Abstract
Energy storage in the form of H2 is in many cases considered to be the best means to store energy coming from intermittent (e.g. wind and solar) renewable energy sources. With localised capacities for renewable energy sources proliferating, a storage system that can match the production rate is urgently required. Proton exchange membrane (PEM) fuel cells and electrolysers may provide the basis for a sustainable H2 production solution that is suited for being coupled to intermittent renewable energy sources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Energy storage in the form of H2 is in many cases considered to be the best means to store energy coming from intermittent (e.g. wind and solar) renewable energy sources. With localised capacities for renewable energy sources proliferating, a storage system that can match the production rate is urgently required. Proton exchange membrane (PEM) fuel cells and electrolysers may provide the basis for a sustainable H2 production solution that is suited for being coupled to intermittent renewable energy sources.
The interest in PEM technology has been revitalised in recent years with the increasing interest in producing green energy; however, there are many challenges to be explored to improve the technology for wider distribution. Furthermore, a high level of understanding of the internal temperature distribution within a PEM system will allow computational modelling to increase in accuracy, aiding the conceptual development of PEM systems.
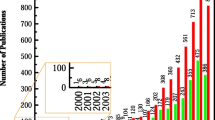
With regard to PEM systems, more than 94,000 fuel cell studies have been published, and almost all water electrolysis methods are also used for fuel cell research, suggesting that water electrolysis research has investigated methods used in fuel cell research [1, 2]. This may be related to fuel cell research encouraging the development of water electrolysis technologies. Therefore, for the context of developing micro-optical sensors for determining temperature distribution in PEM systems, the fuel cell and electrolyser alternatives are virtually identical. Since the PEM fuel cell has developed further than the PEM electrolyser, after a brief description of PEM electrolysers, this chapter will focus more on the fuel cell design.
Hydrogen Production
Traditional Production
The use of H2 as an economically viable energy source has been held back initially by the fact that molecular H2 (unlike fossil fuels) does not readily exist in large scales in nature. Therefore, to obtain H2, energy input is required. Most commonly, H2 is produced by steam reforming of natural gas or other fossil fuels, such as propane, gasoline, diesel, methanol or ethanol [3,4,5]. This is achieved in what is known as a reformer, where high temperature (e.g. 700–1000 °C) water vapour reacts with fossil fuel in the presence of a catalyst generally constructed of nickel [3,4,5]. Although widely used, the steam reforming method of H2 production generally produces low purity H2 with many carbon-containing species (e.g. CO). Furthermore, by using fossil fuels to produce H2, steam reforming does not relieve the dependencies on fossil fuels; therefore, it does not contribute to the energy matrix as a clean, renewable energy storage solution.
Water electrolysis is a method for production of H2 through the electrochemical conversion of water into its two constituents, H2 and O2 [6]. This can yield high-purity H2 due to the lack of complex chemistry occurring.
Electrochemical Production
Water electrolysis is currently the most prominent primary method for sheer H2 manufacturing by electrochemical means, and it is expected that its importance will expand rapidly shortly. Electrolysis of water is based on the movement of electrons supported by an external circuit. The main electrochemical H2 manufacturing techniques are alkaline, proton exchange membrane (PEM) and solid oxide (SO) electrolysers. The input into the electrolyser will be dynamic due to the periodic availability of renewable energy from solar and wind sources. Due to their flexible operation, PEM electrolyser technology is preferential in a renewable energy sense over alkaline and SO techniques.
The main advantages of PEM electrolysers are as follows:
-
There is a possibility of operating the cells at a high current density.
-
High efficiencies can be obtained, even at high current densities.
-
High-purity gases are produced as a result of the high purity water used.
-
They have a high dynamic range.
The main drawbacks of PEM electrolysers are as follows:
-
Capital expenses are still very high.
-
Need for high-purity water.
-
Large-scale systems are not yet developed.
Turning Hydrogen into Electricity
There are many different types of fuel cells, and PEM fuel cells have been developed significantly more than their electrolyser alternatives. Moreover, PEM fuel cells have been developed the most and implemented more due to their capability for generating higher power densities, higher efficiencies, low weight, compact size, lower cost, lower operation temperatures and faster start-up times when compared to the other types of FCs [7,8,9,10].
The PEM is the most essential part of a PEMFC. It must be impermeable to gases and not conduct electrical current, but it must allow passage of protons. Therefore, the membrane acts somewhat like an electrolyte and is placed between two porous, electrically conductive electrodes. These electrodes typically are constructed from carbon-based cloth or paper with platinum catalyst deposited on it. Together, these components make up the membrane electrode assembly (MEA). At both sides of the MEA is where the bipolar plates (BPP) are found. They provide the physical structure of the FC stack, conducting current between single cells, provide control of the temperature via cooling channels, and achieve a uniform reactant gas distribution via their gas channel flow patterns. To avoid corrosion and surface contact resistance, BPPs are generally constructed using graphite composites. Backing layers constructed of polytetrafluoroethylene (PTFE)-coated carbon fibres are known as the gas diffusion layers (GDL). These are used to diffuse the reactant gases and electrons uniformly while limiting the accumulation of liquid water in the MEA. Between the PEM and the GDL is a catalyst layer with catalytic nanoparticles (typically a thin layer of platinum or a platinum-based alloy spread on particles of carbon that are embedded within the polymer electrode). The electrochemical reactions are catalysed in this layer.
The operation principle of the PEM fuel cell is that H2 is supplied to the anode and O2 to the cathode. This is achieved through gas flow channels, resulting in the generation of protons at the anode and current through an external circuit. Moreover, at the anode, H2 is oxidised into protons and electrons. The protons are transported through the PEM, and the electrons through the electrodes, followed by the current collectors, and then through the external electrical circuit to the cathode. The electrons combine with protons and O2 at the cathode electrode to produce water and heat. The overall result of these reactions is the direct current (DC) generation for external usage.
Effects of Temperature and Humidity Within PEMFCs
In order for PEM fuel cells to become a commercial energy production system, several technological challenges regarding thermal management must be resolved [7, 8, 11,12,13,14]. This management includes heat removal from the PEM system generated during regular operation to prevent overheating. The heat can be dissipated using heat exchanging loops or via air convection [15].
The general efficiency of a PEM fuel cell is around 50%, meaning that almost half of the H2 energy is converted into heat. Long-term stability of a PEM fuel cell is, therefore, reliant on thermal management being addressed [16,17,18]. High-resolution analysis of the temperature distribution of the amount of heat generated within the PEM fuel cells will be required to improve their efficiency [19,20,21]. Ideally, each cell within a PEM fuel cell stack should operate with a uniform temperature distribution; however, variations in temperature arise from the inherent design of the manifolds of the stack, the position of single cells within the stack and the method chosen for cooling [22]. Moreover, the maximum cell voltage (theoretical) that a PEM fuel cell can operate is determined by the temperature of the fuel cell [23]; therefore, it can be assumed that an increase in the cell temperature would result in a lower cell voltage in theory [24].
Temperature distributions in PEM fuel cells can be explained as a function of many transport phenomena within the PEM fuel cell, including multicomponent gas transport, charge transport, and two-phase flow and heat transfer, all of which take part in different components of the PEM fuel cell [11]. In order to avoid overheating, the excess heat generated must be removed from the PEM fuel cell. In most PEM fuel cells; the heat is removed by natural convection and radiation. Part of this heat removal is achieved by unused reactants leaving the PEM fuel cell stack, while active cooling is put in place to avoid overheating [25]. In lower temperature PEM fuel cells (operating range of 60–80 °C), a higher temperature can rapidly increase the rate of PEM and catalyst degradation, therefore reducing the stack performance [25,26,27,28,29,30].
Conversely, low temperatures are also not favourable, as they may cause electrode flooding due to a lower water saturation pressure required at a lower temperature, which can cause significant problems about water management within the PEM fuel cell [31]. Furthermore, if there is too much heat energy removed from the PEM fuel cell by active cooling, the kinetics of the chemical reactions within the cell is negatively affected. This can cause the partial pressure of water vapour to increase to a level higher than the saturation pressure, which leads to condensation within the PEM fuel cell, lowering the performance significantly.
It can also become disastrous for the PEM fuel cell if the temperature within becomes is too high. This can occur if the active cooling is not adequate to remove excess heat energy, and the temperature can rise above the allowable operating temperature, which can cause dehydration of the cell, reducing the protonic conductivity of the cell, reducing the stack performance and stability [31, 32]. Localised heat increases within the PEM fuel cell due to an insufficiently designed cooling system can lead to the degradation of the components within the PEM fuel cell, significantly reducing the reliability.
The primary purpose of temperature management in PEM fuel cells is to ensure that the PEM fuel cell stacks operate within what is deemed to be a reliable temperature range and maintaining a uniform distribution of heat [33]. This heat energy can also be recycled, allowing for its effective use for other requirements, improving the efficiency of the PEM fuel cell [34].
Within a PEM fuel cell, an increase in operating temperature up to 80 °C can affect the stack performance positively; however, once the temperature reached 85 °C, the stack performance starts to drop rapidly. This is because the conductivity of the polymer membrane is reduced as there is a lower water content [9, 33]. Therefore, it is critical with regard to the performance of the PEM fuel cell that the temperature is maintained at an ideal operating temperature between 60 and 80 °C. This becomes imperative when the PEM fuel cell is running at high current densities, as heat is generated at a higher rate and needs to be quickly removed.
Distribution of Temperature and Humidity Within PEMFCs
The heat generated within PEM fuel cells generally originates from the cathode side of the catalyst layers, but the membrane and the electrically conductive parts of the PEM fuel cell can also generate heat. Cross-sectional analysis of a PEM fuel cell can show the gradient distribution of heat. The generation of heat in a PEM fuel cell is the result of four factors:
-
1.
The irreversibility of the electrochemical reactions
-
2.
The heat of the reactions
-
3.
The ohmic resistances
-
4.
The condensation of water
The difference between the total chemical energy of the reactants and the maximum of available work is known as entropic heat, in accordance with the Second Law of Thermal Dynamics. The entropic heat is representative of the change in entropy of the electrochemical reactions and must be either supplied to or subtracted from the electrode regions of the PEM fuel cell. What is known as the irreversible heat results from the electrochemical reactions irreversibility within the PEM fuel cell and can be a significant heat generator. Ohmic heating is generated by the inherent resistance of the electrolyte, electrodes, the bipolar plates and the current collecting components within the PEM fuel cell [32, 35, 36]. In addition, the entropic heat associated with the condensation and evaporation of water within the gas diffusion layers must be taken into account.
Heat generation within PEM fuel cells is driven by the electrode thermal resistance properties, the electrochemical rates of reactions, the uniformity of the reactions, the humidity of the reactants and the permeability of the PEM/GDL. The rate at which the heat is generated is proportional to decreases in cell voltages and increases in current densities. These electrical properties are indirectly proportional, because as the current density increases, the cell voltage decreases, and this significantly increases the rate of heat generation within the PEM fuel cell.
The distribution of the heat generated within the PEM fuel cell stack is uneven. Furthermore, the entropy variation imbalance and electrode losses, the exothermic electrochemical reactions that take place at the cathode side significantly influence the heat generation within the PEM fuel cell [21]. Heat conduction is the primary mode of thermal transport through the PEM, while the transfer in the catalyst and gas diffusion layer is significantly influenced by conduction and convection of heat [37]. The convective heat flux direction is the same as the flow direction within the PEM fuel cell; however, the conductive heat flux is in the opposite direction as it is determined by the temperature gradient [31, 37]. The rate of local heat generation affects the durability and performance of the PEM fuel cells, is not straight forward to measure and varies in both through-plane and in-plane directions.
A significant disparity in the understanding of the heat distribution in the through-plane still exists. This is because the heat generation is usually non-uniform within the in-plane direction because of the interrelationship of the water content, local current density, reactant concentration and temperature. The non-uniform distribution of the local heat generation and temperature significantly complicates the cooling of PEM fuel cell stacks [19].
The removal of heat within the PEM fuel cell is achieved using some form of the cooling system or transferred across the faces of the PEM fuel cell stack by conduction and convection. The rate of heat removal is dictated by each component (i.e. PEM, GDL, catalyst layer and BPP) thermal properties. The amount of chemical energy that can be converted into electricity is determined partially by the state of the water produced by the fuel cell (either liquid or gas). The absorption and release of latent heat, water and heat transport are bound to the evaporation and condensation processes of water. These occur in combination with each other due to what is known as a “heat pipe effect.” The vapour pressure saturation point is heavily dependent on local temperatures [38]. The significant contributors to heat generation in the PEM fuel cell are the electrochemical reactions, with the most severe water and heat management problems occurring at the cathode catalyst layer due to its large heat generation. Evaporation of the liquid phase is stimulated by the small pores within the catalyst layers, allowing the flow of water out of the system in the vapour phase, enabling the chemical reactants to diffuse towards their site of reaction. The cathode catalyst layers generated heat can also be conducted through the GDL, BPP and subsequently removed by active cooling. Therefore, the thermal conductivity of the cathode catalyst layer is of significant interest in developing temperature management further [39].
When the high temperature-vaporised water passes through the MPL and GDL, it is slightly cooled, and some water vapour is condensed into liquid water. This improves the heat transfer as latent heat is released during water condensation. Therefore, the thermal gradients within the GDL have a significant effect on water transport and condensation [40].
The gas diffusion layers used for PEM fuel cell construction are highly directionally dependent when it comes to heat transport properties. Moreover, they have different properties for through-plane and in-plane transport directions. When designing the GDL, the heat transfer properties are fundamental for the efficient operation of a PEM fuel cell. The effective thermal conductivity and thermal contact resistance between the GDL and all neighbouring layers must be analysed thoroughly during the design phase [41, 42].
Detailed knowledge of the in situ distribution of temperature within the PEM fuel cell is fundamental for the improvement and optimisation of PEM fuel cell performance and durability. Although through-plane thermal conductivities can be used for predicting temperature distribution, in-plane conductivities are crucial for improving discrepancies between modelled and experimental data [43]. There are two additional fundamental parameters other than the GDL thermal conductivity that significantly influence the PEM fuel cells thermal behaviour. The relative humidity of the feed gas is one of these fundamental parameters, as this has a significant effect on the overall membrane hydration. When the feed gas is fully humidified, the thermal effect is reduced as the membrane maintains full hydration. The other critical parameter is the cell voltage, as this directly controls the current output and therefore the heat generation rate [44].
Research Needs and Measurement Challenges
Managing heat in PEM fuel cells is traditionally secondary in comparison to performance. Despite this, in recent years, it has become a crucial area of improvement for the continuation of performance development [24]. There is a need for more detailed knowledge in the field of PEM fuel cell science and technology development. To understand the challenge, one must understand the geometric levels that a PEM fuel cell is assembled and how temperatures, water and other parameters give an impact.
The core reactor of a PEM fuel cell is the membrane and its anode and catalyst layer (AC and CC). It is in these three layers that the heat is generated. The heat is generated in very thin layers (10–50 μm) and then has to be transported through several thicker layers (200–1000 μm), and this leads to substantial temperature gradients.
A commonly modelled area is indicated with pink and the boundary lines with blue, as shown in Fig. 6.1. The distance between the blue lines here is typically 1000 μm. Using a continuum model with evenly distributed reaction rate and considering different thermal conductivity values [45], one can see that the local temperature variations are up to 14° between the backing reference and the membrane (Fig. 6.2). The research problem is complicated, as the temperature, thermal conductivity and thermal gradients are all within the range of 10–1000 μm. One can see that measuring this temperature requires small sensors.
The understanding of local humidity, temperature and localised gas pressures can be improved by experiments and by modelling. To avoid modelling, which is too empirical and with too many assumptions, high resolution and highly detailed experimental results are needed that cover local temperature and relative humidity in different positions within the PEM fuel cell. The experimental challenge is that catalyst layers are 10–20 μm thick and existing sensors are at least twice as large, and that is before adding corrosion protective coatings. When the sensor (with or without their coating) is larger than the region of interest, one cannot know where data are obtained. Detailed measurements down to the precision of 10 μm are required.
Improved knowledge of data point location for improved understanding of heat and mass transport and improved understanding of ageing mechanisms of a PEM fuel cell is essential in this field. Optical-based sensors that can function on other electrochemical devices with regions thinner than 10 μm is a promising route.
Possibilities for Micro-optical Technologies in PEMFCs
Currently, research into the thermal properties of PEM fuel cells relies on thermocouples that are beyond 100 μm thick when including coatings [45] and the electrical components [46] of the sensor. The challenges with these sensors are twofold. They are susceptible to corrosion-based degradation, and they have a large relative size (the sensor thicknesses influence on the thermal transport within PEM fuel cells is not yet entirely defined). Therefore, there is a requirement for smaller non-electrical sensors to measure temperature within PEM fuel cells.
There is a possibility for micro-optical sensors with small spatial distribution to be designed to undertake fundamental investigation targeting the measurement of temperature within PEM fuel cells. This will allow the research community to have a stronger understanding of the heat distribution within PEM fuel cells. For example, this could improve the understanding of the thermal properties of the PEM fuel cells, allowing the design and manufacture of PEM fuel cell components (e.g. GDL), with significantly improved heat management properties and power performance. The primary requirement of the production of micro-optical sensors that can be used in electrochemical systems is to have an inert sensor that is small enough that it has a negligible effect on the internal components of the PEM fuel cell, which has layers thinner than 10 μm.
References
Ogawa T, Takeuchi M, Kajikawa Y (2018) Analysis of trends and emerging technologies in water electrolysis research based on a computational method: a comparison with fuel cell research. Sustainability 10(2):478
Ogawa T, Takeuchi M, Kajikawa Y (2018) Comprehensive analysis of trends and emerging technologies in all types of fuel cells based on a computational method. Sustainability 10(2):458
Ni M, Leung DYC, Leung MKH (2007) A review on reforming bio-ethanol for hydrogen production. Int J Hydrog Energy 32(15):3238–3247
Rostrup-Nielsen JR, Sehested J, Nørskov JK (2002) Hydrogen and synthesis gas by steam-and CO2 reforming. Adv Catal 47:65–139
Rostrup-Nielsen JR, Rostrup-Nielsen T (2002) Large-scale hydrogen production. CATTECH 6(4):150–159
Lamb JJ, Pollet BG, Burheim OS (2020) Energy storage. In: Energy-smart buildings design: construction and monitoring of buildings for improved energy efficiency. https://doi.org/10.1088/978-0-7503-3259-0ch6. ISBN 978-0-7503-3257-6
Yu S, Jung D (2008) Thermal management strategy for a proton exchange membrane fuel cell system with a large active cell area. Renew Energy 33(12):2540–2548
Eckl R, Zehtner W, Leu C, Wagner U (2004) Experimental analysis of water management in a self-humidifying polymer electrolyte fuel cell stack. J Power Sources 138(1–2):137–144
Pérez-Page M, Pérez-Herranz V (2011) Effect of the operation and humidification temperatures on the performance of a PEM fuel cell stack on dead-end mode. Int J Electrochem Sci 6:492–505
Wang Y, Chen KS, Mishler J, Cho SC, Adroher XC (2011) A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research. Appl Energy 88(4):981–1007
Kandlikar SG, Lu Z (2009) Fundamental research needs in combined water and thermal management within a proton exchange membrane fuel cell stack under normal and cold-start conditions. J Fuel Cell Sci Technol 6(4):44001
Kandlikar SG, Lu Z (2009) Thermal management issues in a PEMFC stack–a brief review of current status. Appl Therm Eng 29(7):1276–1280
Pandiyan S, Jayakumar K, Rajalakshmi N, Dhathathreyan KS (2008) Thermal and electrical energy management in a PEMFC stack–an analytical approach. Int J Heat Mass Transf 51(3–4):469–473
Nolan J, Kolodziej J (2010) Modeling of an automotive fuel cell thermal system. J Power Sources 195(15):4743–4752
Cao T-F, Lin H, Chen L, He Y-L, Tao W-Q (2013) Numerical investigation of the coupled water and thermal management in PEM fuel cell. Appl Energy 112:1115–1125
Yan W-M, Chen C-Y, Mei S-C, Soong C-Y, Chen F (2006) Effects of operating conditions on cell performance of PEM fuel cells with conventional or interdigitated flow field. J Power Sources 162(2):1157–1164
Park YH, Caton JA (2008) Development of a PEM stack and performance analysis including the effects of water content in the membrane and cooling method. J Power Sources 179(2):584–591
Baek SM, Yu SH, Nam JH, Kim C-J (2011) A numerical study on uniform cooling of large-scale PEMFCs with different coolant flow field designs. Appl Therm Eng 31(8–9):1427–1434
Zhang G, Guo L, Ma L, Liu H (2010) Simultaneous measurement of current and temperature distributions in a proton exchange membrane fuel cell. J Power Sources 195(11):3597–3604
Hsiao M-C, Liao S-H, Yen M-Y, Ma C-CM, Lee S-J, Lin Y-F et al (2009) Electrical and thermal conductivities of novel metal mesh hybrid polymer composite bipolar plates for proton exchange membrane fuel cells. In: ASME 2009 7th international conference on fuel cell science, engineering and technology. American Society of Mechanical Engineers, pp 871–878
Yu S, Jung D (2010) A study of operation strategy of cooling module with dynamic fuel cell system model for transportation application. Renew Energy 35(11):2525–2532
Strahl S, Husar A, Puleston P, Riera J (2014) Performance improvement by temperature control of an open-cathode PEM fuel cell system. Fuel Cells 14(3):466–478
Sasmito AP, Birgersson E, Mujumdar AS (2011) Numerical evaluation of various thermal management strategies for polymer electrolyte fuel cell stacks. Int J Hydrog Energy 36(20):12991–13007
Bvumbe TJ, Bujlo P, Tolj I, Mouton K, Swart G, Pasupathi S et al (2016) Review on management, mechanisms and modelling of thermal processes in PEMFC. Hydrog Fuel Cells 1(1):1–20
Islam MR, Shabani B, Rosengarten G, Andrews J (2015) The potential of using nanofluids in PEM fuel cell cooling systems: a review. Renew Sust Energ Rev 48:523–539
Faghri A, Guo Z (2005) Challenges and opportunities of thermal management issues related to fuel cell technology and modeling. Int J Heat Mass Transf 48(19–20):3891–3920
Rodatz P, Büchi F, Onder C, Guzzella L (2004) Operational aspects of a large PEFC stack under practical conditions. J Power Sources 128(2):208–217
Lasbet Y, Auvity B, Castelain C, Peerhossaini H (2006) A chaotic heat-exchanger for PEMFC cooling applications. J Power Sources 156(1):114–118
Banerjee R, See E, Kandlikar SG (2013) Pressure drop and voltage response of PEMFC operation under transient temperature and loading conditions. ECS Trans 58(1):1601–1611
Van den Oosterkamp PF (2006) Critical issues in heat transfer for fuel cell systems. Energy Convers Manag 47(20):3552–3561
Hosseinzadeh E, Rokni M, Rabbani A, Mortensen HH (2013) Thermal and water management of low temperature proton exchange membrane fuel cell in fork-lift truck power system. Appl Energy 104:434–444
Shahsavari S, Desouza A, Bahrami M, Kjeang E (2012) Thermal analysis of air-cooled PEM fuel cells. Int J Hydrog Energy 37(23):18261–18271
Ravishankar S, Prakash KA (2014) Numerical studies on thermal performance of novel cooling plate designs in polymer electrolyte membrane fuel cell stacks. Appl Therm Eng 66(1–2):239–251
Cozzolino R, Cicconardi SP, Galloni E, Minutillo M, Perna A (2011) Theoretical and experimental investigations on thermal management of a PEMFC stack. Int J Hydrog Energy 36(13):8030–8037
Barbir F (2012) PEM fuel cells: theory and practice. Academic, Waltham
Yan Q, Toghiani H, Causey H (2006) Steady state and dynamic performance of proton exchange membrane fuel cells (PEMFCs) under various operating conditions and load changes. J Power Sources 161(1):492–502
See E, Kandlikar SG (2013) Effect of GDL material on thermal gradients along the reactant flow channels in PEMFCs. ECS Trans 58(1):867–880
Ramousse J, Deseure J, Lottin O, Didierjean S, Maillet D (2005) Modelling of heat, mass and charge transfer in a PEMFC single cell. J Power Sources 145(2):416–427
Lopez-Sabiron AM, Barroso J, Roda V, Barranco J, Lozano A, Barreras F (2012) Design and development of the cooling system of a 2 kW nominal power open-cathode polymer electrolyte fuel cell stack. Int J Hydrog Energy 37(8):7289–7298
Ramousse J, Lottin O, Didierjean S, Maillet D (2009) Heat sources in proton exchange membrane (PEM) fuel cells. J Power Sources 192(2):435–441
Wu J, Galli S, Lagana I, Pozio A, Monteleone G, Yuan XZ et al (2009) An air-cooled proton exchange membrane fuel cell with combined oxidant and coolant flow. J Power Sources 188(1):199–204
Karimi G, Li X, Teertstra P (2010) Measurement of through-plane effective thermal conductivity and contact resistance in PEM fuel cell diffusion media. Electrochim Acta 55(5):1619–1625
Sadeghi E, Djilali N, Bahrami M (2011) Effective thermal conductivity and thermal contact resistance of gas diffusion layers in proton exchange membrane fuel cells. Part 1: Effect of compressive load. J Power Sources 196(1):246–254
Zamel N, Li X, Shen J, Becker J, Wiegmann A (2010) Estimating effective thermal conductivity in carbon paper diffusion media. Chem Eng Sci 65(13):3994–4006
Burheim OS, Crymble GA, Bock R, Hussain N, Pasupathi S, Du Plessis A et al (2015) Thermal conductivity in the three layered regions of micro porous layer coated porous transport layers for the PEM fuel cell. Int J Hydrog Energy 40(46):16775–16785
Reum M, Freunberger SA, Wokaun A, Büchi FN (2009) Measuring the current distribution with sub-millimeter resolution in PEFCs II. Impact of operating parameters. J Electrochem Soc 156(3):B301–B310
Acknowledgements
The authors are grateful to the ENERSENSE programme and NTNU Team Hydrogen at the Norwegian University of Science and Technology (NTNU) for supporting and helping on this book project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lamb, J.J., Burheim, O.S., Pollet, B.G. (2020). Hydrogen Fuel Cells and Water Electrolysers. In: Lamb, J., Pollet, B. (eds) Micro-Optics and Energy. Springer, Cham. https://doi.org/10.1007/978-3-030-43676-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-43676-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43675-9
Online ISBN: 978-3-030-43676-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)