of the basement membrane, which have increased both the surface area and allowed apicobasal polarization and differentiation of salivary gland epithelial cells (Soscia et al. 2013). An increase in aquaporin-5, a water channel protein marking acinar differentiation, was also detected in SG cells cultured on higher curvature scaffolds. Further studies with PLGA nanofibers coupled with laminin-111 and chitosan showed that laminin-111 promotes the formation of mature epithelial tight junctions and apicobasal polarization, and, on the other hand, the chitosan antagonizes this phenomenon (Pradhan-Bhatt et al. 2013).
Taken together, current cell-based therapies and tissue engineering studies have provided a promising outlook to regenerate SG and restore the saliva secretory function. However, in order to test these techniques in humans, several hurdles need to be surpassed. To overcome these hurdles, further research steps should include (1) the elimination of xenogeneic elements from transplants for feasible human use to comply with good manufacturing practices; (2) a thorough assessment of histocompatibility barriers; (3) an evaluation of long-term transplant survival and saliva secretion in larger animal models with a better SG human resemblance (i.e., pigs); and (4) an assessment of tumor sensitivity to bioengineered transplants in SG cancer models .
4 3D Bioprinting
One of the major challenges for tissue engineering is to produce a large volume of tissues or organs and to scale them up for clinical applications. The use of 3D bioprinting in regenerative medicine is to address this need for tissues and organs suitable for transplantation. Bioprinting and bioassembly constitute the two major biofabrication setups. Bioprinting allows the precise spatial organization (patterning) of living cells, biomaterials, and bioactive molecules layer by layer, whereas bioassembly facilitates the automated assembly of cell-containing building blocks via cell-driven self-organization or through the preparation of hybrid cell-material building blocks (Moroni et al. 2018).
Only magnetic-based bioprinting and bioassembly have been recently tested for the generation of secretory epithelia in salivary glands (Adine et al. 2018; Ferreira et al. 2019).
4.1 Magnetic-Based Bioprinting
There are two technologies for bioprinting utilizing magnetic forces. The first mode involved suspending cells in paramagnetic liquid via the addition of gadolinium (Gd3+)-based solution which termed as a label-free approach (Turker and Arslan-Yildiz 2018; Abdel Fattah et al. 2016). When a magnetic field is applied, the magnetized fluid is attracted to regions of high-magnetic field gradient, displacing the cells toward regions with a low gradient, a process called as diamagnetophoresis (Abdel Fattah et al. 2016). Since cell patterning through diamagnetophoresis can be controlled and it is nozzle-free, this technique is convenient to rapidly print multicellular spheroids. The technology has been reported to assemble epithelial breast cancer cell lines and can be modified to print multiple cell types (Turker and Arslan-Yildiz 2018). Despite promising results, the major concern over this technology relates to the usage of cytotoxic paramagnetic suspending media. The high concentrations of Gd3+ could be potentially toxic for tissue spheroids, and a certain risk exists for osmotic pressure imbalance due to excessive use of ions in the paramagnetic medium (Parfenov et al. 2018b). Further, this label-free levitation magnetic assembly technology is currently only able to fabricate soft small living blocks due to the size of the magnets underneath each well. Therefore, it is necessary to use either superconducting magnets or apply a higher concentration of the paramagnetic solution to fabricate a larger size of tissue-engineered constructs (Parfenov et al. 2018b).
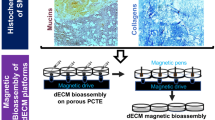
The second magnetic-based bioprinting technology requires cell labeling with magnetic nanoparticles. Souza et al. (Souza et al. 2010) developed a magnetizing solution consisting of poly-L-lysine, iron oxide, and gold nanoparticles named NanoShuttle™ for 3D cell culture formation (Souza et al. 2010; Tseng et al. 2013; Haisler et al. 2013; Timm et al. 2013). Upon magnetic nanoparticle (MNPs) uptake, the cells can be easily directed using mild magnetic forces. Spatial patterning of the 3D cell assembly into the desired morphology is controlled through varying the shape or configuration of the magnetic field. Furthermore, the culture platform can consistently control the size of the spheroids by tuning the following variables: MNP’s concentration, number of cells, and the magnet size. Upon removal of the magnetic field, spheroids rapidly contract in size due to cell rearrangement to reach an equilibrium size and simultaneously produce their own extracellular matrix. This system has already been validated in various cell lines (Souza et al. 2010; Tseng et al. 2013; Haisler et al. 2013; Timm et al. 2013). Furthermore, the developed magnetic 3D cell culture platform has been used in disease modeling of epithelial tissues such as breast cancer (Jaganathan et al. 2014). Given the spheroids contrast with media due to their brown color imparted by the MNPs, the change in size can be imaged at programmed intervals using a mobile device (Timm et al. 2013). As a result, spheroids can be imaged simultaneously in a multi-well plate, thereby improving imaging throughput and efficiency. Due to magnetized spheroids, adding and removing solutions is made easy by the use of magnetic forces to hold down spheroids during aspiration, limiting spheroid loss. Spheroids can also be picked up and transferred between vessels using magnetic tools such as the MagPen™. NanoShuttle™ and magnetic field have been shown to have no compromising effect on cell proliferation, viability, and metabolism as reported in previous publications. Therefore, this technology offers reproducible spheroid formation, scalable, ease of imaging, no specialized equipment or media required, biocompatible, and ready for automation (Timm et al. 2013).
Epithelial cells from the salivary gland are known tightly become packed in the presence of tight junction proteins and exhibit polarization properties (Varghese et al. 2019). However, the majority of the bioengineering studies do not support the development of a neuronal network within the epithelial tissues. Innervation is deemed crucial to maintain and repair SG epithelial cells after radiotherapy damage (Knox et al. 2010). In collaboration with Souza and colleagues, our research team has recently produced innervated and bio-functional SG-like epithelial organoids using the NanoShuttle™ MNPs and its magnetic 3D bioprinting (M3DB) platform (Fig. 1) (Adine et al. 2018). Our first study indicated that M3DB supported the metabolic activity of human dental pulp stem cells (hDPSC) and did not inflicted any cytotoxicity to the cells. Further, M3DB was capable of maintaining hDPSC stemness during a 3-day expansion culture to assemble the cells. Next, our team showed that retinoic acid and FGF10 induced SG epithelial differentiation and created a neuronal network in 11 days of culture. The final 3D bioprinted tissue had not only amylase-secreting secretory epithelia but also functional and responsive neurons in vitro, mimicking the normal SG. Such innervated secretory epithelia secreted salivary amylase and were responsive to both cholinergic (parasympathetic) and β-adrenergic (sympathetic) neurotransmitters. Furthermore, our study showed the bioengineered organoids exhibited tight junction and transepithelial resistance, required for unidirectional saliva flow. This 3D tissue was therefore termed as SG-like organoid as per the above SG features. To determine the regenerative potential, an injured mouse SG model was established after RT damage was induced. SG-like organoids were able to restore branching morphogenesis following transplantation into damaged SG, and neuron integration was established with the native SG neuronal network (Adine et al. 2018). The millimeter-level organoids generated in our study are remarkable but can pose technical challenges in terms of nutrient and oxygen diffusion and upon analyzing or imaging biological mechanisms as a 3D SG in vitro model. Confocal and multiphoton microscopy with multiple detector systems through the spheroid allowed us to provide more biological assessments into the inner cellular core of the organoid. Several proliferative cell compartments were present in both the center and periphery of SG-like organoids after transplantation. With about 1 to 1.2 millimeter in size, vascularization in the organoid is necessary for nutrient and oxygen exchange. Despite this, an apicobasal polarization was challenging to determine due to tightly packed epithelial cells, and vascularization was limited in the developed epithelial organoids. We reported that our organoids have a limited vascular network partially because of the low hematopoietic population of DPSC capable of generating CD31+ cells.
Moreover, our work showed that neurons, as well as SOX2+ cells, are also diminished in the irradiated SG, suggesting that tissue degeneration is due to loss of progenitors and their regulators (Adine et al. 2018). Emmerson et al. (Emmerson et al. 2018) also found that SOX2+ stem cell population is essential for acinar cell maintenance and is capable of replenishing acini after radiotherapy-induced damage. The maintenance of parasympathetic nerves was required to host the SOX2+ population. In our study, SG-like organoid exhibited significant increase in the SOX2+ population in mice ex vivo submandibular glands (Adine et al. 2018). Therefore, we hypothesize that the rescue of epithelial bud growth was due to the survival of SOX2+ cells and the presence of a neuronal (parasympathetic) network in the organoid. In future clinical studies, autologous human DPSC may be isolated from the patient’s impacted tooth, differentiated into SG-like organoids in vitro, and transplanted back to regenerate or repair the damaged SG.
4.2 Magnetic Levitation and 3D Bioassembly
Starting in 2016, our research team developed a user-friendly and reproducible magnetic-based levitation methodology (M3DL) with NanoShuttle™ MNPs for the generation of organoids mimicking native SG cellular compartments and the neuroepithelial communication and secretory function (Ferreira et al. 2019). The M3DL platform used porcine SG primary cells, which supported the formation of epithelial tissue spheres from 24 hours up to 7 days with viable and proliferating cells at both the center and periphery. A 200–250 μm diameter aggregate can be developed after 7 days with a consistent sphere-like shape. More importantly, M3DL-formed organoids did exhibit SG-like secretory functions upon muscarinic stimulation because intracellular calcium activity and salivary amylase secretion were swiftly amplified. This rapid stimulation of the organoid’s secretory activity is an advantage because it is crucial to obtain a clinically relevant saliva secretion in the shortest time possible in patients with dry mouth (Ferreira et al. 2019). These functional mini-glands can potentially be translated to humanized models and be applied in the repair or regeneration of damaged secretory SG epithelia in hyposalivation and dry mouth medical conditions. There is also ongoing works to use these mini-glands as an early in vitro screening tool for drug toxicity or to create in vitro dry mouth models induced by radiotherapy.
In addition, future magnetic levitation studies may need to address phenotypic differences between porcine and human primary cells extracted using various SG cell isolation methodologies, as well as evaluate the effectiveness of these cells in generating functional organoids.
4.3 Scaffold-Assisted Bioprinting
Despite promising progresses, scaffold-assisted bioprinting faces several challenges when laying biomaterials together with cells in a 3D spatial arrangement. This technology is biomaterial-dependent and energy-intensive, making it poorly compatible with some of the intended biological applications. The biomaterial needs to be supportive of both cells and the challenges of the bioprinting process. Thus, a universal biomaterial is required because often each cell type needs to be embedded in a different hydrogel (Kang et al. 2016). More importantly, the common printing methods are intrinsically stressful to live cells, by exposing them to high shear stress, overheating, and/or toxic compounds generated even from initially cell-friendly materials (Nair et al. 2009). If the printing process slows down, then printing large amount of cells may become time-consuming. Also, when epithelial cells from exocrine glands are encapsulated within the bioink, the cells need to both dissolve their biomaterial scaffold and to proliferate to the point where they must contact and produce tight junctions for epithelial polarization and proper fluid secretion. These are among the reasons and challenges of using scaffold-assisted bioprinting techniques in SG epithelial regeneration and organoid development.
At the moment, the future of SG biofabrication may benefit more from the use of a scaffold-free 3D bioprinting or bioassembly approaches until better bioinks are developed to promote epithelial viability and proper epithelial spatial arrangement and polarization. The ideal properties for a SG epithelial-supportive bioink would include printability (viscosity, gelation method, rheology), biocompatibility and material biomimicry, fast degradation kinetics, and nontoxic byproducts, with mechanical/structural properties similar to SG basement membrane molecules (e.g., perlecan). The other important desirable features of a bioink include industrial scalability, availability, short post-printing time for maturation, permeability to oxygen and nutrient transport, and ability to eliminate metabolic waste generated by cells. Hopefully, the emerging 4D bioprinting strategies may be able to overcome the challenges related with the lack of a universal bioink compatible with epithelial organs like the SG.
5 Conclusions
The use of tissue engineering and bioprinting approaches of human tissues or organs for transplantation has developed substantially over the past decade. Many studies have demonstrated promising in vitro and in vivo results to overcome the challenges of achieving innervation, vascularization, and functionalization in bioprinted tissues or organs. Bioengineering the epithelia of the salivary gland is now possible with scaffold-free or with different proteoglycans/hydrogels (e.g., hyaluronic acid, perlecan domain IV, hyaluronic acid, etc.), and innervation can be achieved with a combination of specific cell lines, growth factors (e.g., FGF10, Neurturin, etc.), and bioprinting techniques. However, bioprinted tissues are still restricted to millimeter size constructs with only immature vascular networks. Combining different bioprinting techniques can potentially facilitate the biofabrication of vascularized tissues together with the support of endothelial cells, novel biomaterials, and growth factors. The pathway from organ bioprinting to implantation into a human must be performed within a reasonable amount of time; thus standardized protocols involving patient-specific design, fabrication techniques, maturation processes, surgical operations, and postoperative care are essential for customized functional organ fabrication.
References
Abdel Fattah AR et al (2016) In situ 3D label-free contactless bioprinting of cells through Diamagnetophoresis. ACS Biomater Sci Eng 2(12):2133–2138
Achilli TM, Meyer J, Morgan JR (2012) Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 12(10):1347–1360
Adine C, Ng KK, Rungarunlert S et al (2018) Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 180:52–66
Aframian DJ, Palmon A (2008) Current status of the development of an artificial salivary gland. Tissue Eng Part B Rev 14(2):187–198
Aframian DJ et al (2002) Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng 8(5):871–878
Alaa El-Din Y et al (2018) Potential therapeutic effects of induced pluripotent stem cells on induced salivary gland cancer in experimental rats. Biotech Histochem 94:1–8
Almansoori AA et al (2019) Mesenchymal stem cell therapy in submandibular salivary gland Allotransplantation: experimental study. Transplantation 103:1111
Burford-Mason AP et al (1993) Immunohistochemical analysis of the proliferative capacity of duct and acinar cells during ligation-induced atrophy and subsequent regeneration of rat parotid gland. J Oral Pathol Med 22(10):440–446
Campbell JJ, Watson CJ (2009) Three-dimensional culture models of mammary gland. Organogenesis 5(2):43–49
Elsaadany B et al (2017) Effect of transplantation of bone marrow derived mesenchymal stem cells and platelets rich plasma on experimental model of radiation induced Oral mucosal injury in albino rats. Int J Dent 2017:8634540
Emmerson E et al (2018) Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med 10(3)
Fennema E et al (2013) Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31(2):108–115
Ferreira JN et al (2016) Three-dimensional bioprinting nanotechnologies towards clinical application of stem cells and their Secretome in salivary gland regeneration. Stem Cells Int 2016:7564689
Ferreira JN, Hasan R, Urkasemsin G et al (2019) A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J Tissue Eng Regen Med 13(3):495–508
Fu CY et al (2011) A simple cell patterning method using magnetic particle-containing photosensitive poly (ethylene glycol) hydrogel blocks: a technical note. Tissue Eng Part C Methods 17(8):871–877
Fukuda J et al (2006) Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials 27(30):5259–5267
Groll J, Boland T, Blunk T et al (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8(1):013001
Gronhoj C et al (2018) Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled phase 1/2 trial (MESRIX). Int J Radiat Oncol Biol Phys 101(3):581–592
Hagemann J et al (2017) Spheroid-based 3D cell cultures enable personalized therapy testing and drug discovery in head and neck Cancer. Anticancer Res 37(5):2201–2210
Haisler WL et al (2013) Three-dimensional cell culturing by magnetic levitation. Nat Protoc 8(10):1940–1949
Hegde S, Anuradha A, Sodhi A (2014) Reviving the salivary cells – pathway to new horizon. IJSS Case Reports Rev 1(2):27–29
Hynes K et al (2015) Induced pluripotent stem cells: a new frontier for stem cells in dentistry. J Dent Res 94:1508
Jaganathan H et al (2014) Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci Rep 4:6468
Jakab K et al (2010) Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2(2):022001
Janebodin K, Reyes M (2012) Neural crest-derived dental pulp stem cells function as Ectomesenchyme to support salivary gland tissue formation. Dentistry 2
Jean-Gilles R, Soscia D, Sequeira S et al (2010) Novel modeling approach to generate a polymeric nanofiber scaffold for salivary gland cells. J Nanotechnol Eng Med 1(3):31008
Jeong J et al (2013) Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med 45:e58
Kang HW et al (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319
Kawakami M et al (2013) Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro. Hum Cell 26(2):80–90
Khalili S et al (2010) Bone marrow cells are a source of undifferentiated cells to prevent Sjogren’s syndrome and to preserve salivary glands function in the non-obese diabetic mice. Int J Biochem Cell Biol 42(11):1893–1899
Khalili S et al (2012) Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjogren’s-like disease. PLoS One 7(6):e38615
Khalili S et al (2014) Treatment for salivary gland hypofunction at both initial and advanced stages of Sjogren-like disease: a comparative study of bone marrow therapy versus spleen cell therapy with a 1-year monitoring period. Cytotherapy 16(3):412–423
Knox SM et al (2010) Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329(5999):1645–1647
Kojima T et al (2011) Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope 121(9):1864–1869
Konno M et al (2013) Adipose-derived mesenchymal stem cells and regenerative medicine. Develop Growth Differ 55(3):309–318
Langhans SA (2018) Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol 9:6
Lim JY et al (2013a) Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol 49(2):136–143
Lim JY et al (2013b) Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS One 8(8):e71167
Lin CY et al (2011) Cell therapy for salivary gland regeneration. J Dent Res 90(3):341–346
Lin H, Dhanani N, Tseng H et al (2016) Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol 195(3):788–795
Lombaert IM et al (2006) Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res 12(6):1804–1812
Lombaert IM et al (2008a) Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3(4):e2063
Lombaert IM et al (2008b) Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 14(23):7741–7750
Lombaert I, Movahednia MM, Adine C, Ferreira JN et al (2017) Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells 35(1):97–105
Maimets M et al (2016) Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 6(1):150–162
Maria OM, Zeitouni A, Gologan O et al (2011) Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A 17(9–10):1229–1238
Mironov V, Visconti RP, Kasyanov V et al (2009a) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12):2164–2174
Mironov V et al (2009b) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12):2164–2174
Mishima K et al (2012) Transplantation of side population cells restores the function of damaged exocrine glands through clusterin. Stem Cells 30(9):1925–1937
Moroni L et al (2018) Biofabrication: a guide to technology and terminology. Trends Biotechnol 36(4):384–402
Nair K et al (2009) Characterization of cell viability during bioprinting processes. Biotechnol J 4(8):1168–1177
Nanduri LS et al (2011) Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 99(3):367–372
Nanduri LS et al (2013a) Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol 108(3):458–463
Nanduri LS et al (2013b) Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol 108(3):458–463
Nanduri LS et al (2014) Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep 3(6):957–964
Nelson J, Manzella K, Baker OJ (2013) Current cell models for bioengineering a salivary gland: a mini-review of emerging technologies. Oral Dis 19(3):236–244
Ogawa M, Oshima M, Imamura A et al (2013) Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 4:2498
Ono H et al (2015) Regenerating salivary glands in the microenvironment of induced pluripotent stem cells. Biomed Res Int 2015:293570
Parfenov VA et al (2018a) Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication 10(3):034104
Parfenov VA et al (2018b) Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication 10(3):034104
Pradhan S, Zhang C, Jia X et al (2009) Perlecan domain IV peptide stimulates salivary gland cell assembly in vitro. Tissue Eng Part A 15(11):3309–3320
Pradhan S, Liu C, Zhang C et al (2010) Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryng Head Neck 142(2):191–195
Pradhan-Bhatt S, Harrington DA, Duncan RL et al (2013) Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters. Tissue Eng Part A 19(13–14):1610–1620
Pringle S et al (2016) Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34(3):640–652
Sequeira SJ, Soscia DA, Oztan B et al (2012) The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous plga scaffolds. Biomaterials 33(11):3175–3186
Sfakis L et al (2018) Mesenchymal cells affect salivary epithelial cell morphology on PGS/PLGA Core/Shell nanofibers. Int J Mol Sci 19(4)
Soscia DA, Sequeira SJ, Schramm RA et al (2013) Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 34(28):6773–6784
Souza GR et al (2010) Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol 5(4):291–296
Stewart BW, Wild CW (eds) (2018) World Cancer Report 2018. International Agency for Research on Cancer, France, pp 16–53
Sumita Y et al (2011) Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol 43(1):80–87
Szlavik V et al (2008) Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng Part A 14(11):1915–1926
Takahashi S et al (2004) Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med 33(1):23–29
Tanaka J et al (2018) Generation of orthotopically functional salivary gland from embryonic stem cells. Nat Commun 9(1):4216
Timm DM et al (2013) A high-throughput three-dimensional cell migration assay for toxicity screening with mobile device-based macroscopic image analysis. Sci Rep 3:3000
Tran SD et al (2013) Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS One 8(4):e61632
Tseng H et al (2013) Assembly of a three-dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng Part C Methods 19(9):665–675
Tseng H, Gage JA, Shen T et al (2015) A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci Rep 5:13987
Turker E, Arslan-Yildiz A (2018) Recent advances in magnetic levitation: a biological approach from diagnostics to tissue engineering. ACS Biomater Sci Eng 4(3):787–799
Varghese JJ et al (2019) Salivary gland cell aggregates are derived from self-organization of acinar lineage cells. Arch Oral Biol 97:122–130
Xiao N et al (2014) Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest 124(8):3364–3377
Xu J et al (2012) Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood 120(15):3142–3151
Yamamura Y et al (2013) Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Arch Oral Biol 58(8):935–942
Yi T et al (2016) Single cell clones purified from human parotid glands display features of multipotent epithelial mesenchymal stem cells. Sci Rep 6:36303
Yuan Y-W et al (2013) The ability of transplanted bone marrow-derived cells to differentiate into parenchymal cells of salivary glands. J Hard Tissue Biol 22(4):433–438
Acknowledgments
Our research work was supported by these grants and institutions: Special Task Force for Activating Research (STAR) for Exocrine Gland Biology and Regeneration Research Group, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University, Grant Number: STF 6202432001-1; Chulalongkorn University Faculty of Dentistry Special Funding 2018, Grant Number: DRF62018.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Adine, C., Ferreira, J. (2021). Bioprinting Strategies to Engineer Functional Salivary Gland Organoids. In: Eberli, D., Lee, S.J., Traweger, A. (eds) Organ Tissue Engineering. Reference Series in Biomedical Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-44211-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-44211-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44210-1
Online ISBN: 978-3-030-44211-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences