Abstract
Long bones grow by a process in which chondrocytes within growth plate cartilage near the bone ends synthesize and mineralize a cartilaginous matrix that serves as a template for bone cells. The growth plate consists of several distinct zones, including a reserve zone (RZ) that lies between the epiphyseal subchondral bone plate and the proliferative zone (PZ). Mechanical loading of the growth plate modulates chondrocyte activity and bone growth, but the role of RZ in relation to this is unclear. To explore this, an axisymmetric, large deformation model was developed. In this model, chondrocytes were embedded at four different depths within the RZ between the SB and PZ. Growth plate cartilage was partitioned into sections to represent the RZ and the proliferative/hypertrophic zones and zone of provisional calcification (PC). Chondrocytes were surrounded by a layer of pericellular matrix (PCM). By including or excluding the PCM, we could examine the influence of the PCM on stress-strain distributions within and around the chondrocytes. The volume-averaged height, width and principal tensile strains in the cells and PCM varied with the cell depth within the RZ. The presence of the PCM resulted a 10% decrease in cellular hydrostatic pressure and in a 20% increase in the cellular maximum principal strains, except near the SB plate border where cellular maximum principal strains were amplified by 45%. This suggests that the PCM is a component of the cell’s mechanosensory mechanism and acts to reduce intra-cellular pressure while amplifying cellular strains.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The growth plate or physis consists of a thin layer of hyaline cartilage sandwiched between epiphyseal and metaphyseal bone; it is responsible for most of the longitudinal bone growth by an endochondral ossification mechanism. Growth plate cartilage consists of three main regions: a highly cellular region of proliferative and hypertrophic chondrocytes located in tubular structures aligned with the bone long axis and a region that is more sparsely populated by chondrocytes called the resting zone, or reserve zone, which is located adjacent to the epiphyseal bone [1]. Blood vessels pass through the subchondral bone plate and reserve zone ending at the beginning of the proliferative zone [2, 3], but the RZ itself has no blood supply. In each location throughout the growth plate every chondrocyte is surrounded by an extracellular matrix (ECM). Generally, chondrocytes are responsible for making, maintaining and repairing extracellular matrix in response to mechanical loading. It has been shown that mechanical loading modulates chondrocyte metabolic activities [4]. However, we have a limited understanding of how biomechanical signals are sensed by the cell and what the relevant molecular pathways are. Chondrocyte behavior is partly regulated by the stress-strain state in and around the cell [4,5,6]. The morphology and mechanical properties of the growth plate have been measured at the cell and tissue level by confocal microscopy/fluorescent labeling techniques [7, 8] and atomic force microscopy (AFM) [9, 10]. These studies have revealed differences in the structural and mechanical properties between the zones of the cartilage [9]. It has been shown that the chondrocytes are surrounded by a narrow region of pericellular matrix (PCM) [11, 12].
Chondrocytes and their surrounding PCM and territorial matrix were described by Benninghof as the basic functional units of cartilage and named chondrons using the analogy of osteons in bone [11, 12, 15]. The PCM has been characterized by type IV collagen [13, 14]. Experimental and theoretical studies have reported the mechanical properties of the PCM and extracellular matrix (ECM) within articular cartilage by various techniques, such as micropipette aspiration and atomic force microscopy (AFM) [11, 12, 16, 17] and showed that the PCM mechanical and microstructural properties significantly influence the biomechanical environment of chondrocytes [11, 18, 19]. In contrast to the nonuniformity and mechanical anisotropy of cartilage ECM, the PCM has zonal uniformity and constant mechanical properties at the microscale [20, 21]. There is a large difference between the elastic moduli of chondrocytes, PCM and ECM [4, 7, 22]. Therefore, the stress-strain fields within the cells are expected to differ from the surrounding matrix. Although several biomechanical studies have reported microscale models to study the stress-strain state around the chondrocytes and PCM within articular cartilage ECM, relatively few studies have been published on the deformation and strain of chondrocytes in different zones of growth plate cartilage [8, 23, 24] and to our knowledge none have examined the chondrocytes within the growth plate reserve zone.
The overall goal of this study was to explore the role of the PCM in modulating chondrocyte deformation and stress-strain state as a function of depth within the reserve zone under normal physiological loads. Specifically, we sought to answer the following three questions: (1) How do deformation, strain and stress within the chondrocyte and PCM vary through the depth of the reserve zone? (2) Does the PCM protect reserve zone chondrocytes from excessive stress? (3) Does the PCM amplify or reduce strains in the reserve zone chondrocytes?
2 Method
2.1 Model Description
An axisymmetric linearly elastic microscale model was developed in which circular chondrocytes were embedded within the RZ extracellular matrix (ECM) at four depths between the subchondral bone plate and the proliferative zone. The PCM wall thickness was set to be half of the chondrocyte radius (cell radius = 10 µm, PCM thickness = 5 µm) [25, 26]. Chondrocytes make up only about 10% of the reserve zone volume [7]. Therefore, to simplify the model, we simulated four cells in the RZ in a finite element microscale model using ABAQUS/CAE 2019 (SIMULIA, USA). A chondrocyte was placed in four different locations of the RZ, starting with a location near the subchondral bone (SB) interface and moving along the symmetry axis to a location near the proliferative zone (PZ) interface. Homogeneous isotropic linearly elastic materials (Table 1) were used for all components. The cartilage was unconfined along the perichondrial periphery. This static elastic analysis represents a state during fast loading (short duration relative to the stress relaxation time), such as heal strike in gait, during which the cartilage does not have time to undergo relaxation and creep and the influence of the fluid component is negligible.
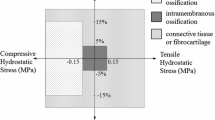
To account for large deformation, the effect of geometrical nonlinearity was considered by turning on NLGEOM. The idealized geometry of our model is shown in Fig. 1, in which each cell is surrounded by a PCM and both are embedded in the ECM of the reserve zone (RZ) along the symmetry axis of the axisymmetric model. The growth plate layer was partitioned into two sections to represent the reserve zone (RZ) and the proliferative/hypertrophic zones (PZ/HZ). The bottom of the model was fixed at the junction between the zone of provisional calcification (PC) and the metaphyseal cancellous bone; and the nodes at the subchondral bone plate surface were constrained to prevent translation in the horizontal direction and a vertical displacement was prescribed equal to 15% of the overall growth plate thickness (height) representing normal physiological loading along the bone long axis (vertical axis). Axisymmetric quadrilateral bilinear, hybrid with constant pressure elements (CAX4H) were used for all parts to avoid hour-glassing and volumetric locking during analysis, which is caused by zero energy deformation modes because nearly incompressible material properties have been assigned for all growth plate regions. A mesh convergence study for the microscale model was done to optimize the mesh. The microscale model was run with all four cells and with a single cell at each depth which confirmed that there were no cell-cell interactions in the stress-strain field.
Overview of the modeling approach. (A) Cross-section of a cylindrical plug of growth plate cartilage, subjected to a displacement of 15% of the overall cartilage thickness. (B) Idealized axisymmetric model consisting of subchondral bone plate (SB), reserve zone (RZ), proliferative/hypertrophic zone (PZ/HZ), provisional calcification (PC). (C) Chondrocyte cell wrapped with a narrow region of pericellular matrix (PCM) embedded in extracellular matrix (ECM).
To evaluate the effect of the PCM on the stress-strain distribution in the chondrocytes, ECM material properties were assigned for the elements in the PCM region to compare it with the model in which chondrocyte, PCM and ECM material properties were defined to be different for each individual location. In addition, a ‘macroscale’ model without cells and PCM was analyzed to evaluate the volume-averaged stresses and strains in the same four cell/PCM locations within the reserve zone by assigning material properties of the ECM to the regions otherwise occupied by the cells and PCM. This allowed for comparisons of stresses and strains over the same cell and PCM regions of the mesh in the microscale cell model as the macroscale cell model devoid of cells and PCM.
2.2 Stress-Strain Measurement
To analyze the interaction between cell and matrix and study the local stress-strain state of the cell, hydrostatic stresses and maximum tensile principal strains of the cells were calculated as the main key mechanobiological factors. The volume averaged hydrostatic compressive stress (volumetric or dilatational stress) and principal tensile strain (related to octahedral shear stress) of the cells and PCM were calculated according to Eqs. (1) and (2), respectively.
where i represent the element number, N is total number of the elements for the cell, Evoli refers to the element volume and \( \sigma_{i} \) is hydrostatic stress for the ith element.
where i is the element number, εi represents the maximum tensile principal strain for each element within the cell and n is the total number of elements for each chondrocyte.
In this paper, the maximum principal strain was used as a substitute for octahedral shear stress since the maximum principal (tensile) strains in the cells and PCM of the reserve zone were found to vary by location in a very similar manner to the octahedral shear stresses. The maximum principal strain results were extracted in terms of logarithmic strains.
Cell height and width engineering strains were calculated based on the change in the horizontal diameter and vertical radius of the cell, respectively before and after compression. PCM height and width strains were also defined as the change in the vertical and horizontal wall thickness, respectively, of the PCM divided by the original wall thickness. This was also done for the reserve zone macroscale model which had no cells or PCM, by using the same mesh used for the microscale model in which cells and PCM were present, but in which the reserve zone was homogenous (assigned Young’s modulus and Poisson’s ratio of the ECM).
3 Results
The results revealed a highly non-homogenous and depth-dependent strain field within and around the cells for the model that included the PCM and the model that excluded the PCM. Figure 2 shows the contour plots of maximum principal tensile strain (logarithmic strain) distribution within and around the cell at different locations within the growth plate reserve zone (RZ). The magnitudes of the cell-averaged hydrostatic stresses and maximum principal tensile strains change throughout the depth of RZ as we move our view point from close to the epiphyseal subchondral bone (cell location 1) to near the PZ/HZ interface (cell location 4) (Fig. 2).
Distribution of maximum principal strains around and within the cell for models (A) with PCM and (B) without PCM (when material properties of ECM are assigned to the PCM, i.e. PCM = ECM), for different locations of the cell within the reserve zone, moving from subchondral bone to proliferative zone, subjected to 15% compression and using CAX4H elements.
3.1 Influence of Cell Location in the Reserve Zone on Chondrocyte Stress and Strain
When the PCM was included in the model, the cell-averaged maximum principal tensile strains within the chondrocytes increased in magnitude from a value of 6% strain near the epiphyseal subchondral bone (SB) to 22% near the proliferative zone (PZ) interface, representing a nearly 4-fold increase (Fig. 3A). Cellular height and width strains followed a similar trend (Fig. 3C). However, the opposite pattern was observed for the cell hydrostatic compressive stresses which decreased from a value 0.12 near the SB to 0.09 MPa near the PZ, an approximate location-dependent decline of 26%. Figure 2 represents how the presence of the PCM changes the stress-strain state around and within the cell.
(A) Cell-averaged hydrostatic stress and maximum principal tensile strain for cells in different locations within the reserve zone moving from subchondral bone (SB) to proliferative zone (PZ) subjected to 15% compression of the growth plate cartilage for two models (with/without PCM). (B) Changes in hydrostatic stress and maximum principal strain due to the presence of the PCM, (C) Chondrocyte height and width strains in locations 1–4, (D) Changes in width and height strains due to the presence of the PCM.
The height and width strains of the matrix within the cell locations in the macroscale model in which there were no cells within the matrix, are compared with the microscale model in which cells and PCM were present, in Fig. 4.
Chondrocyte region height and width strains within each cell location moving from subchondral bone (SB) to proliferative zone (PZ) for: the microscale model in which cells and PCM are present; and the macroscale model in which there are no cells or PCM (‘Chondrocyte Strain’ in this case refers the ECM strain in the macroscale model location of the cell).
3.2 Influence of the Pericellular Matrix on Chondrocyte Stress and Strain
The presence of the pericellular matrix (PCM) resulted in an approximate 20% increase in the cell-averaged maximum principal strains in reserve zone locations 2, 3 and 4 and in a 10–12% decrease in cell-averaged hydrostatic pressure. The influence of the PCM on strains was greatest for the cell located near the SB border where the presence of the PCM amplified the cell-averaged maximum principal strains by 45% (Fig. 3B). Likewise, cellular height strain of the cell closet to the SB was most influenced by the presence of the PCM (Fig. 3D), while the influence of the PCM on cell width strains appeared not to vary much with cell locations (Fig. 3D).
The height and width strains of the PCM followed similar trends to those for cell height and width (Fig. 5). Heights of both cells and PCM decreased under the applied compression, while their widths increased.
PCM region height and width strains within each cell location moving from subchondral bone (SB) to proliferative zone (PZ) for: the microscale model in which cells and PCM are present; and the macroscale model in which there are no cells or PCM (‘PCM Strain’ in this case refers the ECM strain in the macroscale model location of the PCM).
4 Discussion
We developed a computational model to simulate chondrocyte deformation and study chondrocyte–matrix interactions as a function of cell depth within reserve zone of growth plate cartilage under physiological compression. The main objectives of the current study were to characterize the depth-dependent chondrocyte stress-strain distribution within the reserve zone and the influence of the microenvironment of the chondrocytes. Our findings indicate that the pericellular matrix (PCM) amplifies the cell’s internal strains and the cell’s height and width strains, while slightly reducing the cell’s internal hydrostatic stress. The results showed that the state of stress and strain in the reserve zone chondrocytes are significantly depth dependent. The cell-averaged maximum principal strain increased from a minimum near the SB interface and reached maximum near the PZ interface. Cell height and width strains were also smallest at the SB border and greatest at the border with the PZ. This may play a role in preparing the reserve zone chondrocyte for cell alignment in the proliferative zone column of cells (chondron) and for the cell’s function as a daughter cell. In the this study, the presence of PCM with a Young modulus value between that of the chondrocyte and ECM, decreased the cell-averaged hydrostatic stress, while it had the opposite effect on the cellular strains and acted as a strain amplifier, which is similar to the results reported for articular cartilage [4, 11].
In particular, the cell-averaged maximum principal strains, cell height, and cell width strains all increased with cell location from the SB towards the PZ border where they reached maximal values. The addition of the PCM in the model further increased the maximum principal strains, width, and height strains near the PZ by about 20%, 5%, and 16.6%, respectively. The most notable influence of the PCM in amplifying cellular strains occurred for the cell located near the subchondral bone, where cellular strains were at a minimum. This further suggests that the PCM may act as a mechano-transducer and serve as a transitional zone between cell and ECM. Our results revealed that PCM plays a more dominant role in regulating the micro-mechanical strain environment of chondrocytes near the interface with the much ‘stiffer’ subchondral bone.
Experimental and computational studies on PCM biological function and its chemical and mechanical properties, suggest that the PCM chemical composition, and physical and mechanical properties are directly proportional to one another [19]. The PCM can function as a thin barrier between the cell and ECM to filter molecules and regulate the biochemical environment of articular cartilage chondrocytes, and is hypothesized to have a prominent role in cartilage degeneration and osteoarthritis [12, 32]. Computational studies of micromechanical environment of chondrocytes in different zones of articular cartilage imply that the PCM plays a dominant role in modifying the cellular stress-strain state; and any change in PCM properties with aging or cartilage abnormality can significantly affect the biophysical/biomechanical environment of the chondrocytes [18, 33]. Computational modeling of articular cartilage has given rise to the idea that the PCM can act as a mechano-transducer between cells and ECM [4, 11].
Generally, chondrocytes are responsible for making, maintaining and repairing extracellular matrix in response to mechanical loading. Despite the cited foregoing studies on cell-matrix interactions, it is not known how biomechanical signals are transmitted to the cell and transduced–exact molecular mechanisms and pathways are still unknown. One proposed general mechanism by which chondrocytes may sense mechanical signal variations, is through the alteration of cell shape and volume [6]. A confocal microscopy study revealed that the micromechanical environment in articular cartilage is depth and zone dependent and causes an inhomogeneous and anisotropic deformation of the cells [6]. Other studies have proposed that primary cilia on the surface of the cell can act as mechanosensory organelles, which transduce mechanical forces into biological signals [34, 35]. The PCM may function in conjunction with cilia to provide a feasible signal transducer [4, 11]. In articular cartilage, the PCM reduces the local stress of the cell, providing a protective role for the PCM while amplifying the cellular strain [4, 11]. The current study on growth plate cartilage reserve zone suggests a similar role for the PCM in the growth plate reserve zone. Understanding the sequence of events by which cells and ECM can communicate and convert the biomechanical and biochemical signals or signal transduction is still a challenge to investigators. Further evaluation on how PCM components contribute to chondrocyte mechano-transduction in the reserve zone of growth plate cartilage may elucidate factors contributing growth abnormalities and disturbances and enhance our understanding of regulatory mechanical factors that modulate bone growth.
In the current model we have chosen to represent the reserve zone with a thickness slightly more than double the height or thickness of the proliferative and hypertrophic zones combined, based on our histological studies of twenty-day old mixed breed piglet distal ulnar growth plates. The relative thickness of each zone varies by age and location and may influence the reserve zone chondrocyte stress and strain magnitudes. We also chose to model a flat growth plate and need to explore the influence of mammillary processes on reserve zone cell stress and strain and the possible role that reserve zone cells might play in mammillary processes development.
Although macroscale models have been reported of the three zones of the growth plate for various loading conditions [24, 27, 36,37,38,39], to date there has been no model that includes chondrocytes in the reserve zone or of the interaction between reserve zone cells, PCM and ECM. Further computational studies of cell-PCM-ECM interaction in growth plate cartilage may generate new insights into the role played by the micromechanical environment around chondrocytes in the mechano-transduction mechanisms of bone and cartilage growth.
References
Abad, V., Meyers, J.L., Weise, M., Gafni, R.L., Barnes, K.M., Nilsson, O., Bacher, J.D., Baron, J.: The role of the resting zone in growth plate chondrogenesis. Endocrinology 143(4), 1851–1857 (2002)
Brighton, C.T.: The growth plate. Orthop. Clin. North Am. 15(4), 571–595 (1984)
DeCamp, C.E.: The epiphyseal plate: physiology, anatomy, and trauma. Compendium (Yardley, PA) 31(8), 1–11 (2009)
Guilak, F., Mow, V.C.: The mechanical environment of the chondrocyte: a biphasic finite element model of cell–matrix interactions in articular cartilage. J. Biomech. 33(12), 1663–1673 (2000)
Guilak, F.: Compression-induced changes in the shape and volume of the chondrocyte nucleus. J. Biomech. 28(12), 1529–1541 (1995)
Guilak, F., Ratcliffe, A., Mow, V.C.: Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J. Orthop. Res. 13(3), 410–421 (1995)
Amini, S., Veilleux, D., Villemure, I.: Three-dimensional in situ zonal morphology of viable growth plate chondrocytes: a confocal microscopy study. J. Orthop. Res. 29(5), 710–717 (2011)
Amini, S., Veilleux, D., Villemure, I.: Tissue and cellular morphological changes in growth plate explants under compression. J. Biomech. 43(13), 2582–2588 (2010)
Radhakrishnan, P., Lewis, N.T., Mao, J.J.: Zone-specific micromechanical properties of the extracellular matrices of growth plate cartilage. Ann. Biomed. Eng. 32(2), 284–291 (2004)
Campbell, S.E., Ferguson, V.L., Hurley, D.C.: Nanomechanical mapping of the osteochondral interface with contact resonance force microscopy and nanoindentation. Acta Biomater. 8(12), 4389–4396 (2012)
Alexopoulos, L.G., Setton, L.A., Guilak, F.: The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 1(3), 317–325 (2005)
Zhang, Z.: Chondrons and the pericellular matrix of chondrocytes. Tissue Eng. Part B: Rev. 21(3), 267–277 (2014)
Alini, M., Matsui, Y., Dodge, G.R., Poole, A.R.: The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif. Tissue Int. 50(4), 327–335 (1992)
Alexopoulos, L.G., Haider, M.A., Vail, T.P., Guilak, F.: Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 125(3), 323–333 (2003)
Poole, C.A., Glant, T.T., Schofield, J.R.: Chondrons from articular cartilage (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J. Histochem. Cytochem. 39(9), 1175–1187 (1991)
Ferguson, V.L., Bushby, A.J., Boyde, A.: Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J. Anat. 203(2), 191–202 (2003)
Allen, D.M., Mao, J.J.: Heterogeneous nanostructural and nanoelastic properties of pericellular and interterritorial matrices of chondrocytes by atomic force microscopy. J. Struct. Biol. 145(3), 196–204 (2004)
Guilak, F., Alexopoulos, L.G., Haider, M.A., Ting-Beall, H.P., Setton, L.A.: Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann. Biomed. Eng. 33(10), 1312–1318 (2005)
Wilusz, R., Sanchez-Adams, E.J., Guilak, F.: The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 39, 25–32 (2014)
McLeod, M.A., Wilusz, R.E., Guilak, F.: Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J. Biomech. 46(3), 586–592 (2013)
Sanchez-Adams, J., Leddy, H.A., McNulty, A.L., O’Conor, C.J., Guilak, F.: The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 16(10), 451–460 (2014)
Alexopoulos, L.G., Williams, G.M., Upton, M.L., Setton, L.A., Guilak, F.: Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J. Biomech. 38(3), 509–517 (2005)
Sergerie, K., Lacoursière, M.O., Lévesque, M., Villemure, I.: Mechanical properties of the porcine growth plate and its three zones from unconfined compression tests. J. Biomech. 42(4), 510–516 (2009)
Piszczatowski, S.: Geometrical aspects of growth plate modelling using Carter’s and Stokes’s approaches. Acta Bioeng. Biomech. 14(1), 93–106 (2012)
Maggiano, C.: Confocal laser scanning microscopy as a tool for the investigation of tetracycline fluorescence in archaeological human bone. Master thesis, University of Florida (2005)
Korhonen, R.K., Julkunen, P., Wilson, W., Herzog, W.: Importance of collagen orientation and depth-dependent fixed charge densities of cartilage on mechanical behavior of chondrocytes. J. Biomech. Eng. 130(2), 1–11 (2008)
Gao, J., Roan, E., Williams, J.L.: Regional variations in growth plate chondrocyte deformation as predicted by three-dimensional multi-scale simulations. PLoS One 10(4), 1–18 (2015)
Vendra, B.B., Roan, E., Williams, J.L.: Chondron curvature mapping in growth plate cartilage under compressive loading. J. Mech. Behav. Biomed. Mater. 84, 168–177 (2018)
Mente, P.L., Lewis, J.L.: Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 12(5), 637–647 (1994)
Carter, D.R., Mikić, B., Padian, K.: Epigenetic mechanical factors in the evolution of long bone epiphyses. Zool. J. Linn. Soc. 123(2), 163–178 (1998)
Wei, H.W., Sun, S.S., Jao, S.E., Yeh, C., Cheng, C.: The influence of mechanical properties of subchondral plate, femoral head and neck on dynamic stress distribution of the articular cartilage. Med. Eng. Phys. 27(4), 295–304 (2005)
Leddy, H.A., Christensen, S.E., Guilak, F.: Microscale diffusion properties of the cartilage pericellular matrix measured using 3D scanning microphotolysis. J. Biomech. Eng. 130(6), 1–20 (2008)
Guilak, F., Jones, W.R., Ting-Beall, H.P., Lee, G.M.: The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthritis Cartilage 7(1), 59–70 (1999)
Shao, Y.Y., Wang, L., Welter, J.F., Ballock, R.T.: Primary cilia modulate IHH signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50(1), 79–84 (2012)
Seeger-Nukpezah, T., Golemis, E.A.: The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 24(5), 652–661 (2012)
Narváez-Tovar, C.A., Garzón-Alvarado, D.A.: Computational modeling of the mechanical modulation of the growth plate by sustained loading. Theor. Biol. Med. Model. 9(1), 9–41 (2012)
Gao, J., Williams, J.L., Roan, E.: On the state of stress in the growth plate under physiologic compressive loading. Open J. Biophys. 4(1), 13–21 (2014)
Farzaneh, S., Paseta, O., Gómez-Benito, M.J.: Multi-scale finite element model of growth plate damage during the development of slipped capital femoral epiphysis. Biomech. Model. Mechanobiol. 14(2), 371–385 (2015)
Gao, J., Williams, J.L., Roan, E.: Multiscale modeling of growth plate cartilage mechanobiology. Biomech. Model. Mechanobiol. 16(2), 667–679 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Kazemi, M., Williams, J.L. (2020). Chondrocyte and Pericellular Matrix Deformation and Strain in the Growth Plate Cartilage Reserve Zone Under Compressive Loading. In: Ateshian, G., Myers, K., Tavares, J. (eds) Computer Methods, Imaging and Visualization in Biomechanics and Biomedical Engineering. CMBBE 2019. Lecture Notes in Computational Vision and Biomechanics, vol 36. Springer, Cham. https://doi.org/10.1007/978-3-030-43195-2_43
Download citation
DOI: https://doi.org/10.1007/978-3-030-43195-2_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43194-5
Online ISBN: 978-3-030-43195-2
eBook Packages: EngineeringEngineering (R0)