Abstract
The six constituent parts of a full fetal postmortem examination are as follows:
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The six constituent parts of a full fetal postmortem examination are as follows:
-
1.
Photographs
-
2.
Fetal biometry
-
3.
Fetal radiology
-
4.
External clinical examination and phenotype analysis
-
5.
Internal examination (gross/histology)
-
6.
Fetal neuropathology
The first three procedures might be delegated, in their pre-analytic stage, to trained fetopathology technicians.
Only the principal procedures and their roles in performing fetal pathologic examination are described here: covering all aspects and in depth is beyond the scope of this manual.
Part 1: Photographs
Most fetal postmortems are performed in the fresh (unfixed) state following the fetal body being sent to the mortuary. It is important to systematically document findings through a series of external photographs, as well as of all abnormal findings during the autopsy procedure. A specialist macroscopy equipped camera is highly recommended. The camera distance from the stand should be constant allowing precise measurements of various anthropometric/cephalometric distances. A number of computer approaches are available to automatically archive the images in patient’s (preferentially the mother’s) records.
Pre-analytic, external standard photographs, performed in the laboratory by skilled technical staff, include:
-
1.
One photo for parents/family (dressed as the fetus arrives, eventually with belongings, or covered properly for very macerated fetuses), with wristband identification
-
2.
Whole body : anterior (front) view
-
3.
Whole body: back view
-
4.
Whole body : side views
-
5.
Head : frontal view
-
6.
Head: right and left profiles
NB: Particular care should be taken to assuring correct profiles: one should follow the profile line entirely including fronto-nasal angle, columella, subnasion (philtrum), and menton (gnathion). In addition, the helix frequently covers the external meatus (positional effect), and thus auricle should be replaced correctly before taking pictures.
-
7.
Hands and feet : close-up views
This eight-photo series may be expanded additionally for any unusual findings. In very small fetuses, with difficult to evaluate external genitalia, we suggest recording another specific photo of both external genitalia and perineum.
In some instances, i.e., special family request, the photo of the whole fetal body might be taken after an autopsy to clearly document body integrity.
Since parents may contact the hospital and request the photos of their baby several years after an examination, systematic archiving of the photos for families in patients’ (mother’s) records facilitates compliance with this request, and helps to improve the patients’ relationship with medical providers.
Part 2: Fetal Biometry
External body measurements including body weight and length, vertex-coccyx distance, head circumference, and foot length before 29 weeks are biometric parameters for estimating fetal age. In our facility, they are systematically measured before an autopsy by trained and dedicated technicians.
It is worthwhile mentioning that while both imperial and metric systems are used for measurements, the metric system is preferred to standardize clinical fetopathologic reports worldwide.
Once fetal biometric measures are recorded, they should be compared to age-matched tables (see Part II). For non-macerated fetuses, these should be in agreement with expected fetal gestational age. Macerated fetuses, however, show various degrees of shift from the mean, which should be considered in fetal radiology and biometry interpretation. Only assembly of all available predictive data: external biometry, radiology, and histology (skin, kidney, brain) offers an accurate estimate of in utero fetal retention and consequently fetal age (Fig. 1).
Part 3: Fetal Radiology
X-Ray
Fetal radiographs using plain X-ray are an integral part of the standardized fetal postmortem protocol. They are systematically performed and analyzed before external and internal examinations at any fetal age. It is highly advisable that each feto-placental pathology unit has a Faxitron and an image acquisition station, enabling each institution to maintain its own digitalized archives. In addition, there is a clear advantage to performing fetal radiographs by experienced, specialized staff. Three standard views for each fetus include: frontal whole body, limbs (in the same fetal position), and a whole body profile. Exposure time and power in kilovolts are defined for each gestational age from 14 to 41 weeks (Fig. 2).
Each fetopathologic team should determine their protocol for the number of systematic radiographs such as whole body for in utero fetal demises (IUFDs) or miscarriages (SAs), and additional imaging for complex, TOP cases. If a Faxitron is available, performing any additional radiographs is feasible if indicated.
X-rays may also be used after injection studies with contrast in cases of suspected obstructing malformations as in urethral or tracheo-laryngeal stenosis versus atresias. Fine micro-probes frequently create iatrogenic perforations in small fetuses, thus careful intravesical/ intratracheal injections would allow assessment of luminal continuity before any further dissection.
In addition to femoral length, each developing fetus has its own bone maturation criteria. This information may be valuable for determining the precise fetal age (Fig. 3). Only key features for each gestational age are mentioned here. More detailed descriptions are available in the existing literature [1].
At 16 weeks, the vertebral bodies are visible from the C3-S3 level, and the iliac bones are square (Fig. 3a).
At 17 weeks, ischial bones become visible, superior ulnar metaphyses are convex, and second phalanges of fingers are square, except for the fifth fingers, where they are still round.
At 23 weeks, there is complete ossification of vertebral bodies to S4, ischio are vertical, and pubic bones become visible. The calcaneum is present.
At 27 weeks, sacrum is entirely ossified (S5), ischial bones are concave, and pubic bones are well developed. In addition to calcaneus, astragalus is present as well.
At 32 weeks, there are sharp margins of pubic bones, calcaneus and astragalus are well developed.
At 36 weeks, the distal femoral ossification epiphysis (Beclard) is always visible and proximal tibial ossification epiphysis (Todt) might be apparent as well. The sternum is visible, and the coccyx is entirely ossified.
These criteria allow an accurate evaluation of bone maturation and age. Advanced bone maturation may be observed in aneuploïdies.
For an interpretation of various skeletal anomalies, as well as their molecular backgrounds readers are advised to consult the published textbooks in which fetal skeletal anomalies are covered extensively [2]. Expert knowledge in fetal radiology is important to adequately direct fetal autopsies. In chondrodysplasia settings, identifying the disease already by pre-autopsy X-ray may, despite full postmortem consent, reasonably simplify and orient an autopsy to molecular biopsies and bone histologies.
MRI
Virtual fetal autopsy by magnetic resonance imaging (MRI) represents an option if the parents decline invasive fetal autopsy and for specific indications, which may be accompanied by tissue biopsies as appropriate.
Postmortem cross-sectional imaging is becoming established as a useful ancillary method either as a substitute for fetal dissection in cases where invasive autopsy is refused or as a part of the overall postmortem examination [3].
Part 4: External Examination/Fetal Phenotype
Phenotype, according to its current definition, represents an ensemble of physical and behavioral traits of the organism, resulting from the interaction of its genotype with the environment.
While the human phenome project [4] is still in the launch phase, current advances in DNA sequencing technologies and computational methods allow collecting of human phenotype data. It is therefore essential to employ precise and standard semantic terminology. The Human Phenotype Ontology has already more than 10,000 terms describing phenotypic anomalies in humans [5].
Despite quicker diagnoses via exome and genome sequencing in the future, there will remain challenges to understanding diseases through precise phenotyping [6]. Precise phenotypic descriptions allow: (1) stratifying patients into subpopulations, (2) establishing genotype/phenotype correlations, and (3) better understanding of the natural history of disease.
External fetal examinations should be assessed in the same manner as any other clinical genetic examination, however with identification of distinctive physiological signs specific for each age of a developing fetus. It is essential to be guided by these principles in order to avoid unnecessary and confusing false-positive results.
While different parts of the external examination may be performed simultaneously, we present them separately for didactic purposes.
Evaluation of the Degree of Maceration
Several studies on stillborn infants have determined how accurately the time of fetal death can be predicted from the extent of external maceration. Based on retrospective autopsy photographs of 86 stillborns with known time of death, eight gross features correlated reasonably well with specific death-to-delivery times in terms of sensitivity, specificity, and positive predictive values [7] including in increasing time interval order: desquamated skin measuring 1 cm or more in diameter and/or cord discoloration to brown/red (6 h); desquamation involving the skin of multiple body zones: face, back, or abdomen(12 h); desquamation of 5% or more of the body surface (18 h); brown or tan discoloration of the skin, usually involving the abdomen (24 h), and any mummification (2 weeks).
Others have suggested five stages of maceration [8, 9]:
-
1.
None = intrapartum death
-
2.
Slight—skin slippage, rare bullae, little (e.g., scrotum only or single spots of skin loss elsewhere) or no denudation = less than 12 h between death and delivery
-
3.
Mild—focal denudation of multiple regions without other changes = about 12–24 h between death and delivery
-
4.
Moderate—generalized skin maceration/denudation but without significant compressive changes = one to a few days between death and delivery
-
5.
Advanced—compression and/or mummification and/or internal liquefaction = more than a few days between death and delivery
For practical reasons, we think that a simple staging system such as none, slight, moderate, and advanced maceration is generally sufficient. Along with other parameters of fetal maturation (radiologic and histologic), these may allow fine prediction of in utero retention time.
Overall Habitus
General fetal habitus should be carefully observed for:
-
Color and appearance of fetal skin
-
Body proportions
-
In some cases, in addition to dwarfism, fetal growth might be disproportionate, i.e., for upper limbs or even hands. Since these measurements are not routinely included in pre-analytic fetal biometry, it is important not to overlook them at this stage of examination.
-
Fingers and toes for their position, length, and any anomaly.
-
Signs of in utero hypomobility expressed by distal or generalized contractures.
Importantly, any finding should always be correlated with fetal prenatal data. A lack of amniotic fluid by prematurely ruptured membranes can cause the anhydramnios (Potter) sequence which should not be mistaken or described as arthrogryposis, the latter term being reserved for intrinsic fetal neuro-muscular disorders. In the context of ruptured membranes, the terms articular deformities and oligo/anhydramnios sequence would be more appropriate.
Any prenatally diagnosed or newly identified observed anomaly should be systematically and precisely measured and documented photographically.
Clinical Dysmorphologic Evaluation
Fetal dysmorphological study is an essential, albeit complex, part of every fetopathologic examination, fetal morphology evolving considerably with the age. Systematic examination of global fetal impression, i.e., gestalt, is needed, combining standardized terminology (available at http://elementsofmorphology.nih.gov/) and qualitative/descriptive terms. However, inter-observer impressions on fetal dysmorphology varying considerably, pathologic terms should be introduced only after comparison of each measurement with an age-related norm. Additionally, facial measurements should be evaluated in regard to head circumference, since for example normal outer canthal distance for age, in microcephalic fetuses means relative hypertelorism.
The hallmarks for evaluation of fetal cranio-facial dysmorphy , measured by calipers, are presented together with the sources for normal values upon gestational age:
-
1.
Occipito-frontal diameter (Normal values 12–41w from Hansmann 1985) [10]
-
2.
Inner canthal distance (Normal values 27–41w from Merlob et al. 1984) [11]
-
3.
Outer canthal distance (Normal values 27–41w from Merlob et al. 1984) [11]
-
4.
Palpebral fissure length (Normal values 27–41w from Mehes et al. 1974) [12]
-
5.
Philtrum length (Normal values 27–41w from Merlob et al. 1984) [11]
-
6.
Mouth width/Intercommissural distance (Normal values from Merlob et al. 1984) [11]
-
7.
Ear length (Normal values 27–41w from Merlob et al. 1984) [11]
Textbooks are available [13] for further anthropometric measurements.
The goal of dysmorphology evaluation in fetuses is to recognize undiagnosed syndromes and to consequently direct the rest of the fetal examination. Although this is certainly feasible in older fetuses, the task remains difficult in first-trimester terminations, expressing milder facial traits. Moreover, particular attention should be paid to parents’ ethnic backgrounds and general parents’ phenotype. In that context, one should always privilege recording systematically facial measurements and describing, rather than employing straightforward dysmorphology terminology without anthropometric measurements (Fig. 4).
Fetal ears: (a) Microtia in Treacher-Collins syndrome. (b) Normal fetal ear at 30w. (c) Wiedemann–Beckwith syndrome due to a methylation loss at ICR2 at 28w. (d) Trisomy 21 with horizontal crux helicis and rounded ears. (e) CHARGE syndrome due to CHD7 variant. (f) Fryns syndrome. (g) Trisomy 18. (h) Di George syndrome due to 22q11 microdeletion. (i) Cornelia de Lange syndrome showing characteristic profile with hypoplastic ear, prominent philtrum, and unusually long eye lashes
Part 5: Dissection/Internal Examination
Generally, dissection begins by the sharp median inverted Y-shaped incision from the level of the thyroid or sternal notch, bypassing the umbilicus, towards both inguinal regions. This is followed by separating the thoraco-abdominal skin on each side. The pelvic triangle is dissected with blunt scissors to clearly visualize the urinary bladder and umbilical arteries. The thoracic cavity is opened by vertically dissecting rib cartilages from diaphragmatic attachments on both sides upwards, as laterally as possible, to the clavicles, removing median sterno-costal fragment. An exception to this rule represents congenital diaphragmatic hernia/eventrations, where the 12th rib with diaphragmatic attachment should be kept intact, to allow better clinico-radiologic correlations, with precise descriptions, measurements, and classification of an untouched diaphragmatic anomaly (Fig. 5).
In very small fetuses (13–16w) an inverted M-shaped incision (Fig. 6) may be preferred, meaning that secondary to the initial abdominal inverted V incision from the umbilicus, there are common, vertical costo-myo-cutaneous incisions on each side from the inguinal regions up to the clavicles, allowing easier opening by the elevation of a unique flap. In addition, reconstruction after an examination is simpler by turning the whole flap down.
In situ examinations and photographs for any observed anomaly are indicated. The thymus is removed first, and then weighed. Thymus and lung microbiopsies are routinely taken for tissue samples/DNA banking.
The pericardium should be completely removed on both sides to allow clear visualization of pulmonary vessels. Great vessels should be separated from each other from their base, and efferent aortic arteries dissected. Aortic arch, ductus arteriosus, and descending aorta are checked for diameters and position. Ductus arteriosus diameter should be about the same as the preductal part of aortic arch throughout the pregnancy. This notion is important in assessing preductal aortic arch hypoplasias evolving towards coarctation. Before opening, the heart is inspected in situ. In addition to its vasculature, the position of anterior descending coronary arteries indicates ventricular size, and its shift to the right/left or its unusually short path signifies a hypoplastic or univentricular heart.
Opening of the heart follows the blood flow, and should be performed by sequential segmental analysis [14, 15] (Fig. 7).
Fetal heart dissection: Various views during fetal heart examination. View in situ (a) confirming normal (“N”) position of great vessels, and their symmetrical diameters. (b) Moderately rotated to the left, this heart view shows systemic venous return, and the right atrium. (c) Right heart opening along the line depicted by the probe (b). Right auricle, tricuspid valve, and right ventricle are exposed. (d) The probe is dressed to show the next-step dissection to right ventricular outflow. (e) Right ventricular outflow with pulmonary infundibulum, and pulmonary trunk with two ostia for pulmonary artery branches. (f) The probe outlines the left ventricle cut lane from the pulmonary veins (left ones visible), through left auricle, mitral valve to the left ventricle. (g) Left ventricle view. (h) Probe is inserted in ascending aorta showing the next-step cut. (i) Left ventricular outflow showing mitro-aortic continuity and aortic valves with coronary ostia
We recommend dissecting all heart malformations fresh and directly on the photographic stand in order to record all aspects of heart opening from the in situ view to its left ventricular outflow. This might also help later clinico-pathologic correlations with ultrasound expertise on CHDs. Micro instruments, and a magnifying glass may be necessary for the dissection of first-trimester hearts (Fig. 8).
In malformed hearts, in addition to the diameter of the great vessels and their efferent vessels, various other measurements along the dissection are highly recommended:
-
Tricuspid/mitral valve biometry
-
Distance between tricuspid/mitral valve and apex
-
Free wall thicknesses of left/right ventricles
If, for any reason, opening of the heart is postponed, the heart-lung block may be pre-fixed, allowing a possibility of a careful examination of pulmonary vessels, in particular, pulmonary venous return.
Lungs are examined in situ for segmentation and weighed. Samples may be taken for frozen tissue banking.
All abdominal viscera are primarily checked for situs prior to any dissection. After liver, spleen and pancreas examination are completed, the intestines are removed progressively by cutting the mesentery from the duodenum down.
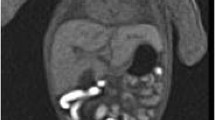
Before removing kidneys and adrenals, peritoneal dissection is necessary in order to allow better observation of renal vasculature and any other underlying anomaly. In autopsies limited to kidneys, in the context of anhydramnios, lumbar incisions allow extraction of the kidneys, and histology with biopsies for DNA studies (Fig. 9). One example of an oriented approach in fetal pathology represents small kidneys. If the renal hypoplasia with or without cystic renal disease is observed grossly, eyes should be dissected, to look for signs of retinal coloboma, identifying the PAX2 gene in renal coloboma syndrome . Eye dissection, when indicated, is performed by sphenoidal windows from the cranial base.
Kidney-limited autopsy in a 25-week fetus terminated for anhydramnios: (a) showing the sole incision line (light blue arrow). (b) Moderately enlarged kidneys on gross/sagittal section. (c) HES (×20) histologic “glomerular crowding” in the cortex. (d) CD10 (×20) An absence of proximal tubules is evident compared to control (e), signing the diagnosis of tubular dysgenesis (homozygous ACE variants identified later)
The uterus and ovaries should be evaluated externally and included for histologic study. Testes after 30 weeks are examined from the inguinal canals, which are at first dilated with blunt scissors prior to removal with forceps.
If any anomaly is present, the thyroid gland should be dissected and included for histology preferentially by the transverse laryngeal cut at the same level. Esophagus and trachea are separated dorsally downward and a probe is inserted to assess esophageal permeability.
Part 6: Neuropathology (Triage and Samplings)
Neuropathologic evaluations should be performed in the following cases:
-
TOPs with any brain malformation seen on prenatal ultrasound scans
-
TOPs without prenatal brain malformation, but in the context of syndromes without evident diagnosis
-
IUFDs in the context of isolated or complex brain anomaly (expectative prenatal approach or parental TOP refusal)
-
Term IUFDs
Pre-fixation in situ is performed systematically by injecting the zinc-formalin (4% formaldehyde) through anterior and posterior fontanels the day before autopsy. It allows better preservation and easier extraction even in cases of severe hydrocephalus. This procedure and other pre-analytic procedures may be performed by the technical staff.
To preserve head appearance as much as possible, the scalp incision is made dorsally from one ear to another. The postero-lateral downward cuts from each end of the previous incisions to the base of the neck allow better assessment of the brainstem and cerebellum. The skin flaps are then reflected frontally and posteriorly to expose cranial bones. Holding the fetal neck with the left hand, with fetal face forward, two incisions are first made on lateral sides of anterior fontanelle, and blunt scissors are carefully introduced, tangentially to the bone plan. The fetal head is rotated 180° and parasagittal incisions are made posteriorly on each side to maintain the falx cerebri. They are continued laterally to the parieto-occipital juncture. At this point blunt scissors are introduced in posterior fossa to cut the cerebellar falx. The fetal head is turned again 180° so that the operator faces the fetal back, and parasagittal incisions are prolonged frontally and fronto-parietally. Sectioning through frontal bone creates bone windows which might easily be removed. At that stage, the fetal head is reflected mildly to the back with a recipient dish held posteriorly. From the olfactory bulbs on, the cranial nerves are resected at the skull base, and the brain is progressively removed from the fetal skull.
If required, the spinal cord is preferentially removed by an anterior approach. In the lumbar region, the vertebral pedicles are divided with scalpel. Blunt scissors are introduced to the medullary canal and its opening upwards on each side exposes the spinal cord. Its removal is from the filum terminale by dissection of posterior nerve roots.
The brain and the spinal cord are fixed in microzinc-zinc formalin solution (formaldehyde 4%). Generally, extended fixation is necessary before a neuropathologic evaluation is performed (see Part III).
Part 7: Banking
For years it was stated that paraffin blocks are sufficient for DNA studies, until it became clear that formalin degrades DNA, reducing quality for most current NGS techniques.
It is consequently of the highest importance to systematically conserve frozen tissue for any ancillary molecular tests. In regard to resistance of autolysis, it is preferable to conserve lymphocyte-rich tissues such as thymus and spleen for DNA studies. Furthermore, fresh tissue biopsies (liver, lung, skin) are also used for various biochemical screens such as for sterol, lysosomal disorders, etc.
In cases of any suspicion of infectious fetopathy, polymerase chain reaction (PCR) probes (simplex or multiplex) may be ordered on frozen fetal tissue.
Part 8: Histology
Unlike gross-fetal organ examination, which closely resembles the postnatal state, varying predominantly by organ size, fetal histology is highly gestational age-dependent with major differences in histogenesis and histology from embryo to birth.
Very specific histological changes appear with each step of development, allowing the determination of developmental stage by studying fetal lungs, kidneys, and skin.
Briefly, fetal lung maturation is divided into four principal stages: (1) pseudoglandular (approx. 9–16 weeks), (2) canalicular (17–26 weeks), (3) saccular (27–32 weeks), and (4) alveolar (after 32 weeks) [16]. From the pseudoglandular stage onwards, successive subdivisions of airway structures occur, resulting in a complete conducting airway system during early childhood. Radial alveolar count (CRA) based on the terminal respiratory unit, i.e., air spaces distal to a terminal respiratory bronchiole, varies from 2.2 at 24–27 weeks to 4.4 at 40 weeks [17]. Although generally used to evaluate lung maturation in diaphragmatic hernia, other causes of lung hypoplasia, or failure to thrive, CRA may also provide information regarding developmental stage.
Even in macerated fetuses, assessment of age by nephrogenesis remains feasible by cortical glomerular generations’ ray count on sagittal renal section, i.e., medullary ray glomerular counting [18]. At the age of 23 weeks, three layers of mature glomeruli in the cortical area are formed. Their number increases progressively by approximately one layer each additional week (Fig. 10).
Additionally, in skin biopsies at the age of 10 weeks no appendages are observed. At about 14 weeks budding of the basal cells appears. Sebaceous glands and hair shafts become visible at 16 weeks. Elongation of eccrine ducts is apparent at 23/24 weeks. Its coiling is seen only after 30 weeks [19].
The retention period in macerated IUFDs is estimated by an extension of the “loss of nuclear basophilia” (LONB) in various organs [20, 21, 22] (Table 1).
On a systematic basis, the following viscera are traditionally examined: lungs, thymus, liver, spleen, pancreas, kidneys, adrenals, and gonads. In fresh stillbirths (<48 h), heart histology (apex) is useful for evaluation of loss of nuclear basophilia (LONB) .
Muscle histology is evaluated from several skeletal muscles, i.e., quadriceps/biceps/trapezius. It is important to take transverse sections to better evaluate fiber size, and to avoid false-positive results.
When indicated, bone histology is most often performed on the epiphyseal growth zone of the long bones, usually femoral or humeral, after decalcification. It is important to closely monitor decalcification procedure, which takes longer in older fetuses, in order to determine the optimal bone softness (for example via a needle test) for longitudinal cutting and embedding. In addition to classical H&E, Alcian Blue staining is frequently used.
Bone sutures or ribs may be sampled when indicated.
Fetal histogenesis is exhaustively described in a number of textbooks [23].
Part 9: Autopsy Report
One should always bear in mind that the autopsy report belongs to the family and will be read by them. It should be concise and written in clear language avoiding unnecessary jargon or terms. Terminology may be provided by the major online databases, allowing international standardization of autopsy reports.
Correlated by the above guidelines, only major or significant pathologic findings should be reported.
If, however, there are prenatally suspected diverse anomalies, absent on fetal pathology exam, they should be described as negative findings to clarify the clinicians’ dilemma.
Concerning the timing of postmortem reports, an initial, provisional report might be issued shortly following the autopsy if major macroscopic abnormalities are documented. In isolated congenital heart malformations (CHM), congenital diaphragmatic hernia (CDH), or the most frequent chondrodysplasias, the X-rays or gross examination findings may be sufficient for the essential elements of the diagnosis. These reports should be provided to the parents as soon as possible. In other cases, the results of all ancillary investigations should be incorporated into an overall final postmortem examination report, with interpretation and comments.
References
Eurin D. Atlas radiographique du squelette foetal normal. Paris: Lavoisier MSP; 1993.
Hall CM, Offiah AC, Forzano F, Lituania M, Fink M, Krakow D. Fetal and perinatal skeletal dysplasias: an atlas of multimodality imaging. 1st ed. London: CRC Press Radcliffe Publishing; 2012.
Dawood Y, Strijkers GJ, Limpens J, Oostra RJ. Novel imagingtechniques to study postmortem human fetal anatomy: a systematic review on microfocus-CT and ultra-high-field MRI. Eur Radiol. 2019;13:1–13.
Freimer N, Sabatti C. The human phenome project. Nat Genet. 2003;34(1):15–21.
Robinson PN, Kôhler S, Bauer S, Seelow D, Horn D, Mundlos S. The human phenotype ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83(5):610–5.
Robinson PN. Deep phenotyping for precise medicine. Hum Mutat. 2012;33:776–80.
Genest DR, Singer DB. Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol. 1992;80(4):593–600.
Pauli RM, Reiser CA, Lebovitz RM, Kirkpatrick SJ. Wisconsin Stillbirth Service Program: I. Establishment and assessment of a community-based program for etiologic investigation of intrauterine deaths. Am J Med Genet. 1994;50(2):116–34.
Pauli RM, Reiser CA. Wisconsin Stillbirth Service Program: II. Analysis of diagnoses and diagnostic categories in the first 1,000 referrals. Am J Med Genet. 1994;50(2):135–53.
Hansmann M. Ultrasonic diagnosis in obstetrics and gynecology. Berlin: Springer; 1985.
Merlob P, Sivan Y, Reisner SH. Anthropometric measurements of the newborn infant 27 to 41 gestational weeks. Birth Defects. 1984;20:7.
Mehes K. Inner canthal and intermammary indices in the newborn infant. J Pediatr. 1974;85:90.
Gripp KW, Slavotinek AM, Hall JG, Allanson JE. Handbook of physical measurements. Oxford: Oxford University Press; 2013.
Anderson RH, Becker AE, Freedom RM, et al. Sequential segmental analysis of congenital heart disease. Pediatr Cardiol. 1984;5:281–7.
Anderson RH, Becker AE. The heart: structure in health and disease. London: Gower Medical Publication; 1992.
Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis. 1984;129:607.
Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35:544–7.
Hinchliffe SA, Sargent PH, Chan YF, van Velzen D, Howard CV, Hutton JL, Rushton DI. “Medullary ray glomerular counting” as a method of assessment of human nephrogenesis. Pathol Res Pract. 1992;188(6):775–82.
Ersch J, Stallmach T. Assessing gestational age from histology of fetal skin: an autopsy study of 379 fetuses. Obstet Gynecol. 1999;94(5 Pt 1):753–7.
Genest DR. Estimating the time of death in stillborn fetuses: II. Histologic evaluation of the placenta; a study of 71 stillborns. Obstet Gynecol. 1992;80(4):585–92.
Genest DR, Williams MA, Greene MF. Estimating the time of death in stillborn fetuses: I Histologic evaluation of fetal organs; an autopsy study of 150 stillborns. Obstet Gynecol. 1992;80(4):575–84.
Jacques SM, Qureshi F, Johnson A, Alkatib AA, Kmak DC. Estimation of time of fetal death in the second trimester by placental histopathological examination. Pediatr Dev Pathol. 2003;6(3):226–32.
Martinovic J. Urinary system: development and diseases. In: Khong TY, Malcomson RDG, editors. Keeling’s fetal and neonatal pathology. Berlin: Springer; 2015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martinovic, J., Sebire, N.J. (2021). Modern Fetal Autopsy Protocol. In: Martinovic, J. (eds) Practical Manual of Fetal Pathology. Springer, Cham. https://doi.org/10.1007/978-3-030-42492-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-42492-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42491-6
Online ISBN: 978-3-030-42492-3
eBook Packages: MedicineMedicine (R0)